Abstract
Background:
Injections into or adjacent to soft tissue structures, including muscle, tendon, bursa, and fascia, for pain relief and an earlier return to play have become common in the field of sports medicine.
Study Design:
Clinical review.
Results:
Corticosteroids, local anesthetics, and ketorolac tromethamine (Toradol) are the most commonly used injectable agents in athletes. The use of these injectable agents have proven efficacy in some disorders, whereas the clinical benefit for others remain questionable. All soft tissue injections performed for pain control and/or an anti-inflammatory effect have potentially serious side effects, which must be considered, especially in the pregame setting.
Conclusions:
The primary concern regarding corticosteroid and local anesthetic injections is an increased risk of tendon rupture associated with the direct injection into the tendon. Intramuscular Toradol injections provide significant analgesia, as well as an anti-inflammatory effect via its inhibitory effect on the cyclooxygenase pathway. The risk of bleeding associated with Toradol use is recognized but not accurately quantified.
Keywords: injection, soft tissue, athletes
Injections into or adjacent to soft tissue structures (including muscle, tendon, bursa, and fascia) for pain relief and an earlier return to play have become common in the field of sports medicine. The majority of these agents provide relief primarily through their local effects (ie, local anesthetics), although at least one medication, ketorolac tromethamine, or Toradol (Roche Pharmaceuticals, Nutley, New Jersey), is a nonsteroidal anti-inflammatory drug (NSAID) that acts systemically as a short-term pain-reducing agent when administered via an intramuscular or intravenous route. Some of these injectable agents have proven efficacy, whereas the clinical benefit of others remains questionable. All soft tissue injections performed for pain control and/or an anti-inflammatory effect have potentially serious side effects that must be considered, especially in the pregame setting. Intra-articular injections have also been used in the sports medicine arena, as extensively discussed elsewhere.8,13,25,60
The purpose of this review is multifactorial: (1) to discuss the types of injuries encountered by the athlete, as well as the basic science of injured soft tissue structures; (2) to describe the acceptable clinical indications for soft tissue injections in this patient cohort; (3) to outline the agents most commonly used for this purpose, including their mechanism of action and potential complications; and (4) to summarize the published outcome studies dealing with this often controversial issue.
Pathophysiology of Soft Tissue Injuries in Athletes
The most common athletic injuries involve periarticular bursae, muscle, tendon, and ligament (see Table 1). Bursitis is inflammation of the fluid-filled sac or potential space that cushions and reduces friction between bone and overlying muscle or skin. Muscle injuries most commonly comprise strains and contusions. Muscle strains occur with activities involving eccentric muscle contraction, such as sprinting and jumping.30,40 Muscle contusions are typically the result of blunt trauma, where a large compressive force is applied to a single area, commonly occurring in the midmuscle belly. Muscle strains and contusions result in partial disruption of the muscle fibers and, subsequently, in variable amounts of muscle necrosis, vascular disruption, hematoma formation, and inflammation.40
Table 1.
Common conditions treated with soft tissue injections in the athlete.a
| Shoulder | Subacromial Bursitis |
|---|---|
| Knee | Pes anserine bursitisPrepatellar bursitisIliotibial band syndromePatellar tendonitis |
| Hip | Trochanteric bursitisIschial bursitisIliac crest contusion (hip pointer) |
| Elbow | Medial epicondylitisLateral epicondylitisOlecranon bursitis |
| Wrist | Carpal tunnel syndromeDeQuervain’s tenosysnovitis |
| Hand | Trigger finger |
| Thigh/leg | Hamstring strainQuadriceps contusion/strainGastrocnemius strain |
| Foot/ankle | Achilles/retrocalcaneal tendonitisSinus tarsi syndromePlantar fasciitisMetatarsal phalangeal sprain (turf toe)Interdigital neuroma (Morton’s neuroma) |
Adapted from Dietzel and Hedlund.19
The repair of muscle injury begins with phagocytosis of necrotic cellular debris. Fibroblasts convert hematoma into granulation connective tissue that provides early strength to the muscle.40 Connective tissue scar strengthens by day 10 after injury, as it changes from predominantly fibronectin to type I collagen.40 Finally, the remodeling stage includes maturation of the muscle repair and contraction and reorganization of the connective tissue scar.40
Tendinopathies are tendon disorders occurring from overuse.54 The histopathological findings can include paratendinitis and/or tendinosis.54,70 Paratendinitis involves acute edema and inflammatory cell infiltration: accurately labeled tenosynovitis in tendons with true synovial sheaths and peritendinitis in tendons without (eg, Achilles tendon). Tendinous tissue has relatively little vascularity and is not prone to inflammation. The more vascular structures of the paratenon are generally the sites of inflammation.
Tendinosis involves focal degeneration of tendon areas with an associated angiofibroblastic reaction.54,61,70 Tendinosis can occur with or without paratendinitis (inflammation). Symptoms may or may not be present with tendinosis. The cause of pain in patients with isolated tendinosis is unclear. Chronic overuse of the muscle-tendon structure leads to microscopic and macroscopic tendon injury.61 Tenocytes lose their reparative ability, resulting in the compromise of collagen cross-linking, the noncollagenous matrix, and the vascular supply of the tendon.54,61,70 Continued activity results in local hypoxia that further compromises the mechanical integrity of the tendon.79 Histologically, these chronic injuries have been shown to lack any evidence of inflammation.44,61,72 Microscopically, these injuries are characterized by fiber disorientation and thinning, collagen degeneration, hypercellularity, and vascular ingrowth.79
Inflammation and the Healing Response
Inflammation induced by sports injury is due to disruption of musculoskeletal tissues by excessive mechanical load or repetitive use. Acute trauma results in vascular disruption, tissue necrosis, and hematoma formation. The extent of the immediate tissue damage defines the zone of primary injury. Further edema and tissue hypoxia owing to the body’s inflammatory response can extend the area of injury to the zone of secondary injury.51 Both corticosteroids and NSAIDs suppress this inflammatory response. Theoretically, this has the potential to limit secondary injury, hasten healing, and allow early rehabilitation; however, the importance of the inflammatory response to complete healing is unclear.8,51
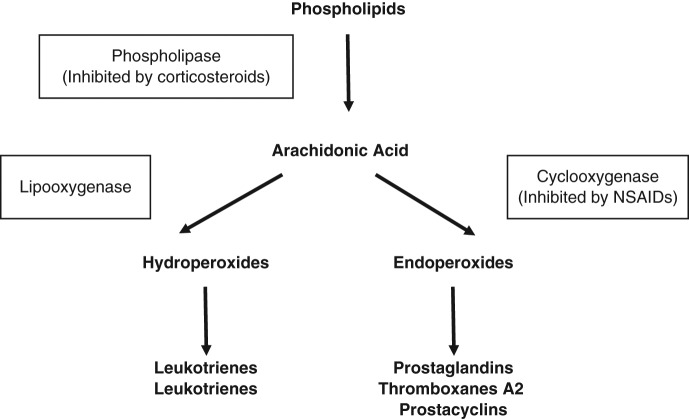
