Abstract
Ultraviolet (UV) radiation from the solar spectrum is a major etiological factor for many cutaneous pathologies including cancer. By understanding changes in cell signaling pathways induced by UVA and UVB, novel strategies for prevention and treatment of UV-related pathologies could be developed. However, much of the information in the literature from various laboratories cannot cross talk because of difficulties associated with the use of ill-defined light sources and physiologically irrelevant light dosimetry. Herein, we have assessed the effect of exposure of normal human epidermal keratinocytes (NHEK) to UVA (2 and 4 J cm−2) or UVB (20 and 40 mJ cm−2) radiation. Employing western blot analysis, we found that exposure of NHEK to UVB, but not UVA, phosphorylates JNK1/2 at Th183/Tyr185, STAT3 at Ser727, AKT at Ser473 and increases c-Fos expression, whereas exposure to UVA, but not UVB, phosphorylates AKT at Thr308. UVB as well as UVA exposure leads to increased phosphorylation of (1) ERK1/2 at Th202/Tyr204; (2) p38 at Th180/Tyr204; (3) STAT3 at Tyr705; (4) mTOR at Thr2448; and (v) p70S6k at Thr421/Ser424; enhanced expression of PI3K (p85) and c-jun; and nuclear translocation of NFκB proteins. These findings could be considered as a beginning for understanding the differential effects of UVA and UVB in the human skin and may have implications both with respect to risk assessment from exposure to solar UV radiation, and to target interventions against signaling events mediated by UVA and UVB.
INTRODUCTION
Solar UV radiation, ubiquitous in the environment, is divided depending on the wavelength, into three regions: short-wave UVC (200–280 nm), mid-wave UVB (280–320 nm), and long-wave UVA (320–400 nm). The amount of UVA radiation reaching the earth surface is approximately 20 times greater than that of UVB and has a deeper penetration in the skin than UVB although the latter is 1000–10 000 times more carcinogenic than UVA (1). UVC, with the highest energy level, is almost completely filtered out and does not penetrate the earth’s atmosphere. Thus, UVB and, to a lesser extent, UVA radiation are responsible for a variety of skin disorders including cancer (1).
UV light has been shown to cause sunburns, photoaging, immunosuppression, angiogenesis, photodermatoses, DNA mutations and photocarcinogenesis (1). UVA is believed to exert its carcinogenic effect through generation of reactive oxygen species (ROS), including superoxide radical, hydrogen peroxide and hydroxyl radical, with singlet oxygen as the major ROS species, which in turn cause oxidative damage of macromolecules including DNA and cellular structures (2). Direct DNA damage by UVB results in the formation of cyclopyrimidine dimers and other photoproducts thought to be involved in the initiation of skin cancer. Studies in mouse model have shown UVA to be a relatively weak initiator of carcinogenesis, but a relatively potent tumor promoter although both UVA and UVB can act as complete carcinogens (2,3).
Following UV exposure, multiple signaling pathways responsible for cell growth, proliferation and survival are activated in the skin cells. Alterations in the signaling molecules involved in UV-mediated stress response give rise to changes in gene expression as well as activation of transcription factors and can be used as potential chemopreventive targets. It is being increasingly appreciated that UV induction of distinct signaling pathways is wavelength-specific (2,4). Thus, it is critical to analyze the mechanisms by which UVA and UVB irradiation affect the function of human skin cells. Much of the information in the literature defining the aberrations in signal transduction pathways induced by UVA and UVB, from various laboratories, cannot cross talk because of the use of ill-defined light sources, physiologically incompatible light dosimetry, and use of different cell populations. The aim of this study was to establish, in normal human epidermal keratinocytes (NHEK), at physiologically relevant UV doses, the expression profiles of cell signaling molecules in response to UVA and UVB irradiation.
MATERIALS AND METHODS
Materials
Extracellular signal-regulated kinases (ERKs)1/2 (phospho-Th202/Tyr204), JNK1/2 (phospo-Th183/Tyr185), p38 (phospho-Th180/Tyr204), NFκB/p65, NFκB/p50, p-IκBα, p-IKKα, STAT3 (phospho-Tyr705), STAT3, mammalian target of rapamycin (mTOR; phospho-Thr2448), p70S6K (phospho-Thr421/Ser424) were obtained from Cell Signaling Technology (Beverly, MA). STAT3 (phospho-Ser727), PI3K (p85), AKT (phospho-Thr308), AKT (phospho-Ser473), AKT, c-Fos, c-jun were obtained from Upstate Biotechnology. Anti-mouse or anti-rabbit secondary antibody horse radish peroxidase conjugate was obtained from Amersham Life Science Inc. (Arlington Heights, IL). Lightshift™ chemiluminiscent EMSA kit was obtained from Pierce (Rockford, IL). The DC BioRad Protein assay kit was purchased from BioRad Laboratories (Herculus, CA). Novex precast Tris-Glycine gels were obtained from Invitrogen (Carlsbad, CA).
