Abstract
Lectins derived from plant and microbial sources constitute a vital class of entry inhibitors that target the oligomannose residues on the HIV envelope gp120. Despite their potency and specificity, success of lectin-based entry inhibitors may be impeded by issues in regards to economical production, formulation and potential mitogenicity. Therefore, there exists a gap in the HIV therapeutics pipeline that underscores the need for mass producible, synthetic, broad-spectrum, and biocomptabile inhibitors of HIV entry. Here, we present the development of a polymeric synthetic lectin, based on benzoboroxole (BzB), which exhibits weak affinity (~25 M−1) for non-reducing sugars, similar to those found on the HIV envelope. High molecular weight BzB-functionalized polymers demonstrated antiviral activity that increased with an increase in ligand density and molecular weight of the polymer construct; revealing that polyvalency improves activity. Polymers showed significant increase in activity from 25 to 75 mol% BzB functionalization with EC50 of 15 μM and 15 nM, respectively. A further increase in mole functionalization to 90% resulted in an increase of the EC50 (59 ± 5 nM), likely due to the elongated rigid structure of the polymer chain compelled by electrostatic repulsion between the boronic acid groups. An increase in molecular weight of the polymer at 50 mol% BzB functionalization showed a gradual but significant increase in antiviral activity, with the highest activity seen with the 382 kDa polymer (EC50 of 1.1 ± 0.5 nM in CEM cells and 11 ± 3 nM in TZM-bl cells). Supplementing the polymer backbone with 10 mol% sulfonic acid not only increased the aqueous solubility of the polymers by at least 50-fold, but also demonstrated a synergistic increase in anti-HIV activity (4.0 ± 1.5 nM in TZM-bl cells), possibly due to electrostatic interactions between the negatively charged polymer backbone and the positively charged V3-loop in the gp120. The benzoboroxole-sulfonic acid copolymers showed no decrease in activity in the presence of a seminal concentration of fructose (p > 0.05). Additionally, the co-polymers exhibit minimal, if any effect on the cellular viability, barrier properties, or cytokine levels in human reconstructed ecto-cervical tissue after 3 days of repeated exposure and did not show pronounced activity against a variety of other RNA and DNA viruses.
Keywords: Synthetic lectins, benzoboroxole, polyvalency, entry inhibitor, HIV
INTRODUCTION
From a therapeutic perspective, each step in the HIV life-cycle provides an opportunity for pharmaceutical intervention. Amongst the initial steps in HIV infection is the binding event that occurs between the gp120 receptor on the viral envelope and surface proteins on the target cell. The HIV envelope is among the most heavily glycosylated proteins known to mankind.1, 2 The gp120 of HIVIIIB has 24 potential N-linked glycosylation sites, of which 13 sites contain complex-type oligosaccharides, and the remaining 11 sites contain hybrid and/or high mannose-type structures.3, 4 Reports on the antiviral activity of lectin-based entry inhibitors suggest that targeting the oligomannose regions of gp120 can potentially produce broad spectrum entry inhibitors capable of inactivating HIV independent of tropism and strains. Prolonged exposure of these agents to virus-infected cells causes cause de-glycosylations on the viral envelope, creating virions that are highly susceptible to neutralization by immunogenic responses. Such mutated virions also lack resistance to other small molecule entry inhibitors.5–8 Therefore, lectin-based gp120 targeted entry inhibitors represent an interesting class of antiretroviral agents that potenatially can inactivate HIV in the vaginal lumen even before they reach susceptible CD4+ cells, preventing male-to-female heterosexual HIV transmission.7, 9
All the entry inhibitors - with the exception of Maraviroc10 and Fuzeon11 - that have been tested in clinical trials have failed, most likely due to non-specific interactions with the cell surface and/or suboptimal efficacy.12–14 Consequently, there are a limited number of clinical candidates that target HIV entry in comparison to agents that target the enzymatic machinery involved in the viral replication.15 Carbohydrate binding proteins (CBP), such as the non-human naturally occurring lectin protein (MBL)16, cyanovirin-N (CV-N)17 and griffithsin18 that bind to the high-mannose oligosaccharides found on gp120, demonstrate the ability to inhibit HIV infection in vitro and ex vivo, irrespective of the cell type, or the co-receptor tropism of the viral strain.19 However, the unacceptably high cost of large scale production and purification of these protein-based lectins, added to their low-stability and plausible immunogenic response,20 may limit their use as a microbicide.21
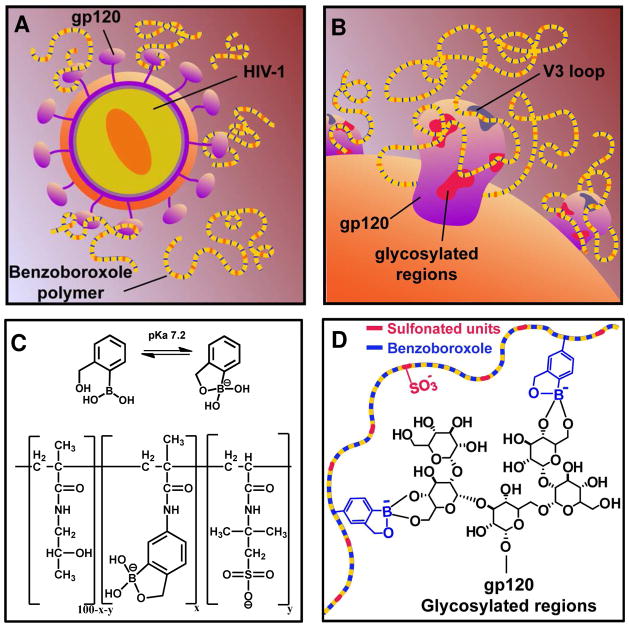
Polyvalency - a mechanism capitalized by CBPs - pertains to simultaneous interactions between multiple-linked ligands and receptors with repeating epitopes, such as those found on the HIV envelope.22 Due to translational entropic advantage23 and steric stabilization, polyvalent interactions may enhance binding affinity of weak ligands.22, 24 Inspired by nature’s adaptation to harness polyvalent interactions as a mechanism to enhance weak binding affinity between ligand-receptor pairs,22 we aimed to develop a synthetic polyvalent inhibitor of the HIV entry by targeting the high-density glycosylation sites on the viral envelope (Fig 1A&B).
Figure 1.
(A) Graphical depiction of the multivalent benzoboroxole-functionalized polymer interacting with the gp120 complex of HIV-1. (B) Schematic illustration of the binding between the polymers (shown in panel C) and the gp120 through interactions between benzoboroxole groups and the gp120 and/or through interactions between the anionic polysulfonate polymer and the cationic peptide fragments in the V3 loop of the gp120. (C) Chemical structure of benzoboroxole and the linear water-soluble polymers containing benzoboroxole and 2-acrylamido-2-methyl-1-propanesulfonic synthesized using a HPMA polymer backbone. (D) Hypothetical scheme of the binding chemistry between the multivalent polymers and the glycosylated regions on the gp120 heterotrimer.
Phenylboronic acids (PBA) form reversible covalent complexes with cis-diols - a common chemical entity found on glycoproteins - e.g. mannose, galactose, N-acetylneuraminic acid, and fucose residues.25 The presence of end-standing multiple mannose residues on each of the hybrid and/or complex-type N-linked glycans of gp120 suggests multiple binding sites for PBA-containing ligands. While PBA-containing polymers have demonstrated the ability to bind to glycosylated proteins, interactions of boronic acids with glycans have yet not been exploited as a mechanism to attenuate HIV entry. This is because the PBA and cis-diol form hydrolytically stable complexes only when the boronic acid exists in its tetrahedral form, mostly prevalent at a pH value greater than the pKa of PBA.26 PBA without any substitutions on the aromatic ring have a pKa of 8.8 and therefore, is found to bind strongly to diols only at alkaline pH’s.27 In order to improve binding at physiological pH researchers have investigated various methods to facilitate the formation of stable tetrahedral boronate species at neutral pH by lowering the pKa,27 through dative bonds formed with boron,28, 29 and multivalency.30 The addition of electron-withdrawing groups such as hydroxymethyl, fluoro and nitro to the phenyl ring of the PBA has been found to improve binding to diols at physiological pH compared to unsubstituted PBA.26, 27, 30, 31 Benzoboroxole (ortho-hydroxymethyl phenylboronic acid; BzB; Fig 1C) demonstrates strong binding to reducing sugars like fructose and is also capable of binding to glycopyranosides, like galactopyranose, a non-reducing sugar structurally similar to those found in the terminal glycan residues on gp120 at neutral pH.32 Owing to the ease of synthesis, stability and improved affinity to terminal sugar residues at neutral pH, this work focused on investigating BzB as a gp120-targeted HIV entry inhibitors.
