Abstract
Context:
Community-associated methicillin-resistant Staphlococcus aureus (CA-MRSA) has become of increasing concern in the athletic setting. Appropriate recognition, treatment, and prevention measures are all paramount to protect individual athletes and teamwide outbreaks.
Evidence Acquisition:
Relevant electronic databases (Medline or PubMed) through 2008 were searched. Articles and studies relevant to this topic were reviewed for pertinent clinical information.
Study Type:
Clinical review.
Results:
CA-MRSA is an increasing problem both in the community at large and in the athletic population.
Conclusion:
Early infections based on methicillin-resistant Staphlococcus aureus are often misidentified, leading to delay in appropriate treatment. A high level of suspicion, prompt recognition, and appropriate treatment can minimize morbidity associated with CA-MRSA. Careful selection of antibiotics in suspected cases is important, with more severe infections requiring hospitalization and intravenous antibiotics. Eradication of bacteria in colonized patients has not yet proven to be effective. Prevention of infections is multifaceted, and it includes education, proper personal hygiene, routine cleaning of equipment, and proper wound care.
Keywords: methicillin-resistant Staphlococcus aureus, treatment, prevention
Methicillin-resistant Staphylococcus aureus (MRSA) was first described in the 1960s as a nosocomial pathogen. In the early 1980s, infections based on community-associated MRSA (CA-MRSA) began emerging. These infections were related to the spread of MRSA from hospitals into the larger community.5 However, since the mid-1990s, a highly pathogenic community-acquired organism without health care association has emerged.23,30 Today the prevalence of CA-MRSA infections are on the rise and becoming a major public health threat.15 These infections are increasing worldwide. In many emergency rooms throughout the United States, this organism is identified as the primary pathogen in skin-related infections.28
The emergence of CA-MRSA poses a unique challenge for the clinician. The increasing prevalence of CA-MRSA in many communities, coupled with the organism’s unique pattern of virulence, antibiotic resistance, and clinical presentation, has important treatment implications.
Case
A professional athlete presents to the trainer with multiple pruritic “spider” bites on the posterior aspect of his upper thigh, acquired while sleeping. The trainer initially treated him symptomatically, and within 24 hours, the affected areas had developed induration and erythema. The team physician evaluated him and administered oral antibiotics. The athlete’s symptoms progressed within 48 hours to include increased pain, surrounding erythema, and multiple abscesses. He was admitted to the hospital, where an incision and drainage procedure was performed. A copious amount of pus was expressed from multiple sites. Intravenous antibiotics were started. Wound culture results revealed a heavy growth of MRSA susceptible to trimethoprim/sulfamethoxazole. He was released from the hospital and followed in the training room. He completed a course of trimethoprim/sulfamethoxazole and returned to full play without residual disability.
Unfortunately, this is not a unique case. The experience of the St Louis Rams (professional football team), as published in the New England Journal of Medicine in 2005, brought to the forefront the emergence of the “superbug” in the athletic arena.24 Other publications (scientific journals and general media) have supported the increasing prevalence of MRSA in the athletic population.1,10,24,26,32
Characteristics of CA-MRSA
CA-MRSA has characteristics that are distinct from those encountered in cases of health care–associated MRSA (HA-MRSA), which suggests that most CA-MRSA isolates did not originate from nosocomial strains. Observed differences have demonstrated that the majority of CA-MRSA isolates carry the mecA gene on the highly mobile type IV staphylococcal chromosomal cassette, whereas the HA-MRSA typically carries the gene on the type II staphylococcal chromosomal cassette.16,29 The mecA gene encodes for the methicillin-resistance penicillin-binding protein. The smaller staphylococcal chromosomal cassette associated with the majority of CA-MRSA has less ability to carry antimicrobial-resistance genes, likely resulting in a greater number of antibiotic susceptibility observed with CA-MRSA.12,16 This is in contrast to the larger type II staphylococcal cassette that is typically carried by the HA-MRSA organisms, allowing for a greater resistance to multiple antimicrobial classes. CA-MRSA strains are typically resistant to beta lactams and 1 or 2 other drug classes. Of increasing concern are multidrug-resistant USA 300 isolates.2,17 The identification of multidrug-resistant CA-MRSA further emphasizes the importance of mandating prevention techniques in training facilities and locker rooms, surveillance, and rapid identification and treatment of skin and soft tissue infections in athletes.
