Abstract
Background:
Therapeutic ultrasound to drive medication (phonophoresis) has been a mainstay in physical therapy. The most common drug used in phonophoresis is hydrocortisone acetate (HA). A number of studies have been done examining phonophoresis in the delivery of HA through the skin to underlying tissues; however, a study has never been done examining the absorption of HA using phonophoresis on human connective tissue.
Hypothesis:
Phonophoresis will facilitate the transmission of HA in human connective tissue.
Study Design:
Randomized controlled study.
Methods:
Twenty-one patients undergoing anterior cruciate ligament reconstruction surgery were randomly assigned to either a sham or true phonophoresis treatment group. The latter group received 6 minutes of 10% HA ultrasound at a point consistent with the gastrocnemius slip of the semitendinosis tendon (treatment site). The sham group received 6 minutes of 10% HA ultrasound to the same area, but the ultrasound was not turned on. The slip and a sample of the distal attachment of the tendon (control) were removed. Samples were analyzed for HA levels.
Results:
Although the mean and median levels of HA found at the treatment site were greater than those of the control site (means, 34.1 vs 22.9 parts per billion; medians, 7 vs 0 parts per billion), the levels of HA found at the treatment site were not significantly greater than those at the control site (P = 0.15). There were no statistically significant differences between the true and sham phonophoresis groups in HA levels (P = 0.80) nor in age, sex, or skin thickness.
Conclusion:
Phonophoresis does not appear to facilitate the absorption of HA in connective tissue when compared with simple absorption (sham).
Clinical Relevance:
Phonophoresis does not appear to enhance the transmission of HA in human connective tissue; therefore, use of phonophoresis should be reconsidered in inflammatory conditions.
Keywords: hydrocortisone acetate, human connective tissue, phonophoresis, ultrasound, absorption
Therapeutic ultrasound is a commonly used modality that utilizes high-frequency sound waves (usually 1 to 3 MHz) that pass through the skin to underlying structures. Ultrasound has been purported to heat tissue, increase blood flow to skin, decrease pain secondarily by decreasing muscle spasm, and promote healing of various tissue.3,12 Therapeutic ultrasound has been shown to be effective as a deep-heating modality.32 Other claims, however, such as the utility of ultrasound as an anti-inflammatory modality, remain controversial.9,14,20,26
Ultrasound is also theorized to assist in driving medications through the skin to underlying tissues.27 This form of ultrasound is referred to as phonophoresis. Although still debated, the mechanism by which ultrasound facilitates absorption of drugs is thought to occur via an alteration of the stratum corneum of the skin. This alteration is postulated to occur as a result of denaturing the structural keratin proteins in the stratum corneum, stripping or delaminating of the cornified layers or the stratum corneum, changing the cell permeability, or altering the lipid-enriched intracellular structure between corneocytes.5 The ability of ultrasound to facilitate the absorption of hydrocortisone acetate (HA) into tendon, nerve, and muscle has been shown in porcine and rat models.18,24 However, studies in canines and humans have questioned the ability of ultrasound to enhance the absorption of HA into muscle.8,25
Phonophoresis has been commonly used in physical therapy practices for many years.3 The most common drug used in phonophoresis is HA in either a 1% or 10% concentration. HA is a naturally occurring corticosteroid that has strong anti-inflammatory properties. HA phonophoresis has been used to treat a variety of inflammatory conditions, such as tendinopathy, tenosynovitis, bursitis, adhesive capsulitis, and carpal tunnel syndrome.29,34 A 1992 review by Newman et al concluded that phonophoresis with hydrocortisone is effective in the treatment of a variety of musculoskeletal injuries by accelerating healing, increasing flexibility, and decreasing pain.29 However, more recent studies have cast doubt on the efficacy of phonophoresis.10,21,22 In a review article in 2007, Goraj-Szczypiorowska et al determined that the dearth of objective research methods and reliable scientific verification does not allow unambiguous determination of the efficacy of phohophoresis.15 Despite these findings, a recent survey revealed that a majority of physical therapists (54.1%) with advanced clinical specialization in orthopaedics continue to use HA phonophoresis for inflammatory conditions.36
HA phonophoresis is frequently used to treat inflammatory conditions of tendons. Although there are several animal and human studies examining the absorption of HA with phonophoresis, no study to date has examined the absorption of HA in human connective tissue. The purpose of this study was to evaluate the effectiveness of phonophoresis to facilitate the absorption of HA into human connective tissue.
