Abstract
We report a case of invasive pulmonary aspergillosis caused by Neosartorya pseudofischeri S. W. Peterson [anamorph Aspergillus thermomutatus (Paden) S. W. Peterson]. The diagnosis was initially based on a positive blood culture for a strain isolated from a neutropenic patient by means of a BACTEC 9050 blood culture system. The final diagnosis was established based on X-ray and computer tomography scan results as well as the detection of Aspergillus antigen in the patient's serum.
CASE REPORT
A 17-year-old male patient with Hodgkin's disease (stage IVB) failed to achieve complete remission after first-line chemotherapy consisting of six courses of a combination of A-doxorubicin, bleomycin, vinblastine, and dacarbazine. A second course of chemotherapy involving minimal treatment with B-carmustine, etoposide, A-cytarabine, and melphalan was initiated. During chemotherapy, the patient was hospitalized in a single isolation room in a hematology ward without any special air filtering.
The patient was discharged in a stable condition 8 days after the initiation of chemotherapy. Five days after discharge (12 days after the initiation of chemotherapy), the patient was readmitted to the hematology ward with neutropenia (leukocyte count, 0.2 × 109/liter), a fever of 38.6°C, and a nonproductive cough. Empirical antibiotic therapy with cefazolin and gentamicin began. Granulocyte colony-stimulating factor was used to accelerate neutrophil recovery. Antifungal prophylaxis was initiated with oral fluconazole (100 mg daily). Routine blood cultures with aerobic (Plus Aerobic/F), anaerobic (Plus Anaerobic/F), and fungal (Mycosis IC/F) broths (each with 10 ml of blood) were incubated in a BACTEC 9050 blood culture system (Becton Dickinson, Cockeysville, Md.). Because the patient had a nonproductive cough, sputum samples were not available for microbiological investigation. No bronchoscopy or lung biopsy could be performed, as the patient had severe thrombocytopenia.
On day 14, the patient's temperature decreased to <37°C. In the microbiology laboratory, growth was detected in fungal blood culture medium, and the two subcultures on Sabouraud dextrose agar were grown at 28 and 37°C. At the higher temperature, conidial heads similar to those of Aspergillus fumigatus were seen, but ascomata were overwhelmingly predominant at the lower temperature. The Aspergillus species most common in clinical practice (A. fumigatus, A. flavus, A. niger, and A. terreus) were excluded. Growth was also detected in aerobic broth, but we did not succeed in obtaining a subculture on blood or chocolate agar. No growth was detected in anaerobic broth. Reference service for identification was obtained from the Centraalbureau voor Schimmelcultures identification service, Utrecht, The Netherlands. The species isolated was identified as Neosartorya pseudofischeri.
On day 18, the patient's fever (>39°C) recurred, the C-reactive protein level was 263 mg/liter, and a chest X-ray showed a nonspecific infiltrate of 3-cm diameter in the right lung. Blood cultures for aerobes, anaerobes, and fungi yielded no growth. A test for Aspergillus galactomannan in serum by a sandwich enzyme-linked immunosorbent assay (Platelia; Bio-Rad Laboratories, Marnes-la-Coquette, France) gave a negative result. The antibiotic regimen was changed to imipenem and amikacin.
Neutropenia resolved at day 22, but a low-grade fever, a cough, and chest pain upon deep inspiration persisted despite the broad-spectrum antibiotic treatment. Aspergillus galactomannan testing of serum was performed and yielded a positive result.
A chest radiograph obtained on day 26 showed the presence of a nodular sign with a halo in the right lung. A definite diagnosis of invasive pulmonary aspergillosis was made. Antibacterial therapy was discontinued, and antifungal treatment was initiated with amphotericin B (0.86 mg/kg of body weight/day) for 16 days.