In the past, our laboratory has shown that BzB ligands are capable of binding to the terminal sugar residues on the N-linked glycans of the gp120 and that multivalent polymers of BzB copolymerized with the water-soluble non-toxic 2-hydroxypropyl methacrylamide (HPMAm) monomer show cross-clade antiviral activity at nanomolar to low micromolar concentrations.33 Based on our investigations to evaluate the effect of mole functionalization of BzB on antiviral activity of the synthetic lectins,33 we chose the 50 mol% BzB polymers for further assessment. However, our previous system involved problems regarding solubility and activity, in addition to the need for biocompatibility evaluations. We hypothesized that incorporation of negatively-charged moieties would improve affinity to gp120 through electrostatic interactions with the positively charged V3 loop, while improving solubility and steric availability of the reactive boronic moieties. This work therefore pertains to modulating the multiplicity, ligand density and charge of ligand presenting a polymer backbone to augment the entry inhibition activity of the synthetic lectins. In the current work, we investigate the effect of - (a) ligand density (BzB mole % incorporation), (b) molecular weight of the BzB50 polymers and (c) activity of the polyvalent synthetic lectins with a sulfonated polymer backbone to inhibit HIV entry. Additionally, we also assayed the influence of fructose and the role of binding kinetics on the antiviral activity of the BzB50 polymers.
Materials and Methods
Alizarin Red S., phenylboronic acid, 2-hydroxymethyl phenylboronic acid and all the diols were purchased from Acros and were used as received. Water was double distilled with a Milli-Q filtration system. 2-Acrylamido-2-methyl- 1-propanesulfonic acid (AMPS) and AIBN was recrystallized from chloroform (dissolved at 60 °C, recrystallized at −80 °C) prior to use in free radical polymerizations. All other chemicals and reagents were purchased from Aldrich (St. Louis, MO) or Acros (Morris Plains, NJ) and used without further purification unless otherwise noted.
1. Colorimetric assay to determine binding constants for boronic acid–diol complexes
An ARS-based binding assay was performed as described by Wang et al.26, 34 Briefly, 10 μM solution of ARS dye was freshly prepared in 100 mM pH 7.4 phosphate buffer. A 2 mM stock solution of BzB was prepared in 10 μM solution of ARS. The pH of both solutions was re-adjusted to 7.4. Using a 96-well plate, varying amounts of BzB solution were added to ARS solution to obtain a range of concentrations of BzB maintaining the concentration of ARS constantly at 10 μM. The plate was allowed to shake for 60 sec prior to measurements. Fluorescence was measured at an excitation wavelength of 485 nm and emission at 620 nM. The experiments were carried out in triplicate. Absorbance spectra were collected over 300–600 nm. The association constant of PBA or BzB for ARS was determined by plotting the inverse of change in fluorescence vs. the inverse of the concentration of boronic acid. The quotient of the y-intercept and the slope of line were used to compute Ka for the boronic acid-ARS complex. To validate the assay we first determined the effect of varying concentrations of PBA on the absorbance and fluorescence intensity of ARS. ARS showed a color change, from red to yellow and an 80-fold increase in fluorescence intensity at pH 7.4. The association constant for the PBA-ARS complex was determined to be 1260 M−1, which is consistent with the values reported in literature.26, 34 Following this, solutions with varying concentrations of the diol (D-fructose, glucose, sialic acid, methyl-β-D-galactopyranoside and methyl-α-D-mannopyranoside) were prepared in 100 mM pH 7.4 phosphate buffers containing 10 μM ARS and 2 mM PBA or BzB. Solutions containing excess of the diol were titrated against the boronic acid-ARS complex. As the diol replaces the ARS to form a boronic acid-diol complex, the concentration of free ARS in the solution increased, thereby showing a marked decrease in fluorescence. This shift in fluorescence was used to compute the association constant for the boronic acid-diol complex.34
2. Polymer Synthesis and characterization
BzB monomers and polymers were synthesized using methods similar to those described previously.33 Briefly, polymers were prepared by free radical polymerization at 25 mol%, 50 mol%, 75 mol% and 90 mol% BzB feed ratio, in the presence of 5 mol% 2,2′-azobisisobutyronitrile (AIBN). Water-soluble and nontoxic 2-hydroxypropylacrylamide was used as a backbone monomer for copolymerization (Fig 1). The reaction mixture was purged with nitrogen and degassed for 1 hr on ice. Following this, the reaction was moved to a 65 °C oil bath for 24 hrs. Polymers were precipitated in anhydrous ether using the fractional precipitation technique. Polymers were centrifuged and purified using dialysis across a 10,000 MWCO membrane using the VivaFlow dialysis system. Polymers with varying molecular weights were synthesized as described above, with 50 mol% feed ratio of BzB and varying feed ratio’s of AIBN (1 mol% – 5 mol%). Polymers with sulfonic acid were also synthesized using conditions described above. The degree of substitution was determined by 1H NMR (Mercury 400 MHz spectrometer, Varian) and found to correlate with the feed ratios (Table 1). Molecular weight was determined by GPC (GPC 1100, Agilent Technologies, Santa Clara, CA) equipped with an organic column (PLgel mixed bed, Polymer Laboratories, Amherst, MA), a differential refractive index detector (BI-DNDC, Brookhaven Instruments, Holtsville, NY) and a multiangle light scattering detector (BI-MwA, Brookhaven Instruments, Holtsville, NY).
Table I.
Association constants of phenylboronic acid and benzoboroxole determined using ARS assay at pH 7.4 in 100 mM phosphate buffer.
| Diol | Association Constant (M−1)
|
|
|---|---|---|
| Phenylboronic acid | Benzoboroxole | |
| ARS | 1260 | 1307 |
| D-fructose | 160 | 664 |
| N-Acetylneuraminic acid | 21 | 160 |
| Glucose | 6 | 21 |
| Methyl-β-D-galactopyranoside | a | 24 |
| Methyl-α-D-mannopyranoside | a | 21 |
Not measurable. Likely below 5 M−1 based on quantitative ARS assay
3. Neutralization assay to evaluate the activity of the synthetic lectins against clinical HIV-1 isoloates
Anti-HIV activity of the synthetic lectin polymers was evaluated using a single-cycle HIV-1 infectivity inhibition assay in TZM-bl cells.33 A well-characterized R5 tropic DU156 (Clade C) virus isolated from a sexually transmitted virus infection and cultured in PBMC was used in the neutralization assay. Additionally, R5 TRO (Clade C) and the pseudotyped X4 WEAU (Clade B) viral strains were assayed to investigate cross-clade and cross-tropism activity. Polymer solutions were prepared at 10 mg/mL in DMEM (4.5 g/L D-glucose, 110 mg/mL sodium pyruvate and L-glutamine, Invitrogen, Carlsbad, CA), with the pH adjusted to 7.5 as necessary, and pasteurized for 5 min at 70°C. After pasteurization, the stock solution was diluted 1:1 with DMEM containing 2X nutrients (DMEM supplemented with 20% fetal bovine serum (Hyclone, Logan, UT), 50 mM HEPES (Gibco/Invitrogen, Carlsbad, CA) and 100 μg/mL gentamicin (Sigma, St. Louis, MO) under sterile conditions. Preparation was designed to pasteurize the polymers without proteins being present in the media. The final media composition after 1:1 dilution was 10% FBS, 25 mM HEPES, and 50 μg/mL gentamicin. The assay was conducted in a 96-well plate such that the polymer solution at the highest concentration was added to the bottom row. From the bottom row of the plate 20 μL of the test sample was added to the prior row with 100 μL of growth media to create a 6-fold dilution. This scheme of serial dilution was repeated five times to obtain a dose-response curve. Appropriate cell controls and virus controls were included in each plate. Following the serial dilutions, 50 μL of cell-free virus (200 TCID50) was added to each well, with the exception of the wells that served as cell control. The plate was then incubated for 1 hr in a 37 °C, 5% CO2 incubator. After the pre-incubation, 100 μL of TZM-bl cell suspension prepared at a density of 1 × 105 cells/mL in growth media containing DEAE dextran (37.5 μg/mL) were added to each well and incubated for an additional 48 hrs. Luminescence was measured after 2 min incubation with Britelite Reagent (PerkinElmer, Waltham, MA). Toxicity of the samples was simultaneously analyzed using an identical plate layout in the absence of virus. To determine loss in cell viability, luminescence from the sample-treated wells was compared to that of the cell controls. EC50 is reported as the concentration of the sample which reduced the relative luminescence units (RLUs) by 50% compared to the virus control. Dose response curves were fit to the percent neutralization data using GraphPad Prism software (Graph Pad, LaJolla, CA).
