Abstract
Background:
Protective athletic mouthguards (PAMs) have been worn in competitive sports for more than 100 years. Today, participants in contact and noncontact sports wear PAMs.
Hypothesis:
Wearing a PAM produces oral injury.
Study Type:
Case series.
Study Design and Methods:
Sixty-two Division I football players voluntarily participated in the study. Before the beginning of the season, each player underwent a thorough oral examination, and all abnormal oral findings were photographed (hyperkeratosis, erythema, ulceration, and combinations thereof). At midseason, 14 players were given complete oral examinations, with all abnormal oral findings documented. At season end, all remaining players (n = 53) had complete oral examinations and photographs taken of abnormal oral findings.
Results:
The preseason examination of 62 players found a total of 85 lesions (1.4 lesions per player) on the gingiva (n = 17), buccal mucosa (n = 60), and palate (n = 8). The 14 midseason players had 28 lesions (2.0 lesions per player) on gingiva (n = 8), buccal mucosa (n = 16), and tongue (n = 4). At season end, the 53 remaining players had 198 lesions (3.7 per player) on the gingiva (n = 96), buccal mucosa (n = 79), tongue (n = 18), and palate (n = 5). In addition, the lesion intensity scores progressively increased over the season. Because the palate did not come into direct contact with the PAM, it was used as an internal control.
Conclusion:
The wearing of a PAM may increase the number and intensity of oral mucosal injuries, which may cause localized soft tissue reactions such as hyperkeratosis, erythema, and ulceration.
Clinical Relevance:
Because the PAM reduces tooth injury but may cause oral lesions, it should be sanitized daily and changed regularly and replaced whenever it becomes sharp and jagged or when the athlete develops an irritation in the mouth.
Keywords: disease transmission, protective athletic mouthguard, oral infections, systemic infections, microorganisms, mouthguard care
Protective athletic mouthguards (PAMs) were initially used by boxers to protect their teeth and to reduce concussions resulting in knockouts.15 Numerous recent reports have noted that although PAMs may not protect against brain injuries during contact sports, they do provide protection for the teeth.9-11,13,17,19 Such positive results have prompted organizations such as the American Dental Association to support the utilization of PAMs for all contact sports2; football teams at all competitive levels have been among the most compliant with the association’s recommendations.1,4,5,12 These results also convinced the Department of the Army in 2004 to mandate the use of PAMs for pugil stick training, rifle/bayonet training, unarmed combat, and confidence/obstacle courses.3
Recent studies of football players,6 hockey players,7 and medical student controls have shown that PAMs harbor a range of pathogenic and opportunistic microorganisms, including yeasts and molds.14 These findings are a concern because current research describes a rise in the number of infections in athletes, from junior to professional levels, and the impact that such infections may have on the athletes’ overall health.8
Even though such studies have examined the physical conditions and microbial contaminations of PAMs, the correlation between developing oral lesions and wearing these devices has not been fully documented.6-8 The following study was performed to answer the following research question: Does the wearing of PAMs induce clinically observable and progressive oral lesions?
Participants and Methods
Sixty-two athletes from a Division I football team volunteered to be research participants. At the beginning of the season, after obtaining informed consent, each participant was given a unique accession number and asked to complete a short health history. To establish a baseline, everyone had a thorough clinical oral examination of 8 sites (gingiva, buccal mucosa, tongue, and palate). These examinations were performed by a board certified oral and maxillofacial pathologist, a pediatric dentist, and a practicing dentist affiliated with a junior ice hockey team. All abnormalities were recorded on a clinical form and photographed using a handheld intraoral digital camera (Claris i310D USB 2.0 Digital Intraoral Camera, Sota Precision Optics, Inc, Orange, California). Abnormal oral findings included single lesions of hyperkeratosis, erythema, ulceration, and all possible combinations thereof. Lesion intensities were scored on a scale from 1 to 7. This established scale has been used by several independent investigators, and it has produced consistent lesion intensity scores.6-8
At the beginning of the season, each participant selected a PAM of his choice, which was either the typical “boil and bite” device or a custom-made device made by the team’s athletic trainers. This study design was independent of the type of PAM selected by the player. Lost or deformed PAMs were replaced upon demand. At midseason, 14 of the 62 players were selected on the basis of their availability (ie, redshirts) for a complete clinical oral examination. With the exception of game time, these players wore PAMs in the same manner as the others. Their oral lesions were scored using the same procedures as the initial examination, and intraoral photographs were made of all abnormalities. At season end, each of the remaining study participants (n = 53) had another thorough clinical oral examination with intraoral photographs made of each abnormality. The intraoral lesions were once again graded using an intensity scale.
