Abstract
A total of 514 Shiga toxin-producing Escherichia coli (STEC) isolates from diarrheic and healthy cattle in Spain were characterized in this study. PCR showed that 101 (20%) isolates carried stx1 genes, 278 (54%) possessed stx2 genes, and 135 (26%) possessed both stx1 and stx2. Enterohemolysin (ehxA) and intimin (eae) virulence genes were detected in 326 (63%) and in 151 (29%) of the isolates, respectively. STEC isolates belonged to 66 O serogroups and 113 O:H serotypes (including 23 new serotypes). However, 67% were of one of these 15 serogroups (O2, O4, O8, O20, O22, O26, O77, O91, O105, O113, O116, O157, O171, O174, and OX177) and 52% of the isolates belonged to only 10 serotypes (O4:H4, O20:H19, O22:H8, O26:H11, O77:H41, O105:H18, O113:H21, O157:H7, O171:H2, and ONT:H19). Although the 514 STEC isolates belonged to 164 different seropathotypes (associations between serotypes and virulence genes), only 12 accounted for 43% of isolates. Seropathotype O157:H7 stx2 eae-γ1 ehxA (46 isolates) was the most common, followed by O157:H7 stx1 stx2 eae-γ1 ehxA (34 isolates), O113:H21 stx2 (25 isolates), O22:H8 stx1 stx2 ehxA (15 isolates), O26:H11 stx1 eae-β1 ehxA (14 isolates), and O77:H41 stx2 ehxA (14 isolates). Forty-one (22 of serotype O26:H11) isolates had intimin β1, 82 O157:H7 isolates possessed intimin γ1, three O111:H- isolates had intimin type γ2, one O49:H- strain showed intimin type δ, 13 (six of serotype O103:H2) isolates had intimin type ɛ and eight (four of serotype O156:H-) isolates had intimin ζ. We have identified a new variant of the eae intimin gene designated ξ (xi) in two isolates of serotype O80:H-. The majority (85%) of bovine STEC isolates belonged to serotypes previously found for human STEC organisms and 54% to serotypes associated with STEC organisms isolated from patients with hemolytic uremic syndrome. Thus, this study confirms that cattle are a major reservoir of STEC strains pathogenic for humans.
Shiga toxin-producing Escherichia coli (STEC), also called verotoxin-producing E. coli (VTEC), strains are the most important recently emerged group of food-borne pathogens. These bacteria can cause severe disease in humans, such as hemorrhagic colitis and hemolytic uremic syndrome (20, 29). Cattle, especially young animals, have been implicated as a principal reservoir of STEC, undercooked ground beef and raw milk being the major vehicles of food-borne outbreaks (2, 5).
Human and bovine STEC strains elaborate two potent phage-encoded cytotoxins called Shiga toxins (Stx1 and Stx2) or verotoxins (VT1 and VT2) (20, 29). In addition to toxin production, another virulence-associated factor expressed by STEC is a protein called intimin, which is responsible for intimate attachment of STEC to intestinal epithelial cells, causing attaching and effacing lesions in the intestinal mucosa (16). Intimin is encoded by the chromosomal gene eae, which is part of a pathogenicity island termed the locus for enterocyte effacement (19). Severe diarrhea (specially hemorrhagic colitis) and hemolytic uremic syndrome were closely associated with STEC types carrying the eae gene for intimin (19, 29).
Differentiation of intimin alleles represents an important tool for STEC typing in routine diagnostics as well as epidemiological and clonal studies. The C-terminal end of intimin is responsible for receptor binding, and it has been suggested that different intimins may be responsible for different host tissue cell tropism (23, 32, 42). Intimin type-specific PCR assays identified 14 variants of the eae gene that encode 14 different intimin types and subtypes (α1, α2, β1, β2, γ1, γ2/θ, δ/κ, ɛ, ζ, η, ι, λ, μ,ν) (1, 6, 10, 18, 26, 36, 37, 42; Blanco et al., submitted for publication). A factor that may also affect the virulence of STEC is the enterohemolysin, also called enterohemorrhagic E. coli hemolysin, which is encoded by the ehxA gene (35).
STEC strains that cause human infections belong to a large number of O:H serotypes (a total of 472 serotypes are listed at our website, http://www.lugo.usc/ecoli). Most outbreaks of hemorrhagic colitis and hemolytic uremic syndrome have been attributed to strains of the enterohemorrhagic serotype O157:H7 (5, 20). However, as STEC non-O157 strains are more prevalent in animals and as contaminants in foods, humans are probably more exposed to these strains. Infections with some non-O157 STEC types, such as O26:H11 or H-, O91:H21 or H-, O103:H2, O111:H-, O113:H21, O117:H7, O118:H16, O121:H19, O128:H2 or H-, O145:H28 or H- and O146:H21 are frequently associated with severe illness in humans, but the role of other non-O157 STEC types in human disease needs further examination (4, 5, 6, 11, 20). Although more than 400 different O:H serotypes of STEC have been isolated from cattle (a total of 435 serotypes are listed at our website, http://www.lugo.usc/ecoli), there is a lack of information regarding associations between serotype, intimin types, and virulence factor profiles among bovine STEC isolates (12, 24, 34, 40).
Thus, the aim of this study was to establish the serotypes, virulence genes, and intimin types of STEC strains isolated from cattle to establish if bovine STEC strains possess the same serotypes and virulence factor profiles as STEC strains that cause human infections.
MATERIALS AND METHODS
E. coli isolates and control strains.
A total of 514 STEC isolates from diarrheic and healthy cattle in Spain were characterized in this study. Only one isolate for each animal was included. E. coli strains used as controls were EPEC-2348 (human, O127:H6, eae-α1), AEEC-IH2498a (human, O125:H6, eae-α2), REPEC-RDEC-1 (rabbit, O15:H-, eae-β1), EPEC-359 (human, O119:H6, eae-β2), STEC-EDL933 (human, O157:H7, stx1 stx2 eae-γ1 ehxA), STEC-VTB308 (bovine, O111:H-, stx1 eae-γ2), STEC-TW07926 (human, O111:H8, stx1 stx2 eae-θ), EPEC-BP12665 (human, O86:H34, eae-δ), AEEC-6044/95 (human, O118:H5, eae-κ), STEC-VTB-286 (bovine, O103:H2, stx1 eae-ɛ), STEC-VTO-50 (ovine, O156:H-, stx1 eae-ζ), AEEC-CF11201 (human, O125:H-, eae-η), AEEC-7476/96 (human, O145:H4, eae-ι), AEEC-68-4 (human, O34:H-, eae-λ), EPEC-373 (human, O55:H51, eae-μ), AEEC-IH1229a (human, O10:H-, eae-ν), and K12-185 (negative for stx1, stx2, eae and ehxA). Strains were stored at room temperature in nutrient broth with 0.75% of agar.
