Abstract
Background:
Prior reports on metabolic derangements observed in distance running frequently have small sample sizes, lack prerace laboratory measures, and report sodium as the sole measure.
Hypothesis:
Metabolic abnormalities—hyponatremia, hypokalemia, renal dysfunction, hemoconcentration—are frequent after completing a full or half marathon. Clinically significant changes occur in these laboratory values after race completion.
Study Design:
Observational, cross-sectional study.
Methods:
Consenting marathon and half marathon racers completed a survey as well as finger stick blood sampling on race day of the National Marathon to Fight Breast Cancer (Jacksonville, Florida, February 2008). Parallel blood measures were obtained before and after race completion (prerace, n = 161; postrace, n = 195).
Results:
The prevalence of prerace and postrace hyponatremia was 8 of 161 (5.0%) and 16 of 195 (8.2%), respectively. Hypokalemia was not present prerace but was present in 1 runner postrace (1 of 195). Renal dysfunction occurred prerace in 14 of 161 (8.7%) and postrace in 83 of 195 (42.6%). Among those with postrace renal dysfunction, 45.8% (38 of 83) were classified as moderate or severe. Hemoconcentration was present in 2 of 161 (1.2%) prerace and 6 of 195 (3.1%) postrace. The mean changes in laboratory values were (postrace minus prerace): sodium, 1.6 mmol/L; potassium, −0.2 mmol/L; blood urea nitrogen, 2.8 mg/dL; creatinine, 0.2 mg/dL; and hemoglobin, 0.3 g/dL for 149 pairs (except blood urea nitrogen, n = 147 pairs). Changes were significant for all comparisons (P < 0.01) except potassium (P = 0.08) and hemoglobin (P = 0.01).
Conclusions:
Metabolic abnormalities are common among endurance racers, and they may be present prerace, including hyponatremia. The clinical significance of these findings is unknown.
Clinical relevance:
It is unclear which runners are at risk for developing clinically important metabolic derangements. Participating in prolonged endurance exercise appears to be safe in the majority of racers.
Keywords: marathon, hyponatremia, renal dysfunction, running, endurance sports
Since its inception in 490 bc,48 the marathon has been an amazing human accomplishment. Recently, participation in marathons and half marathons has gained increasing popularity for a variety of reasons, including the presumed health benefits and the importance of exercise in daily routines.38 Along with this increasing involvement of the general population in marathons, reports have surfaced regarding the injuries and illnesses associated with distance running.47
The most frequent medical complications described in distance exercise are related to hyponatremia.42 Excess hydration with water has been linked to hyponatremia,† although it is uncertain whether this is from hydration before the race or during the race. With progression of exercise-associated hyponatremia, adverse events can occur, including altered sensorium, seizures, pulmonary edema, and even death.4,23,44,53 However, it is unclear from these studies whether and to what degree hyponatremia is present prerace.
The role of hydration in physical activity has been investigated in a variety of studies.‡ The American College of Sports Medicine supports the concept that the salt content of the hydration beverage is an important consideration during physical activity.8 Unfortunately, blanket guidelines on the type or amount of fluid to prevent hyponatremia or renal dysfunction are not possible, given the wide variability in individual sweat rates and renal water excretion, in addition to the range of environmental conditions possible on race day.20
The limitations of prior studies on endurance activity are threefold: Sample sizes are frequently small§; prerace parameters are not measured‖; and sodium is frequently the only reported electrolyte.2,4,9,14,28,32 Additionally, many earlier studies were based on the performance of elite athletes or individuals presenting for medical attention. The associations developed from these data must be critically evaluated in light of these weaknesses.
By collecting blood samples in the prerace environment, any preexisting hematologic or electrolyte disturbances that may increase susceptibility to metabolic derangements in the postrace setting can be identified. Continuing data collection in the postrace setting with parallel blood measures allows detection of changes. These analyses, as set in a large participant population and in persons not necessarily presenting for acute medical attention, are intended to more accurately describe the metabolic effects of participating in distance running.
Methods
This article is an observational cross-sectional study of consenting runners who participated in the annual Mayo Clinic–affiliated National Marathon to Fight Breast Cancer full and half marathons in February 2008. The Institutional Review Board approved this study. The study protocol included all volunteers without regard to race, ethnicity, or sex. Informed consent and a Health Insurance Portability and Accountability Act waiver were used for those who agreed to participate.