Cell culture
Normal human epidermal keratinocytes were obtained from Invitrogen Corporation and the primary cultures were maintained in keratinocyte-SFM medium (Life Technologies, Grand Island, NY) supplemented with 0.1 mm calcium, 0.2% (vol/vol) bovine pituitary extract, EGF (10 ng mL1), insulin (5 µg mL−1), hydrocortisone (5 × 10−7 m), ethanolamine (1 × 10−4 m), phosphoethanolamine (1 × 10−4 M) and l-glutamine. The cells were maintained at 95% humidity in 5% CO2 environment at 37°C. The cells between third and fifth passage were used in the present study.
Exposure of cells to UV irradiation
Normal human epidermal keratinocytes (80% confluent) cultured in keratinocyte-SFM medium were irradiated with UVA (2 and 4 J cm−2) or UVB (20 and 40 mJ cm−2) with a custom designed Research Irradiation Unit (Daavlin, Bryan, OH) that consists of a fixture mounted on fixed legs. Within the exposure unit there are four UVA and UVB lamps, controlled using Daavlin Flex Control Integrating Dosimeters. In this system, dose units can be entered in Joules per centimeter square for UVA or milliJoules per centimeter square for UVB; variations in energy output are automatically compensated to deliver the desired dose. This enables us to expose the cells to accurate dosimetery of UVA and UVB radiation. On the basis of preliminary experiments, cell plates containing 5 mL PBS (10 mm, pH 7.4) were exposed to 2 and 4 J cm−2 UVA or 20 and 40 mJ cm−2 after which PBS was removed and media was added. After 30 or 60 min, cells were harvested and total, cytosolic or nuclear cell lysates were prepared as described elsewhere in the text.
Preparation of total cell lysate
Following treatment of cells with UVA or UVB, the medium was aspirated and the cells were washed twice in PBS (10 mm, pH 7.4). The cells were incubated in 0.4 mL ice-cold lysis buffer (50 mm Tris-HCl, 150 mm NaCl, 1 mm EGTA, 1 mm EDTA, 20 mm NaF, 100 mm Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mm PMSF [pH 7.4]) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III; Calbiochem, La Jolla, CA). The cells were then centrifuged at 14 000 g for 25 min at 4°C and the supernatant (total cell lysate) was collected, aliquoted and stored at −80°C. The protein concentration was determined by the DC BioRad assay using the manufacturer’s protocol (BioRad Laboratories, Herculus, CA).
Preparation of cytosolic and nuclear lysates
Following treatment of cells with UVA or UVB, the medium was aspirated and the cells were washed twice in PBS (10 mm, pH 7.4). The cells were incubated in 0.4 mL ice-cold lysis buffer (10 mm Hepes, 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm DTT, 1 mm PMSF [pH 7.9]) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III; Calbiochem, La Jolla, CA) for 15 min after which 12.5 µL of 10% Nonidet P-40 was added and the contents were mixed on a vortex and then centrifuged for 1 min (14 000 g) at 4°C. The supernatant was saved as cytosolic lysate and stored at −80°C. The nuclear pellet was resuspended in 50 µL of ice-cold nuclear extraction buffer (20 mm Hepes, 0.4 m NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, 1 mm PMSF [pH 7.9]) with freshly added protease inhibitor cocktail for 30 min with intermittent mixing. The cells were centrifuged for 5 min (13 000 g) at 4°C, and the supernatant (nuclear extact) was stored at −80°C. The protein concentration was determined by the BioRad assay as described elsewhere in the text.
Western blot analysis
For western blot analysis, 25–30 µg of protein was resolved over 8–12% polyacrylamide gels and transferred to a nitrocellulose membrane. The blot containing the transferred protein was blocked in blocking buffer (5% nonfat dry milk, 1% Tween 20; in 20 mm TBS, pH 7.6) for 1 h at room temperature followed by incubation with appropriate monoclonal primary anti-body in blocking buffer for 1 h to overnight at 4°C. This was followed by incubation with anti-mouse or anti-rabbit secondary antibody horse radish peroxidase (Amersham Life Sciences, Inc.) for 1 h and then washed several times and detected by chemiluminescence (ECL kit; Amersham Life Sciences, Inc.) and autoradiography using XAR-5 film obtained from Eastman Kodak Co. (Rochester, NY). Densitometric measurements of the band in western blot analysis were performed using digitalized scientific software program UN-SCAN-IT (Silk Scientific Corporation, Orem, UT).