In the assay evaluating the effect of pre-incubation time on the antiviral activity of the synthetic lectins, the assay was conducted using a procedure similar to the one above described, with the exception of the pre-incubation time. The polymer solutions were incubated for 0, 15, 30 and 60 mins with the virus prior to addition of TZM-bl cells. For the assay evaluating the effect of fructose, 30 mg/mL fructose solution was prepared in DMEM media. To the polymer solution, 10 μL of the fructose solution was added along with the virus to create a fructose concentration of 3 mg/mL. The remaining of the steps were performed as described above.
4. Broad-spectrum antiviral assays and activity against laboratory HIV-1 strains
The anti-HIV activity and cytotoxicity were also evaluated against the laboratory HIV-1 strain IIIB and HIV-2 strain ROD in human T-lymphocyte CEM cell cultures. Briefly, virus stocks were titrated in human T-lymphocyte CEM cells and expressed as the 50% cell culture infective dose (CCID50, 1 CCID50 being the virus dose to infect 50% of the cell cultures). CEM cells were suspended in culture medium at ~ 3 × 105 cells/ml and infected with HIV at ~ 100 CCID50. Immediately after viral exposure, 100 μl of the cell suspension was placed in each well of a flat-bottomed microtiter tray containing various concentrations of the test compounds. After a 4-d incubation period at 37 °C, the giant cell formation was microscopically determined. Compounds were tested in parallel for their potential cytostatic effects in uninfected CEM cell cultures.
In a co-cultivation assay, 5 × 104 persistently HIV-1 infected HUT-78 cells (designated HUT-78/HIV-1) were mixed with 5 × 104 SupT1 cells, along with appropriate concentrations of the test compound. After 20 h, marked syncytium formation was noted in the control cell cultures, and the number of syncytia was determined under the microscope. The EC50 was defined as the compound concentration required to prevent syncytium formation by 50%.
The other antiviral assays were based on inhibition of virus-induced cytopathicity in HEL [herpes simplex virus type 1 (HSV-1), HSV-2 (G), vaccinia virus, and vesicular stomatitis virus], Vero (parainfluenza-3, reovirus-1, Coxsackie B4, and Punta Toro virus), HeLa (vesicular stomatitis virus, Coxsackie virus B4, and respiratory syncytial virus) and CrFK (feline corona virus (FIPV) and feline herpes virus) cell cultures. Confluent cell cultures in microtiter 96-well plates were inoculated with 100 CCID50 of virus in the presence of varying concentrations (5,000, 1,000, 200 nM) of the test compounds. Viral cytopathicity was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds.
5. Biocompatibility evaluation in MatTek VEC-100 reconstructed human vaginal tissue
Triplicate solutions of BzB50-AMPS10-HPMA40 at 1 mg/mL were prepared in serum containing media. Polymer solutions were pasteurized at 70 °C for 5 mins. Media containing the polymers were applied to the apical surface of tissues cultured in 24-well plate inserts. Three repeated exposures to the test sample were performed in intervals of 24 h. After 72 h of exposure, tissue viability was determined using MTT assay and change in tissue morphology was evaluated through histological examination of the epithelium. On days 1–3, cytokine levels were assayed using ELISA. Levels for IL-1α, IL- 8, IL-6 and TNF-α were measured in tissue culture supernatant by utilizing human cytokine kits (R&D System). Tissue integrity was monitored through measurements of Transepithelium Electrical Resistance (TEER). Triplicate of control solutions of pHPMA(1 mg/mL), nonoxynol-9 (0.02 mg/mL) and culture medium was also performed.
Results and Discussion
1. Comparative evaluation of the binding affinity of PBA and BzB
To measure the affinity of the boronic acid ligands for reducing and non-reducing sugar residues, we used the ARS-based three-component colorimetric binding assay.34 Affinity for glucose, fructose, sialic acid, methyl-β-D-galactopyranoside and methyl-α-D-mannopyranoside was assayed. To ensure the viability of the assay, the association constant of PBA-ARS was compared to values reported in the literature by Wang and co-workers.34 Table I summarizes the association constants of PBA and BzB for various diols. Comparison between PBA and BzB affinities for simple reducing sugars revealed four-fold stronger affinity of BzB for fructose (~600 M−1) when compared to PBA (160 M−1); however, the affinity of BzB and PBA for glucose was comparable. Unsubstituted PBA demonstrated minimal affinity for non-reducing sugars such as methyl-β-D-galactopyranoside or methyl-α-D-mannopyranoside at neutral pH. In contrast, BzB revealed higher, but still weak, affinity (~25 M−1) for non-reducing complex sugars. To develop a boronic acid based synthetic entry inhibitor for HIV, we have exploited the ability of the BzB to bind to non-reducing sugars,32 which are structurally similar to the terminal sugar moieties found on the high mannose and complex-type N-linked glycans of gp120. We have previously confirmed this observation also using surface plasmon resonance (SPR), in which BzB showed a Kd of ~180 mM for HIVBaL gp120, whereas no measurable binding was observed with PBA.33
Unsubstituted PBA-containing polymers have demonstrated the ability to bind to glycosylated proteins only at alkaline pH; whereas BzB binds to glycosides at neutral pH.29 This difference has been ascribed to the presence of oxygen from the ortho substituent that stabilizes the boronate ester towards hydrolysis28 and decreases the energy barrier for tetrahedral formation.35 While the BzB shows superior binding affinity for sugar residues, when compared to the unsubstituted PBA, the binding is fairly weak for practical applications. In this regard, polyvalency has commonly been exploited to improve binding affinity of weak ligands. This effect is well-known in natural protein-ligand interactions as well as for other synthetic protein mimics.22, 23 Kaur et al. demonstrated almost a double increase of affinity for glucose for a tweezer-like bis-boronic acid molecule compared to a monovalent boronic acid.30 Incorporating ligands into polymer backbones provides a synthetically accessible mechanism for increasing the affinity of ligands for glycoproteins.
2. Polymer synthesis and characterization
Table II summarizes the molecular weight of the polymers synthesized by free-radical polymerization, determined using size exclusion chromatography (Agilent Technologies, Santa Clara, CA) equipped with a PLgel mixed-B column (Polymer Labs, Amherst, MA), a differential refractive index detector (BI-DNDC, Brookhaven Instruments, Holtsville, NY) and a multi-angle light scattering detector (BI-MwA, Brookhaven Instruments, Holstville, NY). Mole functionalization of BzB in the polymer was determined using 1H NMR.
Table II.