Although a number of USA strains of CA-MRSA have been identified, the Centers for Disease Control and Prevention reported at least 3 US strains are more prevalent. Pulsed field gel electrophoresis has identified USA 300 clone as the predominant CA-MRSA strain, whereas USA 100 is more common in HA-MRSA infections.25,28 CA-MRSA strains frequently express genes that encode for a virulence factor known as Panton-Valentine leukocidin. This cytotoxin creates destructive holes in leukocytes and has been associated with soft tissue infections and necrotizing pneumonia.33,35
Risk Factors for Developing CA-MRSA
The Centers for Disease Control and Prevention has developed criteria that can assist in distinguishing HA-MRSA from CA-MRSA.7 Table 1 highlights the most common distinguishing criteria.
Table 1.
Criteria to distinguish between HA-MRSA and CA-MRSA.a
| Criteria | HA-MRSA | CA-MRSA |
|---|---|---|
| Hospitalization within last year | × | |
| Recent surgery | × | |
| Residency in nursing home or skilled-facility | × | |
| Positive culture of MRSA within 48 hours after hospital admission | × | |
| No permanent indwelling catheters or medical device | × | |
| No medical history of MRSA infection or colonization | × |
MRSA, methicillin-resistant Staphylococcus aureus; HA, health care associated; CA, community associated.
CA-MRSA infections have been identified in healthy individuals of all ages who have not had recent exposure to health care facilities.6,30 At-risk populations have been identified and shown to have an increased prevalence of CA-MRSA infections; these populations include day care attendees, military personnel, correctional facility inmates, males having sex with other males, those living in crowded settings, and athletes (Table 2).4,9,18,27
Table 2.
At-risk population for community-associated methicillin-resistant Staphylococcus aureus.
| Athletes |
| Day care attendees |
| Military personnel |
| Inmates at correctional facilities |
| Males having sex with other males |
| Persons living in crowded settings |
Risk of exposure to the organism has been described through transmission routes both direct and indirect. Direct exposure can occur if the athlete comes into contact with a draining lesion or touches an object contaminated by the infected skin. Other, less obvious exposure can occur when the athlete shares an unwashed towel, shaves with a contaminated item, or shares other personal hygiene items. Risk can also increase with athletes who share nonsanitized training equipment, whirlpools, and rehab equipment. MRSA has been shown to survive for days to months on inanimate objects, and cultures obtained from ultrasound gel, countertops, and exercise equipment have been positive for MRSA.8,24 All these common components in the athletic environment place athletes and staff at increase risk of exposure and potential infection.
People who have chronic or recent skin conditions may be at increased risk for MRSA because the natural skin barrier is compromised. Athletic activity often results in skin injury that creates a point of entry for the organism (eg, cuts and abrasions). Athletes who participate in contact sports that have prolonged physical contact (eg, football) are more likely to acquire MRSA infections.
Colonization also may play a role in infectivity. Studies have reported that 25% to 30% of the general population is colonized at any given time with methicillin-sensitive S aureus. Of those colonized, less than 1% are colonized with MRSA.22,30 In addition to the nares, MRSA has been found on skin, pharynx, gastrointestinal tract, axilla, perineum, and rectum (Figure 1).19,31
Figure 1.