RESULTS AND DISCUSSION
UV radiation is arguably the most abundant carcinogen in our environment and a major inducer of DNA damage in the epidermis of the human skin. The epidermal keratinocytes are the prime target of UV and it is essential for their viability that they balance and integrate stress signals and generate appropriate adaptive responses. The degree and type of UV-induced DNA damage depends heavily on the wavelength (5). UVA-induced ROS-dependent lesions are repaired quickly and have low mutagenicity, in contrast to the UVB-inflicted lesions that are repaired more slowly and lead to mutations (6,7). Different mutagenic properties of UVB and UVA are also reflected by their different potential in the induction of skin carcinomas in experimental animals and human studies (7). Although numerous studies have been conducted on the role of UV radiation on skin cells, the impact of UVA and UVB on keratinocytes using physiologically relevant doses is less well understood. The characterization of responses of UV light has mainly been carried out by fibroblasts; however, recent studies have shown that the normal human keratinocyte response to UV is significantly different from that of fibroblasts where the former is more resistant to UVB-induced apoptosis presumably due to the efficient global genome repair, resulting in accelerated removal of UV photoproducts (6). The inherent difficulty in studies using primary keratinocytes is the finite lifespan of these cells. Immortalized keratinocytes have been employed due to ease of propagation and near normal phenotype. However, the transformed cell lines exhibit many phenotypic features not found in normal cells. Even though using immortalized HaCaT keratinocytes as a substitute for normal keratinocytes have provided substantial insight into the signaling pathways in skin cells, caution should be used in the interpretation of these responses. Several molecular defects involving NF-κB signaling pathway were delineated in the immortalized keratinocytes (3). The immortalized cells can escape cell cycle checkpoints resulting in dysregulated cell cycle control, which does not bear relevance to actual human situation (3). This implies that the regulatory control of gene expression in response to UV irradiation may vary with the cell type.
As little as 5 J cm−2 of UVA has been shown to induce polymorphous light eruptions in sensitive individuals; also the same dose can cause rashes in patients suffering from chronic actinic dermatitis (8). Therefore, we selected a UVA dose of 2 and 4 J cm−2 for our experiment. The average minimal erythema dose of broadband UVB for Caucasians is approximately 40 mJ cm−2 (7,9). For our UVB studies, we thus selected the physiologically relevant doses of 20 and 40 mJ cm−2. The differential response of cell signaling proteins was evaluated after 30 and 60 min by exposing NHEK to UVA and UVB at these doses. The protein expression change greater than 1.5-fold, in the UV-treated samples, when compared with the untreated control was considered significant.
Effect of UVA and UVB on MAPKs