Polymer composition and molecular weight distribution.
| Polymer | AIBN (Mole%) | Mole Functionalizationa | Molecular Weightb (kDa) | PDI | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| BzB | AMPS | HPMA | Mw | Mn | |||
| BzB25-HPMA75 | 5.0 | 29 | - | 71 | 103 | 88 | 1.17 |
| BzB75-HPMA25 | 5.0 | 70 | - | 30 | 74 | 50 | 1.48 |
| BzB90-HPMA10 | 5.0 | 91 | - | 8 | 98 | 76 | 1.30 |
| BzB50-HPMA50 | 5.0 | 48 | - | 52 | 110 | 91 | 1.21 |
| 2.5 | 54 | - | 54 | 226 | 159 | 1.42 | |
| 1.0 | 51 | 51 | 382 | 285 | 1.34 | ||
| AMPS10-HPMA90 | 5.0 | - | - | - | 153 | 111 | 1.38 |
| BzB50-AMPS10-HPMA40 | 5.0 | 45 | 10 | 55 | 131 | 109 | 1.20 |
Degree of substitution was determined by 1H NMR and found to correlate with the feed ratios.
Molecular weight was determined by GPC attached to a light scattering and a refractive index detector.
3. Effect of BzB mole functionalization (ligand density) on the entry inhibition activity of the synthetic lectins
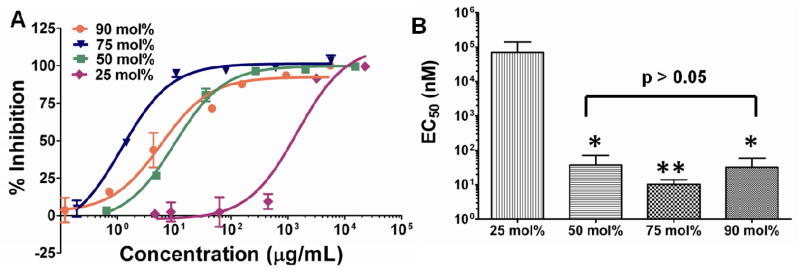
While the binding affinity of BzB for non-reducing sugars determined by the ARS-based colorimetric assay and for gp120 from SPR revealed weak affinity (Table I), high molecular weight polymers with polyvalent presentation of BzB showed ≥ 4 log scale increase in activity (EC50 in low micromolar to nanomolar range). As seen in Fig 2, ~2 log scale increase in antiviral activity was observed with an increase in BzB mole functionalization from 25 mol% to 50 mol% in the polymer (2-tailed Student’s t-test p < 0.001) and further increase in BzB functionalization from 50 mol% to 75 mol% showed only a marginal increase in activity (2-tailed Student’s t-test p = 0.01). On the contrary, an increase in ligand density from 75 mol% to 90 mol% showed a reduction in the ability of the polymers to neutralize HIV entry (2-tailed Student’s t-test p = 0.01), with an anti-HIV activity that was comparable to the 50 mol% BzB-functionalized polymers.
Figure 2.
Effect of BzB mole functionalization on the entry inhibition activity of BzB50 polymers. Results from the single-cycle HIV-1 infectivity inhibition in TZM-bl cells against R5 DU156 (Clade B) isolated from acute STIs. (A) Dose response curve of BzB functionalized HPMA polymers at varying mole incorporation. (B) EC50 was determined by fitting a sigmoidal dose-response curve with variable Hill’s slope. Single factor ANOVA showed significant differences between the groups (p < 0.05). Two tailed Student’s t-test revealed significant increase in activity from 25 to 50 and 75 mol%. Further increase from 75 to 90 mol% showed reduction in activity; EC50 of BzB90-HPMA10 was comparable to EC50 of BzB50-HPMA50 (N = 3, mean ± SD; *p < 0.05; **p < 0.01).
We believe that the increase in antiviral activity with increasing mole functionalization can be explained by at least two distinct mechanisms described by Mammen and co-workers22, 24– polyvalency and steric stabilization. The combined effect of the increase in the number and density of ligands likely improves the probability of binding, thereby enhancing binding and antiviral activity through avidity and polyvalency. As a secondary mechanism, we suspect that, when the BzB presenting polymers bind to gp120, they escort along a large water-swollen polymer drape which possibly makes the viral envelope physically inaccessible for fusion and entry into the host cell.24, 36 If we assume that it is these two mechanisms that collectively determine the activity of synthetic lectins, the gradual increase in activity from 50 to 75 mol% and the decrease in activity from 75 to 90 mol% can be explained through loss in the steric stabilization effect of these polymers. As we increase the ligand density in the polymers has been increased, the negatively charged borons could repel each other, thereby distorting the polymer conformation from an effective random coil structure to a less effective rigid elongated structure.24 On the other hand, increasing the number of available ligands and therefore, points of attachment between the polymer and the gp120 could result in a collapsed polymer structure; compromising the ability of the polymer to sterically stabilize the binding surface.24 Although the relative contribution of the above-mentioned two mechanisms towards antiviral activity is yet to be elucidated, it is reasonable to assume that steric stabilization plays a critical role24 in governing the ability of BzB-functionalized polymer to block HIV entry. Based on these results, further assessments were performed on 50 mol% functionalized BzB polymers.
Depending on the co-receptor tropism and clades, HIV displays significant variations in the different areas on the viral envelope,37, 38 making it important to evaluate cross-clade and –tropism activity of new therapeutic agent.39, 40 For example, agents targeting the positively charged V3 loop of the gp120, such as the non-specific sulfated polymers, show severely compromised activity against R5 tropic strains.41 This is because, the co-receptor tropism of the virus impacts the extent of the positive charge on the gp120 V3 loop, with the CXCR4 co-receptor-tropic HIV-1 strains (X4) exhibiting more positive charges than the CCR5-tropic HIV-1 strains (R5). We have previously reported the antiviral activity of the BzB-HPMA co-polymers at 25, 50 and 75 mol% BzB mole incorporation, against HIV strains across clades and tropism.33 The assays were performed using Clade B - R5 DU156 and Clade C - R5 TRO, isolated from acute sexually transmitted infections, and the pseudotyped Clade B - X4 WEAU. Independent of the ligand density BzB polymers showed comparable antiviral activity across the clades and tropisms of HIV.33 Although, the HIV envelope exhibits domains of heterogeneity across viral strains, many N-linked glycans are believed to be fairly conserved, providing a potential mechanism for the observed broad spectrum activity of lectins.
4. Effect of molecular weight of the BzB50 polymers on the entry inhibition activity of the synthetic lectins
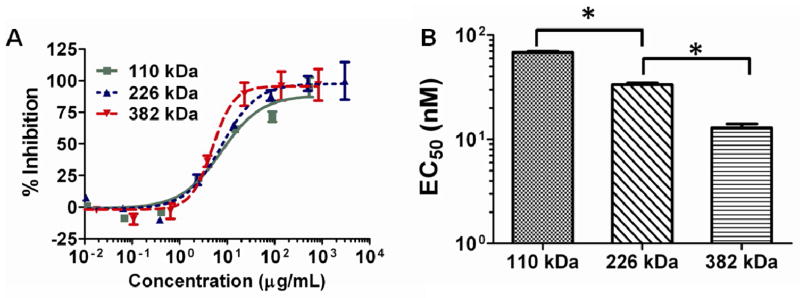
Having an understanding of how ligand density affects activity of the BzB functionalized polymers; we were next interested in exploring the influence of the molecular weight of the polymer at a fixed BzB functionalization on antiviral activity of the synthetic lectins. BzB functionalized polymers at 50 mol% were synthesized using varying concentrations of the initiator, yielding polymers with a range of molecular weights. As shown in Fig 3, EC50’s of the polymers decreased (thus, higher activity) from 68 nM for 132 kDa BzB50 to 12 nM for 382 kDa BzB50 (2-tailed students t-test p = 0.01). Owing to the increased number of ligands available for binding per polymer chain, increase in molecular weight of the polymers at a fixed percent BzB incorporation showed improved activity. However, the improvement in activity found with molecular weight variations was less dramatic than that seen with variation in percent BzB functionalization.