The normal healing process of soft tissue structures has been shown to consist of four phases: inflammation, regeneration, remodeling, and maturation.25 Inflammation results from a variety of events, including dilatation of the local vasculature, increased vascular permeability, exudation of fluids, activation and release of immunological mediators, activation of humoral response mechanisms, and leukocytic migration. Free radicals attack the phospholipase structure of cell membranes, resulting in breakdown of the cell wall and production of arachidonic acid metabolites.51 Arachidonic acid is metabolized to a variety of substances through 2 major pathways.51,58 The lipoxygenase pathway results in production of leukotrienes, whereas the cyclo-oxygenase (COX) pathway results in production of prostaglandins, prostacyclins, and thromboxanes.51,58
The COX-1 (constitutional) pathway plays a role in gastric cytoprotection, vascular homeostasis, platelet aggregation, and kidney function. The COX-2 (inducible) pathway is triggered by the inflammatory response and results in sensitizing pain receptors, elevating body temperature, and recruiting inflammatory cells—the cascade of events seen following athletic injury. Prostaglandins (especially prostaglandin E2) are important in triggering the inflammatory cascade.51 They also appear to cause sensitization of afferent pain nerve receptors.19 Prostaglandin inhibition is primarily via the COX-2 pathway. Thromboxanes (especially thromboxane A2) play a role in regulating blood vessel tone, causing platelet aggregation and clot formation. Thromboxane inhibition is primarily a COX-1 effect and is responsible for the bleeding tendency caused by NSAIDs.22,67 The inhibition of platelet aggregation is generally reversible and dose dependent, whereas aspirin irreversibly binds to platelets, causing longer effects. Corticosteroids and NSAIDs both suppress inflammation through this cascade but at different locations. Corticosteroids inhibit phospholipase A2, which functions in the breakdown of phospholipids to arachidonic acid.51 Thus, it effectively suppresses the COX and lipoxygenase pathways51 (Figure 1).
Figure 1.
The inflammatory cascade. Adapted from Shearn.81
The regeneration stage is characterized by increased cellularity, vascularity, and collagen synthesis. During remodeling, decreased cellularity is noted with increased matrix production. The maturation phase is characterized as the restoration of normal tissue architecture. Corticosteroid and local anesthetics target the inflammatory stage but appear to have some effect on regeneration, remodeling, and maturation.
Corticosteroids
Hydrocortisone acetate was first introduced in the 1949,34 and multiple corticosteroid derivatives have been synthesized from this basic steroid structure. Hollander37,38 was among the first to champion its use, noting that “no other form of treatment has given such consistent local symptomatic relief in so many for so long with so few harmful effects.” The use of oral systemic corticosteroids has constituted an important means of treating various chronic inflammatory/autoimmune disorders— notably, rheumatoid arthritis. However, systemic use of corticosteroids comes at the expense of a variety of documented side effects, including hypertension, glucose intolerance, Cushing’s syndrome, and others.6
Corticosteroid injections have a long history of use in athletics in that they are used to treat the secondary inflammation that results from musculoskeletal trauma. However, the role of inflammation, as an essential step in the healing of musculoskeletal injury, has led some to advise caution in the use of corticosteroids in musculoskeletal injury. The use of local injectable intra-articular, intra-bursal, and peritendinous corticosteroids in general avoids these systemic side effects by targeting a single area. The risks and benefits of intra-articular corticosteroid injections are controversial.8,13,51 Local injections have their own distinct and potentially serious complications.60
Basic Science of Corticosteroids
Corticosteroids produced in vivo are formed from cholesterol-building blocks. Corticosteroids are active in the inflammatory cascade by decreasing production of leukotrienes, prostaglandins, thromboxane A2, and prostacyclin,6 and by stabilizing lysosomal membranes of inflammatory cells, decreasing local vascular permeability, and altering neutrophil chemotaxis and function.6 Corticosteroids are able to pass through cell membranes and bind to nuclear steroid receptors, where they influence RNA transcription and subsequent protein production.6,42
Clinical Use
To maximize the benefit of injection and avoid complications, the sports medicine clinician should be aware of the various corticosteroid agents available and their varying pharmacologic characteristics. Their duration of effect has been shown to be inversely related to the solubility of the agent.12,25 However, when used for soft tissue injection, low-solubility agents (which are favored for joint injections) have a high risk of changes in soft tissue and skin.12,87 The local dose of corticosteroid is not proportional to the effect seen, because excess corticosteroid not retained by local tissue is absorbed into the systemic circulation.13,94 Triamcinolone hexacetonide (Aristospan, Sandoz Inc, Princeton, New Jersey) and triamcinolone acetonide (Kenalog, Bristol-Myers Squibb Co, Princeton, New Jersey) are the least soluble agents.87 Betamethasone sodium acetate (Celestone, Schering-Plough Corp, Kenilworth, New Jersey) and dexamethasone (Decadron, Merck & Co Inc, Whitehouse Station, NJ) are the most soluble agents. Some highly soluble agents can be formulated in suspensions that prolong their duration, such as betamethasone sodium acetate / betamethasone phosphate (Celestone Soluspan, Schering-Plough Corp); methylprednisolone acetate (Depo-Medrol, Pfizer Inc, New York, New York) is most frequently used for soft tissue injections and is of intermediate solubility.12
In 1989, Hill et al35 provided valuable information on the use of corticosteroids by surveying 233 members of the American Academy of Orthopaedic Surgeons. They found that 90% of the responding orthopaedic surgeons used corticosteroid injections and performed an average of 150 intra-articular and 193 extra-articular injections per year. The most commonly injected corticosteroids were betamethasone sodium acetate (Celestone; 28%) and methylprednisolone acetate (Depo-Medrol; 22%). Conditions warranting injection included epicondylitis (93%), shoulder bursitis (91%), greater trochanter bursitis (91%), DeQuervain’s tenosynovitis (87%), bicipital tendonitis (81%), and pes anserine bursitis (78%).
The efficacy of local corticosteroids is an additional source of controversy. Some clinical conditions have been the subject of well-designed randomized controlled trials that show the efficacy of corticosteroid injections, whereas other conditions have only observational studies available.† For example, Levine et al52 reported on the use of local corticosteroid injections in 58 players in the National Football League (NFL) with severe hamstring injuries. They noted improved return to play and found no complications. However, there was no control group for comparison. The favorable natural history of many of these disorders can make results of observational studies difficult to interpret.