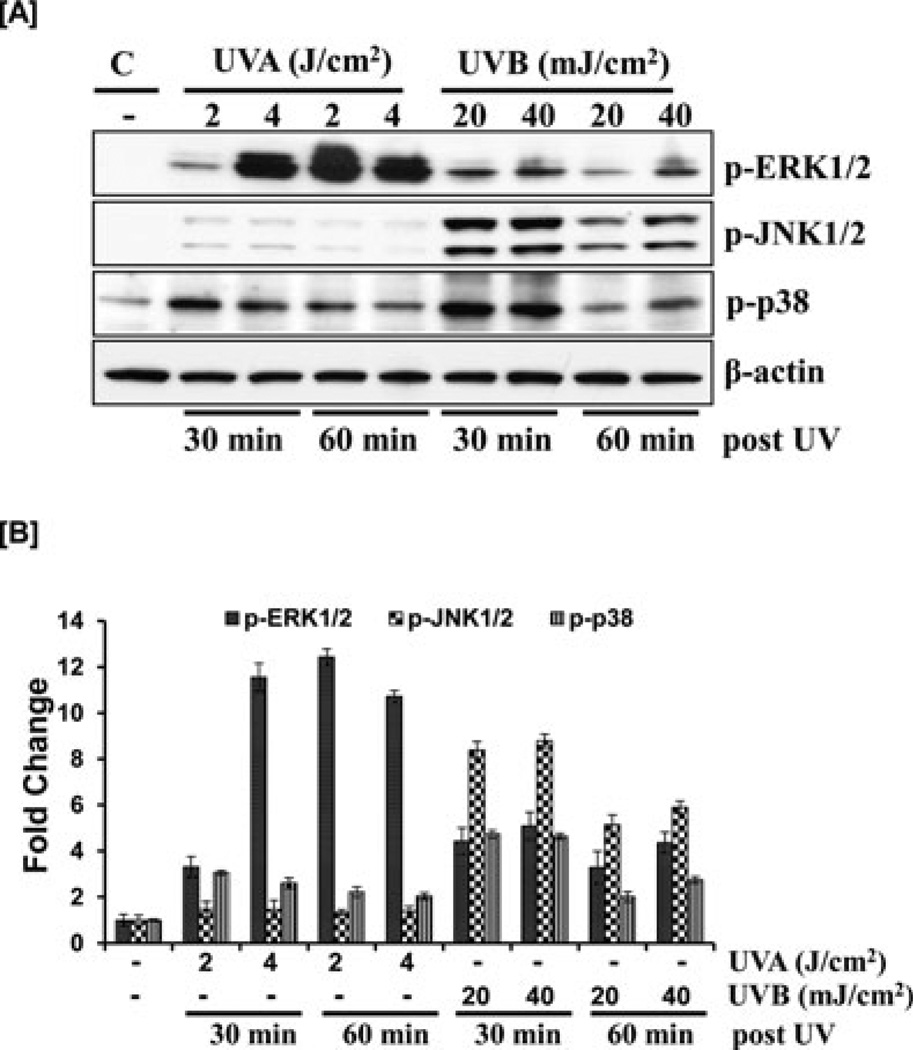
Of the many signaling pathways implicated in UV-induced damage, one is the mitogen-activated protein kinases (MAPK) cascade, a family of evolutionarily highly conserved enzymes that manages the response to growth stimulatory signals, such as insulin or EGF, as well as adverse signals, such as cytotoxic and genotoxic substances or radiation. They include ERKs, stress-activated protein kinases, also known as c-jun terminal kinases (SAPK/JNKs), and the p38-MAPKs. UVA has been shown to induce delayed and sustained ERK activation in HaCaT keratinocytes, in a Ras-dependent manner (10). In addition, significant activation of ERK has been reported post UVB irradiation in HaCaT cells (4,10). To determine whether this distinct pattern of ERK activation is simulated in normal epidermal keratinocytes, we irradiated NHEK with UVA and UVB. We found that UVA exposure for 30 and 60 min resulted in maximal phosphorylation of ERK1/2 at phosphorylation sites T202/Y204 (Fig. 1). UVB exposure also induced ERK phosphorylation, but the effect was not as dramatic as with UVA. Our data show increased phosphorylation of ERK1/2 post 30 min UVB exposure and a progressive decline seen at 60 min (Fig. 1). Our studies demonstrate that although both UVA and UVB result in phosphorylation of ERK, there is significantly more phosphorylation of ERK by UVA at the selected time points than by UVB in NHEK cells.
Figure 1.
Effect of UVA and UVB on MAPKs. (A) As detailed in the Materials and Methods section, the cells were treated with UVA (2–4 J m−2) or UVB (20–40 mJ cm−2) and harvested 30 or 60 min later. Total cell lysates were prepared and 40 µg protein was subjected to SDS polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with essentially similar results. (B) Histogram represents fold change in protein expression levels compared with value for the non UV-treated group, which was set as 1.
JNK plays a critical role in UV-induced apoptotic cell death. It was shown that UVB or UVC, but not UVA, activate JNK in normal keratinocytes (11). Contradicting this study, Chouinard et al. demonstrated that UVA, UVB or UVC failed to activate JNK and ERK MAPKs in these cells (5). To reveal the role of UV in the phosphorylation of JNK in normal human keratinocytes, the cells were irradiated with UVA and UVB and the dose/time-dependent effects were studied. Our experiments with both doses of UVA radiation showed that UVA mediated phosphorylation of JNK at either time point is much less when compared with UVB, which is maximal at 30 min and sustained up to 1 h (Fig. 1).
Rapid activation of p38 can be induced by a variety of cellular stresses, including UV irradiation. Figure 1 shows that p38 phosphorylation was induced by both UVA and B albeit the induction was more pronounced at the earlier time points. Our data are consistent with studies by Chouinard et al. where it was shown that all three UV wavelengths activated p38 in normal keratinocytes with peak activation occurring 2–10 min post UVA, 15–30 min post UVB irradiation and 10–15 min post UVC irradiation (5).