Methods
We modified an experimental design that had previously been used to evaluate the effectiveness of iontophoresis in human connective tissue.19 After obtaining institutional review board approval, 21 patients undergoing anterior cruciate ligament reconstruction surgery using autologous hamstring tendons were recruited into the study. Preoperatively, they were randomly assigned to either a sham (n = 9) or true (n = 12) phonophoresis treatment group. Both patients and their surgeons were blinded to the treatment method. In the preoperative holding area, patients in the true phonophoresis group were treated with 6 minutes of continuous ultrasound at 1 MHz, 1 W/cm2, using 5 g of pharmaceutically prepared 10% HA in a gel mixture. The treatment site was centered over an area 8 cm proximal to the semitendinosus tendon tibial insertion as determined by measuring the distance with a tape measure from the pes anserine (Figures 1 and 2). The treatment area was 5 cm2, consistent with the effective radiating area of the ultrasound unit. This point is consistent with the location of an accessory slip from the semitendinosus to the medial gastrocnemius that must be released during surgical harvest of the semitendinosus tendon (Figure 3, red arrow). One study found this fascial band to be located approximately 8 to 10 cm proximal to the semitendinosus tendon insertion.31 A more recent investigation noted that this band begins 6 to 8 cm and ends 8 to 12 cm proximal to the semitendinosus tendon insertion. The average width of this band was 2.5 cm.35 Patients in the sham group received the same treatment except with sham ultrasound; that is, the ultrasound machine was not turned on. All patients had skinfold thickness measured at the treatment site using calipers (Figure 4). Both groups were treated the morning of their surgeries. The time the treatment concluded was recorded.
Figure 1.
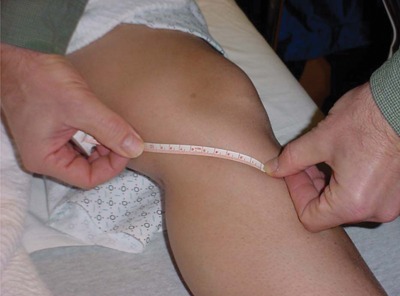
Measuring to establish the treatment site.
Figure 2.
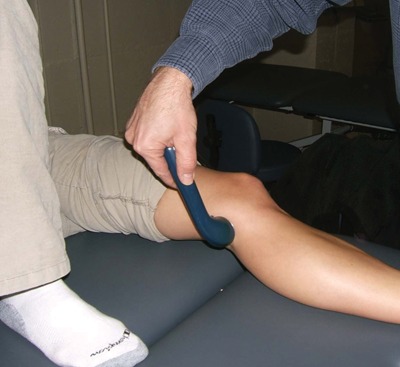
Performing the phonophoresis treatment.
Figure 3.
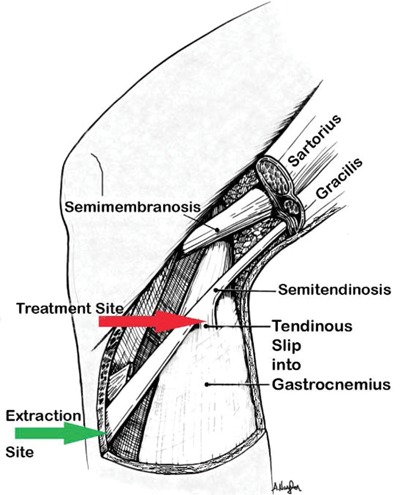
Location of tendon slip (red arrow, treatment site) and control site (green arrow).
Figure 4.
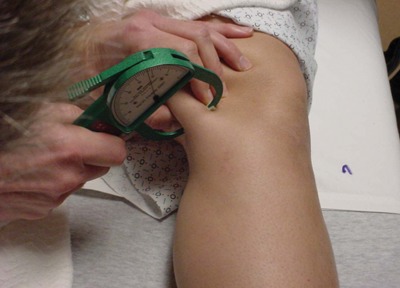
Measuring skinfold thickness at treatment site.
