Abstract
Individuals with antemortem preservation of cognition who show autopsy evidence of at least moderate Alzheimer disease (AD) pathology suggest the possibility of brain reserve, that is, functional resistance to structural brain damage. This reserve would, however, only be relevant if the pathologic markers correlate well with dementia. Using data from the Nun Study (n = 498) and the Adult Changes in Thought (ACT) Study (n = 323), we show that Braak staging correlates strongly with dementia status. Moreover, participants with severe (Braak stage V–VI) AD pathology who remained not demented represent only 12% (Nun Study) and 8% (ACT study) of nondemented subjects. Comparison of these subjects to those who were demented revealed that the former group was often significantly memory impaired despite not being classified as demented. Most of these nondemented participants showed only stage V neurofibrillary pathology and frontal tangle counts that were slightly lower than a comparable (Braak stage V) dementia group. In summary, these data indicate that, in individuals with AD-type pathology who do not meet criteria for dementia, neocortical neurofibrillary tangles are somewhat reduced and incipient cognitive decline is present. Our data provide a foundation for helping to define additional factors that may impair, or be protective of, cognition in older adults.
Keywords: Adult Changes in Thought Study, Alzheimer disease, brain reserve, dementia, Nun Study, presymptomatic, preclinical
INTRODUCTION
Observations of individuals with Alzheimer disease (AD) pathology without dementia have led to the concept of “brain reserve” as a way of describing relative resistance to cognitive decline despite the presence of histopathologic disease defining features of AD (plaques and tangles) in the brain (1, 2). These individuals have been variously described as showing pathologic aging, presymptomatic aging, preclinical AD, asymptomatic AD, presymptomatic AD, or (as used here) nondemented but with AD pathology (NDAP) (3–7). Although rarely a person with definite pathologic features of AD may have remained cognitively intact antemortem, many studies suggest that it is much more likely that a person with substantial amyloid plaque and neurofibrillary tangle (NFT) accumulation would eventually develop dementia (8–11). However, the NDAP group is an informative group to study for understanding genetic or environmental factors that may explain their significantly better cognitive performance compared with pathologically similar groups.
Pathologic lesions of AD are thought to accumulate continuously for many years before sufficient brain pathologic alterations, presumably including synaptic and neuronal loss, lead to clinical manifestations (12, 13). An individual with more brain reserve might, therefore, accumulate more lesions before synaptic and neuronal loss result in cognitive impairment than someone with less reserve. Our goal was to develop a definition of brain reserve that focuses on neuropathology to describe an NDAP group that functioned at a higher level than expected for a given amount of AD pathology. This pathologic definition does not distinguish between structural brain reserve (e.g. increased synaptic density) and cognitive reserve (i.e. “cognitive coping”) (14, 15). Defining a functionally significant clinicopathological diagnosis of AD in the context of brain reserve is complicated by the presence of additional structural abnormalities and conditions, such as normal pressure hydrocephalus and metabolic diseases, and by overlapping disease processes, such as Lewy body disease and vascular disease, all of which contribute to reduced cognition (16, 17). Another difficulty in defining brain reserve based on neuropathologic criteria is that categorizing clinical and pathologic features creates artificial separations when the disease process may more appropriately be considered a continuum. In a biologic sense, pathologic changes do not fit neatly into predefined categories of possible, probable, and definite AD (18). For example, because Braak staging is based on the presence of pathologic markers in specific locations rather than on specific quantitative criteria, an advanced Braak stage IV case may be very similar to an early Braak stage V case if only rare neocortical NFTs are present. Moreover, individual neuropathologists have different thresholds for these categories (19). Because the disease may progress inconsistently across individuals, artificial categories of both cognitive ability and neuropathologic stages can be misleading. A good neuropathologic definition of brain reserve would take into account these complicating factors.
MATERIALS AND METHODS
Study Population
The design of the Nun Study has been described in detail (20). Briefly, it is a cohort study of 678 Catholic sisters living in the United States who were born before 1917 and who were recruited in 1991 at age 75 or older. Participants with dementia at the start of the study were included, and all participants underwent multiple annual waves of neuropsychologic testing, as well as detailed neuropathologic data collection at autopsy. The analyses were based on a surveillance period ending in July 2006. Brain donation occurred for 542 (95%) of the participants who died during this period and 498 had complete neuropathologic assessments at the time of this study.