Participants
Registrants for the race were eligible for participation, were informed about this volunteer study via e-mail, and approached during the Health Expo in the 2 days before the race. Racers who consented were identified by a brightly colored sticker on their race bib numbers. Participants were included without regard to the degree of running experience or medical history. Individuals younger than 18 years of age were excluded.
Procedures
Parallel blood measures were obtained prerace (morning) and postrace (immediately after completion). Venous blood was tested via a finger stick and Nova Stat Profile Critical Care Xpress blood analyzers (Nova Biomedical Corporation, Waltham, Massachusetts). Calibration and quality control of the Nova machine were performed automatically and met industry standards. Finger stick was via small sterile lancets similar to those used for testing venous glucose.
Outcome Measures
The main outcome measures were hyponatremia, hypokalemia, renal dysfunction, and hemoconcentration before and after the race. Secondary outcome measures included the mean changes in these metabolic parameters.
Sample Size Considerations
The target enrollment for this study was 250 runners, based on an expected volunteer rate of 5% of the 5000 race participants. The enrollment was limited by logistics of staffing and resources. After allowing for the loss of up to 20% owing to inadequate blood samples or failure to present for analysis (final n = 200), power calculations determined that the prevalence of metabolic abnormalities could be estimated (as a binomial proportion) to within a 3% to 7% margin of error for values between 5% and 95%.
Analysis
To ensure accurate and complete data collection, a random 5% audit was conducted. Staff were blinded to runner-identifying information during data entry by using race/bib numbers as numeric identifiers.
Subsequent analysis was performed by dividing runners into 2 groups by race distance (Figure 1). Approximately equal numbers of pairs were available for paired data set analysis (79 half marathon pairs vs 70 full marathon pairs).
Figure 1.
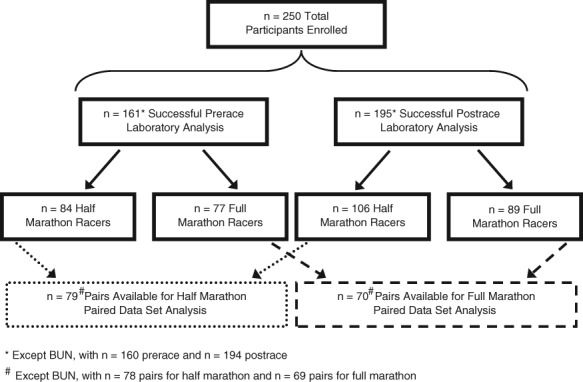
Data flow in the prerace and postrace setting by race distance.
The Wilcoxon signed-rank test for paired data was used to determine if statistically significant changes in these parameters occurred from prerace to postrace. Paired data set analyses therefore allowed for examination of within-subject changes.
For the purposes of analyses, laboratory abnormalities were defined (Table 1) in part on the basis of prior reports3,11,45,52 and laboratory cutoffs. Additionally, Table 2 lists the characteristics of study participants according to race distance.
Table 1.
Laboratory abnormality definitions.
| Hyponatremia, sodium | < 135 mmol/L |
| Moderate | ≤ 130 |
| Severe | ≤ 125 |
| Hypokalemia, potassium | < 3.6 mmol/L |
| Moderate | ≤ 3.0 |
| Severe | ≤ 2.5 |
| Renal dysfunction, BUN / Cra | > 21 / > 1.1 mg/dL |
| Moderate | ≥ 30 / ≥ 1.4 |
| Severe | ≥ 40 / ≥ 2.0 |
| Hemoconcentration, hemoglobin | |
| Females | > 15.5 g/dL |
| Males | > 17.0 |
Blood urea nitrogen/creatinine.
Table 2.
Characteristics of study participants according to race distance.
| Race Distance | ||||
|---|---|---|---|---|
| All Participants | Half | Full | P | |
| Sex, malea | 73/250 (29%) | 33/125 (26%) | 40/125 (32%) | 0.33 |
| Age, yearsb | 46 (20-71) | 45 (24-71) | 46 (20-70) | 0.73 |
| Finish time, minutesb | 240 (90.6-398.7) | 166.1 (90.6-358.6) | 333.7 (194.4-398.7) | < 0.01 |
| Pace, minutes/mileb | 12.7 (6.9-27.4) | 12.7 (6.9-27.4) | 12.7 (7.4-15.2) | 0.47 |
Categorical variable reported as fraction (percentage), with comparison between half and full marathoners performed with the Fisher exact test.