During surgery, the tibial insertion of the semitendinosus tendon was exposed through an oblique incision over the pes anserine. The insertion was sharply divided off the tibia, and a sample of the distal tendon was removed as a control sample (Figure 3, green arrow). A whip stitch of No. 2 Ethibond suture was then placed in the distal end of the tendon. Blunt dissection was then performed to mobilize the semitendinosus tendon until the accessory slip to the gastrocnemius could be identified. This accessory slip was sharply divided 6 to 8 mm from the main tendon. The semitendinosus tendon was then harvested using a tendon stripper. Finally, the accessory slip was removed as a treatment sample (Figure 3, red arrow). The difference in time between the treatment and tissue extraction was noted, and the samples were then stored at –47°C until testing was performed.
Both samples for each patient were then analyzed for HA levels using high-performance liquid chromatography (Agilent, Santa Clara, California) coupled to a triple-quadrupole mass spectrometry (Applied Biosystems, Foster City, California). An assay was developed for the measurement of HA and hydrocortisone. During the development, the method performance was shown to be 8 to 200 parts per billion (ppb) for the linearity range, and the lower limit of quantitation was defined at 8 ppb. The recovery of HA from human connective tissue was approximately 100%. The laboratory was blinded to the group assignment. Levels of HA in the control site (resting levels) were then subtracted from levels in the treatment site to yield a net HA level for each patient. This was done because cortisol is naturally found throughout the body, including connective tissue. By subtracting the level of cortisone in the control site from that of the treatment site, we could more confidently say that the difference in the cortisone levels between the 2 sites (net HA levels) would be attributed to the phonophoresis.
Statistical Analysis
The Wilcoxon signed rank test was used to compare HA concentrations for treatment and control sites for the 21 patients. A Fisher exact test was used to compare the sex distributions for the 2 groups. Wilcoxon rank sum tests were used to compare age, skin thickness, time between treatment and tissue extraction, and net HA levels between the treatment and sham groups. All analyses used SAS 9.1 and a significance level of P < 0.05. Based on a previous study using the same procedure to detect dexamethasone using iontophoresis, a power analysis demonstrated that sample sizes of 20 in each group were needed to detect an effect size19 of 0.91.
Results
There were no statistically significant differences between the sham group and the true phonophoresis groups in age, sex, or skin thickness. A significant difference (P = 0.02) was found in the time between treatment and tissue extraction, with the sham group having a longer average period (187 vs 118 minutes) (Table 1). Of the 21 patients, 16 had higher HA levels in the treatment site than at the control site. Although the mean and median levels of HA found at the treatment site were greater than those of the control site (means, 34.1 vs 22.9 ppb; medians, 7 vs 0 ppb), the levels of HA found at the treatment site were not significantly greater than at the control site (P = 0.15). The ranges of HA found in the treatment site were 0 to 206.9 ppb and in the control site, 0 to 298.5 ppb. At the treatment site, 1 patient had no detectable HA in the connective tissue, compared with 11 with no detectable HA at the control site. There was no statistically significant difference in net HA levels between the true and sham phonophoresis groups (P = 0.80).
Table 1.
Group comparisons.
| Control | Treatment | P | |
|---|---|---|---|
| Age, y | |||
| Mean ± SD | 33.4 ± 9.0 | 38.1 ± 8.9 | 0.31a |
| Median (range) | 31 (21, 48) | 39 (22, 52) | |
| Skin, mm | |||
| Mean ± SD | 19.5 (4.8) | 19.6 (7.5) | 0.94a |
| Median (range) | 20 (14, 28) | 18 (10, 31) | |
| Time, min | |||
| Mean ± SD | 187 ± 91 | 118 ± 18 | 0.02a |
| Median (range) | 155 (120, 393) | 113 (93, 145) | |
| Difference between experiment and control sites, parts per billion | |||
| Mean ± SD | 24.7 ± 51.1 | 1.1 ± 35.7 | 0.80a |
| Median (range) | 0 (–43, 106) | 5.4 (0, 71) | |
| Sex, n (%) | |||
| Women | 5 (56) | 8 (67) | 0.67b |
| Men | 4 (44) | 4 (33) |
Wilcoxon rank sum test.
Fisher exact test.