The Adult Changes in Thought (ACT) study is an ongoing prospective community-based study of brain aging and dementia. ACT has enrolled approximately 3,700 individuals from Group Health Cooperative, a managed health care provider of approximately 23,000 persons in King County, Washington. Approximately 20% of enrolled individuals who die undergo an autopsy with detailed neuropathologic examination (21). The present study analyzes the initial 323 consecutive ACT autopsies.
Neuropathologic Data
Brain weights were recorded fresh for Nun Study participants and fixed for ACT study participants. For both studies, Braak staging and Consortium to Establish a Registry for Alzheimer Disease (CERAD) scoring were performed according to consensus criteria (22–24). Stains included hematoxylin and eosin, modified Bielschowsky and Gallyas histochemistry for plaques and tangles, and α-synuclein (mouse monoclonal anti–α-synuclein from Novacastra [NCL-ASN, clone KM51]) immunohistochemistry for Lewy body staining. Detailed counts of pathologic features correlating with dementia, including neuritic plaque and NFT counts in multiple sections of limbic cortex and isocortex, were available in an organized, well maintained database for correlation with the clinical variables accumulated during the 16 to 18 years of the studies. Neuropathologic data, such as NFT and neuritic plaque burden, were collected in 8-Hm-thick sections in a manner consistent with CERAD and Braak grading schemes. Specific recommendations described in 1997 consensus recommendations (Reagan criteria) were followed with regard to Bielschowsky and Gallyas staining of neocortical (temporal, parietal, frontal, and primary visual and association) and hippocampal sections sampled for determination of Braak stage (22). The occipital lobe section contained areas 17 (striate), 18 (parastriate), and 19 (peristriate), all of which were used in Braak staging and in generating quantitative occipital cortex data. Many other sections described as optimal in Reagan consensus criteria were also sampled. The primary neuropathologist for the Nun Study (William Markesbery) generated mean regional plaque and tangle counts in selected fields using previously published methodology. Briefly, the 5 subjectively highest concentration fields were counted for neuritic plaques and for NFTs (25). The neuropathologist was blinded to clinical information except age. Regional neuritic plaque and NFT counts were not performed for the ACT study. Supplementary restricted analyses eliminated cases with defined disease processes, other than AD, that could contribute to dementia. These included gross cystic infarcts, neocortical Lewy bodies, hippocampal sclerosis and cerebral metastatic carcinoma.
Cognitive Testing
Nun Study participants were assessed annually for cognitive and physical function. The last cognitive assessment before death was used in these analyses. The cognitive test battery administered to participants in the Nun Study included the measures compiled in the CERAD neuropsychologic assessment battery (26, 27). This battery assesses memory, language, visuospatial ability, concentration, and orientation and was administered by 2 trained field gerontologists in the building in which the participants lived. Tests included Boston naming (BNT), verbal fluency (VRBF), word list learning (WRL), word list recognition (WRCO), word list recall (WRCL), constructional praxis (CNPR), and the Mini–Mental Status Examination (MMSE). We assessed 5 basic (feeding, dressing, standing, walking, and toileting) and 5 instrumental (reading, using the telephone, telling time, taking medication, and handling money) activities of daily living. Performance-based measures (28, 29) were used for all activities except toileting, where independence was determined from the nursing reports for participants receiving nursing care and self-reports for the remaining participants.
Dementia status was determined using a cognitive status 6 level rating scheme developed by the Nun Study as previously reported (30). This neuropsychologic definition substitutes for a diagnosis rendered by a neurologist using accepted criteria. Briefly, subjects who met clinical criteria for dementia demonstrated impairments in memory and at least one other cognitive domain, as well as a decline in cognitive function. They were also impaired on activities of daily living. Participants were judged to be cognitively intact if they did not show impairments in activities of daily living or any cognitive tests. The mean ± SD number of years between the last cognitive assessment and death was 0.94 ± 0.49 years for the NDAP group and 0.79 ± 0.53 years for the AD group; these did not differ significantly between the groups. The mean duration between last assessment and death did not differ significantly between the Braak zero group and the entire sample of 498.