Production and detection of Shiga toxins (verotoxins) in Vero and HeLa cells.
For production of Shiga toxins, one loopful of each isolated colony was inoculated in 50-ml Erlenmeyer flasks containing 5 ml of tryptone soy broth (pH 7.5) with mitomycin C and incubated for 20 h at 37°C (shaken at 200 rpm) and then centrifuged (6,000 × g) for 30 min at 4°C. The Vero and HeLa cell culture assays were performed with nearly confluent cell monolayers grown in plates with 24 wells. At the time of assay, the growth medium (RPMI with polymyxin sulfate) was changed (0.5 ml per well) and 75 μl of undiluted culture supernatant was added. Cells were incubated at 37°C in a 5% CO2 atmosphere and the morphological changes in cells were observed after 24 and 48 h of incubation with a phase contrast inverted microscope (8, 9).
Detection of virulence genes by PCR.
Bacteria were harvested from tryptone soy agar, suspended in 250 μl of sterile water, incubated at 100°C for 5 min to release the DNA, and centrifuged. The supernatant was used in the PCR as described below. Base sequences and predicted sizes of amplified products for the specific oligonucleotide primers used in this study are shown in Table 1. The majority of oligonucleotide primers were designed by us according to the nucleotide sequences of the virulence genes (Blanco et al., submitted). Multiplex PCR was used only for detection of stx1 and stx2 genes. Isolates positive for the eae gene with the EAE-1 and EAE-2 primers were afterwards analyzed with all different variant primers.
TABLE 1.
PCR primer and conditions for amplification of STEC virulence genes
| Gene | Primers | Oligonucleotide sequence (5′-3′)c | Fragment size (bp) | Annealing temp (°C) | Primer coordinates | GenBank accession no. |
|---|---|---|---|---|---|---|
| stx1 | VT1-A | CGCTGAATGTCATTCGCTCTGC | 302 | 55 | 113-134 | M17358 |
| VT1-B | CGTGGTATAGCTACTGTCACC | 394-414 | ||||
| stx2 | VT2-A | CTTCGGTATCCTATTCCCGG | 516 | 55 | 50-69 | M59432 |
| VT2-B | CTGCTGTGACAGTGACAAAACGC | 543-565 | ||||
| ehxA | HlyA1 | GGTGCAGCAGAAAAAGTTGTAG | 1,551 | 60 | 238-259 | X79839 |
| HlyA4 | TCTCGCCTGATAGTGTTTGGTA | 1767-1788 | ||||
| eaea | EAE-1 | GGAACGGCAGAGGTTAATCTGCAG | 775 | 55 | 1441-1460 | AF022236 |
| EAE-2 | GGCGCTCATCATAGTCTTTC | 2193-2215 | ||||
| eaeb | EAE-F | ATTACTGAGATTAAGGCTGAT | 682 | 55 | 1978-1998 | AF022236 |
| EAE-RB | ATTTATTTGCAGCCCCCCAT | 2640-2659 | ||||
| eae-α1 | EAE-FB | AAAACCGCGGAGATGACTTC | 820 | 60 | 1909-1928 | AF022236 |
| EAE-A | CACTCTTCGCATCTTGAGCT | 2709-2728 | ||||
| eae-α2 | IH2498aF | AGACCTTAGGTACATTAAGTAAGC | 517 | 60 | 2099-2122 | AF530555 |
| IH2498aR | TCCTGAGAAGAGGGTAATC | 2597-2615 | ||||
| eae-β | EAE-FB | AAAACCGCGGAGATGACTTC | 830 | 64 | 1909-1928 | AF453441 |
| EAE-B | CTTGATACACTTGATGACTGT | 2718-2738 | ||||
| eae-β1 | EA-B1-F | CGCCACTTAATGCCAGCG | 811 | 60 | 1928-1945 | AF453441 |
| EAE-B | CTTGATACACCTGATGACTGT | 2718-2738 | ||||
| eae-β2 | EA-B2-F | CCCGCCACTTAATCGCACGT | 807 | 60 | 1929-1948 | AF043226 |
| EAE-B | CTTGATACACCTGATGACTGT | 2715-2735 | ||||
| eae-γ1 | EAE-FB | AAAACCGCGGAGATGACTTC | 804 | 60 | 1909-1928 | AF071034 |
| EAE-C1 | AGAACGCTGCTCACTAGATGTC | 2691-2712 | ||||
| eae-γ2/θ | EAE-FB | AAAACCGCGGAGATGACTTC | 808 | 58 | 1909-1928 | AF025311 |
| EAE-C2 | CTGATATTTTATCAGCTTCA | 2697-2716 | ||||
| eae-δ/κ | EAE-FB | AAAACCGCGGAGATGACTTC | 833 | 60 | 1909-1928 | U66102 |
| EAE-D | CTTGATACACCCGATGGTAAC | 2721-2741 | ||||
| eae-ɛ | EAE-FB | AAAACCGCGGAGATGACTTC | 722 | 66 | 1909-1928 | AF116899 |
| LP5 | AGCTCACTCGTAGATGACGGCAAGCG | 2605-2630 | ||||
| eae-ζ | Z1 | GGTAAGCCGTTATCTGCC | 206 | 62 | 2062-2079 | AF449417 |
| Z2 | ATAGCAAGTGGGGTGAAG | 2250-2267 | ||||
| eae-η | EAE-FB | AAAACCGCGGAGATGACTTC | 712 | 62 | 1899-1918 | AJ308550 |
| LP8 | TAGATGACGGTAAGCGAC | 2593-2610 | ||||
| eae-ι | EAE-FB | AAAACCGCGGAGATGACTTC | 807 | 66 | 1909-1928 | AJ308551 |
| LP7 | TTTATCCTGCTCCGTTTGCT | 2695-2715 | ||||
| eae-λ | 68.4F | CGGTCAGCCTGTGAAGGGC | 466 | 64 | 2061-2079 | AF530557 |
| 68.4R | ATAGATGCCTCTTCCGGTATT | 2506-2526 | ||||
| eae-μ | FV373F | CAACGGTAAGTCTCAGACAC | 443 | 64 | 114-133 | AJ579305 |
| FV373R | CATAATAAGCTTTTTGGCCTACC | 534-556 | ||||
| eae-ν | IH1229aF | CACAGCTTACAATTGATAACA | 311 | 60 | 269-289 | AJ579306 |
| IH1229aR | CTCACTATAAGTCATACGACT | 559-579 | ||||
| eae-ξ | EAE-FB | AAAACCGCGGAGATGACTTC | 468 | 66 | 1909-1928 | AF116899 |
| B49R | ACCACCTTTAGCAGTCAATTTG | 363-384 | AJ582912 |
Universal oligonucleotide primer pair EAE-1 and EAE-2 with homology to the 5′ conseved region of eae (detects all types of eae variants described at the moment).