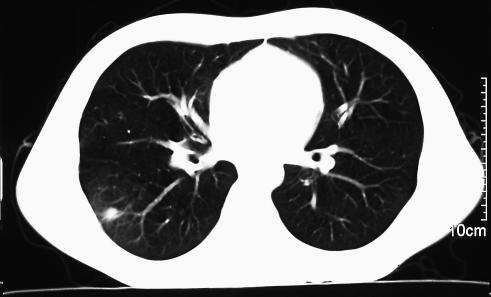
The treatment resulted in rapid clinical improvement. On the eighth day of treatment with amphotericin B, no Aspergillus galactomannan was detected in serum. After the patient was discharged from the hospital, antifungal therapy was continued with oral itraconazole (200 mg/day). After 2 weeks, a chest computer tomography (CT) scan showed a 9-mm-diameter round lesion with decreased attenuation (Fig. 1). Oral treatment with itraconazole was continued for 5 weeks. On day 46 (day 20 of the antifungal therapy), a serum sample was tested for Aspergillus galactomannan with a negative result, and the patient remained symptom free.
FIG. 1.
Patient's CT scan on day 33 after chemotherapy.
The patient relapsed 6 months later, and chemotherapy was begun again. There was no evidence of invasive fungal infection.
Species of the genus Neosartorya are rare human pathogens. To date, only a few cases of opportunistic infection caused by N. fischeri, N. pseudofischeri, and N. hiratsukae have been described (1, 5). Six previous cases of human infection caused by Neosartorya pseudofischeri S. W. Peterson [anamorph Aspergillus thermomutatus (Paden) S. W. Peterson] have been recorded in medical literature. Isolates examined by Peterson (9) and cases described or reviewed by Padhye et al. (8) connected N. pseudofischeri with pulmonary disease, osteomyelitis, mycotic keratitis, endocarditis, and unspecified aspergillosis. In two of the cases reviewed by Padhye et al. (8), the species involved had originally been reported under other Neosartorya names (4, 12). Recently, a case of N. pseudofischeri peritonitis in a peritoneal dialysis patient was described (7).
It is very likely that infections by Neosartorya species are underdiagnosed in clinical practice because white aspergilli are often regarded as contaminants in laboratory practice. The same opinion has previously been stated by Guarro and coauthors (5). White, nonsporulating aspergilli and aspergilli covered with white ascomata should be regarded as pathogenic rather than nonpathogenic. White, sporulating aspergilli are due to mishandling of specimens or laboratory contamination more often than they are due to infection. All such isolates should be examined carefully and identified to the species level by using conventional cultures on malt extract, Czapek Dox, or oatmeal agar.
The species belonging to Neosartorya are thermophilic and closely related to A. fumigatus, the most common species causing invasive mold infections. N. pseudofischeri has whitish, fast-growing colonies at 37 and 45°C. Conidiophores with mature conidia of the anamorph A. thermomutatus morphologically resemble A. fumigatus, but their conidia are grayish yellow instead of deep bluish green overall.
Our isolate of N. pseudofischeri was identified by its morphological characters on malt extract and oatmeal agars. The culture on malt extract yielded abundant growth of ascomata at 25°C. The final identification of the ascoma, indicating the sexual state, was made by using scanning electron microscopy. The characteristic ascospore wall ornamentation of N. pseudofischeri, that is, a pattern of nonanastomosing, elongate ridges formed from wall tissue not showing any reticulate pattern, was seen (Fig. 2). The ornamentation is located in the central part of the convex spore wall. The distal part of the hemisphere and the area between the ridges are smooth. Other species of Neosartorya with ornamented ascospore walls include N. fischeri, N. spinosa, and N. hiratsukae. N. fischeri has a reticulate pattern of anastomosing ridges on its ascospore wall. The N. spinosa ascospore wall bears spiny ornamentation. N. hiratsukae ascospores have a reticulate ornamentation resembling that of N. fischeri. These two species differ in their colony characteristics on Czapek Dox agar (5).
FIG. 2.