Continuous variable reported as median (range), with comparison between half and full marathoners performed with the Wilcoxon rank-sum test.
Results
Enrollment limit was met early on the second day of the 2-day Health Expo. Although target enrollment was met without difficulty, almost one-fifth of enrolled runners did not present for finger stick sampling: 18% prerace (46 of 250) and 19% postrace (47 of 250). An additional 46 fingerstick samples were lost (39 prerace and 7 postrace samples) during the process of laboratory analysis because of insufficient sample volume. A total of 161 and 195 records remained for prerace and postrace analyses, respectively.
On race day, at 8:00 am just before the start of the race, the temperature was 64°F (17.8°C) with 88% humidity. At 2:00 pm, the maximum temperature of the day was 76°F (24.4°C) with 43% humidity. Our study sample had 29% male participation: 26% in the half marathon group and 32% in the full marathon group.6
In sum, 165 individuals responded to the athletic history survey question regarding number of previous half marathons completed: Values ranged from 1 to 57 prior races, with a mean of 8 and a median of 4. For the number of prior full marathons completed, 133 individuals responded: range, 1 to 187 prior races; mean, 12; median, 4 races. With regard to runners’ nonsteroidal anti-inflammatory drug usage, 101 of 250 (40.4%) reported regular usage, and 42 of 250 (16.8%) reported using an nonsteroidal anti-inflammatory drug on the morning of the race or during the race.
The overall means for paired data (ie, within-subject changes) were similar to all participant means (Table 3): Prerace and postrace values for potassium, creatinine (Cr), and hemoglobin were identical. Mean prerace sodium was similar (138.4 mmol/L for all participants and 138.3 mmol/L for paired runners) and was identical postrace (140.0 mmol/L). Blood urea nitrogen (BUN), however, was slightly higher in paired runners both prerace and postrace (12.9 mg/dL and 15.7 mg/dL, respectively) when compared with the all-participants group prerace and postrace (12.8 mg/dL and 15.4 mg/dL). Similar findings were observed when examined by race distance (Tables 4 and 5).
Table 3.
Laboratory measures: Paired data only.a
| Mean Values | ||
|---|---|---|
| Laboratory Measure | Prerace | Postrace |
| Sodium, mmol/L | 138.3 (3.5) | 140.0 (4.5) |
| Potassium, mmol/L | 5.4 (1.2) | 5.2 (1.1) |
| Blood urea nitrogen, mg/dL | 12.9 (3.5) | 15.7 (4.2) |
| Creatinine, mg/dL | 0.9 (0.2) | 1.1 (0.3) |
| Hemoglobin, g/dL | 13.7 (1.1) | 13.9 (1.2) |
n = 149 pairs, except blood urea nitrogen (n = 147 pairs).
Table 4.
Half marathon: Paired data only.a
| Mean Values | ||
|---|---|---|
| Laboratory Measure | Prerace | Postrace |
| Sodium, mmol/L | 137.9 (4.3) | 140.5 (3.8) |
| Potassium, mmol/L | 5.4 (1.1) | 5.4 (1.2) |
| Blood urea nitrogen, mg/dL | 13.3 (4.1) | 15.2 (4.2) |
| Creatinine, mg/dL | 0.9 (0.3) | 1.1 (0.4) |
| Hemoglobin, g/dL | 13.6 (1.3) | 13.6 (1.2) |
n = 79 pairs, except blood urea nitrogen (n = 78 pairs).
Table 5.
Full marathon: Paired data only.a
| Mean Values | ||
|---|---|---|
| Laboratory Measure | Prerace | Postrace |
| Sodium, mmol/L | 138.8 (2.1) | 139.5 (5.1) |
| Potassium, mmol/L | 5.5 (1.2) | 5.1 (1.0) |
| Blood urea nitrogen, mg/dL | 12.4 (2.7) | 16.2 (4.1) |
| Creatinine, mg/dL | 0.9 (0.2) | 1.1 (0.3) |
| Hemoglobin, g/dL | 13.7 (0.9) | 14.2 (1.2) |
n = 70 pairs, except blood urea nitrogen (n = 69 pairs).
More half marathoners (5 of 84, 6.0%) than full marathoners (3 of 77, 3.9%) were hyponatremic prerace, with 1 half marathoner severely hyponatremic. At the completion of the race, 3 times as many full marathoners were hyponatremic (12 of 89, 13.5%; 4 of 106, 3.8%; respectively). Hypokalemia was essentially nonexistent; only 1 runner postrace was mildly hypokalemic. Mean values of potassium were high (> 5 mmol/L) in all subgroups, more so prerace, presumably as a result of hemolysis during finger stick blood sampling.