Discussion
Topically applied 10% HA, either with or without US, did not significantly increase the cortisone levels in connective tissue at the treatment site over the level at the control site. Phonophoresis did not appear to facilitate the absorption of HA into human connective tissue. A comparison of the sham and experimental groups showed the median differences in HA between the treatment and control sites to be 5.4 ppb (Table 1). This means that the application of ultrasound netted a median increase of only 0.0054 mg/kg of hydrocortisone in the connective tissue when compared with the sham treatment. The minimal concentration of hydrocortisone necessary to have an anti-inflammatory effect in animal models has been shown to be 78 mg/kg,11 a level that is more than 4 orders of magnitude greater than what we found between the sham and experimental groups. A power analysis showed that 20 patients per group were needed to demonstrate a significant effect (if one existed). However, since the median difference in HA concentrations found in the connective tissue between the sham and experimental groups in our first 21 patients did not approach clinically significant concentrations, we concluded that there was no reason to continue the study.
Our finding that phonophoresis did not facilitate absorption of HA into human connective tissue is in agreement with several other studies. Byl et al compared the effect of dexamethasone (0.334%) and HA (10%) on collagen deposition as measured by the amount of hydroxyproline deposited in polytetrafluroethylene tubes following topical application, injection, or phonophoresis in humans.6 There was no measurable reduction in collagen deposition (an effect of corticosteroids) in the submuscular or subtendinous areas with those who received phonophoresis. Only those tubes treated with hydrocortisone injection showed a reduction in collagen deposition. A canine model found that absorption of 5% and 10% HA was enhanced with phonophoresis through the stratum corneum into the epidermis, but no HA was found in the knee joint or muscle.8 In a human model, Bare et al investigated the phonophoretic delivery of 10% HA through the epidermis by measuring serum cortisol concentrations.1 One week apart, each patient received both phonophoresis (10% HA gel coupling medium over an area of 50 cm2 for 5 minutes at 1.0 W/cm2 and 1 MHz) and a control treatment with only ultrasound to the volar forearm. Serum HA levels were measured from an antecubital vein at 0, 5, and 15 minutes posttreatment. No rise in serum cortisol concentration was detected following HA phonophoresis. Finally, Kuntz performed a randomized controlled study with an ultrasound (sham) group and a 10% HA phonophoresis (treatment) group.25 Ultrasound at 1 MHz, 1.0 W/cm2, continuous for 7 minutes, was administered over the vastus lateralis muscle. The contralateral limb served as the control for both groups. HA levels were analyzed immediately after treatment from a vastus lateralis muscle biopsy of both limbs. No significant difference in HA levels was found in either group (treatment or sham). Our study used methods similar to Kuntz’s and found no benefit to ultrasound increasing HA absorption; however, we did find a slight increase in HA levels over the control connective tissue in both groups. We conclude that a small amount of HA is able to diffuse through the skin but that there is no additional benefit from concomitant ultrasound treatment.
Other authors have shown an ability of phonophoresis to facilitate absorption of corticosteroids. Fellinger and Schmidt showed that ultrasound could transport hydrocortisone across an avascular membrane and concluded that phonophoresis is an effective treatment for the arthritic hand.13 Griffin and Touchstone showed that ultrasound enables the penetration of HA into skeletal paravertebral muscle and nerves in swine.18 They found an increase of 146% in HA levels in neural tissue and 100% increase in muscle tissue. There was no increase in systemic cortisol levels. The highest concentration of HA in muscle was found when the animals were treated at the lowest intensity (0.1 W/cm2) for the longest period (51 minutes). Using serum HA concentration levels as an outcome measure, Saliba et al found that phonophoresis delivered significantly higher concentrations of dexamethasone than did occlusive dressings in humans. They used serum concentration levels of dexamethasone as their outcome measure.33 Koeke et al found that ultrasound stimulated the acceleration of tissue repair processes and induced the transdermal delivery of hydrocortisone in a therapeutic concentration on the rat tendon.24
Possible explanations for differences in findings among these studies include (1) differences in the transmissivity of the delivered drug (HA vs dexamethasone), (2) differences in absorption of the target tissue (tendon vs nerve, muscle, or serum), and (3) differences in the dermal properties of the species (human vs swine or mouse). Transmissivity in ultrasound is defined as the percentage of the ultrasound energy that the coupling medium is able to transmit. For ultrasound to have its purported desired effects on the skin, the conducting medium needs to transmit ultrasound energy.7 According to Cameron and Monroe, 1% hydrocortisone powder in ultrasound gel had poor transmission (29%) compared with ultrasound gel alone (96%) or ultrasound lotion (90%). Specifically, 1% and 10% hydrocortisone cream were found to have no transmission (0%). Byl et al also found HA to have poor transmissivity.6 Because differences may exist in the ability of different target tissues to absorb HA, our study examined connective tissue, which is a frequent target tissue for HA phonophoresis treatments. Likewise, while there are known differences in the properties of different animal skin,2,4,16,28,30 our model looked at the clinically important human skin.