In the ACT study, participants were followed every 2 years with a protocol-based examination and assessment that used the MMSE and the Cognitive Assessment Screening Instrument (CASI). A CASI score of 85 or less triggered a dementia workup. Dementia was diagnosed by consensus conference and followed the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria (31). The ACT participants were stratified based on clinical dementia status and most recent CASI score preceding death. The ACT participants do not undergo subsequent cognitive evaluations once a consensus diagnosis of dementia is rendered. Therefore, the time from last testing until death is longer for demented individuals. Those individuals without a dementia diagnosis and who underwent protocol-based examination within 2 years of death were considered not demented.
Apolipoprotein E
For the Nun Study, apolipoprotein E (ApoE) genotyping was determined by buccal swabs collected from participants who were living at the time of the original study, or from frozen or paraffin-embedded tissue samples from deceased participants (32). Blood samples were used for the ACT study (33).
Statistical Analysis
Statistical analyses were performed using PASW Statistics 18, Release Version 18.0.2 (SPSS, Inc, Chicago, IL). Means were reported as mean ± SD or SEM. Spearman correlations were performed to assess associations between various key pathologic features of AD and performance on cognitive tests. One-way analysis of variance was used to compare the means of cognitive groups, with post hoc Scheffé tests in case of equal variances or Tamhane tests in case of unequal variances. W2 tests were used to compare categorical data, with Fisher exact tests as appropriate. Statistical significance was considered as p < 0.05.
Demographic and Brain Morphologic Features
Comparison of groups designed to represent classic AD, NDAP and healthy aging (defined as Braak zero) for both the Nun Study and ACT study revealed that the NDAP group seemed to be somewhat intermediate between classic AD and Braak zero participants with respect to education, brain weight, and likelihood of having an ApoE-ε4 allele (Table 1). However, there were no statistically significant differences between NDAP and either AD or Braak zero groups with respect to demographic and brain morphologic features. In contrast, AD and Braak zero groups were different with regard to ApoE-ε4 status and age of death but not brain weight or education. Brain weights were not compared between Nun Study and ACT participants because all Nun Study participants are female and Nun Study brain weights were based on fresh tissue, whereas ACT brain weights were based on fixed tissue.
Table 1. Demographic and Brain Morphologic Features.
Demographic and brain morphologic features of Nun Study and Adult Changes in Thought participants, restricted sample (without alternative explanations for dementia). The NDAP groups are not significantly different from either the AD or the Braak zero groups.
| AD | NDAP | Braak Zero | ||||
|---|---|---|---|---|---|---|
| Study | NS (n = 46) | ACT (n =26) | NS (n = 15) | ACT (n = 14) | NS (n =11) | ACT (n =10) |
| Description | Braak Stage V–VI Demented | Braak Stage V–VI not Demented | Braak Stage Zero not Demented | |||
| Age at death, y | 90.98±5.54 | 89.93±6.03 | 90.80±5.22 | 89.15±6.91 | 86.13±6.05 | 81.56±5.70 |
| Education, y | 15.43±3.28 | 12.63±3.56 | 16.13±2.77 | 13.69±2.75 | 16.73±1.85 | 16.33±3.81 |
| Brain weight, g | 1072.18±157.59 | 1136.74±122.50 | 1113.33±118.74 | 1254.00±103.97 | 1143.00±97.87 | 1291.33±167.53 |
| ApoE4 (>1 allele), % | 45.2* | 28.3† | 21‡ | 23.1 | 9 | 28.6§ |
Mean and SD are shown, except as indicated. ACT, Adult Changes in Thought study; AD, Alzheimer disease; ApoE4, apolipoprotein E-ε4; NDAP, nondemented with Alzheimer pathology; NS, Nun Study.