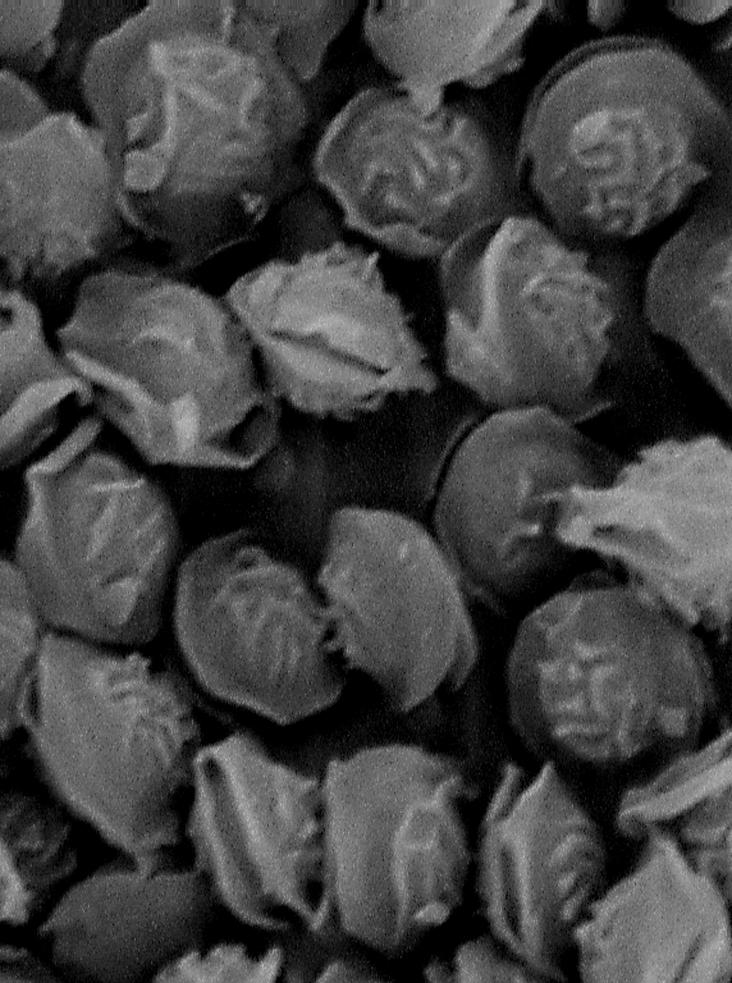
Ascospores of N. pseudofischeri CBS 110899 (=TFC 2002-19) under scanning electron microscopy. Magnification, ×12,300.
The identity of our strain was confirmed by molecular identification. Genomic DNA of the ribosomal internal transcribed spacer (ITS) region, including ITS1, 5.8S, and ITS2, was sequenced. In comparison with data available in GenBank, the sequence of our isolate was found to be 98% similar to that of an isolate deposited in the Agricultural Research Service Culture Collection, National Center of Agricultural Utilization Research, Peoria, Ill. (NRRL 180; GenBank accession number AF459729).
Living cultures of the case strain are deposited in the Centraalbureau voor Schimmelcultures (CBS 110899) and in the Tartu Fungal Collection, Institute of Zoology and Botany, Estonian Agricultural University, Tartu, Estonia (TFC 2002-19).
The isolation of Aspergillus spp. from blood is usually related to a central venous catheter infection, to cardiac surgery, or, for neutropenic patients, to pulmonary infection. Positive blood cultures have been reported for one case of pulmonary aspergilloma (10). The rarity of the isolation of Aspergillus spp. from blood derives from the specific pathology of invasive aspergillosis. Aspergillus can invade peripheral blood vessels and cause thrombosis, stopping the continuous flow of blood to the affected site and preventing the release of hyphal fragments via blood flow (3). It can be speculated that the probability of introducing hyphal fragments into circulating blood is higher during the initial stage of blood vessel wall involvement, when the vessel is still partly open, than in later stages of involvement. In theory, in the intravascular infection of central blood vessels, hyphal fragments can be continuously released, but it is clearly more likely that intermittent hyphal release takes place (3). This means that the optimal time period for obtaining successful blood cultures is in the early stage of the infection. A series of blood culture attempts with an appropriate medium and culture system may be needed for successful diagnosis.