Figure 3.
Effect of molecular weight on the entry inhibition activity of BzB50 polymers. Results from the single-cycle HIV-1 infectivity inhibition in TZM-bl cells against R5 DU156 (Clade B) isolated from acute STIs. (A) Dose response curve of BzB50-HPMA50 polymers at varying molecular weights and (B) EC50 determined by fitting a sigmoidal dose-response curve with variable Hill’s slope. Two tailed Student’s t-test was conducted (N = 3, mean ± SD; *p < 0.05). Significant increase in activity was observed with increase in MW of the polymer from 110 to 226 kDa and further to 382 kDa (p =0.03 and 0.01, respectively). Highest activity was observed with 382 kDa MW polymer (11.3 ± 2.9 nM)
5. Antiviral activity of BzB50 polymers against viruses bearing glycosylated envelopes
Entry inhibition activity of lectins originates from their ability to bind to glycans spiked on the viral surface. Several of the enveloped viruses bear carbohydrate shields on the envelope,42 which could be effectively targeted using lectins that non-specifically bind to carbohydrates. Therefore, we and others in the field have tested the activity of plant, bacterial and/or synthetic lectins against lentiviruses43 (HIV-1, HIV-2, SIV, MSV), RNA viruses44 (VSV, Coxsackie virus B4, and respiratory syncytial virus; and parainfluenza type 3 virus, reovirus type 1, Sindbis virus, and Punta Toro virus, Ebola, Coronaviruses, Influenza viruses), DNA viruses43 (herpesvirus such as cytomegalovirus) with glycosylated envelopes. Table III summarizes the inhibitory concentration of BzB50,382 kDa against the viruses we tested. We notably observed activity solely against HIV. Except for HIV, none of the other viruses were inhibited by the BzB polymers even at concentration 100-fold higher than the EC50 for HIV. These results are in contrast with UDA that inhibit viruses such as HIV-1, HIV-2, RSV, Influenza, CMV, SIV and FIV. CV-N - an extensively reviewed and tested CBP derived from the cyanobacterium Nostoc ellipsosporum demonstrated marked inhibition of viruses such as HIV-139, 45, SIV, Ebola44, HCV46, Influenza A virus47 and HSV. The HIV-specific activity we observed with the synthetic lectins can be attributed to the fact that no other virus that was tested in this assay exhibited comparable extent of glycosylation sites and/or a similar nature of the glycans (i.e. high-mannose-type) as HIV. Since, the interactions between a polyvalent construct such as the BzB-based synthetic lectin and the viral envelope depends on a multitude of factors such as the density of glycosides on the viral surface, the degree of oligomerization of the glycoprotein, spatial arrangement of saccharides and flexibility or both, it is conceivable that the synthetic lectins selectively inhibit viruses, in this particular case HIV and not just all types of viruses that bear a glycosylated envelope.
Table III.
Antiviral activity of BzB50-HPMA50 (382 kDa) against viruses with a glycosylated envelope
| Virus | EC50 (nM)+ | Virus | EC50 (nM)+ |
|---|---|---|---|
| HIV-1 | 1.1 | Herpes simplex virus-1 (KOS)c | > 1000 |
| Feline Corona Virus (FIPV)a | 1000 | Herpes simplex virus-2 (G)c | > 1000 |
| Feline Herpes Virusa | 5000 | Herpes simplex virus-1TK (KOS)c | > 1000 |
| Punta Toro virusb | > 40 | Vesicular stomatitis virusc,d | >1000 |
| Parainfluenza-3 virusb | > 40 | Respiratory syncytial virusd | > 1000 |
| Reovirus-1b | > 40 | Coxsackie virus B4d | > 1000 |
| Vaccinia virusc | > 1000 |
50% Effective concentration or compound concentration producing 50%inhibition of virus-induced cytopathic effect
Crandell-Rees Feline Kidney cells (CRFK cells)
Vero cells
HEL cells
HeLa cells
6. Incorporation of sulfonic acid in the polymer backbone affords a synergistic increase in viral entry inhibition activity of BzB50 polymers
Lead polymers from the above studies with an antiviral activity of ~ 10 nM (BzB75, 75kDa and BzB50, 382kDa) showed poor-aqueous solubility (< 5 mg/mL). Considering that the activity of the BzB polymers could be compromised in the presence of proteins and other components in the vaginal environment, improving aqueous solubility of the polymers may be required in order to deliver the required drug concentrations. Kataoka et al. and others have demonstrated that the incorporation of amines into a PBA-containing polymer could lower the pKa of the boronic acid to around 7, thereby enhancing solubility.48, 49 Incorporation of amines was also utilized by Winblade et al., who reductively aminated 4-formylphenylboronic acid to a poly-lysine-PEG backbone. The pKa of this polymer was below 6.36, 50 To address the poor aqueous solubility of the polymers at high benzoboroxole functionalization (≥ 50 mol%) and/or high molecular weight polymers (≥ 125 kDa), we have synthesized polymers with 10 mole% 2-Acrylamido-2-methylpropane sulfonic acid (AMPS), which is an anionic monomer. Incorporation of AMPS into the copolymer adds charge to the otherwise neutral HPMA backbone, increasing the aqueous solubility of the polymer at neutral pHs. Nearly 100-fold increase in aqueous solubility was observed with the incorporation of AMPS.
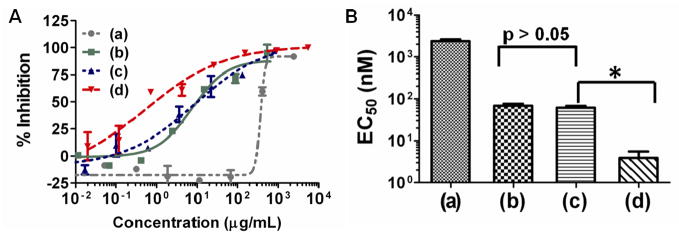
Since both AMPS and BzB bind to gp120, we tested both polymers individually, as a physical mixture and a co-polymer of AMPS and BzB. BzB was functionalized at 50 mol% and AMPS at 10 mol% in a HPMA polymer. As shown in the dose response curve shown in Fig 4, the physical mixture of the two polymers - BzB50 and AMPS10 - did not demonstrate any significant increase in activity (2-tailed student’s t-test p = 0.70). This data suggests that the presence of AMPS10 only in the polymer solution has no influence on the binding of BzB to gp120. In contrast, when the AMPS and BzB were co-polymerized, the resulting polymer showed a more than 2 log scale decrease in EC50 (2-tailed student’s t-test p = 0.004). We believe that the presence of (a) simultaneous ionic and covalent interactions; (b) steric stabilization; and (c) entropic advantage with multiple binding events - may contribute to the observed synergistic improvement in antiviral activity of the co-polymers over the physical mixture. The charged sulfonated polymer backbone likely facilitates initial ionic interactions with the V3 loop on gp120; thereby sterically facilitating covalent interactions between the BzB on the polymer and the glycans on gp120. This implies that, irrespective of the binding affinity, the binding event would bring along a large polymer drape to the viral surface, thus inhibiting interactions with the host cell. The BzB-AMPS co-polymers show activity at concentrations (~ 1 to 4 nM) comparable to the CBP, such as CV-N (~ 0.6 nM). The compound has also been evaluated for its potential to prevent syncytium formation in co-cultures of persistently HIV-1IIIB infected HUT-78 and uninfected Sup T1 cells, and found to display an EC50 of 50 ± 2.6 nM.
Figure 4.