Renal dysfunction (BUN > 21 or Cr > 1.1 mg/dL) was present in 8.7% of racers prerace and in 42.6% of racers postrace. A greater number and percentage of half marathoners had preexisting renal dysfunction (9 of 84, 10.7%) than did full marathoners (5 of 77, 6.5%). Postrace renal dysfunction was prevalent for both race distances, with a slightly greater percentage of full marathoners meeting criteria for overall renal dysfunction (43 of 89, 48.3%, with BUN > 21 or Cr > 1.1 mg/dL) and moderate renal dysfunction (21 of 89, 23.6%, with BUN > 30 or Cr > 1.4 mg/dL). Of all participants with postrace renal dysfunction, 45.8% (38 of 83) were classified as moderate or severe.
Hemoconcentration (similar to hyponatremia and renal dysfunction) was more prevalent among half marathoners prerace (2 of 84, 2.4%) than full marathoners (0 of 77, 0.0%). Postrace prevalence was higher in full marathoners than in half marathoners (4 of 89, 4.5%; 2 of 106, 1.9%, respectively).
All laboratory values except potassium increased postrace. For potassium, no mean change was observed in the half marathon group (P = 0.72), whereas negative mean changes were observed in the full marathon (P = 0.04) and all participant groups (P = 0.08), but these differences were not statistically significant.
The mean change in sodium (postrace minus prerace) was statistically significant in the half marathon group and among all participants (P < 0.01 for both) but not in the full marathon group (P = 0.31). The estimated mean change in BUN and Cr was statistically significant for all groups (P < 0.01). Full marathoners had a mean change double that of half marathoners: BUN increased 3.8 mg/dL and 1.9 mg/dL and Cr, 0.2 mg/dL and 0.1 mg/dL, for full and half marathoners, respectively. No mean change was observed for hemoglobin among half marathoners (0.0 g/dL, P = 0.56), whereas a statistically significant mean change was observed among full marathoners (0.6 g/dL, P < 0.01). Among all participants, the mean change in hemoglobin was 0.3 g/dL (P = 0.01).
Analysis of the overall means of laboratory measures of all participants by sex revealed that men had higher levels of Cr and hemoglobin before and after the race (P < 0.01). Postrace men had slightly higher sodium levels as well (P = 0.01). When analysis was limited to paired data only, both women and men had within-subject changes for BUN and Cr that were statistically significant (P < 0.01). Additionally, both women and men had increases in sodium levels (P = 0.01).
When limited to half marathon participants, overall means revealed similar differences by sex. Men had statistically significant higher Cr levels (postrace only) and hemoglobin levels (prerace and postrace) (P < 0.01). Paired data analysis by sex for half marathon participants revealed statistically significant within-subject changes only for women’s sodium and BUN levels (P < 0.01).
Full marathon overall laboratory means by sex revealed similar findings as for all-participant data analysis: Men had higher Cr and hemoglobin levels in both prerace and postrace settings (P < 0.01). Paired data only for full marathoners by sex revealed statistically significant within-subject changes for BUN (women and men, P < 0.01) and Cr (women, P < 0.01; men, P = 0.01).
Prevalence of metabolic abnormalities by sex revealed that postrace, men were more likely to have moderate renal dysfunction, both in the all-participants group and full marathon group (P < 0.01). No other statistically significant differences in rates of metabolic abnormalities by sex were observed.
Discussion
This observational cross-sectional study of volunteer runners examined several laboratory measures that are essential in the normal physiologic regulation of the body.1 Despite a large and growing body of literature on endurance sports, it is unclear what degree of laboratory derangements are well tolerated by runners without untoward effects.9,19 Although metabolic derangments were not uncommon—particularly, renal dysfunction—the clinical significance of these derangments is not known.
The overall means of all laboratory measures were within normal limits by our definitions, with the exception of postrace Cr in the full marathon group (1.2 mg/dL).33,38,52 The effect of participating in prolonged endurance exercise for the general population appears to be safe in the majority of racers.31,36,50
There was a subgroup of individuals in our study who did not have normal laboratory findings before the race start. Few other studies have examined prerace sodium levels9,28,51 without noting prerace hyponatremia. This study suggests that some runners are hyponatremic before starting the race: 5 of 84 of half marathoners (6.0%), 3 of 77 of full marathoners (3.9%), and 8 of all 161 racers (5.0%).