Because mold isolation from blood is rare, the validation of different blood culture systems for fungal isolation is difficult. In published reports of Aspergillus fungemia, the blood culture system used for detection is often not disclosed. There are few documented reports that the BACTEC series 9000 system is suitable for the isolation of molds from blood (11). The mean time required to obtain a positive blood culture with Aspergillus infections has been found to be 8.5 days; this result was based on a study of various culture systems (3). In the present case, the BACTEC 9050 system with Mycosis IC/F medium yielded a positive result as early as 48 h after inoculation. N. pseudofischeri grew in a subculture on Sabouraud dextrose agar. The Plus Aerobic/F medium also showed growth after 48 h. The subcultures from the aerobic bottle were done on blood agar and chocolate agar, but unfortunately, no growth was observed on these plates after 48 h and they were mistakenly discarded.
A diagnosis of invasive aspergillosis is not easy to establish. The isolation of Aspergillus from blood is unlikely, and contamination should be suspected, especially in cases in which there has been no previous suspicion of systemic fungal infection. Duthie and Denning (3) have reviewed 34 cases of pseudofungemia due to Aspergillus spp. described in medical literature, mainly in connection with mishandled cultures in the laboratory or in the ward. There were no instances of fungal contamination seen in our laboratory while this case was investigated. We repeated the blood culture twice to confirm the diagnosis, but results remained negative. The case described here, however, is considered sufficiently reliably confirmed for reporting based on the combination of (i) the single strain isolated from a positive blood culture, (ii) the positive result obtained with the Aspergillus galactomannan detection system, and (iii) the radiographic and CT findings for the lung.
A significant recent development in the diagnosis of invasive aspergillosis has been the availability of the Aspergillus cell wall galactomannan detection enzyme-linked immunosorbent assay kit (Platelia; Bio-Rad). This kit shows high sensitivity (60 to 100%) and specificity (81 to 99%) (P. Verweij, Antigen detection in diagnosis of invasive aspergillosis [http://www.aspergillus.man.ac.uk/secure/diagnosis/verweij.html]). It was designed for the detection of the antigen of common pathogenic Aspergillus species but, as demonstrated in the present case, can also be useful for the detection of invasive infection caused by N. pseudofischeri. In the present case, the patient's serum was tested for Aspergillus galactomannan four times, on days 6, 10, 20, and 33 after the positive blood culture results were obtained, and gave optical density indices of 0.7, 1.63, 0.8, and 0.5, respectively. According to the manufacturer's recommendations, only indices higher than 1.5 should be considered positive, and by this criterion, our patient's serum should have first been considered positive on day 10 after culture isolation. Some authors, however, have proposed lowering the cutoff value from 1.5 to 1.0 for patients with prolonged neutropenia or even to 0.7 for allogeneic stem cell transplant patients (6, 13). Our findings of lower indices (0.7 and 0.8) in a case of proven infection with a positive blood culture support these proposals.
Since clinical findings of pulmonary aspergillosis are nonspecific, the Invasive Fungal Infection Cooperative Group (IFICG) of the European Organization for Research and Treatment of Cancer and the Mycosis Study Group (MSG) of the National Institute of Allergy and Infectious Diseases have defined criteria for the diagnosis of proven, probable, and possible invasive fungal infections. By these IFICG-MSG criteria, any fungus recovered from blood in a case in which compatible clinical findings and predisposing host factors are present should be considered sufficient for the diagnosis of an invasive fungal infection. Because of the need for a rapid response in cases involving immunocompromised patients, the standard for diagnosis is necessarily lower than the standard for proof. The Aspergillus antigenemia detected in two consecutive plasma samples, in the context of the other evidence presented for our case, would be accepted as giving sufficient corroboration to yield a proven diagnosis by IFICG-MSG criteria (2). The present case should therefore be considered a proven case of N. pseudofischeri infection.