Missing data, n = 4
Missing data, n = 6
Missing data, n = 1
Missing data, n = 3
RESULTS
Correlation Between Pathologic Markers and Cognitive Tests
Spearman correlations were performed to determine the neuropathologic features in specific subregions that correlated best with cognitive tests relevant to specific brain subregions A subset of these correlations supports the selection of overall Braak score rather than regional plaque or tangle counts (Table 2). Correlations tended to be somewhat stronger in a small subset of participants who did not have overlapping disease processes, such as infarcts and Lewy body disease. We designated this group as the “restricted group.” Although there were subtle differences in correlations of various aspects of cognition with various types (diffuse or neuritic plaques [data not shown] or tangles) and sites of disease markers, no particular type or site of pathology was identified as more strongly correlated with any aspect of cognition or overall cognitive function than Braak stage. Therefore, we chose to examine a group of Nun Study participants with Braak stage V–VI pathology to find a group that performed better than expected on cognitive tests. Criteria were as follows: Braak stage V–VI pathology and clinical dementia for the AD group, Braak stage V–VI without clinical dementia for the NDAP group, and Braak stage zero without clinical dementia for the Braak zero group.
Table 2. Correlations Between Neurofibrillary Pathology and Cognitive Performance by Whole or Restricted Group (Nun Study).
MMSE, Mini-Mental State Examination; NFT, neurofibrillary tangles per squared millimeter.
| COGNITIVE PERFORMANCE | ||||||
|---|---|---|---|---|---|---|
| Word Recall | Verbal Fluency | MMSE | ||||
| Neurofibrillary pathology | Whole | Restricted* | Whole | Restricted* | Whole | Restricted* |
| BRAAK STAGE | −0.57 | −0.68 | −0.48 | −0.55 | −0.52 | −0.60 |
| MEAN NFTS | ||||||
| Frontal cortex | −0.39 | −0.45 | −0.42 | −0.51 | −0.52 | −0.62 |
| Hippocampus | −0.49 | −0.60 | −0.39 | −0.48 | −0.45 | −0.54 |
| Temporal cortex | −0.58 | −0.64 | −0.48 | −0.51 | −0.54 | −0.58 |
| Occipital cortex | −0.42 | −0.44 | −0.40 | −0.39 | −0.42 | −0.39 |
The restricted group consists only of participants without alternative or coexisting pathologies as likely contributing factors to dementia.
Pathologic Grade and Dementia Status
The majority (71%/76%) of Nun Study/ACT participants, respectively, with dementia showed Braak stage III pathology or higher, with the largest groups (50%/51%) classified as stage V–VI (Tables, Supplemental Digital Content 1 and 2, http://links.lww.com/NEN/A267 and http://links.lww.com/NEN/A268). Nondemented participants most commonly showed Braak changes less than stage III. The proportion of nondemented participants with Braak stage less than III was 52%/64% Nun Study/ACT Study. The largest number among this group was not those with stage 0 changes, but instead those with stage I–II pathology: only 17 (6.7%)/13 (6%) of nondemented participants in the Nun Study/ACT study showed essentially no NFT pathology (Tables, Supplemental Digital Content 1 and 2, http://links.lww.com/NEN/A267 and http://links.lww.com/NEN/A268).
Prevalence of Poor Clinical-Pathologic Correlation
Although the numbers were small, some individuals showed apparent mismatches of pathologic changes to cognitive status. Only 3 participants with dementia from the Nun Study and 3 from the ACT study showed a Braak stage of zero, although 29% and 23% of participants with dementia from the Nun Study and ACT study, respectively, showed stage II changes or less. In contrast, 8% and 12% of ACT and Nun Study participants, respectively, who were not demented were categorized as Braak stages V–VI (NDAP) (Tables, Supplemental Digital Content 1 and 2, http://links.lww.com/NEN/A267 and http://links.lww.com/NEN/A268).
Participants with alternative clear pathologic reasons for dementia were removed from the analyses to determine whether AD pathology was more strongly associated with clinical dementia in this subset (Tables 3 and 4). The most striking result of this analysis was that of the original 71 Nun Study and 25 ACT participants with dementia and Braak stage II or less, only 12 Nun Study and 3 ACT participants remained in the restricted sample for whom there was no clear neuroanatomic or clinical-neuropathologic explanation for dementia (Tables 3 and 4). In this restricted subsample, only 11%/10% of Nun Study/ACT participants with Braak stage V or VI pathology were not demented (NDAP).