The half marathon group had a higher percentage of racers with hyponatremia, renal dysfunction, and hemoconcentration before the race. This group may have been less experienced and perhaps less prepared and may have started the race overhydrated (hyponatremia) or underhydrated (renal dysfunction or hemoconcentration). Recognized risk factors for exercise-associated hyponatremia (but not necessarily renal dysfunction or hemoconcentration) include female sex2,4,14,22,41 and event inexperience.22,39,53
Although the primary outcome measures (hyponatremia, renal dysfunction, and hemoconcentration) were less prevalent prerace among full marathoners than half marathoners, full marathoners had higher rates (13.5%, 48.3%, and 4.5%, respectively) than did half marathoners (3.8%, 37.7%, and 1.9%, respectively) upon completion of the race. The 13.5% rate of exercise-associated hyponatremia among full marathoners postrace (8.2% rate among all runners) warrants further discussion. Runners appear to be drinking excessively. Similar rates (9% and 13%) have been cited in recent studies from North American marathons,2,22 but in other regions of the world (and in events with fewer aid stations) lower rates have been reported (0%-4%).28,34,36,50 Aid stations were positioned approximately every mile along the course of the marathon, and this ease of access to fluids is a possible link to exercise-associated hyponatremia.40,43 Aggressive hydration strategies may lead to excessive fluid consumption. The median finishing time for full marathoners in our study was 333.7 minutes, or just over 5.5 hours. Finishing times of greater than 4 hours2,19,23,26,43,45 have been cited as a risk factor for the development of exercise-associated hyponatremia.2,18,22,25,41,43 Potassium fluctuations, however, are generally negligible among all racers.31,34,36,50,51
We are not able to report whether the renal dysfunction observed in this study was clinically significant, because we do not know how many participants sought medical attention for dehydration, renal failure, or other associated complications. Prior reports have demonstrated small elevations of serum Cr concentrations as a result of endurance exercise.25,31,34,50 In this study, high rates of “moderate” renal dysfunction were found in the postrace setting among half and full marathoners, but this may be due to our definition of renal dysfunction. Because a glomerular filtration rate was not calculated, we are unable to conclude whether these observed abnormalities would persist at such rates if age, weight, and race were incorporated into renal function calculations.17,30,38
Why half marathoners had a statistically significant mean increase in sodium levels (P < 0.01) versus full marathoners (P = 0.31) is unclear. Although the clinical significance of these findings is unknown, an increase in sodium levels31,32 may represent a potential marker of dehydration or volume contraction. Change in hemoglobin, however, as a marker of dehydration was larger in the full marathon group than in the half marathon group (0.6 g/dL vs 0.0 g/dL), and this difference was statistically significant in only the full marathon group (P < 0.01). Presumably, race distance may play a significant role in observed rates of hemoglobin change, but again, it is unclear if these findings have clinical significance.
The mean changes observed in the markers of renal dysfunction (BUN and Cr) were statistically significant (P < 0.01) across all groups, but the clinical significance is again questionable. The cause of a mean change in BUN (3.8 mg/dL) and Cr (0.2 mg/dL) for the full marathon group, which was double that of the half marathon group (BUN 1.9 mg/dL, Cr 0.1 mg/dL), is uncertain. Although the degree of change in BUN and Cr appears to be race-distance related, we cannot conclude whether this association is linear or whether it applies to distances longer than a full marathon.
Sex differences in metabolic findings occur frequently. The higher prerace Cr observed in men among all participants and in the full marathon group likely represents differences in weight and may not persist if glomerular filtration rate is used. Similar reasoning explains consistently higher hemoglobin levels in men across all groups. Interestingly, the sex difference in Cr did not occur in the half marathon group (P = 0.07); however, the number of men available for this comparison was small (n = 21).
For paired data analysis by sex, both women and men frequently had statistically significant changes for markers of renal function (BUN and Cr). For the half marathon group, women had increases in BUN (P < 0.01) and Cr (P = 0.01) that were not observed among men—likely a function of the small paired data sample size of men (n = 19) available for comparison.