Consistent benefits have been shown from the use of corticosteroids in several areas.‡ A systematic review of multiple randomized studies of corticosteroid injections for lateral epicondylitis found improved short-term results (< 6 weeks), compared to local anesthetic or conservative treatment.84 In a single randomized controlled trial, similar short-term improvement was seen for medial epicondylitis.86 Interestingly, this improvement occurred despite the absence of histological evidence indicating inflammation in these conditions.4,47,61,72 Corticosteroid injections for DeQuervain’s tenosynovitis have been shown to be effective in more than 80% of cases, on the basis of a pooled literature review.74 Corticosteroid injections for trigger finger were more effective than local anesthetic injections in 2 randomized studies, with efficacy rates of 60% to 64% (compared to 16% to 20% for local anesthetic injection).49,59
Observational studies have demonstrated the rates of success with corticosteroid injections for several disorders.7,11,80 Conclusions from these studies are suspect, however, because they lack control groups. Injections for pes anserine bursitis are more effective than NSAIDs, with significant improvement in 70% of patients.11 Observational studies of corticosteroid injections for trochanteric bursitis show efficacy of 77% at 1 week and 69% at 6 weeks.80 Corticosteroid injections for Morton’s neuroma can provide at least short-term relief in about half of patients.7
Corticosteroid injections for Achilles tendinosis/tendinopathy appear to be of no benefit, having the equivalent efficacy of local anesthetic injections.16 A systematic review by Koester et al45 in 2007 found “little reproducible evidence” that subacromial corticosteroid injections were effective in treating rotator cuff disease. Randomized controlled trials of corticosteroid injections for plantar heel pain have also shown little to no short-term benefit.15
General recommendations for corticosteroid injections include use only after other nonsurgical treatments (ie, exercise, rest, oral anti-inflammatory medications) have failed and only when a discrete, palpable localization of the symptom source is identified.51 No more than 3 injections should be used, spaced several weeks apart, with repeat injections given only if previously injections provided relief.51 A period of rest or protection after the injection should be used.51 Corticosteroid injections given immediately after injury, before a competitive event, or in the presence of infection should be avoided.8,42,51 Direct injections into tendons or ligaments should never be performed owing to the risk of tendon rupture.
Side Effects and Complications
Knowing the potential side effects of corticosteroid injections is important to avoid complications and to provide adequate informed consent before their administration. Corticosteroids are generally contraindicated in the setting of a drug allergy, infection, or fracture or in areas at high risk for tendon rupture. Potential local side effects include tendon/ligament weakening or rupture, postinjection pain flare, soft tissue / subcutaneous fat atrophy, and skin hypopigmentation.60 Tendon/ligament weakening and rupture are the most significant side effect.60 Direct injection into a tendinous structure should be avoided. This side effect has the potential to cause major morbidity in the competitive athlete. Postinjection steroid flare generally occurs in the 24 to 36 hours following injection, and it is thought to be due to crystal-induced synovitis caused by precipitation of preservatives in the injected suspension.41 Fat atrophy and skin hypopigmentation are more likely at superficial injection sites, and they usually result from overinfiltration of subcutaneous tissues.51 Fat atrophy has been reported in 3% to 14% of injections. Fat atrophy and skin depigmentation generally have a delayed onset, occurring between 6 and 12 weeks after injection, and are in some cases permanent.48,51,78 Fat atrophy and skin depigmentation are more likely after injection of superficial targets and after injection with corticosteroids of low solubility.
Less common side effects include infection, vascular injury, and postinjection neuritis.19 Infection is a rare but potential side effect at any injection site and is best prevented with appropriate aseptic technique. Injection into bursal structures seems to result in the highest risk for infection,51 most likely due to a failure to clinically differentiate an early bursal infection from aseptic inflammation. Postinjection neuritis is a complication of direct injection into a nerve. Avoidance of vascular and nerve injury is avoided with proper technique and knowledge of local anatomy.
In the study by Hill et al,35 89% of orthopaedic surgeon responders reported observing a complication of corticosteroid injections in their practice. Most common complications included subcutaneous fat atrophy (64%), skin depigmentation (54%), and tendon rupture (39%). In a review of 124 corticosteroid injections in athletes, Leadbetter51 reported 4 cases of skin depigmentation, 4 instances of intratendinous/intrabursal corticosteroid precipitation (documented at subsequent surgery), and 1 case of subcutaneous atrophy. In a meta-analysis summarizing 25 studies (983 injections), Nichols60 noted a 5.5% complication rate (excluding pain). The most common side effects included skin atrophy (2.4%), skin depigmentation (0.8%), localized erythema and warmth (0.7%), and facial flushing (0.6%). Postinjection pain was noted in 9.7% of patients.
Systemic side effects of corticosteroid injections include vasovagal reactions, facial flushing, hyperglycemia, anaphylaxis, leukemoid reactions, and osteonecrosis.51,77,94 Corticosteroid injection into soft tissue structures results in more systemic absorption than does intra-articular injection.13 In diabetic patients, hyperglycemia has been shown to persist up to 5 days after a single soft tissue injection.94 Leukemoid reactions to local injections have also been observed resulting in elevated white blood cells counts as high as 20 000 white blood cells per cubic millileter,51 as has a single case of femoral osteonecrosis following multiple soft tissue injections.77
Intratendinous injections of corticosteroids have detrimental effects that can facilitate tendon rupture.5,60 Direct injection into rabbit Achilles tendons resulted in collagen necrosis, decreased tensile strength, and long-term structural changes.5 In a meta-analysis of animal studies, 4 of 10 showed deleterious effects of local corticosteroids injections on tendon.60
Peritendinous injections of corticosteroids have resulted in negative effects.57 Peritendinous injection of rabbit Achilles tendons with corticosteroids decreased failure stress, total energy absorbed, and increased total strain, similar to the negative effects of intra-tendinous injection.39 Martin et al55 also demonstrated that peritendinous injections result in altered mechanical properties. However, these properties returned to normal by 14 days postinjection.55 McWhorter et al57 found no significant histological effects of peritendinous injections of rat Achilles tendons.