Effect of incorporation of sulfonic acid on the entry inhibition activity of BzB50 polymers. Results from the single-cycle HIV-1 infectivity inhibition in TZM-bl cells on (a) AMPS10-HPMA90, (b) BzB50-HPMA50, (c) physical mixture of AMPS10-HPMA90 and BzB50-HPMA50 and (d) co-polymer BzB50-AMPS10-HPMA40 against R5 DU156 (Clade B) isolated from acute STIs. (A) Dose response curve of the polymers and (B) EC50 determined by fitting a sigmoidal dose-response curve with variable Hill’s slope. Two tailed Student’s t-test was conducted (N = 3, mean ± SD; p < 0.05). AMPS10 showed an EC50 of 2361 nM ± 60 (~350 μg/mL). Physical mixture of BzB50-HPMA50 and AMPS10-HPMA90 showed no significant increase in antiviral activity over BzB50-HPMA50 (p = 0.70). However, copolymers of HMPBA and AMPS at 50 and 10 mol% functionalization, respectively, showed a dramatic increase in antiviral activity with EC50 of 4.0 nM ± 1.6 (~1 μg/mL), suggesting the presence of synergistic activity between HMPBA and AMPS (* p = 0.004).
Sulfated anionic polymers have previously been evaluated as a microbicide that targets the V3 loop on gp120.51 We are aware of the issues associated with the sulfonated polymers for microbicide applications which include toxicity due to non-specific binding, tropism-specific activity41 and loss of antiviral activity in the presence of semen.51 In an attempt to address the above issues, we synthesized polymers with small amounts of AMPS co-polymerized with the biocompatible HPMAm; most likely reducing potential toxicity. Furthermore, the primary mechanism of action of the synthetic lectins is through the ability of BzB to covalently bind to gp120, which demonstrates clade and tropism independent activity 33. The ionic interactions between AMPS and the V3 loop is an auxiliary mechanism of action which augments the overall activity of the BzB-based synthetic lectins. In summary, incorporation of AMPS in BzB50 yields a polymer with enhanced aqueous-solubility and generates a heteromeric polyvalent presentation of ligands with superior binding to gp120.
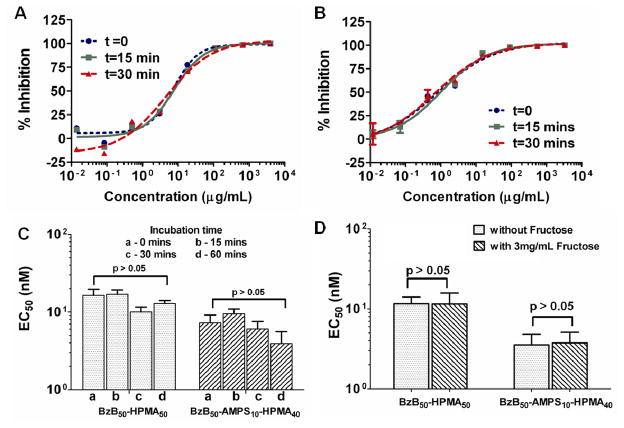
7. Effect of binding kinetics on the activity of the synthetic lectins
While the BzB polymers retained activity in the presence of seminal concentrations of fructose, we were next interested in asking whether the activity of these polymers is governed by thermodynamics alone or whether binding kinetics also influences the entry inhibition activity of the BzB. To evaluate the effect of binding kinetics, the BzB-APMS co-polymer samples were pre-incubated with the virions for 0, 15, 30 and 60 mins. As shown in Fig 5, irrespective of the incubation time, the co-polymer samples showed 50% inhibition of HIV at ~ 4.5 ± 2.0 nM (2-tailed student’s t-test p > 0.05). Unlike the α-(1–3)- and α-(1–6)-D-mannose oligomer-specific plant lectins, whose antiviral activity is up to 20-fold more pronounced upon a 1 hr pre-incubation time with the virus,43 the BzB-based synthetic lectins show superior and rapid inhibition of HIV entry, irrespective of pre-incubation with the virus particles.
Figure 5.
Effect of incubation time and fructose on the entry inhibition activity of BzB50 polymers. Dose response curves of (A) BzB50-HPMA50 (382 kDa) and (B) BzB50-AMPS10-HPMA40 against R5 DU156 as a function of pre-incubation time. (C) EC50 of both polymers as function of pre-incubation time. Using single factor ANOVA, independent of the incubation time, no significant difference in EC50 was observed (p = 0.72 and 0.55). (D) EC50 of BzB50-HPMA50 and BzB50-AMPS10-HPMA40 in the presence of seminal concentration of fructose. Both polymers showed no significant loss in antiviral activity in the presence of fructose. Using 2-tailed, Student’s t-test p = 0.44 and 0.53 for BzB50-HPMA50 and BzB50-AMPS10-HPMA40, respectively.
8. Effect of seminal concentrations of fructose on the activity of the synthetic lectins
Several antiviral agents such as sulfated polymers,51 intended to be delivered vaginally show compromised or complete loss of antiviral activity in the presence of seminal fluid. Seminal fluid is a rich source of proteins, enzymes, salts, immune cells and sugars.52 Of these, the presence of high concentrations of simple sugars such as fructose especially may raise concerns for lectin-based therapeutics. As seen by our results from the ARS assay, BzB show exceptionally high affinity for fructose. Therefore, we tested the ability of the BzB50-AMPS10-HPMA40 co-polymers and BzB50-HPMA50 (383 kDa) to neutralize HIV entry in the presence of average seminal fructose concentration (3 mg/mL) of fructose. As shown in Fig 5d, activity of the synthetic lectins was fully preserved in the presence of fructose, suggesting that fructose from seminal fluid would not compromise the antiviral activity of the synthetic lectins (2-tailed student’s t-test p > 0.05). This feature of the synthetic lectins is especially attractive, as some of the microbicides tested in the past including CV-N39 have shown compromised activity in the presence of seminal fluid.51
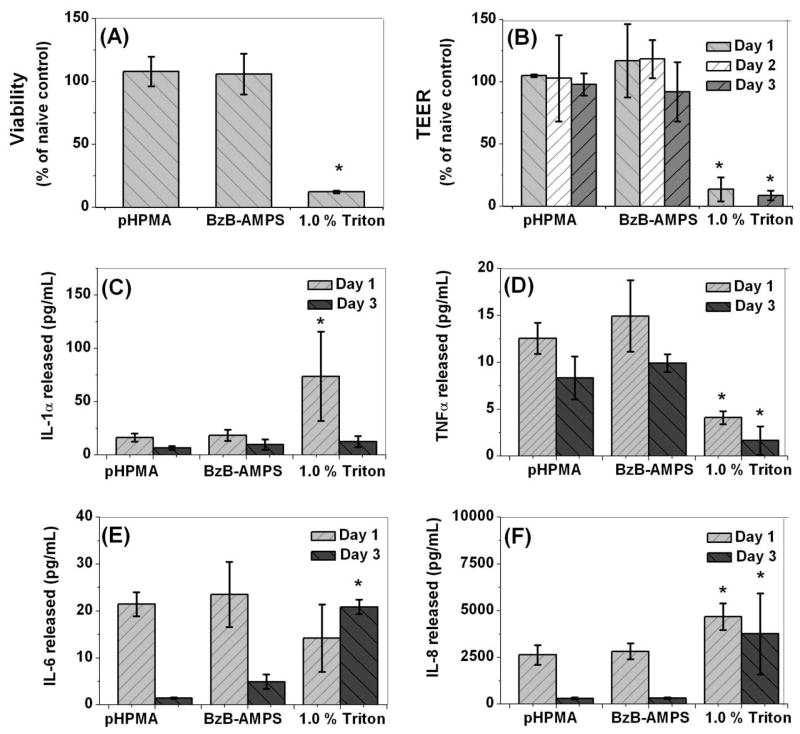
9. Biocompatibility evaluation in MatTek VEC-100 reconstructed human vaginal tissue
Enabling this research for microbicide development requires careful determination of how interactions of BzB polymers with the epithelial cells of cervicovaginal tissue may impact their viability and cytokine production. Owing to the ability of the BzB to bind to glycoproteins and the ability of sulfonic acid to non-specifically bind to positively charged surfaces; it is critical to assess the safety of the BzB-AMPS co-polymers in vaginal tissue. The VEC-100 tissue purchased from MatTek Corporation serves as a suitable model for evaluating the tissue-level toxicity of topical microbicides.53 As can be seen in Fig 6, BzB-AMPS co-polymers showed no significant loss in tissue viability when compared to the non-toxic HPMA control even at concentrations ~ 1000-fold the in vitro EC50, after 72 hours of exposure (≥ 90% viability). The irritation potential of test polymers were evaluated by ELISA for inflammatory cytokines associated with mucosal toxicity (i.e. IL-8, IL-1, IL-6, TNFα) using human cytokine kits (R&D System). Our investigations on biocompatibility revealed no significant alterations in tissue viability, cytokine levels, tissue morphology or the barrier properties of the epithelium. In summary, these results suggest that polymers functionalized with BzB moieties, are likely to be non-toxic and are likely to have a high therapeutic index; allowing higher concentrations of the polymer to be dosed safely, thereby minimizing the impact of competing binding sites that may be present in the vaginal environment.