Given that no significant differences except for renal dysfunction were observed between women and men in rates of metabolic abnormalities, we can conclude that both sex are equally likely (or unlikely) to develop such abnormalities. The significantly higher rates of moderate renal dysfunction postrace among men in the all-participant group and full marathon group (P < 0.01) may have resulted in part because of our cutoff definitions of renal dysfunction. Despite this, men were nearly 6 times (all participants) or 9 times (full marathon group) as likely as women to develop moderate renal dysfunction. Differences in race pace25 and hydration strategies36 might be causes of this sex-based discrepancy in the observed renal parameters.
Although the study methodology is a strength, this design has limitations. Runners were not selected at random for participation; they were self-selected. This introduces selection bias because these individuals are potentially more interested in the health outcomes associated with distance running. Second, there were incomplete data sets because some participants presented only prerace or postrace and not for both; therefore, this limited the paired data set analysis. Also limiting paired data analysis was the loss of 39 “quantity not sufficient” blood samples in the prerace setting—likely due to the colder, morning conditions and associated peripheral vasospasm in the runners’ fingers. Note that the number of “quantity not sufficient” samples in the postrace setting was only 7, presumably because blood samples were much easier to obtain from the warmer digits of runners who had just completed the race. Third, data were not collected on the degree of effort (ie, running most of the race, walking most of the race, or both). Race pace could affect the observed laboratory values. A small number of participants (< 15) identified themselves as “walkers,” but they were included in the analysis as runners.
Conclusions
Metabolic abnormalities are not uncommon among endurance racers, and they may be present prerace, including hyponatremia. The clinical significance of these findings is unknown, and participation in distance running events appears to be safe for the majority of racers.
Footnotes
References
- 1. Adolph EF. Physiological Regulations. Lancaster, PA: Jacques Cattell Press; 1943:502 [Google Scholar]
- 2. Almond CS, Shin AY, Fortescue EB, et al. Hyponatremia among runners in the Boston Marathon. N Engl J Med. 2005;352(15):1550-1556 [DOI] [PubMed] [Google Scholar]
- 3. Asadollahi K, Beeching N, Gill G. Hyponatraemia as a risk factor for hospital mortality. QJM. 2006;99(12):877-880 [DOI] [PubMed] [Google Scholar]
- 4. Ayus JC, Varon J, Arieff AI. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners. Ann Intern Med. 2000;132(9):711-714 [DOI] [PubMed] [Google Scholar]
- 5. Below PR, Mora-Rodríguez R, González-Alonso J, Coyle EF. Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med Sci Sports Exerc. 1995;27(2):200-210 [PubMed] [Google Scholar]
- 6. Breast Cancer Marathon Results for the 2008 Breast Cancer Marathon. http://www.breastcancermarathon.com/run/2008-results/
- 7. Burgess WA, Davis JM, Bartoli WP, Woods JA. Failure of low dose carbohydrate feeding to attenuate glucoregulatory hormone responses and improve endurance performance. Int J Sport Nutr. 1991;1(4):338-352 [DOI] [PubMed] [Google Scholar]
- 8. Casa DJ, Clarkson PM, Roberts WO. American College of Sports Medicine roundtable on hydration and physical activity: consensus statements. Curr Sports Med Rep. 2005;4(3):115-127 [DOI] [PubMed] [Google Scholar]
- 9. Chorley J, Cianca J, Divine J. Risk factors for exercise-associated hyponatremia in non-elite marathon runners. Clin J Sport Med. 2007;17(6):471-477 [DOI] [PubMed] [Google Scholar]
- 10. Coggan AR, Coyle EF. Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. J Appl Physiol. 1987;63(6):2388-2395 [DOI] [PubMed] [Google Scholar]