Table 3. Categorical Classification of Braak Stage, CERAD Score (Nun Study).
Categorical classification of Braak Stage, CERAD score, and NIA Reagan designation by dementia status for Nun Study participants, restricted sample without alternative explanations for dementia. Italicized numbers indicate 35 cases with dementia and 115 cases without dementia that were unclassifiable by Reagan criteria. CERAD, Consortium to Establish a Registry for Alzheimer Disease.
| Braak Stage | |||||
|---|---|---|---|---|---|
| CERAD | 0 | I–II | III–IV | V–VI | Total |
| Demented | |||||
| 0 | 1 | 5 | 2 | 0 | 9 |
| A | 0 | 1 | 4 | 0 | 5 |
| B | 0 | 4 | 7 | 10 | 21 |
| C | 0 | 1 | 8 | 36 | 45 |
| Total | 1 | 11 | 21 | 46 | 80 |
| Not demented | |||||
| 0 | 6 | 34 | 4 | 1 | 45 |
| A | 2 | 5 | 5 | 4 | 16 |
| B | 3 | 24 | 4 | 6 | 37 |
| C | 0 | 17 | 15 | 4 | 36 |
| Total | 11 | 70 | 28 | 15 | 134 |
Table 4. Categorical Classification of Braak Stage, CERAD Score (ACT Study).
Categorical classification of Braak Stage, CERAD score, and NIA Reagan designation by dementia status for Adult Changes in Thought participants, restricted sample without alternative explanations for dementia. Italicized numbers indicate 20 cases with dementia and 86 cases without dementia that were unclassifiable by Reagan criteria. CERAD, Consortium to Establish a Registry for Alzheimer Disease.
| Braak Stage | |||||
|---|---|---|---|---|---|
| CERAD | 0 | I–II | III–IV | V–VI | Total |
| Demented | |||||
| 0 | 1 | 2 | 3 | 1 | 7 |
| A | 0 | 0 | 2 | 3 | 5 |
| B | 0 | 0 | 3 | 8 | 11 |
| C | 0 | 0 | 1 | 14 | 15 |
| Total | 1 | 2 | 9 | 26 | 38 |
| Not demented | |||||
| 0 | 4 | 29 | 7 | 1 | 41 |
| A | 4 | 24 | 25 | 0 | 53 |
| B | 2 | 9 | 19 | 3 | 33 |
| C | 0 | 2 | 4 | 10 | 16 |
| Total | 10 | 64 | 55 | 14 | 143 |
Characteristics of Participants With Poor Clinical-Pathologic Correlation
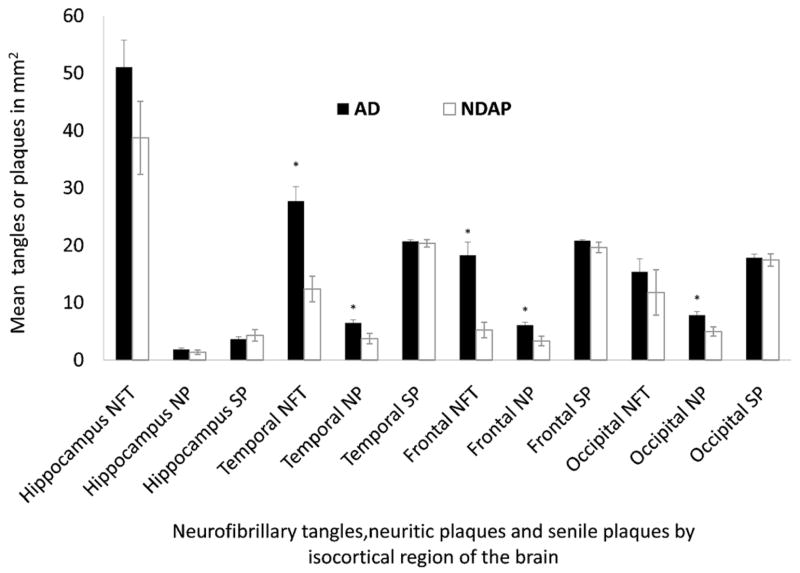
Clinical and neuropathologic features of the NDAP group in the Nun Study differed from participants with high pathology that were demented (AD group) (Figs. 1 and 2). A comparison of the 2 high-pathology groups showed that, in frontal lobe and temporal cortex, NFT counts were significantly lower in the NDAP group versus the AD group (temporal NFT range, 2.4–31.7 for NDAP and 5.8–57.3 for AD, p < 0.0001; frontal NFT range, 0–16 for NDAP and 1.7–59.4 for AD, p < 0.0001) (Fig. 1). In general, there was no significant difference between the AD and NDAP groups with regard to hippocampal NFT pathology, possibly because of a ceiling effect of limbic pathology. The number of senile plaques (defined as diffuse plus neuritic plaques) in the NDAP group was not significantly different from those in the AD group in any isocortical region. In contrast, neuritic plaque counts were lower in the NDAP group versus the AD group in all isocortical regions examined, including Brodmann area 9 of the frontal cortex and area 18 to 19 peristriate/parastriate occipital cortex; the only exception was the hippocampus.