Figure 6.
Safety evaluation of BzB50-AMPS10-HPMA40 on VEC-100 reconstructed human ectocervical tissue after three repeated exposures. pHPMA and Triton served as non-toxic and toxic controls, respectively. (A) Percentage viability of the tissue determined using the MTT assay at the end of day 3, (B) TEER measurements graphed as percentage of the no-treatment control for days 1–3. (C–F) Pro-inflammatory cytokine IL-1α, TNFα, IL-6 and IL-8 release for days 1–3. Tissues treated with BzB50-AMPS10-HPMA40 showed no symptoms of loss in tissue viability or elevation in cytokine levels when compared to pHPMA-treated non-toxic control. (N=3, Mean ± SD, 2-tailed Student’s t-test; * p < 0.05).
Conclusion
This work is relevant to the development of new agents capable of rendering the virus inactive during female-to-male heterosexual transmission. An ideal microbicide candidate must exhibit potency, cross-clade broad spectrum activity, selective inhibition of the pathogen, mass producible and biocompatible. While current research on CBPs suggests that they possess several of the above favorable properties to qualify as potential microbicide candidates, their success is largely impeded by the potential mitogenic properties, cost of production and isolation and purification in mass quantities. In this regard, our approach of developing synthetic lectins using the BzB moieties meet all of the above criteria of an ideal microbicide. The BzB can readily be incorporated into a variety of biocompatible polymer backbones and polymeric constructs which can be synthetically modified to enhance activity and specificity. Additionally, the BzB-based synthetic lectins also present an affordable and scalable product that could be delivered to the pandemic regions.
Acknowledgments
We thank David Montefiori (Duke University) for providing lab space, viral strains, assay reagents and the TZM-bl cell line for the neutralization studies. We thank Julie I. Jay for polymer samples (BzB25 and BzB75) and Molli Kiser for graphic design of Fig 1. This work was supported by the NIH, grant number R21-AI062445, and The Bill & Melinda Gates Foundation Grand Challenge Exploration awarded to Dr. Patrick F. Kiser, the K.U. Leuven Program Financing (PF/10/018) and the “Fonds voor Wetenschappelijk Onderzoek” (no. G.0485.08) to Dr. Jan Balzarini.
References
- 1.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393(6686):705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 2.Poignard P, Saphire EO, Parren PW, Burton DR. gp120: Biologic aspects of structural features. Annu Rev Immunol. 2001;19:253–274. doi: 10.1146/annurev.immunol.19.1.253. [DOI] [PubMed] [Google Scholar]
- 3.Gallaher WR, Ball JM, Garry RF, Martin-Amedee AM, Montelaro RC. A general model for the surface glycoproteins of HIV and other retroviruses. AIDS Res Hum Retrovir. 1995;11(2):191–202. doi: 10.1089/aid.1995.11.191. [DOI] [PubMed] [Google Scholar]
- 4.Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265(18):10373–10382. [PubMed] [Google Scholar]
- 5.Balzarini J, Van Laethem K, Hatse S, Vermeire K, De Clercq E, Peumans W, Van Damme E, Vandamme AM, Bolmstedt A, Schols D. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J Virol. 2004;78(19):10617–27. doi: 10.1128/JVI.78.19.10617-10627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balzarini J, Van Laethem K, Hatse S, Froeyen M, Van Damme E, Bolmstedt A, Peumans W, De Clercq E, Schols D. Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and Galanthus nivalis. Mol Pharmacol. 2005;67(5):1556–1565. doi: 10.1124/mol.104.005082. [DOI] [PubMed] [Google Scholar]
- 7.Balzarini J, Van Laethem K, Hatse S, Froeyen M, Peumans W, Van Damme E, Schols D. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV gp120: A new therapeutic concept to hit the achilles heel of HIV. J Biol Chem. 2005;280(49):41005–41014. doi: 10.1074/jbc.M508801200. [DOI] [PubMed] [Google Scholar]
- 8.Balzarini J, Van Laethem K, Peumans WJ, Van Damme EJM, Bolmstedt A, Gago F, Schols D. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J Virol. 2006;80(17):8411–8421. doi: 10.1128/JVI.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balzarini J. Large-molecular-weight carbohydrate-binding agents as HIV entry inhibitors targeting glycoprotein gp120. Curr Opin HIV AIDS. 2006;1(5):355–360. doi: 10.1097/01.COH.0000239846.36076.2c. [DOI] [PubMed] [Google Scholar]
- 10.Vandekerckhove L, Verhofstede C, Vogelaers D. Maraviroc: integration of a new antiretroviral drug class into clinical practice. J Antimicrob Chemother. 2008;61(6):1187–1190. doi: 10.1093/jac/dkn130. [DOI] [PubMed] [Google Scholar]
- 11.Makinson A, Reynes J. The fusion inhibitor enfuvirtide in recent antiretroviral strategies. Curr Opin HIV AIDS. 2009;4(2):150–158. doi: 10.1097/COH.0b013e32832498d8. [DOI] [PubMed] [Google Scholar]
- 12.Pirrone V, Wigdahl B, Krebs FC. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res. 2011;90(3):168–182. doi: 10.1016/j.antiviral.2011.03.176. [DOI] [PubMed] [Google Scholar]
- 13.Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 14.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Jentsch U, Pool R, Chisembele M, Kapiga S, Mutemwa R, Vallely A, Palanee T, Sookrajh Y, Lacey CJ, Darbyshire J, Grosskurth H, Profy A, Nunn A, Hayes R, Weber J. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376(9749):1329–1337. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene WC. The brightening future of HIV therapeutics. Nat Immunol. 2004;5(9):867–871. doi: 10.1038/ni0904-867. [DOI] [PubMed] [Google Scholar]
- 16.Sato Y, Hirayama M, Morimoto K, Yamamoto N, Okuyama S, Hori K. High mannose-binding lectin with preference for the cluster of {alpha}1-2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J Biol Chem. 2011;286(22):19446–19458. doi: 10.1074/jbc.M110.216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd M, Gustafson K, McMahon J, Shoemaker R, O’Keefe B, Mori T, Gulakowski R, Wu L, Rivera M, Laurencot C, Currens M, Cardellina J, 2nd, Buckheit R, Jr, Nara P, Pannell L, Sowder R, 2nd, Henderson L. Discovery of cyanovirin-N, a novel human immunodeficiency virus- inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41(7):1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori T, O’Keefe BR, Sowder RC, 2nd, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, Jr, McMahon JB, Boyd MR. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280(10):9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 19.Dey B, Lerner DL, Lusso P, Boyd MR, Elder JH, Berger EA. Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J Virol. 2000;74(10):4562–9. doi: 10.1128/jvi.74.10.4562-4569.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micewicz ED, Cole AL, Jung CL, Luong H, Phillips ML, Pratikhya P, Sharma S, Waring AJ, Cole AM, Ruchala P. Grifonin-1: a small HIV-1 entry inhibitor derived from the algal lectin, Griffithsin. PLoS One. 2010;5(12):e14360. doi: 10.1371/journal.pone.0014360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balzarini J, Van Laethem K, Daelemans D, Hatse S, Bugatti A, Rusnati M, Igarashi Y, Oki T, Schols D. Pradimicin A, a carbohydrate-binding nonpeptidic lead compound for treatment of infections with viruses with highly glycosylated envelopes, such as human immunodeficiency virus. J Virol. 2007;81(1):362–373. doi: 10.1128/JVI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammen M, Choi S-K, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37(20):2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Kitov PI, Bundle DR. On the nature of the multivalency effect: A thermodynamic model. J Am Chem Soc. 2003;125(52):16271–16284. doi: 10.1021/ja038223n. [DOI] [PubMed] [Google Scholar]