Nucleotide sequence accession number.
The nucleotide sequence of the isolate obtained in this study has been deposited in GenBank under accession number AY330710.
Acknowledgments
We thank Mart Rahi for assistance with scanning electron microscopy and Irja Saar for assistance with DNA sequencing.
This study was supported by Estonian Scientific Foundation grant 4688.
REFERENCES
- 1.de Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures/Universitat Rovira i Virgili, Utrecht, The Netherlands.
- 2.Donnelly, P. 2002. Symptoms and diagnosis of nosocomial fungal infections—state-of-the-art. Eur. J. Med. Res. 7:192-199. [PubMed] [Google Scholar]
- 3.Duthie, R., and D. W. Denning. 1995. Aspergillus fungemia: report of two cases and review. Clin. Infect. Dis. 20:598-605. [DOI] [PubMed] [Google Scholar]
- 4.Gerber, J., J. Chomicki, J. W. Brandsberg, R. Jones, and K. J. Hammermann. 1973. Pulmonary aspergillosis caused by Aspergillus fischeri var. spinosus. Am. J. Clin. Pathol. 60:861-866. [DOI] [PubMed] [Google Scholar]
- 5.Guarro, J., E. G. Kallas, P. Godoy, A. Karenina, J. Gene, A. Stchigel, and A. L. Colombo. 2002. Cerebral aspergillosis caused by Neosartorya hiratsukae, Brazil. Emerg. Infect. Dis. 8:989-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbrecht, R., V. Letscher-Bru, C. Oprea, B. Lioure, J. Waller, F. Campos, O. Villard, K. L. Liu, S. Natarajan-Ame, P. Lutz, P. Dufour, J. P. Bergerat, and E. Candolfi. 2002. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J. Clin. Oncol. 20:1898-1906. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto, N., H. Shiraga, K. Takahashi, K. Kikuchi, and K. Ito. 2002. Successful treatment of Aspergillus peritonitis in a peritoneal dialysis patient. Pediatr. Nephrol. 17:243-245. [DOI] [PubMed] [Google Scholar]
- 8.Padhye, A. A., J. H. Godfrey, F. W. Chandler, and S. W. Peterson. 1994. Osteomyelitis caused by Neosartorya pseudofischeri. J. Clin. Microbiol. 32:2832-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson, S. W. 1992. Neosartorya pseudofischeri sp. nov. and its relationship to other species in Aspergillus section Fumigati. Mycol. Res. 96:547-554. [Google Scholar]
- 10.Seki, M., S. Maesaki, K. Hashiguchi, Y. Tomiyama, K. Tomono, T. Tashiro, and S. Kohno. 2000. Aspergillus fumigatus isolated from blood samples of a patient with pulmonary aspergilloma after embolization. Intern. Med. 39:188-190. [DOI] [PubMed] [Google Scholar]
- 11.Siemann, M., and G. Rabenhorst. 1998. Detection of fungemia from blood cultures using the BACTEC 9240 instrument. Zentbl. Bakteriol. 287:53-55. [DOI] [PubMed] [Google Scholar]
- 12.Summerbell, R. C., L. de Repentigny, C. Chartrand, and G. St.-Germain. 1992. Graft-related endocarditis caused by Neosartorya fischeri var. spinosa. J. Clin. Microbiol. 30:1580-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulusakarya, A., E. Chachaty, J. M. Vantelon, A. Youssef, C. Tancrede, J. L. Pico, J. H. Bourhis, P. Fenaux, and J. N. Munck. 2000. Surveillance of Aspergillus galactomannan antigenemia for invasive aspergillosis by enzyme-linked immunosorbent assay in neutropenic patients treated for hematological malignancies. Hematol. J. 1:111-116. [DOI] [PubMed] [Google Scholar]