- 11. Couchoud C, Pozet N, Labeeuw M, Pouteil-Noble C. Screening early renal failure: cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int. 1999;55(5):1878-1884 [DOI] [PubMed] [Google Scholar]
- 12. Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61(1):165-172 [DOI] [PubMed] [Google Scholar]
- 13. Coyle EF, Montain SJ. Carbohydrate and fluid ingestion during exercise: are there trade-offs? Med Sci Sports Exerc. 1992;24(6):671-678 [PubMed] [Google Scholar]
- 14. Davis DP, Videen JS, Marino A, et al. Exercise-associated hyponatremia in marathon runners: a two-year experience. J Emerg Med. 2001;21(1):47-57 [DOI] [PubMed] [Google Scholar]
- 15. Davis JM, Welsh RS, Alerson NA. Effects of carbohydrate and chromium ingestion during intermittent high-intensity exercise to fatigue. Int J Sport Nutr Exerc Metab. 2000;10(4):476-485 [DOI] [PubMed] [Google Scholar]
- 16. Davis JM, Welsh RS, De Volve KL, Alderson NA. Effects of branched-chain amino acids and carbohydrate on fatigue during intermittent, high-intensity running. Int J Sports Med. 1999;20(5):309-314 [DOI] [PubMed] [Google Scholar]
- 17. Felig P, Cherif A, Minagawa A, Wahren J. Hypoglycemia during prolonged exercise in normal men. N Engl J Med. 1982;306(15):895-900 [DOI] [PubMed] [Google Scholar]
- 18. Frizzell RT, Lang GH, Lowance DC, Lathan SR. Hyponatremia and ultramarathon running. JAMA. 1986;255(6):772-774 [PubMed] [Google Scholar]
- 19. Hew-Butler T, Anley C, Schwartz P, Noakes T. The treatment of symptomatic hyponatremia with hypertonic saline in an Ironman triathlete. Clin J Sport Med. 2007;17(1):68-69 [DOI] [PubMed] [Google Scholar]
- 20. Hew-Butler T, Ayus JC, Kipps C, et al. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med. 2008;18(2):111-121 [DOI] [PubMed] [Google Scholar]
- 21. Hew-Butler T, Jordaan E, Stuempfle KJ, et al. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J Clin Endocrinol Metab. 2008;93(6):2072-2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hew TD, Hew TD, Chorley JN, Cianca JC, Divine JG. The incidence, risk factors, and clinical manifestations of hyponatremia in marathon runners. Clin J Sport Med. 2003;13(1):41-47 [DOI] [PubMed] [Google Scholar]
- 23. Hillman M. Another close call with hyponatremia. http://www.restonrunners.org/special/hyponatremia/KCGuevera-CloseCall.php
- 24. Hsieh M, Roth R, Davis DL, Larrabee H, Callaway CW. Hyponatremia in runners requiring on-site medical treatment at a single marathon. Med Sci Sports Exerc. 2002;34(2):185-189 [DOI] [PubMed] [Google Scholar]
- 25. Irving RA, Noakes TD, Buck R. Evaluation of renal function and fluid homeostasis during recovery from exercise-induced hyponatremia. J Appl Physiol. 1991;70(1):342-348 [DOI] [PubMed] [Google Scholar]
- 26. Kang J, Robertson RJ, Goss FL, et al. Effect of carbohydrate substrate availability on ratings of perceived exertion during prolonged exercise of moderate intensity. Percept Mot Skills. 1996;82(2):495-506 [DOI] [PubMed] [Google Scholar]
- 27. Maughan RJ, Leiper JB, Shirreffs SM. Restoration of fluid balance after exercise-induced dehydration: effects of food and fluid intake. Eur J Appl Physiol Occup Physiol. 1996;73(3-4):317-325 [DOI] [PubMed] [Google Scholar]
- 28. Mettler S, Rusch C, Frey WO, Bestmann L, Wenk C, Colombani PC. Hyponatremia among runners in the Zurich Marathon. Clin J Sport Med. 2008;18(4):344-349 [DOI] [PubMed] [Google Scholar]
- 29. Nicholas CW, Williams C, Lakomy HK, Phillips G, Nowitz A. Influence of ingesting a carbohydrate-electrolyte solution on endurance capacity during intermittent, high-intensity shuttle running. J Sports Sci. 1995;13(4):283-290 [DOI] [PubMed] [Google Scholar]
- 30. Noakes TD. Overconsumption of fluids by athletes. BMJ. 2003;327(7407):113-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noakes TD, Carter JW. Biochemical parameters in athletes before and after having run 160 kilometres. S Afr Med J. 1976;50(40):1562-1566 [PubMed] [Google Scholar]
- 32. Noakes TD, Norman RJ, Buck RH, Godlonton J, Stevenson K, Pittaway D. The incidence of hyponatremia during prolonged ultraendurance exercise. Med Sci Sports Exerc. 1990;22(2):165-170 [PubMed] [Google Scholar]