Universal oligonucleotide primer pair EAE-F and EAE-RB with homology to the 3′ variable region of eae (detects all types of eae variants described at the moment).
HlyA1 and Hly4 primer pair was designed by Schmidt et al. (35). The remaining primer pairs were designed by us according to the nucleotide sequences of the genes (10; Blanco et al., submitted).
Amplification of bacterial DNA was performed with 30-μl volumes containing 7 μl of the prepared sample supernatant; 150 ng of the oligonucleotide primers; 0.2 mM (each) dATP, dGTP, dCTP, and dTTP; 10 mM Tris-HCl (pH 8.8); 1.5 mM MgCl2; 50 mM KCl; and 1 U of Biotaq DNA polymerase (Bioline, United Kingdom). The conditions for the PCR were 94°C for 2 min for initial denaturation of DNA within the sample, followed by 35 cycles of 94°C for 1 min (denaturation), 55°C to 66°C (see Table 1) for 1 min (primer annealing), and 72°C for 1 min (DNA synthesis) performed with a thermal cycler (model PCR express; Hybaid, United Kingdom). The amplified products were visualized by standard submarine gel electrophoresis with 10 μl of the final reaction mixture on a 2% agarose gel in TBE buffer (89 mM Tris, 89 mM boric acid, 2.5 mM EDTA). The samples were electrophoresed for 20 to 40 min at 130 V. Amplified DNA fragments of specific sizes were located by UV fluorescence after being stained with ethidium bromide. Molecular size markers (HaeIII digest of φx174DNA) (Promega) were included in each gel (10).
Sequencing of the eae genes.
The nucleotide sequence of the amplification products was determined by the dideoxynucleotide triphosphate chain termination method of Sanger, with the BigDye Terminator v3.1 cycle sequencing kit with an ABI 3100 genetic analyzer (Applied Bio-Systems).
Phylogenetic analyses.
Genetic distances and phylogenetic trees of eae sequences were calculated and constructed with the CLUSTAL W program (38) included in the EMBL software (http://www.ebi.ac.uk/clustalw/).
Serotyping.
The determination of O and H antigens was carried out by the method described by Guinée et al. (14) employing all available O (O1 to O181) and H (H1 to H56) antisera. All antisera were obtained and absorbed with the corresponding cross-reacting antigens to remove the nonspecific agglutinins. The O antisera were produced in the Laboratorio de Referencia de E. coli, Departamento de Microbioloxía e Parasitoloxía, Facultade de Veterinaria, Universidade de Santiago de Compostela (Lugo, Spain, http://www.lugo.usc.es/ecoli), and the H antisera were obtained from the Statens Seruminstitut (Copenhagen, Denmark).
Nucleotide sequence accession number.
The eae-ξ sequence of strain B49 O80:H- was deposited in the European Bioinformatics Institute (EMBL Nucleotide Sequence Database) and has been assigned accession number AJ582912.
RESULTS
Virulence genes.
A total of 514 STEC isolates from diarrheic and healthy cattle in Spain were characterized in this study. PCR showed that 101 (20%) isolates carried stx1 genes, 278 (54%) possessed stx2 genes, and 135 (26%) possessed both stx1 and stx2. Enterohemolysin (ehxA) and intimin (eae) virulence genes were detected in 326 (63%) and in 151 (29%) of the isolates, respectively.
Serotypes and seropathotypes.
STEC isolates belonged to 66 O serogroups and 113 O:H serotypes (including 23 new serotypes). However, 67% were of one of these 15 serogroups (O2, O4, O8, O20, O22, O26, O77, O91, O105, O113, O116, O157, O171, O174, and OX177) and 52% of isolates belonged to only 10 serotypes (O4:H4, O20:H19, O22:H8, O26:H11, O77:H41, O105:H18, O113:H21, O157:H7, O171:H2, and ONT:H19). Although the 514 STEC isolates belonged to 164 different seropathotypes (associations between serotypes and virulence genes), only 12 accounted for 43% of isolates (Table 2). Seropathotype O157:H7 stx2 eae ehxA (46 isolates) was the most common, followed by O157:H7 stx1 stx2 eae ehxA (34 isolates), O113:H21 stx2 (25 isolates), O22:H8 stx1 stx2 ehxA (15 isolates), O26:H11 stx1 eae ehxA (14 isolates), and O77:H41 stx2 ehxA (14 isolates). The majority 85% of bovine STEC isolates belonged to serotypes previously found among human STEC strains and 54% to serotypes associated with STEC strains isolated from patients with hemolytic uremic syndrome.
TABLE 2.