In summary, MAPK pathway, a major component of the primary adaptive response of keratinocytes against UV toxicity, was differentially activated by UVA and UVB. UVB irradiation preferentially phosphorylated JNK1/2 within 30 min of exposure whereas ERK1/2 and p38 were activated by both UVA and UVB. UVA exposure resulted in greater phosphorylation of ERK1/2 as compared with UVB; however, the latter may be a residual effect that was initiated within minutes of exposure to UVB and sustained up to an hour (12).
Effect of UVA and UVB on the AP-1 proteins
The activator protein-1 (AP-1) transcription factor family is made up of homodimers or heterodimers of Jun and Fos proteins. We studied the changes in expression of c-jun and c-Fos following UV exposure to human epidermal keratinocytes. Immunoblot analysis revealed that there was marked induction of c-jun by UVA as well as UVB at both time points. Interestingly, c-Fos was induced only by UVB at these time points and we did not observe any significant induction of the protein in UVA treated keratinocytes (Fig. 2).
Figure 2.
Effect of UVA and UVB on AP-1 proteins. (A) As detailed in the Materials and Methods section, the cells were treated with UVA (2–4 J m−2) or UVB (20–40 mJ cm−2) and harvested 30 or 60 min later. Total cell lysates were prepared and 40 µg protein was subjected to SDS polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with essentially similar results. (B) Histogram represents fold change in protein expression levels compared with value for the non UV-treated group, which was set as 1.
There are conflicting data in the literature regarding the effect of UVB irradiation on c-jun expression. Time-dependent studies did not find any significant change in c-jun protein levels post UVB (50 mJ cm−2) exposure in primary keratinocytes (13). However, when c-jun levels were analyzed in primary keratinocytes exposed to 5000 mJ cm−2 of UVB, strong induction of protein was observed as early as 1 h after irradiation, which persisted up to 4 h post irradiation, and thereafter returned to its initial value 48 h post irradiation (14). In other studies, UVB produced strong induction of c-jun and c-Fos transcripts in several cells, including human primary keratinocytes. Interestingly, UVB irradiation (50 mJ cm−2) has been reported to induce the expression of c-jun, jun-B and c-Fos in HaCaT keratinocytes with maximal induction noted 1–3 h post UVB exposure (14).
Effect of UVA and UVB on STAT 3
The phosphorylation of signal transducer and activator of transcriptions (STATs) at the tyrosine residue has generally been accepted as a prerequisite for their DNA binding and transactivation, although growth factors and cytokines induce phosphorylation of STATs on both tyrosine and serine residues (15). Studies in other model systems clearly demonstrate that UV is a potent inducer of STAT3, although there is limited data on the effect of UVA and UVB on STAT3 protein expression in normal human keratinocytes (16). We determined the level of expression of phosphorylated as well as total STAT3 protein in NHEK upon exposure to UVA and UVB using monoclonal antibodies. Interestingly, STAT3 was phosphorylated at Tyr705 by both UVA and UVB at 30 and 60 min of exposure (Fig. 3). However, UVA failed to phosphorylate STAT3 at Ser727 inducing no change in its status at both time points. Conversely, distinct phosphorylation at the serine residue was visible by UVB after 30 min and was sustained up to 1 h. The total STAT levels remained unchanged during these events.
Figure 3.
Effect of UVA and UVB on STAT3. (A) As detailed in the Materials and Methods section, the cells were treated with UVA (2–4 J m−2) or UVB (20–40 mJ cm−2) and harvested 30 or 60 min later. Total cell lysates were prepared and 40 µg protein was subjected to SDS polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with essentially similar results. (B) Histogram represents fold change in protein expression levels compared with value for the non UV-treated group, which was set as 1.
Bito et al. have suggested STAT3 activation as one of the initial events in UV-exposed human skin along with generation of ROS and subsequent DNA damage. They have shown that UVB exposure resulted in the activation of STAT3 30 min post irradiation, which was sustained for 6 h and returned to the baseline 12 h after UVB exposure. However, the UVB doses used in these studies ranged from 4000 to 8000 mJ cm−2 (17). Our data show that even at much lower doses STAT3 is phosphorylated by UVB at both tyrosine and serine residues. Moreover, in our experimental conditions, UVA phosphorylation of STAT3 in primary human keratinocytes occurred only at the tyrosine residue, at the selected time points.