There are numerous case reports and case series of tendon rupture following corticosteroid injections.60 The use of triamcinolone appears to be associated with the highest risk of tendon rupture.60 The most commonly reported ruptures involve the plantar fascia and Achilles tendon, although ruptures of other tendons have been reported, including quadriceps tendon, patellar tendon, triceps, and lateral epicondyle extensor attachment.60 Despite a need for caution, case series do not prove cause-effect relationships between corticosteroid injection and tendon rupture. Chronic tendinopathies are prone to tendon rupture.47,54,70 Corticosteroids may play a role in progression of tendon damage or in masking the symptoms of tendon damage, allowing for further mechanical stress.51 Although most authors recommend a period of rest (1-2 weeks) and avoidance of strenuous activity following corticosteroid injection,51 no studies have adequately addressed this topic. Injections immediately before competition are generally contraindicated.42,51 In addition, the maximal number of injections and the required period between injections have not been determined. Recommendations in this area rely primarily on expert opinion.66
A meta-analysis, pooling case reports of 95 major complications in 18 studies, found that the most frequent major complications were ruptures of plantar fascia (53.7%), patellar/quadriceps tendon (9.5%), Achilles tendon (8.4%), biceps tendon (8.4%), and subcutaneous atrophy (7.4%). Tendon rupture has also occurred at the biceps femoris, tibialis anterior, triceps, and finger flexors.60
Local Anesthetics
Local anesthetics decrease nerve conduction through the blockade of sodium channels, which disrupts axonal nerve conduction.22,90 They are classified as esters or amides, according to the type of linkage between the aromatic ring and side chain. Ester agents include cocaine, procaine, tetracaine, benzocaine, and 2-chloroprocaine. Their clinical use is limited for local anesthesia, owing to their relative instability in solution as well as their high rates of allergic reaction. Amide agents are more commonly used in practice and so include lidocaine, bupivacaine, prilocaine, and mepivacaine.90
Local anesthetic injections allow for an immediate assessment of pain relief for therapeutic purposes and as a confirmatory diagnostic method when the source of discomfort and injury is in question. The most commonly used agents for local injections are as follows: 1% lidocaine (Xylocaine, APP Pharmaceuticals, Wilmington, Delaware), 2% lidocaine, 0.25% bupivacaine (Marcaine, Hospira Inc, Lake Forest, Illinois), and 0.5% bupivacaine. Lidocaine has a rapid onset (1-2 minutes) and short duration (1 hour), whereas bupivacaine has a slower onset (30 minutes) and a longer duration (8 hours).87 The clinician should be aware of the signs and symptoms of anesthetic toxicity: flushing, hives, chest or abdominal discomfort, nausea, cardiac arrhythmia, and seizure.12
Local anesthetics can be used alone or in combination with corticosteroids for the combined effect of immediate relief of local pain and the longer-lasting therapeutic effect of the steroid agent. The combination also increases the distribution area of the agent, avoiding high concentrations of corticosteroid in a small area. Manufacturers recommend against mixing corticosteroid agents with lidocaine because of the risk of flocculation (clumping) and the precipitation of steroid crystals,12 which is not clinically observed with any frequency.12 Inspecting the combination for gross crystal precipitation before injection is recommended.
Reports of the use of local anesthetics for analgesic effect in sports injuries is uncommon. Orchard described the use of local anesthetics in Australian Rules football and rugby.63,64 In sum, 221 local painkilling injections were administered for game-day pain relief over 6 years (joint and soft tissue).63 An average of 1.7 players (10.7% of all team members) took the field with the aid of a bupivacaine injection (with or without epinephrine injection) per game.63 Eighty-six injections (38.9% of all injections) were performed for injuries occurring during the course of a game. The most common sites of injection were the rib, iliac crest, acromioclavicular joint, finger/thumb, and ankle. Six major complications and 11 minor complications resulted: distal clavicle osteolysis following acromioclavicular joint injection (n = 2), partial rupture of the Achilles tendon following local injection for tendinopathy, chronic adductor tendinopathy following local injection for a partial tear, prepatellar bursal infection, and carpal/radiocarpal degenerative disease after local injection for a scapholunate ligament tear done in an attempt to delay surgery until the offseason. Minor complications included inadvertent blocks of the lateral femoral cutaneous nerve following an iliac crest hematoma injection (n = 3), inadvertent partial sensory nerve blocks of the ankle (n = 2), worsening of a first metacarpal fracture and sternoclavicular sprain, and rupture of the plantar fascia. They concluded that low-risk, high-benefit injections can be achieved at the following areas: acromioclavicular joint, fingers, second through fifth metacarpals, ribs/sternum, iliac crest, and plantar fasciitis. Of note, subsequent plantar fascia rupture was not clinically significant in this population. Injections of ankle sprains, tendon injuries, prepatellar/olecranon bursitis, first metacarpal injuries, and radiocarpal injuries were described as being high risk.
The International Rugby Board has subsequently banned painkilling injections.64 Most other governing bodies, including the NFL and the National Collegiate Athletic Association, leave their use to the discretion of the treating team physician.64 It is the responsibility of the physician and medical staff to appropriately educate the athlete on the risks and benefits of anesthetic injection and to avoid reckless injections around regional motor or sensory nerves, major weightbearing joints, and in or around fractures. A 1992 NFL Players Association survey found that 45% of players received local anesthetic injections immediately before competition at some point during their careers.64 Current information on the prevalence of their use in the NFL has not been reported. Orchard63 documented 5 litigation cases concerning the long-term negative consequences of local anesthetic injections in American professional sports (including the NFL and the National Basketball Association). As with corticosteroids, local anesthetic injection into injured tendon, ligament, and muscle is likely to be associated with an increased risk of rupture or worsening of the traumatic or degenerative process.63 Removal of pain as a feedback mechanism exposes the already compromised structure to the extreme forces seen with sporting competition.
Toradol
NSAIDs are among the oldest and most widely prescribed drugs in medicine. The first NSAID was extracted from the bark of willow trees.92 Since then, numerous NSAIDs have been developed with varying pharmacokinetics. NSAIDs have anti-inflammatory and analgesic effects and can be classified as either acidic or nonacidic.67 Acidic NSAIDs are further subdivided into carboxylic acids and enolic acids, which include Toradol. No single NSAID has been shown to be consistently more effective than another.8,51
Toradol is the only NSAID currently available in an intramuscular or intravenous form in the United States since its approval by the Food and Drug Administration in 1990. Although it is available for oral use, intramuscular administration is most prevalent in the sports medicine arena. In its intramuscular form, it is a potent analgesic, with rapid onset of pain relief within 10 minutes, with peak concentration and clinical effects seen approximately 45 minutes after injection, and with a half-life of 6.5 hours.68 It is 99% plasma bound and has hepatic metabolism and renal excretion. Its oral dosage is 10 mg, whereas the injectable dosage is 30 mg.68 A recommended maintenance dose for adults who weigh more than 110 pounds (50 kg) and are less than 65 years old is 30 mg every 6 hours.68
Basic Science of NSAIDs
NSAIDs are generally weak acids, and they bind avidly to albumin. The basic chemical structure of NSAIDs consists of an aromatic ring with an acidic functional side group.67 It is this hydrophobic portion that makes the liquid formulation of NSAIDs difficult to synthesize.67 NSAIDs generally inhibit the COX pathway, thus preventing the formation of prostaglandin, prostacyclin, and thromboxane.51,58,67 Nonselective NSAIDs, such as Toradol, block the COX-1 and COX-2 pathways. However, the lipoxygenase pathway is not affected.