The data were analyzed using various mathematical and statistical models, including but not limited to the following: means, standard deviation, standard error, t test, analysis of variance, and frequency distribution.
Results
Overall, at the beginning of the season, 47 of 62 participants (75.8%) had 85 oral lesions (1.4 lesions per volunteer). This represented a preseason baseline before PAM usage, and it was consistent with similar preseason studies of ice hockey players of roughly the same age. These volunteers had a total of 17 gingival lesions, 60 buccal mucosal lesions, 8 palatal lesions, and zero tongue lesions. The midseason oral examination chosen on the basis of volunteers’ availability (redshirts; n = 14) revealed 28 lesions (2.0 lesions per player), 8 gingival lesions, 16 buccal mucosal lesions, zero palatal lesions, and 4 tongue lesions. Although the findings of the midseason examinations were not used for statistical analysis, they did demonstrate a progressive increase in the number of lesions per person.
By the end of the season, 53 remaining volunteers (96%) had a total of 198 lesions (3.7 lesions per player), representing 96 gingival lesions, 79 buccal mucosal lesions, 5 palatal lesions, and 18 tongue lesions. There was not only an overall increase in the number of lesions but a notable distribution with the gingiva having the greatest number of lesions. The tongue and the buccal mucosa also increased in the number of lesions, reflective of their contact with the PAMs. The decrease in the number of palatal lesions is consistent with its lack of contact with the PAMs. At season end, there was also an increase in the overall intensity scores. These findings were independent of the type of PAMs (boil and bite versus custom-made) that the players used. Based on the absolute number of lesions or the intensity scores, there were statistically significant differences between the number of lesions and their intensity scores at the beginning of the season versus the end of the season (t test, P < .01). With the exception of the palate, analysis of variance found that all sites demonstrated statistically significant differences between the preseason and season-end lesion numbers and intensity scores. No statistical differences were noted between the right and left sides of the mouth.
Discussion
The results of this study confirm that the wearing of PAMs has a significant influence in producing oral lesions and may have a significant influence in producing oral disease. Although the statistical analyses demonstrate significance, the photographs of the PAMs (Figure 1) and the oral lesions (Figures 2-5) are even more compelling. For example, Figure 2 is typical of lesion progression observed in an individual from preseason to season end. Although this lesion is described as being hyperkeratotic, it is a combination of parakeratin layer thickening and Malpighian cell layer spongiotic edema. The net effect could have been an irritation-induced change in the mucosa as a result of wearing a PAM. When compared with either the erythematous lesion or the ulcerative lesion, this type of lesion has less probability of microbial invasion.
Figure 1.
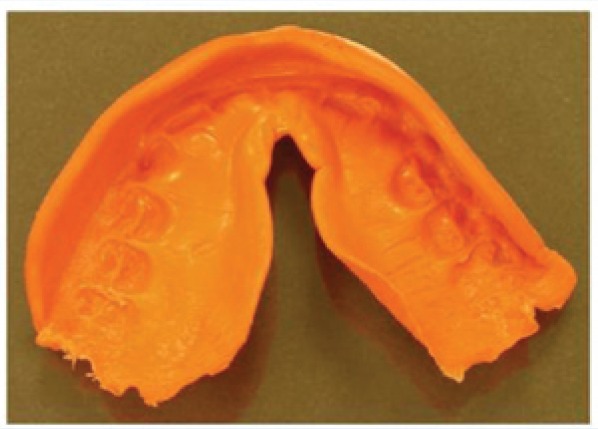
Typical example of protective athletic mouthguard worn by a football player for the entire season. Note the rough and jagged edges, which are close to the pterygoid plexus of veins in the mouth that could facilitate direct access into the vascular system.
Figure 2.
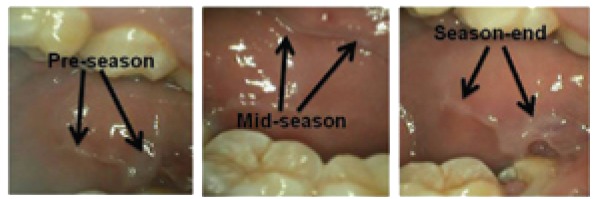
Pathology (hyperkeratosis; black arrows) in the posterior buccal mucosa. Note the progressive thickening of the mucosa in response to the constant rubbing and suction generated by the protective athletic mouthguard.