Effect of UVA and UVB on PI3K/AKT signaling
UV irradiation causes oxidative stress that can lead to apoptotic cell death (18). There are various pathways that can protect keratinocytes from apoptosis, and one of them is the phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway. It has been reported that UVA does not induce cell cycle arrest in NHEK up to 40 kJ m−2 (400 J cm−2) presumably due to UVA-induced EGFR-dependent AKT activation (18). UV activation of PI3K/AKT pathway is thought to be initiated by ROS and prolonged by feedback activation of p38 induced by released cytokines in response to UV irradiation (19). To examine if PI3K is differentially activated upon UV exposure, we probed cell lysates with antibody to PI3Kp85, a regulatory subunit of PI3K. Both UVA and UVB increased the expression of PI3Kp85, although the effect was more pronounced by UVB at both time points, whereas maximum induction by UVA was seen with the higher dose at 1 h (Fig. 4). Next, we examined the effect of UVA and UVB on the phosphorylated and total AKT levels in the NHEK. Our data showed that UVB causes significant phosphorylation of AKT at Ser473 at both time points, whereas only UVA causes the protein to phosphorylate at Thr308 with little or no effect by UVB, at either time point. The total AKT levels remain unchanged with both UV wavelengths (Fig. 4).
Figure 4.
Effect of UVA and UVB on PI3K and AKT. (A) As detailed in the Materials and Methods section, the cells were treated with UVA (2–4 J m−2) or UVB (20–40 mJ cm−2) and harvested 30 or 60 min later. Total cell lysates were prepared and 40 µg protein was subjected to SDS polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with essentially similar results. (B) Histogram represents fold change in protein expression levels compared with value for the non UV-treated group, which was set as 1.
Effect of UVA and UVB on mTOR and p70S6K
To see if UVA and UVB can induce phosphorylation of mTOR and p70S6kinase (p70S6K) in NHEK at our selected doses, we exposed the cells to UVA and UVB for 30 and 60 min and probed the cell lysates with phosphospecific antibodies. As observed in Fig. 5, both UVA and UVB exposure resulted in phosphorylation of mTOR at Thr2448 with maximum induction occurring at 1 h, both with UVA and UVB. Consistent with mTOR phosphorylation, UV radiation also caused marked phosphorylation of p70S6K at Thr421/Ser424, but here maximum induction occurred within 30 min of exposure by both UVA and UVB, and with UVB the phosphorylation seemed to diminish at 1 h (Fig. 5).
Figure 5.
Effect of UVA and UVB on mTOR and p70S6K. (A) As detailed in the Materials and Methods section, the cells were treated with UVA (2–4 J m−2) or UVB (20–40 mJ cm−2) and harvested 30 or 60 min later. Total cell lysates were prepared and 40 µg protein was subjected to SDS polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with essentially similar results. (B) Histogram represents fold change in protein expression levels compared with value for the non UV-treated group, which was set as 1.
There is limited data on the effect of UV irradiation on mTOR/ p70S6K signaling in normal epidermal keratinocytes. UVB has been shown to induce mTOR activation in the immortalized HaCat keratinocytes in a TSC2-dependent manner (20). Using mouse epidermal cells, Huang et al. have shown that exposure to UV irradiation leads to a similar increase in p70S6K phosphorylation at the Threonine/Serine residues (21). Our data demonstrates that both wavelengths of UV activated mTOR/ p70S6K signaling in NHEK suggesting that UV-mediated modulation of signaling molecules is part of the global cell response to UV-damage in epidermal keratinocytes.
Effect of UVA and UVB on the NFκB proteins
Previously, we showed that UVB irradiation results in the activation of IKKα protein, which in turn phosphorylates and degrades IκBα protein in epidermal keratinocytes (22). In addition, the effect of UVA on nuclear factor κ B (NFκB) signaling has been well studied in HaCat keratinocytes (23,24). Herein, we examined the differential effect of UV irradiation on NFκB proteins, in normal human keratinocytes. We prepared cytosolic and nuclear lysates of NHEK exposed to UVA and UVB irradiation and examined various components of NFκB signaling. Western blot analysis revealed that both UV wavelengths resulted in increased phosphorylation of IKKα and IκBα proteins, at our selected time points. UVA as well as UVB exposure resulted in the activation and nuclear translocation of the functionally active subunits NFκB/p50 and NFκB/p65 in normal keratinocytes (Fig. 6).
Figure 6.
Effect of UVA and UVB on NFκB proteins. (A) As detailed in the Materials and Methods section, the cells were treated with UVA (2–4 J m−2) or UVB (20–40 mJ cm−2) and harvested 30 or 60 min later. Cytoplasmic and nuclear lysates were prepared and 40 µg protein was subjected to SDS polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin (cytoplasmic) and lamin (nuclear). The immunoblots shown here are representative of three independent experiments with essentially similar results. (B) Histogram represents fold change in protein expression levels compared with value for the non UV-treated group, which was set as 1.