Seropathotypes (serotypes and virulence genes) of 514 bovine STEC isolates
| Serotype | Total no. of strains | stx1 | stx2 | eae | ehxA | Serotype | Total no. of strains | stx1 | stx2 | eae | ehxA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O2:H27a | 7 | − | + | − | − | |||||||
| O2:H29b | 5 | − | + | − | − | |||||||
| O4:H4 | 1 | − | + | − | + | |||||||
| O4:H4 | 10 | − | + | − | − | |||||||
| O6:H8c | 1 | + | + | − | + | |||||||
| O6:H34a | 1 | − | + | − | + | |||||||
| O7:H6a | 1 | + | − | − | − | |||||||
| O8:H2b | 5 | + | + | − | + | |||||||
| O8:H2b | 1 | + | − | − | − | |||||||
| O8:H2b | 2 | − | + | − | + | |||||||
| O8:H2b | 1 | − | + | − | − | |||||||
| O8:H8 | 1 | + | − | − | − | |||||||
| O8:H19b | 1 | + | + | − | + | |||||||
| O8:H19b | 1 | − | + | − | + | |||||||
| O8:H19b | 1 | − | + | − | − | |||||||
| O9:H-b | 1 | + | − | − | + | |||||||
| O15:H16 | 1 | − | + | − | − | |||||||
| O15:H19c | 1 | + | + | − | − | |||||||
| O15:H27a | 1 | − | + | − | − | |||||||
| O17:H18a | 1 | + | + | − | + | |||||||
| O17:H18a | 1 | + | − | − | + | |||||||
| O20:H7a | 1 | + | − | − | − | |||||||
| O20:H19b | 10 | + | + | − | + | |||||||
| O20:H19b | 2 | − | + | − | + | |||||||
| O20:H19b | 6 | − | + | − | − | |||||||
| O22:H8b | 15 | + | + | − | + | |||||||
| O22:H8b | 2 | + | + | − | − | |||||||
| O22:H8b | 1 | + | − | − | + | |||||||
| O22:H8b | 1 | + | − | − | − | |||||||
| O22:H8b | 4 | − | + | − | + | |||||||
| O22:H8b | 2 | − | + | − | − | |||||||
| O22:H16a | 1 | − | + | − | + | |||||||
| O22:H16a | 1 | − | + | − | − | |||||||
| O26:H-b | 1 | + | − | + (β1) | + | |||||||
| O26:H-b | 2 | − | + | + (β1) | + | |||||||
| O26:H11b | 14 | + | − | + (β1) | + | |||||||
| O26:H11b | 8 | + | − | + (β1) | − | |||||||
| O26:H11b | 1 | + | − | − | + | |||||||
| O28:H9c | 1 | + | + | − | + | |||||||
| O38:H21a | 1 | − | + | − | + | |||||||
| O39:H21 | 1 | + | + | − | − | |||||||
| O39:H48a | 1 | − | + | − | − | |||||||
| O41:H2a | 3 | − | + | − | + | |||||||
| O41:H14 | 1 | + | − | − | − | |||||||
| O41:H51c | 1 | + | − | − | − | |||||||
| O49:H-b | 1 | + | − | + (δ) | + | |||||||
| O60:H19a | 1 | − | + | − | + | |||||||
| O64:H-c | 4 | + | − | − | − | |||||||
| O64:H34c | 1 | − | + | − | − | |||||||
| O65:H16a | 1 | − | + | − | − | |||||||
| O74:H28c | 1 | + | − | − | − | |||||||
| O77:H41a | 5 | + | + | − | + | |||||||
| O77:H41a | 14 | − | + | − | + | |||||||
| O77:H41a | 2 | − | + | − | − | |||||||
| O79:H- | 1 | + | + | − | + | |||||||
| O80:H-a | 2 | + | − | + (ξ) | + | |||||||
| O81:H28c | 1 | + | + | − | + | |||||||
| O81:H28c | 1 | + | + | − | − | |||||||
| O81:H31a | 1 | + | + | − | + | |||||||
| O82:H8a | 7 | + | + | − | + | |||||||
| O84:H2a | 1 | + | − | + (ζ) | + | |||||||
| O88:H8 | 1 | + | + | − | + | |||||||
| O88:H25a | 1 | − | + | − | + | |||||||
| O90:H-a | 1 | + | − | − | − | |||||||
| O91:H19c | 1 | + | − | − | + | |||||||
| O91:H19c | 1 | − | + | − | − | |||||||
| O91:H21b | 2 | + | + | − | + | |||||||
| O91:H21b | 6 | − | + | − | + | |||||||
| O96:H-a | 1 | + | + | − | + | |||||||
| O103:H-b | 2 | + | − | + (ɛ) | + | |||||||
| O103:H2b | 5 | + | − | + (ɛ) | + | |||||||
| O103:H2b | 1 | + | − | − | + | |||||||
| O103:H21a | 1 | + | − | + (ɛ) | − | |||||||
| O104:H21b | 1 | + | + | − | + | |||||||
| O104:H21b | 1 | + | + | − | − | |||||||
| O105:H18b | 12 | + | + | − | + | |||||||
| O105:H18b | 3 | + | − | − | + | |||||||
| O110:H2 | 1 | − | + | − | − | |||||||
| O111:H-b | 3 | + | − | + (γ2) | + | |||||||
| O113:H-a | 3 | − | + | − | − | |||||||
| O113:H4a | 1 | + | + | − | − | |||||||
| O113:H4a | 7 | − | + | − | − | |||||||
| O113:H7a | 1 | + | − | − | − | |||||||
| O113:H21b | 1 | + | + | − | − | |||||||
| O113:H21b | 7 | − | + | − | + | |||||||
| O113:H21b | 25 | − | + | − | − | |||||||
| O116:H-a | 1 | − | + | − | + | |||||||
| O116:H21a | 9 | − | + | − | + | |||||||
| O117:H28a | 2 | + | + | − | − | |||||||
| O118:H-b | 2 | + | − | + (β1) | + | |||||||
| O118:H16b | 2 | + | − | + (β1) | + | |||||||
| O123:H16c | 2 | + | − | − | − | |||||||
| O126:H-a | 1 | + | − | + (β1) | + | |||||||
| O126:H20a | 4 | + | + | − | + | |||||||
| O127:H21a | 1 | − | + | − | − | |||||||
| O128:H-b | 4 | + | − | − | − | |||||||
| O132:H-a | 1 | + | − | − | − | |||||||
| O136:H- | 1 | + | − | − | − | |||||||
| O136:H- | 1 | − | + | + (ɛ) | − | |||||||
| O136:H12 | 3 | + | − | − | − | |||||||
| O136:H16 | 2 | + | − | − | − | |||||||
| O136:H16 | 1 | − | + | − | − | |||||||
| O138:H48c | 1 | + | − | + (ζ) | − | |||||||
| O138:H48c | 1 | + | − | − | − | |||||||
| O140:H21c | 1 | − | + | − | + | |||||||
| O141:H2a | 1 | + | + | − | + | |||||||
| O141:H8a | 1 | + | + | − | − | |||||||
| O141:H8a | 3 | − | + | − | + | |||||||
| O146:H21a | 1 | − | + | − | + | |||||||
| O146:H32c | 1 | + | − | − | + | |||||||
| O148:H8c | 1 | − | + | − | − | |||||||