There are several potential criticisms of our in vivo human model. First, there was a relatively long period between treatment and tissue harvest. It is possible that HA did penetrate the connective tissue but diffused away during the interval before tissue harvest. However, for HA to exert the desired anti-inflammatory effects, we would expect that detectable levels would need to remain in the tissue for hours or days. Second, the stress of surgery could have initiated a native HA release that would have masked any effect from the phonophoresis. Our patients all had peripheral nerve blocks and general anesthesia, which should diminish the “stress response” to surgery. In fact, we found very low control tissue HA levels in most of our patients. Third, we had a statistically significant difference between the true and sham phonophoresis groups in time between treatment and tissue harvest. It is possible that the differences in time could have affected the concentrations of HA. However, the correlation of HA concentration and time was −0.17 (P = 0.69) in the sham group and −0.13 (P = 0.70) in the true phonophoresis group, with an overall correlation of −0.20 (P = 0.41). It appears that time had no effect on HA concentrations in either group so that the differences in time between the groups were not a factor. Fourth, the lack of difference in HA levels between the control and treatment sites could have occurred by cross-contamination of the control site from the treatment site or by simple diffusion of the HA from the treatment site to the control site. However, all tissues were extracted in a manner that pulled the control site out of the surgical portal first so that the control site never touched the treatment site. Furthermore, the distance between the control site and the treatment site was 8 cm, rendering the possibility of diffusion unlikely. A final limitation is that our model looked only at the ability of phonophoresis to promote absorption into human connective tissue. It is possible that there may be a benefit of phonophoresis in other target tissues.
Clinical studies also differ to whether or not phonophoresis is effective. Several older studies have shown a benefit of HA phonophoresis at reducing pain in inflamed tissue.17,23 A 1992 review by Newman concluded that phonophoresis with HA is effective in the treatment of a variety of musculoskeletal injuries by accelerating healing, increasing flexibility, and decreasing pain.29 Conversely, more recent studies have not shown a benefit for HA phonophoresis in treating carpal tunnel syndrome lateral epicondylitis, or adhesive capsulitis.10,21,22 In a 2007 review, Goraj-Szczypiorowska et al concluded that the lack of objective research methods and reliable scientific verification does not allow unambiguous determination of the efficacy of phonophoresis.15
In summary, our model demonstrates that HA is absorbed through human skin to underlying connective tissue. However, treatment using phonophoresis with a 10% hydrocortisone gel did not facilitate the absorption of HA over topical hydrocortisone alone (sham). Ultrasound did not improve the absorption of the hydrocortisone into the targeted connective tissue. Given the results of this study, we cannot recommend HA phonophoresis for treatment of inflammatory conditions of connective tissues.