A recent study has shown that UVA mediated activation of NFκB signaling is stronger in HaCaT keratinocytes when compared with primary human keratinocytes. Because of the difference in the response observed in the normal and immortalized keratinocytes it was recommended that findings obtained from the latter should be extended with caution to primary keratinocytes and human epidermis (25). Realizing this, we have attempted to establish a response profile in normal keratinocytes exposed to UV irradiation, which may then allow for comparison with studies in other model systems.
In summary, we found that exposure of NHEK to UVB, but not UVA, phosphorylates JNK1/2 at Th183/Tyr185, STAT3 at Ser727, AKT at Ser473 and increases c-Fos expression, whereas exposure to UVA, but not UVB, phosphorylates AKT at Thr308. UVB as well as UVA exposure leads to increased phosphorylation of (1) ERK1/2 at Th202/Tyr204; (2) p38 at Th180/Tyr204; (3) STAT3 at Tyr705; (4) mTOR at Thr2448; and (5) p70S6k at Thr421/Ser424; enhanced expression of PI3K (p85) and c-jun; and activation and nuclear translocation of NFκB proteins.
Taken together, our results demonstrate that there is a significant diversity in the biological response of keratinocytes to different wavelengths of UV. There seems to be a commonality as well as selectivity in the manner that keratinocytes respond to the UVA and UVB spectrum of solar radiation resulting in the activation of cellular targets. Moreover, our data is consistent with the realization that UV-mediated effects depend on the cellular context, time period and the dosimetry used. By integrating the above experimental data with further studies, a model for UV wavelength-specific induction of different signaling networks can be obtained. A better understanding of the molecular mechanisms and the differential effects of UVA and UVB on human skin would help unravel the etiology of different skin related diseases including cancer and have implications with respect to risk assessment from exposure to solar radiation, and to target interventions against signaling events mediated by UVA and UVB.
Footnotes
This paper is part of the Special Issue in Commemoration of the 70th birthday of Dr. David R. Bickers.
REFERENCES
- 1.Afaq F, Mukhtar H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006;15:678–684. doi: 10.1111/j.1600-0625.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 2.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat. Rev. Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi V, Qin JZ, Denning MF, Choubey D, Diaz MO, Nickoloff BJ. Abnormal NF-kappaB signaling pathway with enhanced susceptibility to apoptosis in immortalized keratinocytes. J. Dermatol. Sci. 2001;26:67–78. doi: 10.1016/s0923-1811(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Tang Q, Gonzales MS, Bowden GT. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene. 2001;20:3921–3926. doi: 10.1038/sj.onc.1204530. [DOI] [PubMed] [Google Scholar]
- 5.Chouinard N, Valerie K, Rouabhia M, Huot J. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. Biochem. J. 2002;365:133–145. doi: 10.1042/BJ20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Errico M, Lemma T, Calcagnile A, Proietti De Santis L, Dogliotti E. Cell type and DNA damage specific response of human skin cells to environmental agents. Mutat. Res. 2007;614:37–47. doi: 10.1016/j.mrfmmm.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Lisby S, Gniadecki R, Wulf HC. UV-induced DNA damage in human keratinocytes: quantitation and correlation with long-term survival. Exp. Dermatol. 2005;14:349–355. doi: 10.1111/j.0906-6705.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 8.Hampton PJ, Farr PM, Diffey BL, Lloyd JJ. Implication for photosensitive patients of ultraviolet A exposure in vehicles. Br. J. Dermatol. 2004;151:873–876. doi: 10.1111/j.1365-2133.2004.06098.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaidbey KH, Agin PP, Sayre RM, Kligman AM. Photoprotection by melanin—a comparison of black and Caucasian skin. J. Am. Acad. Dermatol. 1979;1:249–260. doi: 10.1016/s0190-9622(79)70018-1. [DOI] [PubMed] [Google Scholar]
- 10.He YY, Huang JL, Chignell CF. Delayed and sustained activation of extracellular signal-regulated kinase in human keratinocytes by UVA: implications in carcinogenesis. J. Biol. Chem. 2004;279:53867–53874. doi: 10.1074/jbc.M405781200. [DOI] [PubMed] [Google Scholar]