Figure 5.
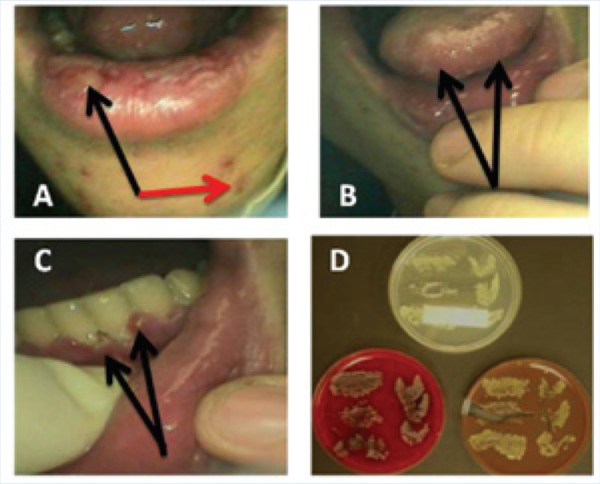
Oral and perioral pathology of protective athletic mouthguard wearer at season end: A, lesions consistent with necrotizing labiitis (black arrow) and facial acne (red arrow); B, lesions consistent with necrotizing glossitis (black arrows); C, lesions consistent with acute necrotizing gingivitis (black arrows); D, a culture of predominantly Staphylococcus spp. resulting from touching the protective athletic mouthguard surfaces and depths onto chocolate agar (lower right) and blood agar (lower left). The Sabouraud dextrose agar (top) demonstrates Candida spp. Both these microorganisms could have substantially contributed to these disease processes.
Figure 3 is an example of hyperkeratosis that would tend to be associated with direct trauma (thickening of the parakeratin layer only and/or metaplasia to orthokeratin). This figure also shows the type of marginal gingivitis (erythema) that would be associated with direct contact between the tissue and the PAMs. Of even greater importance is the carious tooth in this figure with obvious pulpal involvement. Although there is no evidence that the PAMs caused the caries, this open tooth could act as a potential portal of entry for microorganisms residing in the PAMs. In addition, at the root end of this tooth would be a granulomatous reaction, with the proliferation of small, thin-walled vessels (arterioles and venules). The hydraulic forces generated by a contaminated PAM and its interaction with the carious tooth could cause direct access of microbes and toxins into these vessels and ultimately into the entire vascular system.
Figure 3.
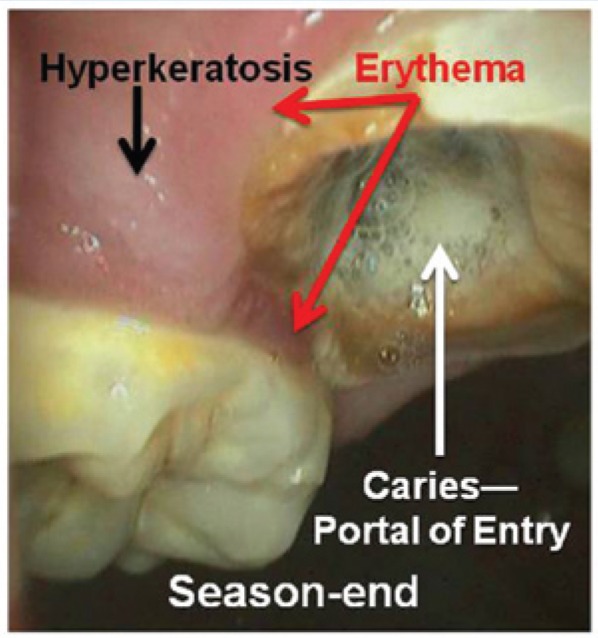
Example of pathology noted at season end. The preexisting carious tooth (white arrow) with its associated pulpal involvement provides a portal for microbial invasion, resulting in possible systemic involvement. Such carious teeth also occur in nonathletes; however, the risk of microbial invasion and vascular dissemination may be enhanced by wearing a contaminated protective athletic mouthguard. Note the presence of hyperkeratosis (black arrow) and erythema (red arrow) of the gingiva (combined lesional intensity score, 4).