| O149:H1c | 1 | + | − | − | − | |||||||
| O150:H-a | 1 | + | − | + (ζ) | + | |||||||
| O156:H-a | 3 | + | − | + (ζ) | + | |||||||
| O156:H-a | 1 | − | + | + (ζ) | + | |||||||
| O156:H-a | 5 | − | + | − | − | |||||||
| O156:H19c | 1 | − | + | − | − | |||||||
| O156:H25a | 1 | + | − | + (ζ) | + | |||||||
| O157:H7b | 34 | + | + | + (γ1) | + | |||||||
| O157:H7b | 46 | − | + | + (γ1) | + | |||||||
| O157:H7b | 2 | + | − | + (γ1) | + | |||||||
| O162:H4a | 1 | − | + | − | + | |||||||
| O162:H7c | 1 | + | + | − | + | |||||||
| O162:H7c | 1 | − | + | − | − | |||||||
| O162:H21c | 1 | − | + | − | + | |||||||
| O162:H21c | 3 | − | + | − | − | |||||||
| O162:H28c | 1 | + | + | − | − | |||||||
| O163:H19b | 3 | − | + | − | + | |||||||
| O163:H21c | 1 | + | + | − | + | |||||||
| O163:H21c | 1 | + | − | + (ɛ) | − | |||||||
| O163:H21c | 1 | − | + | − | + | |||||||
| O165:H21a | 1 | − | + | + (ɛ) | + | |||||||
| O166:H28a | 1 | − | + | − | − | |||||||
| O167:H21c | 1 | + | − | − | − | |||||||
| O168:H8 | 1 | + | − | − | − | |||||||
| O168:H8 | 2 | − | + | − | − | |||||||
| O171:H2a | 20 | − | + | − | − | |||||||
| O171:H25 | 1 | + | − | − | + | |||||||
| O171:H25 | 3 | − | + | − | − | |||||||
| O174:H-b | 3 | + | + | − | + | |||||||
| O174:H-b | 2 | − | + | − | − | |||||||
| O174:H2b | 5 | + | + | − | + | |||||||
| O174:H2b | 2 | − | + | − | + | |||||||
| O174:H2b | 1 | − | + | − | − | |||||||
| O174:H21b | 6 | − | + | − | − | |||||||
| OX177:H-a | 5 | − | + | + (β1) | + | |||||||
| OX177:H11 | 5 | + | − | + (β1) | + | |||||||
| OX178:H11c | 1 | + | + | − | + | |||||||
| OX178:H11c | 1 | − | + | − | + | |||||||
| OX178:H19a | 2 | + | + | − | + | |||||||
| ONT:H-b | 1 | − | + | + (β1) | + | |||||||
| ONT:H-b | 5 | − | + | − | + | |||||||
| ONT:H-b | 1 | − | + | − | − | |||||||
| ONT:H2a | 3 | + | + | − | + | |||||||
| ONT:H2a | 2 | − | + | − | + | |||||||
| ONT:H8a | 1 | + | + | − | + | |||||||
| ONT:H19a | 13 | − | + | − | + | |||||||
| ONT:H19a | 3 | − | + | − | − | |||||||
| ONT:H21a | 1 | + | + | − | − | |||||||
| ONT:H21a | 1 | − | + | − | − | |||||||
| ONT:H25b | 1 | + | − | + (ζ) | + | |||||||
| ONT:H32c | 1 | + | − | − | − |
Serotype previously found in human STEC.
Serotype previously associated with human STEC strains that cause hemolytic uremic syndrome.
New serotype not found in STEC in previous studies.
Typing of eae (intimin) genes.
Forty-one strains of serotypes O26:H11, O26:H-, O118:H16, O118:H-, O126:H-, OX177:H11, OX177:H-, and ONT:H- had intimin β1, 82 strains of serotype O157:H7 possessed intimin γ1, three strains of serotype O111:H- had intimin type γ2, one strain of serotype O49:H- showed intimin type δ, 13 strains of serotypes O103:H2, O103:H-, O103:H21, O136:H-, O163:H21, O165:H21 and O165:H25 had intimin type ɛ and eight strains of serotypes O84:H2, O138:H48, O150:H-, O156:H-, O156:H25 and ONT:H25 had intimin ζ. We have identified a new variant of the eae intimin gene designated ξ (xi) in two isolates of serotype O80:H-.
Sequence comparison and evolutionary analysis of E. coli intimin genes.
An approximately 682-bp fragment of the 3′ variable region of the eae gene was amplified with universal eae primers EAE-F and EAE-RB from the STEC-B49 O80:H- strain and sequenced. As the eae sequence of STEC-B49 strain (accession number AJ582912) represents a new variant of the intimin gene, it has been designated eae-ξ (xi) in order to follow the Greek alphabet.
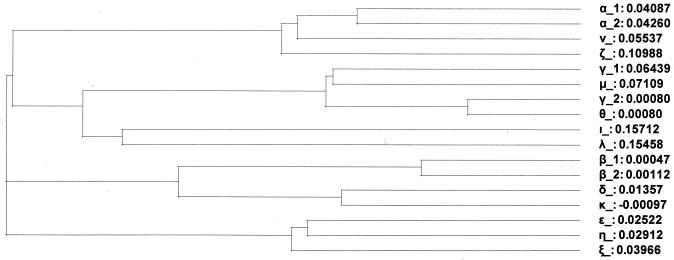
We determined the genetic relationship of the 3′ variable region of the eae-ξ gene (accession number AJ582912, fragment size 600 bp, primer coordinates 1 to 600) and the remaining 16 eae variants: α1 (AF022236, 635 bp, 2002 to 2636, aligned score 60%), α2 (AJ579368, 635 bp, 2002 to 2636, 54%), β1 (AF453441, 635 bp, 2002 to 2636, 64%), β2 (AF043226, 629 bp, 2005 to 2633, 63%), γ1 (AF071034, 620 bp, 2002 to 2621, 58%), γ2 (AF025311, 623 bp, 2002 to 2624, 59%), δ (U66102, 638 bp, 2002 to 2639, 61%), ɛ (AF116899, 644 bp, 2002 to 2645, 93%), ζ (AF449417, 629 bp, 2002 to 2630, 58%), η (AJ308550, 644 bp, 1992 to 2635, 91%), θ (AF449418, 623 bp, 2002 to 2624, 59%), ι (AJ308551, 632 bp, 2002 to 2633, 63%), κ (AJ308552, 635 bp, 1992 to 2636, 62%), λ (AF530557, 632 bp, 2002 to 2633, 56%), μ (AJ579305, 622 bp, 1 to 622, 59%), and ν (AJ579306, 635 bp, 16 to 650, 60%). Since the nucleotide sequences analyzed were of different lengths (600 to 644 bp), we used CLUSTAL W for optimal sequence alignment. Thus, the eae-ξ sequence is similar to eae-ɛ (identity of 93%) and eae-η (identity of 91%) sequences. The phylogenetic tree (Fig. 1) supports the data of the sequence identity values. Phylogenetic analyses revealed five groups of the closely related intimin genes: (i) α1, α2, ζ and ν; (ii) β1, β2, δ and κ; (iii) γ1, γ2, θ and μ; (iv) ι and λ; and (v) ɛ, η and ξ.