- 11.Adachi M, Gazel A, Pintucci G, Shuck A, Shifteh S, Ginsburg D, Rao LS, Kaneko T, Freedberg IM, Tamaki K, Blumenberg M. Specificity in stress response: epidermal keratinocytes exhibit specialized UV-responsive signal transduction pathways. DNA Cell Biol. 2003;22:665–677. doi: 10.1089/104454903770238148. [DOI] [PubMed] [Google Scholar]
- 12.Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001;176:110–117. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- 13.Andersson E, Rosdahl I, Torma H, Vahlquist A. Differential effects of UV irradiation on nuclear retinoid receptor levels in cultured keratinocytes and melanocytes. Exp. Dermatol. 2003;12:563–571. doi: 10.1034/j.1600-0625.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 14.Brellier F, Marionnet C, Chevallier-Lagente O, Toftgard R, Mauviel A, Sarasin A, Magnaldo T. Ultraviolet irradiation represses PATCHED gene transcription in human epidermal keratinocytes through an activator protein-1-dependent process. Cancer Res. 2004;64:2699–2704. doi: 10.1158/0008-5472.can-03-3477. [DOI] [PubMed] [Google Scholar]
- 15.Ramsauer K, Sadzak I, Porras A, Pilz A, Nebreda AR, Decker T, Kovarik P. p38 MAPK enhances STAT1-dependent transcription independently of Ser-727 phosphorylation. Proc. Natl Acad. Sci. USA. 2002;99:12859–12864. doi: 10.1073/pnas.192264999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahsan H, Aziz MH, Ahmad N. Ultraviolet B exposure activates Stat3 signaling via phosphorylation at tyrosine705 in skin of SKH1 hairless mouse: a target for the management of skin cancer? Biochem. Biophys. Res. Commun. 2005;333:241–246. doi: 10.1016/j.bbrc.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 17.Bito T, Sumita N, Masaki T, Shirakawa T, Ueda M, Yoshiki R, Tokura Y, Nishigori C. Ultraviolet light induces Stat3 activation in human keratinocytes and fibroblasts through reactive oxygen species and DNA damage. Exp. Dermatol. 2010;19:654–660. doi: 10.1111/j.1600-0625.2010.01084.x. [DOI] [PubMed] [Google Scholar]
- 18.Jean C, Hernandez-Pigeon H, Blanc A, Charveron M, Laurent G. Epidermal growth factor receptor pathway mitigates UVA-induced G2/M arrest in keratinocyte cells. J. Invest. Dermatol. 2007;127:2418–2424. doi: 10.1038/sj.jid.5700863. [DOI] [PubMed] [Google Scholar]
- 19.Zhang QS, Maddock DA, Chen JP, Heo S, Chiu C, Lai D, Souza K, Mehta S, Wan YS. Cytokine-induced p38 activation feedback regulates the prolonged activation of AKT cell survival pathway initiated by reactive oxygen species in response to UV irradiation in human keratinocytes. Int. J. Oncol. 2001;19:1057–1061. doi: 10.3892/ijo.19.5.1057. [DOI] [PubMed] [Google Scholar]
- 20.Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, Tamakloe T, Chu WM, Guan KL, Wan Y. AMP-activated protein kinase contributes to UV- and H2O2-induced apoptosis in human skin keratinocytes. J. Biol. Chem. 2008;283:28897–28908. doi: 10.1074/jbc.M804144200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Huang C, Li J, Ke Q, Leonard SS, Jiang BH, Zhong XS, Costa M, Castranova V, Shi X. Ultraviolet-induced phosphorylation of p70(S6K) at ThR(389) and ThR(421)/SeR(424) involves hydrogen peroxide and mammalian target of rapamycin but not Akt and atypical protein kinase C. Cancer Res. 2002;62:5689–5697. [PubMed] [Google Scholar]
- 22.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez Ruderisch H, Schwarz C, Shang J, Tebbe B. Trioxsalen in the presence of UVA is able to induce nuclear factor kappa B binding activity in HaCaT keratinocytes. Skin Pharmacol. Appl. Skin Physiol. 2002;15:335–341. doi: 10.1159/000064538. [DOI] [PubMed] [Google Scholar]
- 24.Shang J, Schwarz C, Sanchez Ruderisch H, Hertting T, Orfanos CE, Tebbe B. Effects of UVA and L-ascorbic acid on nuclear factor-kappa B in melanocytes and in HaCaT keratinocytes. Skin Pharmacol. Appl. Skin Physiol. 2002;15:353–359. doi: 10.1159/000064541. [DOI] [PubMed] [Google Scholar]
- 25.Pastore S, Lulli D, Potapovich AI, Fidanza P, Kostyuk VA, Dellambra E, De Luca C, Maurelli R, Korkina LG. Differential modulation of stress-inflammation responses by plant polyphenols in cultured normal human keratinocytes and immortalized HaCaT cells. J. Dermatol. Sci. 2011;63:104–114. doi: 10.1016/j.jdermsci.2011.04.011. [DOI] [PubMed] [Google Scholar]