Acknowledgments
We would like to acknowledge Betty Skipper, PhD, for her contributions in the statistics of this paper, and to Sam VanBlarcom for his edits and his omnipresent dedication to grammatical correctness
References
- 1. Bare A, McAnaw M, Pritchard A, et al. Phonophoretic delivery of 10% hydrocortisone through the epidermis of humans as determined by serum cortisol concentrations. Phys Ther. 1996;76:738-749 [DOI] [PubMed] [Google Scholar]
- 2. Bartek MJ, LaBudde JA, Miabach HI. Skin permeability in vivo: comparison in rat, rabbit, pig and man. J Investig Dermatol. 1972;58(1):114-123 [DOI] [PubMed] [Google Scholar]
- 3. Belanger AY. Evidence-Based Guide to Therapeutic Physical Agents. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:223-261 [Google Scholar]
- 4. Bronaugh RL, Stewart RF, Wester RC, Bucks D, Mailbach HI, Anderson J. Comparison of percutaneous absorption of fragrances by humans and monkeys. Food Chem Toxicol. 1985;23(1):111-114 [DOI] [PubMed] [Google Scholar]
- 5. Byl N. The use of ultrasound as an enhancer for transcutaneous drug delivery: phonophoresis. Phys Ther. 1995;75:539-553 [DOI] [PubMed] [Google Scholar]
- 6. Byl N, McKenzie A, Haliday B, et al. The effects of phonophoresis with corticosteroids: a controlled pilot study. J Orthop Sports Phys Ther. 1993;18:590-600 [DOI] [PubMed] [Google Scholar]
- 7. Cameron MH, Monroe LG. Relative transmission of ultrasound by media customarily used for phonophoresis. Phys Ther. 1992;72:142-148 [DOI] [PubMed] [Google Scholar]
- 8. Davick JP, Martin RK, Albright JP. Distribution and deposition of tritrated cortisol using phonophoresis. Phys Ther. 1998;68:1672-1675 [DOI] [PubMed] [Google Scholar]
- 9. De Deyne PG, Kirsch-Volders M. In vitro effects of therapeutic ultrasound on the nucleus of human fibroblasts. Phys Ther. 1995;75(7):629-634 [DOI] [PubMed] [Google Scholar]
- 10. de Entrambasaguas M, Máñez I, Girona G, López-Santoveña F, Poyatos J. Steroid injection, wrist splinting and phonophoresis in carpal tunnel syndrome. Rehabilitación. 2006;40(4):193-200 [Google Scholar]
- 11. Dhawan BN, Srimal RC. Anti-inflammatory and some other pharmacological effects of 3,4-trans-2,2 dimethyl-3-phenyl-4-[β-pyrrolidinoethoxy)-phenyl]-7-methoxy chroman (Centchroman). Br J Pharmacol. 1973;49:64-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Draper DO, Harris ST, Schulthies S, et al. Hot-pack and 1 MHz ultrasound treatments have additive effect on muscle temperature increase. J Athl Train. 1998;33:21-24 [PMC free article] [PubMed] [Google Scholar]
- 13. Fellinger K, Schmidt J. Klinik und Therapie des Chronischem. Vienna, Austria: Maudrich; 1954:549-554 [Google Scholar]
- 14. Goddard DH, Revell PA, Cason J, Gallagher S, Currey HL. Ultrasound has no anti-inflammatory effect. Ann Rheum Dis. 1983;42(5):582-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goraj-Szczypiorowska B, Zając L, Skalska-Izdebska R. Evaluation of factors influencing the quality and efficacy of ultrasound and phonophoresis treatment. Ortop Traumatol Rehabil. 2007;9(5):449-458 [PubMed] [Google Scholar]
- 16. Grabau JH, Dong L, Mattie DR, Jepson GW, McDougal JN. Comparison of Anatomical Characteristics of the Skin of Several Laboratory Animals. Newton Centre, MA: Geo-Centers Inc; 1995:35 [Google Scholar]
- 17. Griffin JE, Echternach JL, Price RE, Touchstone JC. Patients treated with ultrasonic driven hydrocortisone and with ultrasound alone. Phys Ther. 1967;47:594-601 [DOI] [PubMed] [Google Scholar]
- 18. Griffin JE, Touchstone JC. Low-intensity phonophoresis of cortisol in swine. Phys Ther. 1968;48(12):1336-1344 [DOI] [PubMed] [Google Scholar]
- 19. Gurney AB, Wascher DC. Absorption of dexamethasone sodium phosphate in human connective tissue using iontophoresis. Am J Sports Med. 2008;36(4):753-759 [DOI] [PubMed] [Google Scholar]