- 33. Noakes TD, Sharwood K, Speedy D, et al. Three independent biological mechanisms cause exercise-associated hyponatremia: evidence from 2,135 weighed competitive athletic performances. Proc Natl Acad Sci U S A. 2005;102(51):18550-18555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Page AJ, Reid SA, Speedy DB, Mulligan GP, Thompson J. Exercise-associated hyponatremia, renal function, and nonsteroidal antiinflammatory drug use in an ultraendurance mountain run. Clin J Sport Med. 2007;17(1):43-48 [DOI] [PubMed] [Google Scholar]
- 35. Ray ML, Bryan MW, Ruden TM, Baier SM, Sharp RL, King DS. Effect of sodium in a rehydration beverage when consumed as a fluid or meal. J Appl Physiol. 1998;85(4):1329-1336 [DOI] [PubMed] [Google Scholar]
- 36. Reid SA, Speedy DB, Thompson JM, et al. Study of hematological and biochemical parameters in runners completing a standard marathon. Clin J Sport Med. 2004;14(6):344-353 [DOI] [PubMed] [Google Scholar]
- 37. Roberts WO. A 12-year profile of medical injury and illness for the Twin Cities Marathon. Med Sci Sports Exerc. 2000;32(9):1549-1555 [DOI] [PubMed] [Google Scholar]
- 38. Running USA Track and field running information center. http://www.runningusa.org Accessed January 7, 2011
- 39. Shapiro SA, Ejaz AA, Osborne MD, Taylor WC. Moderate exercise-induced hyponatremia. Clin J Sport Med. 2006;16(1):72-73 [DOI] [PubMed] [Google Scholar]
- 40. Sharwood K, Collins M, Goedecke J, Wilson G, Noakes T. Weight changes, sodium levels, and performance in the South African Ironman Triathlon. Clin J Sport Med. 2002;12(6):391-399 [DOI] [PubMed] [Google Scholar]
- 41. Speedy DB, Noakes TD, Rogers IR, et al. Hyponatremia in ultradistance triathletes. Med Sci Sports Exerc. 1999;31(6):809-815 [DOI] [PubMed] [Google Scholar]
- 42. Speedy DB, Noakes TD, Schneider C. Exercise-associated hyponatremia: a review. Emerg Med. 2001;13(1):17-27 [DOI] [PubMed] [Google Scholar]
- 43. Speedy DB, Rogers IR, Noakes TD, et al. Diagnosis and prevention of hyponatremia at an ultradistance triathlon. Clin J Sport Med. 2000;10(1):52-58 [DOI] [PubMed] [Google Scholar]
- 44. Steinberg D. Running the risk of too much water: hyponatremia can sometimes lead to death for marathoners. Washington Post. October 24, 2003:A1 [Google Scholar]
- 45. Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334(22):1448-1460 [DOI] [PubMed] [Google Scholar]
- 46. Utter A, Kang J, Nieman DC, et al. Effect of carbohydrate substrate availability on ratings of perceived exertion during prolonged running. Int J Sport Nutr. 1997;7(4):274-285 [DOI] [PubMed] [Google Scholar]
- 47. van Gent RN, Siem D, van Middelkoop M, van Os AG, Bierma-Zeinstra SM, Koes BW. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. Br J Sports Med. 2007;41(8):469-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vandraanen W. The history of the marathon. http://www.exercisetherighttoread.org/historyofmarathon.pdf Accessed January 7, 2011
- 49. Welsh RS, Davis JM, Burke JR, Williams HG. Carbohydrates and physical/mental performance during intermittent exercise to fatigue. Med Sci Sports Exerc. 2002;34(4):723-731 [DOI] [PubMed] [Google Scholar]
- 50. Wharam PC, Speedy DB, Noakes TD, Thompson JM, Reid SA, Holtzhausen LM. NSAID use increases the risk of developing hyponatremia during an Ironman triathlon. Med Sci Sports Exerc. 2006;38(4):618-622 [DOI] [PubMed] [Google Scholar]
- 51. Whiting PH, Maughan RJ, Miller JD. Dehydration and serum biochemical changes in marathon runners. Eur J Appl Physiol Occup Physiol. 1984;52(2):183-187 [DOI] [PubMed] [Google Scholar]
- 52. Yip R, Johnson C, Dallman PR. Age-related changes in laboratory values used in the diagnosis of anemia and iron deficiency. Am J Clin Nutr. 1984;39(3):427-436 [DOI] [PubMed] [Google Scholar]
- 53. Young M, Sciurba F, Rinaldo J. Delirium and pulmonary edema after completing a marathon. Am Rev Respir Dis. 1987;136(3):737-739 [DOI] [PubMed] [Google Scholar]