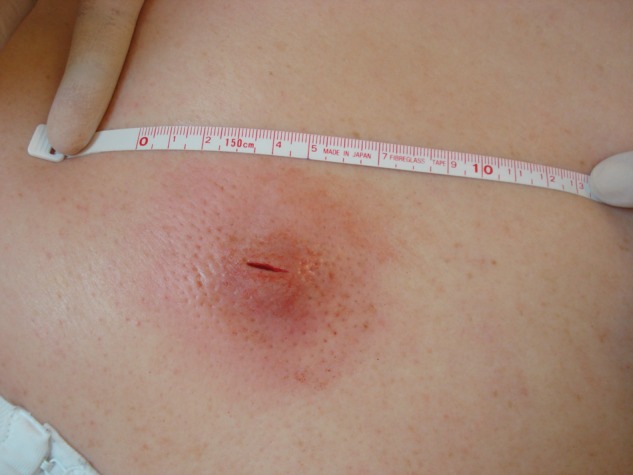
Weight lifter requiring incision and drainage of a furuncle that cultured positive for methicillin-resistant Staphylococcus aureus.
Clinical Findings
CA-MRSA soft tissue infections may clinically present as cellulitis, folliculitis, furuncles, carbuncles, and abscesses.14 The infection often begins as a mild superficial infection of the skin, which may look harmless at onset but rapidly develop into large abscesses within 24 to 48 hours (Figures 2 and 3). The lesions are often mistaken for spider bites.20 Although skin and soft tissue infections are more common with CA-MRSA, osteomyelitis, otitis media, respiratory tract, and blood sepsis can occur.21
Figure 2.
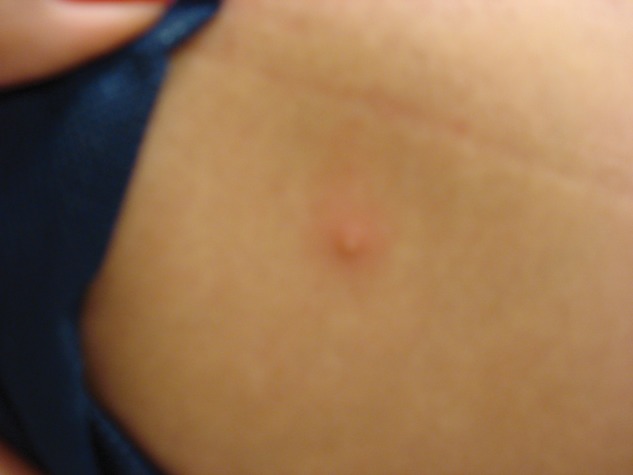
Division I athlete with recurrent infections colonized with methicillin-resistant Staphylococcus aureus.
Figure 3.
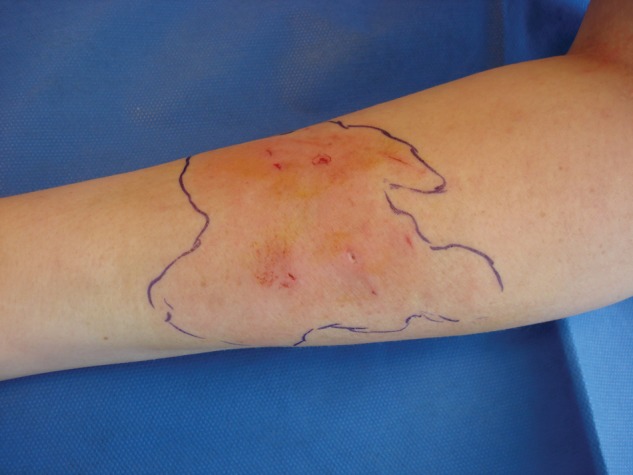
A typical presentation of a patient who developed a rapid, spreading cellulitis with multiple small pustules and abscesses within 24 hours.
Treatment
The clinician must maintain a high index of suspicion when confronted with skin and soft tissue infections. It is important to culture all purulent skin lesions. The information obtained from the culture guides antibiotic selection and provides information about community prevalence of MRSA. The primary treatment of an abscess or purulent skin lesion is that of incision and drainage. Although incising and draining the lesion (Figure 4) is usually adequate to resolve the infection, it is sometimes insufficient, and empiric antibiotic treatment may be necessary. Empiric antibiotic therapy may include clindamycin, trimethoprim-sulfamethoxazole or tetracycline.11 When selecting an antibiotic before the availability of culture results, the physician must take into account susceptibility data from the community. Using antibiotics following the incision and drainage of the lesion is left to the discretion of the treating physician. Factors that determine the need for antibiotics include the size of the lesion, cellulitis, age of the patient (very young or elderly), fever, signs of systemic infection, and comorbid conditions. MRSA should be included in the differential in all skin and soft tissue infection but especially in rapidly progressing infections that are not responding to the empiric antimicrobial treatment. Admission to the hospital for intravenous antibiotics may be needed in these situations (Figure 5).13
Figure 4.