- 20. Hashish I, Harvey W, Harris M. Anti-inflammatory effects of ultrasound therapy: evidence for a major placebo effect. Br J Rheumatol. 1986;25(1):77-81 [DOI] [PubMed] [Google Scholar]
- 21. Hoppenrath T, Ciccone CD. Is there evidence that phonophoresis is more effective than ultrasound in treating pain associated with lateral epicondylitis? Phys Ther. 2006;86(1):136-140 [PubMed] [Google Scholar]
- 22. Jewell DV, Riddle DL, Thacker LR. Interventions associated with an increased or decreased likelihood of pain reduction and improved function in patients with adhesive capsulitis: a retrospective cohort study. Phys Ther. 2009;89(5):419-429 [DOI] [PubMed] [Google Scholar]
- 23. Klienkort JA, Wood AF. Phonophoresis with 1 percent versus 10 percent hydrocortisone. Phys Ther. 1975;55:1320-1324 [DOI] [PubMed] [Google Scholar]
- 24. Koeke PU, Parizotto NA, Carrinho PM, Salate AC. Comparative study of the efficacy of the topical application of hydrocortisone, therapeutic ultrasound and phonophoresis on the tissue repair process in rat tendons. Ultrasound Med Biol. 2005;31(3):345-350 [DOI] [PubMed] [Google Scholar]
- 25. Kuntz AR, Griffiths CM, Rankin JM, Armstrong CW, McLoughlin TJ. Cortisol concentrations in human skeletal muscle tissue after phonophoresis with 10% hydrocortisone gel. J Athl Train. 2006;41(3):321-324 [PMC free article] [PubMed] [Google Scholar]
- 26. Leung MC, Ng GY, Yip KK. Effect of ultrasound on acute inflammation of transected medial collateral ligaments. Arch Phys Med Rehabil. 2004;85(6):963-966 [DOI] [PubMed] [Google Scholar]
- 27. Mitragotri S, Blankschtein D, Langer R. Transdermal drug delivery using low-frequency sonophoresis. Pharm Res. 1996;13(3):411-420 [DOI] [PubMed] [Google Scholar]
- 28. Moody RP, Franklin CA, Ritter L. Dermal absorption of the phenoxy herbicides 2,4-D, 2,4-D amine, 2,4-D isooctyl, and 2,4,5-T in rabbits, rats, rhesus monkeys, and humans: a cross-species comparison. J Toxicol Environ Health. 1990;29:237-245 [DOI] [PubMed] [Google Scholar]
- 29. Newman JT, Nellermoe MD, Carnett JL. Hydrocortisone phonophoresis: a literature review. J Am Podiatr Med Assoc. 1992;82(8):432-435 [DOI] [PubMed] [Google Scholar]
- 30. Ngawhirunpat T, Opanasopit P, Prakongpan S. Comparison of skin transport and metabolism of ethyl nicotinate in various species. Eur J Pharm Biopharm. 2004;58(3):645-651 [DOI] [PubMed] [Google Scholar]
- 31. Pagnani MJ, Warner JJP, O’Brien SJ, et al. Anatomic considerations in harvesting the semitendinosus and gracilis tendons and a technique of harvest. Am J Sports Med. 1993;21:565-571 [DOI] [PubMed] [Google Scholar]
- 32. Rose S, Draper D, Schulthies S, et al. The stretching window part two: rate of thermal decay in deep muscle following 1-MHz ultrasound. J Athl Train. 1996; 31:139-143 [PMC free article] [PubMed] [Google Scholar]
- 33. Saliba S, Mistry DJ, Perrin DH, Gieck J, Weltman A. Phonophoresis and the absorption of dexamethasone in the presence of an occlusive dressing. J Athl Train. 2007;42(3):349-354 [PMC free article] [PubMed] [Google Scholar]
- 34. Stratford PW, Levy DR, Gauldie S, Miseferi D, Levy K. The evaluation of phonophoresis and friction massage as treatments for extensor carpi radialis tendinitis: a randomized controlled trial. Physiother Can. 1989;41(2):93-99 [Google Scholar]
- 35. Tuncay I, Kucuker H, Uzun I, et al. The fascial band from semitendinosus to gastrocnemius: the critical point of hamstring harvesting. Acta Orthop. 2007;78:361-363 [DOI] [PubMed] [Google Scholar]
- 36. Wong RA, Schumann B, Townsend R, Phelps CA. A survey of therapeutic ultrasound use by physical therapists who are orthopaedic certified specialists. Phys Ther. 2007;87(8):986-994 [DOI] [PubMed] [Google Scholar]