FIG. 1.
Phylogenetic tree of the intimin variant genes constructed with the CLUSTAL W program. Numbers on the branches denote the genetic distance. Phylogenetic analyses revealed five groups of the closely related intimin genes: (i) α1, α2, ζ, and ν; (ii) β1, β2, δ, and κ; (iii) γ1, γ2, θ, and μ; (iv) ι and λ; and (v) ɛ, η, and ξ.
DISCUSSION
The results of our previous studies indicate that STEC colonization is widespread among cattle in Spain. Between 1993 and 1995, 1,069 healthy cattle were examined for STEC colonization. STEC-positive animals were found in 95% of the farms examined and the estimated proportion of positive cattle in each farm ranged from 0 to 100%. The overall prevalence rates of STEC colonization were estimated to be 37% in calves and 27% in cows. In Spain, stx2 and stx1 stx2 isolates were present in similar proportions in calves and cows. In contrast, stx1 and eae isolates were more commonly recovered from calves than from cows. STEC O157:H7 was detected in only 8 (0.7%) of the 1,069 animals investigated. Interestingly, the majority of eae non-O157 STEC and the eight STEC O157:H7 were isolated from calves, confirming that young animals are the most important reservoir of highly pathogenic eae STEC (5, 8, 9).
In a survey realized between 1998 and 1999, STEC O157:H7 was isolated from 55 (12%) of 471 feedlot calves (4 to 8 months of age). Although only a mean of three animals per herd were sampled, STEC O157:H7 isolates were detected in 32 (22%) of 145 feedlots examined. When we sampled a higher number of animals (9 to 56), STEC O157:H7 was found in 3 (60%) of five feedlots investigated. Individual prevalences in the three positive feedlots were 23% (13 of 56), 22% (two of nine) and 8% (1 of 13). Farms were visited only in one occasion to collect fecal samples (5). While the total numbers of healthy and diarrheic calves positive for STEC did not vary significantly, a significantly higher percentage of Stx1-producing E. coli was found in diarrheic calves, suggesting a pathogenic role in neonatal calf diarrhea (5, 7). Similar prevalences of STEC were found in other countries (3, 15, 21, 22, 25, 33, 39, 41, 43, 44).
In the present work, we have established the serotypes, virulence genes, and intimin types of STEC isolated from cattle in previous studies (5, 7, 8, 9) to know if bovine STEC possess the same serotypes and virulence factor profiles that STEC strains that cause human infection. STEC isolates belonged to 66 O serogroups and 113 O:H serotypes (including 23 new serotypes). However, 67% were of one of these 15 serogroups (O2, O4, O8, O20, O22, O26, O77, O91, O105, O113, O116, O157, O171, O174, and OX177) and 52% of isolates belonged to only 10 serotypes (O4:H4, O20:H19, O22:H8, O26:H11, O77:H41, O105:H18, O113:H21, O157:H7, O171:H2, and ONT:H19), including 10 serotypes also found among STEC strains that cause human infections and six serotypes associated with hemolytic uremic syndrome (O20:H19, O22:H8, O26:H11, O105:H18, O113:H21, and O157:H7). Similarly, among the 20 serotypes most frequently isolated in cattle or cattle products in Canada by Johnson et al. (17), 18 have been isolated from humans, and 11 of these are serotypes associated with bloody diarrhea/hemorrhagic colitis and/or hemolytic uremic syndrome. Pradel et al. (33) in France, Beutin et al. (3), and Montenegro et al. (25) in Germany, Wells et al. (39) in the United States, and Parma et al. (28) in Argentina have also found that many STEC isolates recovered from cattle belong to serotypes previously associated with human disease. Like other authors (12, 17, 24, 40), we have observed that bovine and human STEC isolates of the same serotype have similar known virulence-associated properties.
The eae gene, which has been shown to be necessary for attaching and effacing activity, encodes a 94- to 97-kDa outer membrane protein which is termed intimin (16, 20). Numerous investigators have underlined the strong association between the carriage of the eae gene and the capacity of STEC strains to cause severe human disease, especially hemolytic uremic syndrome (1, 27, 29). This important virulence gene was detected in 100% of STEC O157:H7 and in 17% of non-O157 bovine isolates assayed in the present study. Nevertheless, production of intimin is not essential for pathogenesis, because a number of sporadic cases of hemolytic uremic syndrome have been caused by eae-negative non-O157 STEC strains. Thus, STEC O104:H21 and O113:H21 strains lacking the eae gene were responsible for an outbreak and a cluster of three hemolytic uremic syndrome cases in the United States and Australia, respectively (30, 31). Furthermore, recently Paton and Paton (31) described a novel megaplasmid-encoded adhesin (Saa) which we have detected in some of bovine STEC strains lacking the eae gene (data not published). This adhesin may be an important virulence factor of eae-negative STEC strains capable of causing severe diseases in humans.
Analysis of the nucleotide sequences of the intimin genes from different STEC and enteropathogenic E. coli strains has shown a high degree of homology in the 5′ two-thirds of the genes and a significant degree of heterogeneity in the 3′ one-third of the genes (1, 13). Fourteen variants of the eae gene were identified by intimin type-specific PCR assays with oligonucleotide primers complementary to the 3′end of the specific intimin genes that encode the intimin types α1, α2, β1, β2, γ1, γ2/θ, δ/κ, ɛ, ζ, η, ι, λ, μ, and ν (1, 6, 10, 18, 26, 27, 36, 37, 42; Blanco et al., submitted).