NSAIDs affect neutrophil function, including aggregation, migration, lysosomal enzyme release, oxidative phosphorylation, and the chemotactic response.8,21,22,51 The inhibition of the COX-1 pathway is responsible for the major side effects of NSAIDs, including decreased cytoprotection in the gastrointestinal tract, alteration in regulation of renal blood flow and water resorption, and increased bleeding owing to decreased platelet adhesiveness.8,21,22,51 The pain produced by inflammation is thought to be due to sensitization of peripheral nociceptors.21,67 The analgesic effect of NSAIDs is due to decreased sensitivity of afferent nerve receptors caused by reduction in prostaglandin levels.21,67 NSAIDs have also been shown to inhibit the NF-KB (nuclear factor–kappa B) system,46,75,89 which has been shown to play a role in the activation of cytokines, the initiation of the inflammatory process, and the recruitment of leukocytes.
Bone injury also results in an inflammatory response that culminates in fracture healing. Prostaglandins (especially, prostaglandin E2) play a major role in fracture repair.58 Multiple studies1,3,24,36,56 have demonstrated the negative impact of a variety of NSAIDs on fracture healing in animals. In humans, various retrospective studies have shown an association between delayed union/nonunion and the use of NSAIDs44 on fractures of the femur,31 tibia,10 clavicle,43 and acetabulum,9 as well as on spinal fusion.18,32,73 Two retrospective studies have demonstrated that Toradol significantly increased the risk of nonunion after spinal arthrodesis.32,73 Therefore, use of this agent in athletes recovering from a fracture, including stress fractures, should be carefully considered.58,96
The effect of NSAIDs on soft tissue injuries is more controversial. Animal studies have produced contradictory results.2,17,23,33 Many human studies demonstrate variable small improvements in pain relief and earlier return to play.58,95 A consistent beneficial result from an anti-inflammatory has not been demonstrated.
The effect of NSAIDs on chronic tendon injury or tendinopathies is controversial. NSAIDs may function primarily as an analgesic in these conditions owing to the absence of inflammatory cells.58,71,95 Their value in acute peritendinitis and tenosynovitis, such as De Quervain’s tenosynovitis, may be through an analgesic and anti-inflammatory effect.50,58
The effect of NSAIDs on ligament healing is unclear, with basic science evidence again being contradictory. Several animal studies have demonstrated positive and negative effects, but most have shown no significant effect on ligamentous healing.17,23,33 Human studies have shown NSAIDs to be effective in decreasing pain and allowing faster return to play after ankle sprains, with no significant long-term benefits.58,65,83 As with tendinopathies, the positive clinical effect of NSAIDs on acute ligamentous sprains may relate solely to their analgesic effects.
The effect of NSAIDs on muscle healing is also controversial. Muscle injury results in tissue necrosis and hematoma formation. The inflammatory response is responsible for the degradation of this tissue to allow muscle regeneration.40 Animal studies have shown that NSAID use improves contractile force and increases maximal failure force early after injury, whereas, histologically, it results in a delay in collagen deposition, degradation of damaged tissue, and muscle regeneration.2,62 In human studies, NSAIDs have generally been shown to decrease pain, improve early muscle strength recovery, and allow early return to sport.58,65,95 There are no known long-term positive or negative effects of NSAIDs.58 Short courses (7-14 days) of high doses of various NSAIDs,29,69,76,93 including Toradol,69 can be effective in preventing heterotopic ossification after total hip arthroplasty. A similar protection against the formation of myositis ossificans after major muscle contusions is speculated but lacks definitive evidence.58
Clinical Use
Limited published information is available on Toradol use in athletics. Toradol use is common in the NFL, as described in a 2002 study by Powell et al.68 In their survey of NFL medical staffs during the 2000 season, 28 of 30 responding teams used intramuscular Toradol in the pregame setting. An average of 15 players per team received Toradol injections, up to once a week. The majority of teams (24 of 27) allowed 1 injection per week throughout the duration of the season. Most medical staffs believed that Toradol provided 50% to 75% pain relief that lasted 1 to 2 days. Six of the 28 teams that used Toradol reported minor complications: gastrointestinal irritation, generalized soreness, and muscle injuries (including strains and worsening contusions). Significant bleeding and renal complications were not reported, but several teams saw “psychological addiction”68 to game-day Toradol injections. Reports on Toradol usage since 2000 are not available, and current levels of use are unknown. Data are not available on the use of Toradol in other professional or collegiate sports, but its use is speculated.68
Toradol is contraindicated for a traumatic or stress fracture at high risk for nonunion (eg, fifth metatarsal, navicular, anterior tibia) or chronic muscle injury.58 Other contraindications include a hypersensitivity to NSAIDs (triad syndrome of NSAID hypersensitivity), allergic rhinitis, and nasal polyps. Such individuals are at high risk for serious reactions.26 Toradol should also be avoided in patients with peptic ulcer disease, hepatic disease (hepatitis, cirrhosis), and diminished renal function.
Side Effects and Complications
The side effects of Toradol are the same as those for other NSAIDs and so reflect the systemic nature of its action. Gastrointestinal side effects include dyspepsia and, more seriously, gastrointestinal bleeding and ulceration. Gastrointestinal side effects are apparent in up to 20% of individuals with extended use, with peptic ulcer disease developing in 1% to 2%. These medications can also worsen asthma in at-risk individuals.21 Side effects are more likely with higher doses and longer duration of use, as well as in individuals whose comorbidities put them at increased risk.21,28,88 Toradol use exceeding 5 days is associated with an increased risk of renal and gastrointestinal side effects, which has led to the recommendation that its administration not exceed this duration.28,88
NSAIDs have also been associated with renal dysfunction, including acute renal failure.28 They can decrease the glomerular filtration rate by up to 11% in normal healthy adults subjected to strenuous exercise, dehydration, and salt restriction.27 In the volume-depleted state, the kidney is in a prostaglandin-dependent state and thus more likely to react adversely to NSAIDs. This setting is particularly relevant to the athlete who is involved in strenuous competition. Of equal concern are the possible renal complications of Toradol use in athletes without routine laboratory testing.68
As with the majority of other NSAIDs, Toradol is associated with a reduction in platelet function and blood clotting.14,20,82,85,91 Toradol decreases platelet aggregation and thromboxane production.91 Because Toradol affects only platelet function, it does not change the prothrombin time, partial thromboplastin time, or platelet count.85 Similar to aspirin, a single dose of Toradol can increase bleeding time in healthy patients (0% to 60%).20,82,85 Despite this elevation, bleeding times generally remain within the normal range (up to 10 minutes) in healthy individuals. Toradol binding to platelets is reversible; that is, platelet function and bleeding times return to baseline values within 24 hours of discontinuation.14 This is in contrast to aspirin, which may cause platelet dysfunction for up to 10 days.67
The clinical relevance of the prolongation of bleeding times in unclear20,82,85,91 because it has not correlated with operative blood loss.53,88 The combination of Toradol with additional oral NSAIDs (either prescription or over-the-counter) has not been studied but has the potential to increase bleeding and renal complications.21 The risks are also unknown of Toradol use with various supplements and herbal medications commonly used by athletes.