Figure 4 is an excellent example of combination lesions (lesion score, 7) seen at the oral examination given at season end but not observed at the preseason examination. The changes seen in the oral cavity of this football player could certainly incriminate the PAMs as a major factor for such changes. Of all the participants, the athlete seen in Figure 5 had the most severe and acute condition that may be associated with his jagged PAM. The entire mouth became involved in the process, and the isolation of predominantly Staphylococcus aureus from the PAMs supported the clinical findings.
Figure 4.
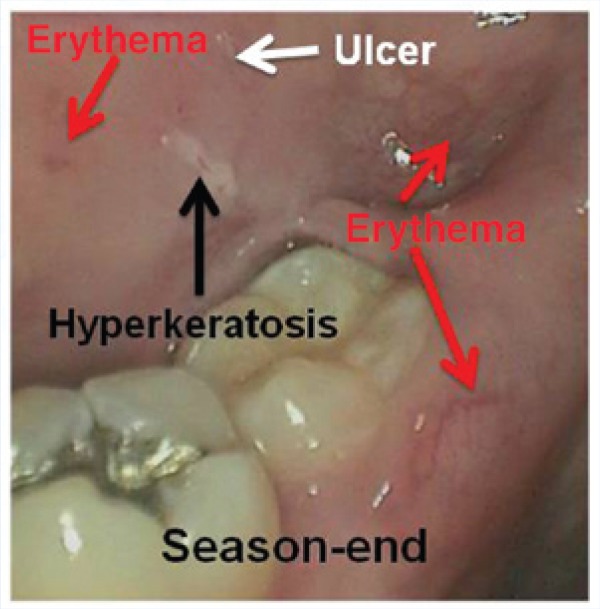
Example of integrated oral disease at season end induced by usage of the protective athletic mouthguard, showing all 3 types of lesions for an intensity score of 7 (hyperkeratosis, black arrow; erythema, red arrows; ulcer, white arrow). None of these lesions were noted during the preseason examination. The partially erupted third molar (wisdom tooth) has an associated pericoronitis (a microbial infection) that was not present preseason. The contaminated protective athletic mouthguards that produced the other 3 lesions could have also contributed to the pericoronitis.
Of course, there may have been other causes for the changes seen in the volunteers, such as tobacco use and poor oral hygiene. Although all participants were asked questions regarding these potential causes, the veracity and reliability of their answers are suspect, in part, because of their concern that such information would be passed along to the coaching staff, even though each participant was assured otherwise. In addition, lesions caused as such would have been clinically observed at the preseason examination.
If the results of the present study are compared with the overall incidence of oral lesion development in a general population, a wide disparity is noted. In a study of 17 235 adults aged 17 and older (including tobacco users), the hard palate had the highest percentage of lesions (25.9%), followed by the gingiva (20.4%), the tongue (14.2%), and the buccal mucosa (9.2%).18 In the present study, the percentage of palatal lesions was 12.9%, which decreased to 8.9% by the end of the study. The percentage of gingival lesions was 16.0% at the beginning of the study, and it increased to 73.2% by the end of the study, whereas tongue lesions went from 0% at the beginning of the study to 32.1%. Buccal lesions increased from 66.1% to 85.7% by the end of the study. Furthermore, a study of 4098 participants from Turin, Italy (general population), showed an even lower incidence of oral trauma, adding credence to the finding that wearing PAMs produces oral lesions.16
PAMs may produce oral lesions; as such, the corollary question becomes, what should be done with these devices to reduce this problem? First, a PAM should be regarded as a therapeutic device and discarded when it becomes distorted or develops sharp and jagged edges (or after 14 days of regular use).
Furthermore, the surfaces of all volunteers’ PAMs were cultured, and they yielded 339 bacterial isolates, 20 yeast isolates, and 108 fungal/mold isolates (Glass et al, unpublished data, 2008). Although the bacterial and fungal isolates could be related to tissue infections, the molds could initiate exercise-induced asthma and allergies.8 Therefore, athletes should be advised to replace the device whenever they develop any type of oral lesion or respiratory distress. In addition, the overall condition of the oral cavity should be monitored on an ongoing basis with the use of any PAM.
Third, because of its propensity to become a microbial reservoir,6,7 the PAM should be sanitized on a daily basis, using one of the commercially available antimicrobial denture-cleansing solutions.
Acknowledgments
Original protocol approved by the Institutional Review Board, Oklahoma State University Center for Health Sciences (No. 2002003, February 11, 2002; approved amendment No. 5, August 20, 2008).
Footnotes
No potential conflict of interest declared.