In the present study, we have identified a new intimin variant, designated intimin type ξ (xi) to follow the Greek alphabet. The eae-ξ (xi) sequence of our bovine STEC-B49 O80:H- strain is almost identical (>99%) to the eae genes sequenced by K. G. Zehmke (accession numbers AJ276416, AJ275092, AJ275103, and AJ275107, unpublished data) from four STEC strains isolated from calves in Germany and Belgium. As the eae-ξ sequence is similar to the eae-ɛ variant, K. G. Zehmke assumed that his bovine STEC strains were positive for intimin type ɛ. Our phylogenetic analysis revealed that the eae-ξ gene is closely related to intimin genes eae-ɛ and eae-η. As in human strains, the intimin types β1 and γ1 are the most frequently found among bovine STEC isolates. The intimin β1 was mainly found among strains belonging to serotype O26:H11, whereas the intimin type γ1 was detected in all 82 STEC O157:H7 assayed.
Seropathotypes O26:H11 stx1 eae-β1, O157:H7 stx1 stx2 eae-γ1 and O157:H7 stx1 stx2 eae-γ1 are the most frequently observed in STEC strains that cause human infections in Spain (6). These highly virulent seropathotypes were detected in 102 bovine STEC strains characterized in the present study. Although our and other authors' results indicate that STEC strains of human and animal origin with the same serotype are similar in relation to the presence of known virulence-associated factors (4, 11, 12, 24, 34), the results of Boerlin et al. (11) suggest that STEC isolates from humans form a different population from those found in the bovine reservoir or that they are only a subpopulation of the latter. Thus, future studies are necessary to establish if animal and human strains represent the same clones or are only related subpopulations.
Acknowledgments
We thank Monserrat Lamela for skillful technical assistance.
This work was supported by grants from the Fondo de Investigación Sanitaria (grants FIS G03-025-COLIRED-O157 and FIS 98/1158), the Xunta de Galicia (grant PGIDIT02BTF26101PR), the Comisión Interministerial de Ciencia y Tecnología (grants CICYT-ALI98-0616 and CICYT-FEDER-1FD1997-2181-C02-01), and the European Commission (FAIR program grants CT98-4093 and CT98-3935). A. Mora and G. Dahbi acknowledge the Xunta de Galicia and the Agencia Española de Cooperación Internacional (AECI) for research fellowships.
REFERENCES
- 1.Adu-Bobie, J., G. Frankel, C. Bain, A. G. Goncalves, L. R. Trabulsi, G. Douce, S. Knutton, and G. Dougan. 1998. Detection of intimins α, β, γ, and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J. Clin. Microbiol. 36:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, G. L., J. Hollingsworth, and J. G. Morris. 1996. Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol. Rev. 18:29-51. [DOI] [PubMed] [Google Scholar]
- 3.Beutin, L., D. Geier, H. Steinrück, S. Zimmermann, and F. Scheutz. 1993. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J. Clin. Microbiol. 31:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutin, L. 1999. Escherichia coli O157 and other types of verocytotoxigenic E. coli (VTEC) isolated from humans, animals and food in Germany, p. 121-145. In C. S. Stewart and H. J. Flint (ed.), Escherichia coli O157 in farm animals. CABI Publishing, Wallingford, United Kingdom.
- 5.Blanco, J., M. Blanco, J. E. Blanco, A. Mora, M. P. Alonso, E. A. González, and M. I. Bernárdez. 2001. Epidemiology of verocytotoxigenic Escherichia coli (VTEC) in ruminants, p. 113-148. In G. Duffy et al. (ed.), Verocytotoxigenic Escherichia coli. Food & Nutrition Press Inc., Trumbull, N.J.
- 6.Blanco, J. E., M. Blanco, M. P. Alonso, A. Mora, G. Dahbi, M. A. Coira, and J. Blanco. 2004. Serotypes, virulence genes and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from human patients: prevalence in Lugo (Spain) from 1992. through 1999. J. Clin. Microbiol. 42:311-319. [DOI] [PMC free article] [PubMed]
- 7.Blanco, M., J. Blanco, J. E. Blanco, and J. Ramos. 1993. Enterotoxigenic, verotoxigenic and necrotoxigenic Escherichia coli isolated from cattle in Spain. Am. J. Vet. Res. 54:1446-1451. [PubMed] [Google Scholar]
- 8.Blanco, M., J. E. Blanco, J. Blanco, E. A. González, A. Mora, C. Prado, L. Fernández, M. Rio, J. Ramos, and M. P. Alonso. 1996. Prevalence and characteristics of Escherichia coli serotype O157:H7 and other verotoxin-producing E. coli in healthy cattle. Epidemiol. Infect. 117:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco, M., J. E. Blanco, J. Blanco, A. Mora, C. Prado, M. P. Alonso, M. Mouriño, C. Madrid, C. Balsalobre, and A. Juárez. 1997. Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet. Microbiol. 54:309-319. [DOI] [PubMed] [Google Scholar]
- 10.Blanco, M., J. E. Blanco, A. Mora, J. Rey, J. M. Alonso, M. Hermoso, J. Hermoso, M. P. Alonso, G. Dhabi, E. A. González, M. I. Bernárdez, and J. Blanco. 2003. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from healthy sheep in Spain. J. Clin. Microbiol. 41:1351-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brett, K. N., M. A. Hornitzky, K. A. Bettelheim, M. J. Walker, and S. P. Djordjevic. 2003. Bovine non-O157 Shiga toxin 2-containing Escherichia coli isolates commonly possess stx2-EDL933 and/or stx2vhb subtypes. J. Clin. Microbiol. 41:2716-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gannon, V. P. J., M. Rashed, R. K. King, and E. J. Golsteyn Thomas. 1993. Detection and characterization of the eae gene of Shiga-like toxin-producing Escherichia coli with polymerase chain reaction. J. Clin. Microbiol. 31:1268-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guinée, P. A. M., W. H. Jansen, T. Wadström, and R. Sellwood. 1981. Escherichia coli associated with neonatal diarrhoea in piglets and calves. Curr. Top. Vet. Anim. Sci. 13:126-162. [Google Scholar]
- 15.Guth, B. E. C., I. Chinen, E. Miliwebsky, A. M. F. Cerqueira, G. Chillemi, J. R. C. Andrade, A. Baschkier, and M. Rivas. 2003. Serotypes and Shiga toxin genotypes among Escherichia coli isolated from animals and food in Argentina and Brazil. Vet. Microbiol. 92:335-349. [DOI] [PubMed] [Google Scholar]