The bleeding risk of Toradol is an utmost concern in collision sports such as football. Even a small increase in bleeding risk can exacerbate high-risk injuries such as concussions, spinal cord, spleen, and kidney trauma. Pre-game Toradol injections can increase this risk, although it has not been objectively studied. Therefore, these injections should be carefully considered in the competitive setting with a thorough discussion of the potential risks and complications before the game situation, when the athlete can make an informed decision not influenced by the emotional ramifications of an impending competitive event.
Conclusion
Soft tissue injections of corticosteroids, local anesthetics, and Toradol are commonly used in sports medicine. Knowledge of their mechanism, efficacy, and complications is essential for proper use. Local injections of corticosteroids have the potential to decrease inflammation. Anesthetic agents, similar to corticosteroids, are typically used to lessen the pain of musculoskeletal injury to facilitate athletic participation. The primary concern with corticosteroid and anesthetic injections adjacent to tendons is an increased risk of tendon rupture. Intramuscular Toradol provides significant analgesia as well as an anti-inflammatory effect via its inhibitory effect on the cyclooxygenase pathway. The risk of bleeding associated with Toradol use is recognized but not adequately quantified, thereby raising concern regarding its use in collision sports.
Footnotes
References
- 1. Allen H, Wase A, Bear W. Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand. 1980;51:595-600 [DOI] [PubMed] [Google Scholar]
- 2. Almekinders LC, Gilbert JA. Healing of experimental muscle strains and the effects of nonsteroidal anti-inflammatory medication. Am J Sports Med. 1986;14(4):303-308 [DOI] [PubMed] [Google Scholar]
- 3. Altman RD, Latta LL, Keer R, et al. Effect of nonsteroidal anti-inflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9:392-400 [DOI] [PubMed] [Google Scholar]
- 4. Armstrong T, Devor W, Borschel L, Contreras R. Intracarpal steroid injection is safe and effect for short-term management of carpal tunnel syndrome. Muscle Nerve. 2004;29(1):82-88 [DOI] [PubMed] [Google Scholar]
- 5. Balasubramaniam P, Prathap K. The effect of injection of hydrocortisone into rabbit calcaneal tendons. J Bone Joint Surg Br. 1972;54:729-734 [PubMed] [Google Scholar]
- 6. Baxter JD, Forsham PH. Tissue effects of glucocorticoids. Am J Med. 1972;53(5):573-589 [DOI] [PubMed] [Google Scholar]
- 7. Bennett GL, Graham CE, Mauldin DM. Morton’s interdigital neuroma: a comprehensive treatment protocol. Foot Ankle Int. 1995;16(12):760-763 [DOI] [PubMed] [Google Scholar]
- 8. Buckwalter JA. Current concepts review: pharmacological treatment of soft-tissue injuries. J Bone Joint Surg Am. 1995;77(12):1902-1914 [DOI] [PubMed] [Google Scholar]
- 9. Burd T, Hughes M, Anglen J. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700-705 [PubMed] [Google Scholar]
- 10. Butcher CK, Marsh DR. Nonsteroidal anti-inflammatory drugs delay tibial fracture union. Injury. 1996;27:375 [Google Scholar]
- 11. Calvo-Alen J, Rua-Figueroa I, Erausquin C. Anserine bursitis treatment: local corticosteroid injection against NSAID: a prospective study. Rev Esp Reumatol. 1993;20:13-15 [Google Scholar]
- 12. Cardone DA, Tallia AF. Joint and soft tissue injection. Am Fam Physician. 2002;66(2):283-288 [PubMed] [Google Scholar]
- 13. Cole BJ, Schumaker R. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13(1):37-46 [DOI] [PubMed] [Google Scholar]
- 14. Concannon MJ, Meng L, Welsh CF, Puckett CL. Inhibition of perioperative platelet aggregation using Toradol (ketorolac). Ann Plast Surg. 1993;3: 264-266 [DOI] [PubMed] [Google Scholar]
- 15. Crawford F, Thomson CE. Intervention for treating plantar heel pain. Cochrane Database Syst Rev. 2000;3:CD000416. [DOI] [PubMed] [Google Scholar]
- 16. DaCruz DJ, Geeson M, Allen MJ, Phair I. Achilles paratendinitis: an evaluation of steroid injection. Br J Sports Med. 1988;22(2):64-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dahners LE, Gilbert JA, Lester GE, Taft TN, Payne LZ. The effect of a nonsteroidal anti-inflammatory drug on the healing of ligaments. Am J Sports Med. 1988;16(6):641-646 [DOI] [PubMed] [Google Scholar]
- 18. Deguchi M, Rapoff AJ, Zdeblick TA. Posterolateral fusion for isthmic spondylolisthesis in adults: analysis of fusion rate and clinical results. J Spinal Disord. 1998;11(6):459-464 [PubMed] [Google Scholar]
- 19. Dietzel DP, Hedlund EC. Injections and return to play. Curr Sports Med Rep. 2004;3:310-315 [DOI] [PubMed] [Google Scholar]
- 20. Dordoni P, Della Ventura M, Stefanelli A, et al. Effect of ketorolac, ketoprofen and nefopam on platelet function. Anaesthesia. 1994;49:1046-1049 [DOI] [PubMed] [Google Scholar]
- 21. Dugowson CE, Gnanashanmugam P. Nonsteroidal anti-inflammatory drugs. Phys Med Rehabil Clin N Am. 2006;17:347-354 [DOI] [PubMed] [Google Scholar]
- 22. Ekman EF, Koman LA. Acute pain following musculoskeletal injuries and orthopaedic surgery: mechanisms and management. J Bone Joint Surg Am. 2005;86(6):1316-1327 [PubMed] [Google Scholar]
- 23. Elder Cl, Dahners LE, Weinhold PS. A cyclooxygenase-2 inhibitor impairs ligament healing in the rat. Am J Sports Med. 2001;29(6):801-805 [DOI] [PubMed] [Google Scholar]
- 24. Elves M, Bayley I, Roylance P. The effect of indomethacin upon experimental fractures in the rat. Acta Orthop Scand. 1982;53:35-41 [DOI] [PubMed] [Google Scholar]
- 25. Fadale PD, Wiggins ME. Corticosteroid injections: their use and abuse. J Am Acad Orthop Surg. 1994;2:133-140 [DOI] [PubMed] [Google Scholar]
- 26. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24;113-124 [DOI] [PubMed] [Google Scholar]
- 27. Farquhar WB, Morgan AL, Zambraski EJ, Kenney WL. Effects of acetaminophen and ibuprofen on renal function in the stressed kidney. J Appl Physiol. 1999;86:598-604 [DOI] [PubMed] [Google Scholar]