References
- 1. Berg R, Berkey D, Tang J, Altman D. Knowledge and attitudes of Arizona high-school coaches regarding oral-facial injuries and mouth guard use among athletes. J Am Dent Assoc. 1998;129:1425-1432 [DOI] [PubMed] [Google Scholar]
- 2. Bureau of Dental Health Education and Bureau of Economic Research and Statistics Mouth protectors: 1962 and the future. J Am Dent Assoc. 1963;66:539-543 [Google Scholar]
- 3. dela Cruz GG, Knapik JJ, Birk MG. Evaluation of mouthguards for the prevention of orofacial injuries during United States Army basic military training. Dent Traumatol. 2008;24:86-90 doi: 10.1111/j.1600-9657.2006.00500.x 10.1111/j.1600-9657.2006.00500.x [DOI] [PubMed] [Google Scholar]
- 4. De Wet G, Badenhorst R, Rossouw L. Mouthguards for rugby players at primary school level. J Dent Assoc S Afr. 1981;36:249-253 [PubMed] [Google Scholar]
- 5. Garon M, Merkle A, White T. Mouth protectors and oral trauma: a study of adolescent football players. J Am Dent Assoc. 1986;112:663-665 [DOI] [PubMed] [Google Scholar]
- 6. Glass RT, Bullard JW, Conrad RS. The contamination of protective mouthguards: a characterization of the microbiota found in football players’ protective mouthguards as compared to the oral microbiota found in first-year medical students. J Amer Dent Inst Cont Educ. 2006;93:23-38 [Google Scholar]
- 7. Glass R, Bullard J, Goodson L, Conrad R. Microbial contamination of protective mouthguards in hockey players: an in vivo study. Compend Cont Educ Dent. 2001;22:1093-1108 [PubMed] [Google Scholar]
- 8. Glass R, Wood C, Bullard J, Conrad S. Possible disease transmission by contaminated mouthguards in two young football players. Gen Dent. 2007;55: 436-440 [PubMed] [Google Scholar]
- 9. Heintz W. Mouth protectors: a progress report. J Am Dent Assoc. 1968;77: 632-636 [DOI] [PubMed] [Google Scholar]
- 10. Labella C, Smith B, Sigurdsson A. Effect of mouthguards on dental injuries and concussions in college basketball. Med Sci Sports Exerc. 2002;34:41-44 [DOI] [PubMed] [Google Scholar]
- 11. McNutt T, Shannon S, Wright J, Feinstein J. Oral trauma and adolescent athletes: a study of mouth protectors. Pediatr Dent. 1989;11:209-213 [PubMed] [Google Scholar]
- 12. Moon D, Mitchell D. An evaluation of commercial protective mouthpieces for football players. J Am Dent Assoc. 1961;62:568-572 [DOI] [PubMed] [Google Scholar]
- 13. Morrow R, Seals R, Jr, Barnwell G, Jr, Day E. Report of a survey of oral injuries in male college and university athletes. Athl Train JNATA. 1991;26:338-342 [Google Scholar]
- 14. Murray PR, Rosenthal KS, Kobayashi GS, Pfaller MA. Medical Microbiology. 4th ed. St Louis, MO: Mosby; 2002 [Google Scholar]
- 15. Patrick DG, van Noort R, Found MS. Scale of protection and the various types of sports mouthguard. Br J Sports Med. 2005;39:278-281 doi: 10.1136/bjsm.2004.012658 10.1136/bjsm.2004.012658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pentenero M, Broccoletti R, Carbone M, Conrotto D, Gandolfo S. The prevalence of oral mucosal lesions in adults from the Turin area. Oral Dis. 2008;14:356-366 [DOI] [PubMed] [Google Scholar]
- 17. Sane J. Comparison of maxillofacial and dental injuries in four contact team sports: American football, bandy, basketball, and handball. Am J Sports Med. 1988;16:647-652 [DOI] [PubMed] [Google Scholar]
- 18. Shulman JD, Beach MM, Rivera-Hidalgo F. The prevalence of oral mucosal lesion in the U.S. adults: data from the Third National Health and Nutrition Examination Survey, 1988-1994. J Am Dent Assoc. 2004;135:1279-1286 [DOI] [PubMed] [Google Scholar]
- 19. Stenger J, Lawson E, Wright J, Ricketts J. Mouthguards: protection against shock to the head, neck and teeth. J Am Dent Assoc. 1964;69:273-281 [DOI] [PubMed] [Google Scholar]