- 16.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Karper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, R. P., R. C. Clarke, J. B. Wilson, S. Read, K. Rahn, S. A. Renwick, K. A. Sandhu, D. Alves, M. A. Karmali, H. Lior, S. A. Mcewen, J. S. Spika, and C. L. Gyles. 1996. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J. Food Prot. 59:1112-1122. [DOI] [PubMed] [Google Scholar]
- 18.Jores, J., K. Zehmke, J. Eichberg, L. Rumer, and L. H. Wieler. 2003. Description of a novel intimin variant (type ζ) in the bovine O84:NM verotoxin-producing Escherichia coli strain 537/89 and the diagnostic value of intimin typing. Exp. Biol. Med. 228:370-376. [DOI] [PubMed] [Google Scholar]
- 19.Kaper, J. B., S. Elliott, V. Sperandio, N. T. Perna, G. F. Mayhew, and F. R. Blattner. 1998. Attaching and effacing intestinal histopathology and the locus of enterocyte effacement, p. 163-182.. In J. B. Kaper and A. D. O′Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 20.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, H., J. Shimada, M. Nakazawa, T. Morozumi, T. Pohjanvirta, S. Pelkonen, and K. Yamamoto. 2001. Prevalence and characteristics of Shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl. Environ. Microbiol. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mainil, J. G., E. R. Jacquemin, A. E. Kaeckenbeeck, and P. H. Pohl. 1993. Association between the effacing (EAE) gene and the Shiga-like toxin-encoding genes in Escherichia coli isolates from cattle. Am. J. Vet. Res. 54:1064-1068. [PubMed] [Google Scholar]
- 23.McGraw, E. A., J. Li, R. K. Selander, and T. S. Whittam. 1999. Molecular evolution and mosaic structure of alpha, beta, and gamma intimins of pathogenic Escherichia coli. Mol. Biol. Evol. 16:12-22. [DOI] [PubMed] [Google Scholar]
- 24.Meng, J., S. Zhao, and M. P. Doyle. 1998. Virulence genes of shiga toxin-producing Escherichia coli isolated from food, animals and humans. Int. J. Food Microbiol. 45:229-235. [DOI] [PubMed] [Google Scholar]
- 25.Montenegro, M. A., M. Bülte, T. Trumpf, S. Aleksic, G. Reuter, E. Bulling, and R. Helmuth. 1990. Detection and characterization of fecal verotoxin-producing Escherichia coli from healthy cattle. J. Clin. Microbiol. 28:1417-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen, E. M., and M. T. Andersen. 2003. Detection and characterization of verocytotoxin-producing Escherichia coli by automated 5′ nuclease PCR assay. J. Clin. Microbiol. 41:2884-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marchès, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parma, A. E., M. E. Sanz, J. E. Blanco, J. Blanco, M. R. Viñas, and M. Blanco. 2000. Virulence genotypes and serotypes of verotoxigenic Escherichia coli isolated from cattle and foods in Argentina. Eur. J. Epidemiol. 16:757-762. [DOI] [PubMed] [Google Scholar]
- 29.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paton, A. W., M. C. Woodrow, R. M. Doyle, J. A. Lanser, and J. C. Paton. 1999. Molecular characterization of a shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paton, A. W., and J. C. Paton. 2002. Direct detection and characterization of shiga toxigenic Escherichia coli by multiplex PCR for stx1, stx2, eae, ehxA, and saa. J. Clin. Microbiol. 40:271-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips, A. D., and G. Frankel. 2000. Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J. Infect. Dis. 181:1496-1500. [DOI] [PubMed] [Google Scholar]
- 33.Pradel, N., V. Livrelli, C. De Champs, J. B. Palcoux, A. Reynaud, F. Scheutz, J. Sirot, B. Joly, and C. Forestier. 2000. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J. Clin. Microbiol. 38:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandhu, K. S., R. C. Clarke, K. McFadden, A. Brouwer, M. Louie, J. Wilson, H. Lior, and C. L. Gyles. 1996. Prevalence of the eaeA gene in verotoxigenic Escherichia coli strains from dairy cattle in Southwest Ontario. Epidemiol. Infect. 116:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 66:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Son, W. G., T. A. Graham, and V. P. J. Gannon. 2002. Immunological characterization of Escherichia coli O157:H7 intimin γ1. Clin. Diagn. Lab. Immunol. 9:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarr, C. L., and S. Whittam. 2002. Molecular evolution of the intimin gene in O111 clones of pathogenic Escherichia coli. J. Bacteriol. 184:479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells, J. G., L. D. Shipman, K. D. Greene, E. G. Sowers, J. H. Green, D. N. Cameron, F. P. Downes, M. L. Mantin, P. M. Griffin, S. M. Ostroff, M. E. Potter, R. V. Tauxe, and I. K. Wachsmuth. 1991. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing Escherichia coli from dairy cattle. J. Clin. Microbiol. 29:985-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wieler, L. H., E. Vieler, C. Erpenstein, T. Schlapp, H. Steinrück, R. Bauerfeind, A. Byomi, and G. Baljer. 1996. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J. Clin. Microbiol. 34:2980-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson, J. B., S. A. McEwen, R. C. Clarke, K. E. Leslie, R. A. Wilson, D. Waltner-Toews, and C. L. Gyles. 1992. Distribution and characteristics of verocytotoxigenic Escherichia coli isolated from Ontario dairy cattle. Epidemiol. Infect. 108:423-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, W. L., B. Köhler, E. Oswald, L. Beutin, H. Karch, S. Morabito, A. Caprioli, S. Suerbaum, and H. Schmidt. 2002. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J. Clin. Microbiol. 40:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zschöck, M., H. P. Hamann, B. Kloppert, and W. Wolter. 2000. Shiga-toxin-producing Escherichia coli in faeces of healthy dairy cows, sheep and goats: prevalence and virulence properties. Lett. Appl. Microbiol. 31:203-208. [DOI] [PubMed] [Google Scholar]