- 28. Feldmann HI, Kinman JL, Berlin JA, et al. Parenteral Ketorolac: the risk for acute renal failure. Ann Intern Med. 1997;126:193-199 [DOI] [PubMed] [Google Scholar]
- 29. Fijn R, Koorevaar RT, Brouwers JR. Prevention of heterotopic ossification after total hip replacement with NSAIDs. Pharm World Sci. 2003;25(4): 138-145 [DOI] [PubMed] [Google Scholar]
- 30. Garrett WE. Muscle strain injuries. Am J Sports Med. 1996;24(6)(suppl): S2-S8 [PubMed] [Google Scholar]
- 31. Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, DeBoer P. Nonunion of the femoral diaphysis: the influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000;82:655-658 [DOI] [PubMed] [Google Scholar]
- 32. Glassman SD, Rose SM, Dimar JR, Puno RM, Campbell MJ, Johnson JR. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23(7):834-838 [DOI] [PubMed] [Google Scholar]
- 33. Hanson CA, Weinhold PS, Afshari HM, Dahners LE. The effect of analgesic agents on the healing rat medial collateral ligament. Am J Sports Med. 2005;33(5):674-679 [DOI] [PubMed] [Google Scholar]
- 34. Hench PS. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dihydrocortisone: compound E) and of a pituitary adrenocorticotropic hormone on rheumatoid arthritis: preliminary report. Mayo Clin Proc. 1949;24:181-197 [PubMed] [Google Scholar]
- 35. Hill JJ, Jr, Trapp RG, Colliver JA. Survey on the use of corticosteroid injections by orthopaedists. Contemp Orthop. 1989;18:39-45 [Google Scholar]
- 36. Hogevold H, Grogaard B, Reikeras O. Effects of short-term treatment with corticosteroids and indomethacin on bone healing: a mechanical study of osteotomies in rats. Acta Orthop Scand. 1992;63:607-611 [DOI] [PubMed] [Google Scholar]
- 37. Hollander JL. Intra-articular hydrocortisone in arthritis and allied conditions: a summary of two years’ clinical experience. J Bone Joint Surg Am. 1953;35:983-990 [PubMed] [Google Scholar]
- 38. Hollander JL, Jessar RA, Brown EM. Intra-synovial corticosteroid therapy: a decade of use. Bull Rheum Dis. 1961;11:23-40 [PubMed] [Google Scholar]
- 39. Hugate R, Pennypacker J, Saunders M, Juliano P. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am. 2004;86:794-801 [DOI] [PubMed] [Google Scholar]
- 40. Jarvinen TA, Jarvinen TL, Kariainen M, Kalimo H, Jarvinen M. Muscle Injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764 [DOI] [PubMed] [Google Scholar]
- 41. Kahn CB, Hollander JL, Schumacher HR. Corticosteroid crystals in synovial fluid. JAMA. 1970;211(5):807-809 [PubMed] [Google Scholar]
- 42. Kerlan RK, Glousman RE. Injections and techniques in athletic medicine. Clin Sports Med. 1989;3:541-560 [PubMed] [Google Scholar]
- 43. Khan M. Fracture healing: role of NSAIDs. Am J Orthop. 1997;26:413. [PubMed] [Google Scholar]
- 44. Koester MC, Spindler KP. Pharmacologic agents in fracture healing. Clin Sports Med. 2005;25(1):63-73 [DOI] [PubMed] [Google Scholar]
- 45. Koester MC, Dunn WR, Kuhn JE, Spindler KP. The efficacy of subacromial corticosteroid Injection in the treatment of rotator cuff disease: a systematic review. J Am Acad Orthop Surg. 2007;15:3-11 [DOI] [PubMed] [Google Scholar]
- 46. Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956-959 [DOI] [PubMed] [Google Scholar]
- 47. Kraushaar BS, Nirchl RP. Current concepts review: tendinosis of the elbow (tennis elbow): clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81:259-278 [PubMed] [Google Scholar]
- 48. Kumar N, Newman RJ. Complications of intra- and peri-articular steroid injections. Br J Gen Pract. 1999;49(443):465-466 [PMC free article] [PubMed] [Google Scholar]
- 49. Lambert MA, Morton RJ, Sloan JP. Controlled study of the use of local steroid injection in the treatment of trigger finger and thumb. J Hand Surg [Br]. 1992;17(1):69-70 [DOI] [PubMed] [Google Scholar]
- 50. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg [Br]. 2001;26:258-260 [DOI] [PubMed] [Google Scholar]
- 51. Leadbetter WB. Anti-inflammatory therapy in sports injury. Clin Sports Med. 1995;14(2):353-410 [PubMed] [Google Scholar]
- 52. Levine WN, Bergfeld JA, Tessendorf W, et al. Intramuscular corticosteroid Injection for hamstring injuries. Am J Sports Med. 2000;28(3):297-300 [DOI] [PubMed] [Google Scholar]
- 53. Lind SE. The bleeding time does not predict surgical bleeding. Blood. 1991;77:2547-2552 [PubMed] [Google Scholar]
- 54. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy.1098:14(8):840-843 [DOI] [PubMed] [Google Scholar]
- 55. Martin DF, Carlson CS, Berry J, Smith BP. Effect of injected versus iontophoretic corticosteroid on the rabbit tendon. South Med J. 1999;92(6):600-608 [DOI] [PubMed] [Google Scholar]
- 56. Martin GJ, Jr, Boden SD, Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti- inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine. 1999;24(21):2188-2193 [DOI] [PubMed] [Google Scholar]
- 57. McWhorter W, Francis RS, Heckmann RA. Influence of local steroid injections on traumatized tendon properties: a biomechanical and histological study. Am J Sports Med. 1991;19:435-439 [DOI] [PubMed] [Google Scholar]
- 58. Mehallo CJ, Drezner JA, Bytomski JR. Practical management: nonsteroidal antiinflammatory drug (NSAID) use in athletic injuries. Clin J Sport Med. 2006;16(2):170-174 [DOI] [PubMed] [Google Scholar]
- 59. Murphy D, Failla JM, Koniuch MP. Steroid versus placebo injection for trigger finger. J Hand Surg [Am]. 1995;20(4):628-631 [DOI] [PubMed] [Google Scholar]
- 60. Nichols AW. Complications associated with the use of corticosteroids in the treatment of athletic injuries. Clin J Sport Med. 2005;15(5):370-375 [DOI] [PubMed] [Google Scholar]
- 61. Nirschl RP, Pettrone FA. Tennis elbow: the surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832-839 [PubMed] [Google Scholar]
- 62. Obremsky WT, Seaber AV, Ribbeck BM, Garrett WE. Biomechanical and histologic assessment of a controlled muscle strain injury treated with piroxicam. Am J Sports Med. 1994;22(4):558-561 [DOI] [PubMed] [Google Scholar]