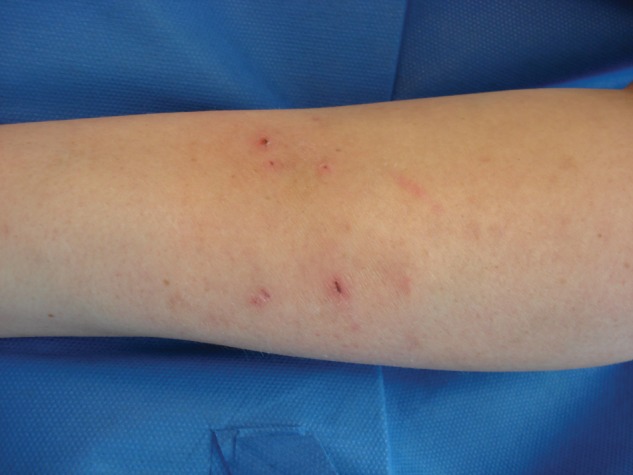
Resolving cellulitis after incision and drainage and antibiotic treatment.
Figure 5.
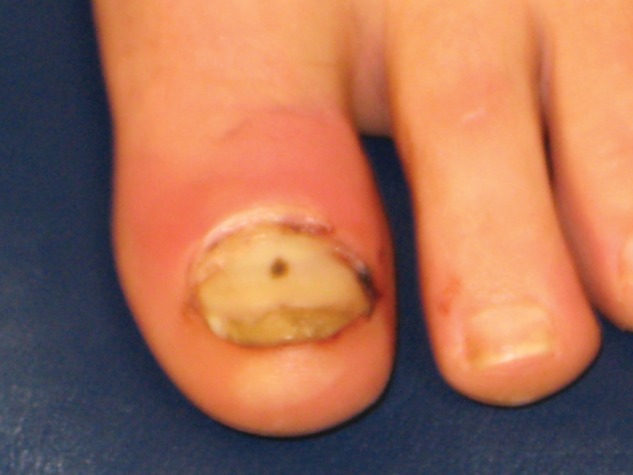
Division I athlete requiring hospitalization and intravenous vancomycin for toe infection related to methicillin-resistant Staphylococcus aureus.
Proper identification and management of skin and soft tissue infections is imperative in reducing morbidity and mortality. The majority of these infections resolve without consequence, but deaths have been reported.6
Prevention
A multidisciplinary approach is necessary to fully implement prevention strategies (see Table 3). This approach should include all athletic personnel and family members. Education is the key to prevention. Staff and athletes must be educated about MRSA and the importance of good hygiene practices. The athlete should inform the medical staff about skin lesions no matter how innocuous they appear.
Table 3.
Athletic facility prevention.
| Educate staff and athletes. |
| Enforce hand washing. |
| Use soap dispensers rather than bar soap. |
| Shower immediately after workouts. |
| Avoid sharing personal items: towels, water bottles, shavers, combs/brushes, etc. |
| Disinfect whirlpools, hot tubs, showers, and exercise equipment according to manufacturing specifications. |
| Establish routine cleaning schedules for the equipment. |
| Wash and dry clothing according to manufacturer’s specifications. |
| Report all skin lesions to medical staff. |
| Perform proper wound care and coverage. |
| Use antibiotics appropriately. |
MRSA has been shown to survive on surfaces for hours and even months,8 which further emphasizes that proper cleansing of all equipment is imperative in reducing the potential spread of the infection. Universal precautions should be enforced at all times, with a strong focus on proper hand washing. Stringent daily disinfecting practices should be employed in the training room, locker room, showers, and common areas. It may be helpful to have an infectious disease specialist review disinfecting policies, inspect a facility’s training room and locker room, and make additional prevention recommendations.