- 24.Mammen M, Dahmann G, Whitesides GM. Effective inhibitors of hemagglutination by influenza virus synthesized from polymers having active ester groups - insight into mechanism of inhibition. J Med Chem. 1995;38(21):4179–4190. doi: 10.1021/jm00021a007. [DOI] [PubMed] [Google Scholar]
- 25.Taylor ME, Drickamer K. Introduction to glycobiology. 2. Oxford University Press; 2006. [Google Scholar]
- 26.Springsteen G, Wang BH. A detailed examination of boronic acid-diol complexation. Tetrahedron. 2002;58(26):5291–5300. [Google Scholar]
- 27.Yan J, Springsteen G, Deeter S, Wang B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols-it is not as simple as it appears. Tetrahedron. 2004;60(49):11205–11209. [Google Scholar]
- 28.Cai SX, Keana JFW. o-Acetamidophenylboronate esters stabilized toward hydrolysis by an intramolecular O-B interaction: potential linkers for selective bioconjugation via vicinal diol moieties of carbohydrates. Bioconj Chem. 1991;2:317–322. doi: 10.1021/bc00011a004. [DOI] [PubMed] [Google Scholar]
- 29.Dowlut M, Hall DG. An improved class of sugar-binding boronic acids, soluble and capable of complexing glycosides in neutral water. J Am Chem Soc. 2006;128(13):4226–4227. doi: 10.1021/ja057798c. [DOI] [PubMed] [Google Scholar]
- 30.Kaur G, Fang H, Gao X, Li H, Wang B. Substituent effect on anthracene-based bisboronic acid glucose sensors. Tetrahedron. 2006;62(11):2583–2589. [Google Scholar]
- 31.Mulla HR, Agard NJ, Basu A. 3-methoxycarbonyl-5-nitrophenyl boronic acid: high affinity diol recognition at neutral pH. Bioorg Med Chem Lett. 2004;14(1):25–27. doi: 10.1016/j.bmcl.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Bérubé M, Dowlut M, Hall DG. Benzoboroxoles as efficient glycopyranoside-binding agents in physiological conditions: structure and selectivity of complex formation. J Org Chem. 2008;73(17):6471–6479. doi: 10.1021/jo800788s. [DOI] [PubMed] [Google Scholar]
- 33.Jay JI, Lai BE, Myszka DG, Mahalingam A, Langheinrich K, Katz DF, Kiser PF. Multivalent benzoboroxole functionalized polymers as gp120 glycan targeted microbicide entry inhibitors. Mol Pharm. 2010;7(1):116–129. doi: 10.1021/mp900159n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Springsteen G, Wang B, Alizarin Red S. as a general optical reporter for studying the binding of boronic acids with carbohydrates. Chem Commun. 2001;(17):1608–1609. doi: 10.1039/b104895n. [DOI] [PubMed] [Google Scholar]
- 35.Bhat KL, Howard NJ, Rostami H, Lai JH, Bock CW. Intramolecular dative bonds involving boron with oxygen and nitrogen in boronic acids and esters: a computational study. J Mol Struc. 2005;723(1–3):147–157. [Google Scholar]
- 36.Winblade ND, Schmökel H, Baumann M, Hoffman AS, Hubbell JA. Sterically blocking adhesion of cells to biological surfaces with a surface-active copolymer containing poly(ethylene glycol) and phenylboronic acid. J Biomed Mat Res. 2002;59(4):618–631. doi: 10.1002/jbm.1273. [DOI] [PubMed] [Google Scholar]
- 37.Cutalo JM, Deterding LJ, Tomer KB. Characterization of glycopeptides from HIV-I(SF2) gp120 by liquid chromatography mass spectrometry. J Am Soc Mass Spectrom. 2004;15(11):1545–1555. doi: 10.1016/j.jasms.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X, Borchers C, Bienstock RJ, Tomer KB. Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry. 2000;39(37):11194–11204. doi: 10.1021/bi000432m. [DOI] [PubMed] [Google Scholar]
- 39.Buffa V, Stieh D, Mamhood N, Hu Q, Fletcher P, Shattock RJ. Cyanovirin-N potently inhibits human immunodeficiency virus type 1 infection in cellular and cervical explant models. J Gen Virol. 2009;90(1):234–243. doi: 10.1099/vir.0.004358-0. [DOI] [PubMed] [Google Scholar]
- 40.Rogers KM, Heise M. Modulation of cellular tropism and innate antiviral response by viral glycans. J Innate Immun. 2009;1(5):405–412. doi: 10.1159/000226422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moulard M, Lortat-Jacob H, Mondor I, Roca G, Wyatt R, Sodroski J, Zhao L, Olson W, Kwong PD, Sattentau QJ. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J Virol. 2000;74(4):1948–60. doi: 10.1128/jvi.74.4.1948-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vigerust DJ, Shepherd VL. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 2007;15(5):211–218. doi: 10.1016/j.tim.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balzarini J, Schols D, Neyts J, Van Damme E, Peumans W, De Clercq E. Alpha-(1-3)- and alpha-(1-6)-D-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob Agents Chemother. 1991;35(3):410–406. doi: 10.1128/aac.35.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrientos LG, O’Keefe BR, Bray M, Sanchez A, Gronenborn AM, Boyd MR. Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antiviral Res. 2003;58(1):47–56. doi: 10.1016/s0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 45.Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Human Retrovir. 2004;20(1):11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- 46.Helle F, Wychowski C, Vu-Dac N, Gustafson KR, Voisset C, Dubuisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. The Journal of biological chemistry. 2006;281(35):25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 47.O’Keefe BR, Smee DF, Turpin JA, Saucedo CJ, Gustafson KR, Mori T, Blakeslee D, Buckheit R, Boyd MR. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother. 2003;47(8):2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kataoka K, Miyazaki H, Okano T, Sakurai Y. Sensitive glucose-induced change of the lower critical solution temperature of poly[N,N-(dimethylacrylamide)-co-3-(acrylamido)-phenylboronic acid] in physiological saline. Macromolecules. 1994;27(4):1061–1062. [Google Scholar]
- 49.Morokoshi S, Ohhori K, Mizukami K, Kitano H. Sensing capabilities of colloidal gold modified with a self-assembled monolayer of a glucose-carrying polymer chain on a glass substrate. Langmuir. 2004;20(20):8897–8902. doi: 10.1021/la049201x. [DOI] [PubMed] [Google Scholar]
- 50.Winblade ND, Nikolic ID, Hoffman AS, Hubbell JA. Blocking adhesion to cell and tissue surfaces by the chemisorption of a poly-l-lysine-graft-(poly(ethylene glycol); phenylboronic acid) copolymer. Biomacromolecules. 2000;1(4):523–533. doi: 10.1021/bm000040v. [DOI] [PubMed] [Google Scholar]
- 51.Patel S, Hazrati E, Cheshenko N, Galen B, Yang H, Guzman E, Wang R, Herold BC, Keller MJ. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis. 2007;196(9):1394–1402. doi: 10.1086/522606. [DOI] [PubMed] [Google Scholar]
- 52.Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26(4):459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 53.Ayehunie S, Cannon C, Lamore S, Kubilus J, Anderson DJ, Pudney J, Klausner M. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol in Vitro. 2006;20(5):689–698. doi: 10.1016/j.tiv.2005.10.002. [DOI] [PubMed] [Google Scholar]