- 63. Orchard JW. Benefits and risks of using local anesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36:209-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Orchard JW. Is it safe to use local anesthetic painkilling injections in professional football? Sports Med. 2004;34(4):209-219 [DOI] [PubMed] [Google Scholar]
- 65. Petrella R, Ekman EF, Schuller R, Fort JG. Efficacy of celecoxib, a COX-2-specific inhibitor, and naproxen in the management of acute ankle sprain: results of a double-blind, randomized controlled trial. Clin J Sport Med. 2004;14:225-231 [DOI] [PubMed] [Google Scholar]
- 66. Phillips B, Ball C, Sackett D. Levels of Evidence. Oxford, UK: Centre for Evidence-Based Medicine; 2001 [Google Scholar]
- 67. Phillips WJ, Currier BL. Analgesic pharmacology: II specific analgesics. J Am Acad Orthop Surg. 2004;12:221-233 [DOI] [PubMed] [Google Scholar]
- 68. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;4:191. [DOI] [PubMed] [Google Scholar]
- 69. Pritchett JW. Ketorolac prophylaxis against heterotopic ossification after hip replacement. Clin Orthop Relat Res. 1995;314:162-165 [PubMed] [Google Scholar]
- 70. Puddu G, Ippolito E, Postacchini F. A classification of Achilles tendon disease. Am J Sports Med. 1976;4(4):145-150 [DOI] [PubMed] [Google Scholar]
- 71. Rahusen FT, Weinhold PS, Almekinders LC. Nonsteroidal anti-inflammatory drugs and acetaminophen in the treatment of an acute muscle injury. Am J Sports Med. 2004;32(8):1856-1859 [DOI] [PubMed] [Google Scholar]
- 72. Regan W, Wold LE, Coonrad R, Morrey BF. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992;20(6):746-749 [DOI] [PubMed] [Google Scholar]
- 73. Reuben SS, Ablett D, Kaye R. High dose nonsteroidal anti-inflammatory drugs compromise spinal fusion. Can J Anaesth. 2005;52(5):506-512 [DOI] [PubMed] [Google Scholar]
- 74. Richie CA, Briner WW. Corticosteroid injection for the treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16(2):102-106 [DOI] [PubMed] [Google Scholar]
- 75. Roman-Blas JA, Jimenez SA. NF-KB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839-848 [DOI] [PubMed] [Google Scholar]
- 76. Romano CL, Duci D, Romano D, Mazza M, Meani E. Celecoxib versus indomethacin in the prevention of heterotopic ossification after total hip arthroplasty. J Arthroplasty. 2004;19(1):14-18 [DOI] [PubMed] [Google Scholar]
- 77. Roseff R, Canoso JJ. Femoral osteonecrosis following soft tissue corticosteroid infiltration. Am J Med. 1984;77(6):1119-1120 [DOI] [PubMed] [Google Scholar]
- 78. Rostron PK, Calver RF. Subcutaneous atrophy following methylprednisolone injection in Osgood-Schlatter epiphysitis. J Bone Joint Surg Am. 1979;61(4):627-628 [PubMed] [Google Scholar]
- 79. Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87(1):187-202 [DOI] [PubMed] [Google Scholar]
- 80. Shbeeb MI, O’Duffy JD, Michet CJ, Matteson EL. Evaluation of glucocorticoid injection for the treatment of trochanteric bursitis. J Rheumatol. 1996;23(12):2104-2106 [PubMed] [Google Scholar]
- 81. Shearn MA. Nonsteroidal anti-inflammatory agents; nonopiate analgesics; drugs used in gout. In: Katzung BG, ed. Basic and Clinical Pharmacology. 2nd ed. Los Altos, CA: Lange Medical Publications; 1984:402 [Google Scholar]
- 82. Singer, et al. Ketorolac and bleeding times. Am J Emergency Med. 2003;21(5):431-442 [Google Scholar]
- 83. Slatyer MA, Hensley MJ, Lopert R. A randomized controlled trial of piroxicam in the management of acute ankle sprain in Australian Regular Army recruit. Am J Sports Med. 1997;25:544-553 [DOI] [PubMed] [Google Scholar]
- 84. Smidt N, Assendelt WJ, van der Windt DA, Bouter LM. Corticosteroid injections for lateral epicondylitis: a systematic review. Pain. 2002;96(1-2):23-40 [DOI] [PubMed] [Google Scholar]
- 85. Spowart K, Greer IA, McLaren M, et al. Haemostatic effects of ketorolac with and without concomitant heparin in normal volunteers. Thromb and Haemost. 1988;60:382-386 [PubMed] [Google Scholar]
- 86. Stahl S, Kaufman T. The efficacy of an injection of steroids for medial epicondylitis: a prospective study of sixty elbows. J Bone Joint Surg Am. 1997;79(11):1648-1652 [DOI] [PubMed] [Google Scholar]
- 87. Stephens MB, Beutler AI, O’Connor FG. Musculoskeletal injections: a review of the evidence. Am Fam Physician. 2008;78(8):971-976 [PubMed] [Google Scholar]
- 88. Strom BL, Berlin JA, Kinman PW, et al. A post-marketing surveillance study: parenteral ketorolac and risk of gastrointestinal bleeding and operative site bleeding. JAMA. 1996;275:376-382 [PubMed] [Google Scholar]
- 89. Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057-2072 [DOI] [PubMed] [Google Scholar]
- 90. Tetzlaff JE. The pharmacology of local anesthetics. Anesthesiol Clin North America. 2000;18(2):217-233 [DOI] [PubMed] [Google Scholar]
- 91. Thwaites BK, Nigus DB, Bouska GW, Mongan PD, Ayala EF, Merrill GA. Intravenous ketorolac tromethamine worsens platelet function during knee arthroscopy under spinal anesthesia. Anesth Analg. 1996;82(6):1176-1181 [DOI] [PubMed] [Google Scholar]
- 92. Vane JR. The fight against rheumatism: from willow bark to COX-1 sparing drugs. J Physiol Pharmacol. 2000;51(4-1):573-586 [PubMed] [Google Scholar]
- 93. Vielpeau C, Joubert JM, Hulet C. Naproxen in the prevention of heterotopic ossification after total hip replacement. Clin Orthop Relat Res. 1999;369:279-288 [DOI] [PubMed] [Google Scholar]
- 94. Wang AA, Hutchinson DT. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. J Hand Surg [Am]. 2006;31(6):979-981 [DOI] [PubMed] [Google Scholar]
- 95. Weiler JM. Medical modifiers of sports injury: the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in sports soft-tissue injury. Clin Sports Med. 1992;11(3):625-644 [PubMed] [Google Scholar]
- 96. Wheeler P, Batt ME. Do non-steroidal anti-inflammatory drugs adversely affect stress fracture healing? A short review. Br J Sports Med. 2005;39:65-69 [DOI] [PMC free article] [PubMed] [Google Scholar]