Recurrent MRSA Infections: Presurgical Screening and Decolonization Techniques
There is currently no consensus concerning the ideal patient population to screen or the optimal decolonization protocol. Over the course of many years, researchers have investigated numerous decolonization regimens. Decolonization agents have included skin washes and topical and systemic antimicrobial treatments. To date, decolonization efforts have failed to eliminate recolonization. Decolonization with agents such as mupirocin nasal ointment and antiseptic body washes has been studied and may be appropriate in colonized individuals who have recurrent MRSA infections or who are at high risk for postoperative infections.3,34 Clinicians who are considering screening presurgical patients for decolonization are advised to consult with hospital infectious disease specialists. Studies are ongoing to further define the ideal decolonization agent (or agents).
Return-To-Sports Criteria
Athletes with MRSA skin or soft tissue infections require prompt treatment and close monitoring of their infections. Exclusion from participation depends on the severity of the infection, the ability to appropriately cover the wound, and the specific sport. Athletes with evidence of spreading cellulitis or systemic symptoms (ie, fever and chills) should be restricted from sports participation. Athletes with contained infections but no systemic symptoms may be able to participate, although they are typically handled on a case-by-case basis. Following the identification of a MRSA infection, the sports medicine team should review and comply with the return-to-sports guidelines developed by the governing sports organization. Full compliance with the guidelines protects the athlete and the other participants.
Summary
Medical providers face an increasing challenge of CA-MRSA prevalence in the treatment and care of athletes. Therefore, a high degree of clinical suspicion is required to adequately identify and diagnose the infection. Best practices include involving all members of the athletic staff (athletes, athletic trainers, and team physicians) in the monitoring and treatment of these infections. In addition, it is imperative that early detection and aggressive treatment of CA-MRSA infections be implemented to limit morbidity and mortality. Incision and drainage of abscesses and culture-directed antibiotic treatment are the mainstays of treatment. Hospitalization may be necessary for individuals with systemic symptoms or worsening infections (for intravenous antibiotics).
Footnotes
No potential conflict of interest declared.
References
- 1. Bartlett PC, Martin RJ, Cahill BR. Furunculosis in a high school football team. Am J Sports Med. 1982;10:371-374 [DOI] [PubMed] [Google Scholar]
- 2. Binh AD, Chambers HF, Graber CJ, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148(4): 249-257 [DOI] [PubMed] [Google Scholar]
- 3. Boyce JM. MRSA patients: proven methods to treat colonization and infection. J Hosp Infect. 2001;48(suppl A): S9-S14 [DOI] [PubMed] [Google Scholar]
- 4. Buescher ES. Community-acquired methicillin-resistant Staphylococcus aureus in pediatrics. Curr Opin Pediatr. 2005;17;67-70 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control Community-acquired methicillin-resistant Staphylococcus aureus infections: Michigan. MMWR Morb Mortal Wkly Rep. 1981;30:185-187 [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. JAMA. 1999;282:1123-1125 [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention Community-associated MRSA information for clinicians. http://www.cdc.gov/ncidod/dhqp/ar_mrsa_ca_clinicians.html. Accessed July 14, 2009
- 8. Centers for Disease Control and Prevention Environmental management of staph and MRSA in community settings. http://www.cdc.gov/ncidod/dhqp/ar_mrsa_Enviro_Manage.html. Accessed July 14, 2009
- 9. Centers for Disease Control and Prevention Methicillin-resistant Staphylococcus aureus in correctional facilities: Georgia, California, and Texas, 2001-2003. MMWR Morb Mortal Wkly Rep. 2003;52:992-995 [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention Methicillin-resistant Staphylococcus aureus infections among competitive sports participants: Colorado, Indiana, Pennsylvania and Los Angeles County, 2000-2003. MMWR Morb Mortal Wkly Rep. 2003;53:793-795 [PubMed] [Google Scholar]
- 11. Center for Disease Control and Prevention. Strategies for clinical management of MRSA in the community: [Accessed July 14, 2009];2006 Mar; http://www.cdc.gov/ncidod/dhqp/pdf/ar/CAMRSA_ExpMtgStrategies.pdf.
- 12. Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7(2):178-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen P, Grossman M. Management of cutaneous lesions associated with an emerging epidemic: community-acquired methicillin-resistant Staphylococcus aureus skin infections. J Am Acad Dermatol. 2004;51:132-135 [DOI] [PubMed] [Google Scholar]
- 14. Cohen P, Kurzrock R. Community acquired methicillin-resistant Staphylococcus aureus skin infection: an emerging clinical problem. J Am Acad Dermatol. 2004; 50:277-280 [DOI] [PubMed] [Google Scholar]
- 15. Crum NF, Lee RU, Thornton MS, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943-951 [DOI] [PubMed] [Google Scholar]
- 16. Daum RS, Ito T, Hiramatsa K, et al. A novel methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002;186:1344-1347 [DOI] [PubMed] [Google Scholar]
- 17. Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA 300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet. 2006;367:731-739 [DOI] [PubMed] [Google Scholar]
- 18. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39:971-979 [DOI] [PubMed] [Google Scholar]
- 19. Faden H, Ferguson S. Community-acquired methicillin-resistant Staphylococcus aureus and intra-family spread of pustular disease. Pediatr Infect Dis J. 2001;20:554-555 [DOI] [PubMed] [Google Scholar]
- 20. File TM. Impact of community acquired methicillin-resistant Staphylococcus aureus in the hospital setting. Cleve Clin J Med. 2007;74(suppl 4):S6-S11 [DOI] [PubMed] [Google Scholar]
- 21. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444 [DOI] [PubMed] [Google Scholar]
- 22. Graham PL, Lin SX, Larson ELA. US population-based survey of Staphylococcus aureus colonization. Ann Intern Med. 2006;144:318-325 [DOI] [PubMed] [Google Scholar]
- 23. Gorak EJ, Yamada SM, Brown JD. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin Infect Dis. 1999;29:797-800 [DOI] [PubMed] [Google Scholar]
- 24. Kazakova S, Hageman J, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352:468-475 [DOI] [PubMed] [Google Scholar]
- 25. King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicilling-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309-317 [DOI] [PubMed] [Google Scholar]
- 26. Lindenmayer J, Schoenfeld S, O’Grady R, Carney J. Methicillin resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med. 1998;158:895-899 [DOI] [PubMed] [Google Scholar]
- 27. Lu D, Holtom P. Community-associated methicillin-resistant Staphylococcus aureus, a new player in sports medicine. Curr Sports Med Rep. 2005;4:265-270 [DOI] [PubMed] [Google Scholar]
- 28. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674 [DOI] [PubMed] [Google Scholar]
- 29. Rice L. Antimicrobial resistance in gram positive bacteria. Am J Med. 2006;119(6)(suppl 1): S11-S19 [DOI] [PubMed] [Google Scholar]
- 30. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors . Clin Infect Dis. 2002;36:131-139 [DOI] [PubMed] [Google Scholar]
- 31. Samad A, Banerjee D, Carbarns N, Ghosh S. Prevalence of methicillin- resistant Staphylococcus aureus colonization in surgical patients on admission to a Welsh hospital. J Hosp Infect. 2002;51(1):43-46 [DOI] [PubMed] [Google Scholar]
- 32. Stacey AR, Endersby KE, Chan PC, Marples RR. An outbreak of methicillin resistant Staphylococcus aureus infection in a rugby football team. Br J Sports Med. 1998;32:153-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vandenesch F, Naimi T, Enright MC, et al. Community acquired methicillin resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201 [DOI] [PubMed] [Google Scholar]
- 35. Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired methicillin resistant Staphylococcus aureus: an emerging threat. Lancet Infec Dis. 2005;5:275-286 [DOI] [PubMed] [Google Scholar]