Figure 1.
Neuropathologic comparison of Nun Study participants with Braak stage V–VI pathologic changes and clinical dementia (AD group) to those without dementia (NDAP group). AD indicates Alzheimer disease; NDAP, nondemented with Alzheimer pathologic symptom; NFT, neurofibrillary tangles; NP, neuritic plaques; SP, senile plaques. Mean and SEM are shown. *p < 0.05, AD versus NDAP group.
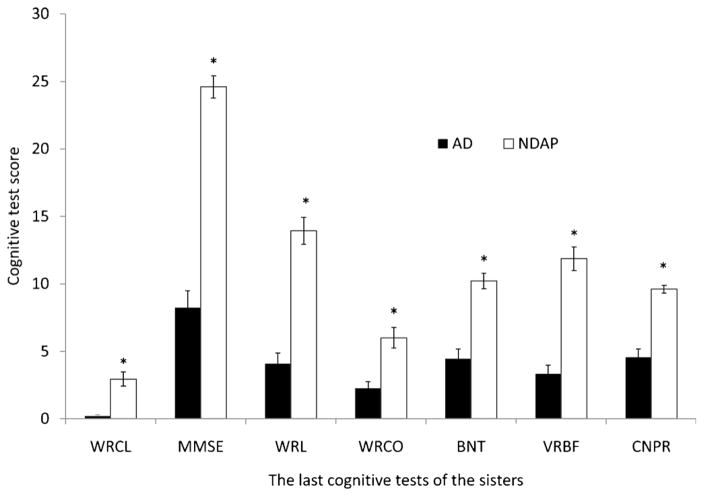
Figure 2.
Neuropsychologic profile comparison of Nun Study participants with Braak stage V–VI pathologic changes and clinical dementia (AD group) to those without dementia (NDAP group). AD indicates Alzheimer disease; BNT, Boston naming test; CNPR, constructional praxis score; NDAP, nondemented with Alzheimer pathology; MMSE, Mini–Mental Status Examination; VRBF, verbal fluency; WRCL, word list recall; WRL, word list memory; WRCO, word recognition. Mean and SEM are shown. *p < 0.05, AD versus NDAP group.
Although individuals in the NDAP group were, by definition, not demented, cognition was far from intact in this group. Performance on cognitive tests at the last assessment before death indicated that all of the 15 NDAP participants showed some impairment on cognitive testing. The NDAP participants were significantly different from a Braak stage 0, nondemented comparison group with regard to all cognitive tests with the exception of BNT, VRBF, and CNPR, which, although lower, failed to reach significance (comparisons not shown) (WRCL range, 0–6 for NDAP and 6–8 for Braak zero group, p < 0.0001; MMSE range 18–29 for NDAP and 27–30 for Braak zero group, p = 0.0005; WRL range, 8–20 for NDAP and 17–24 for Braak zero, p < 0.0001; WRCO range, 2–10 for NDAP and 8–10 for Braak zero, p = 0.0003). However, mean cognitive scores for the NDAP group were significantly better than the AD group in all categories shown (Fig. 2).
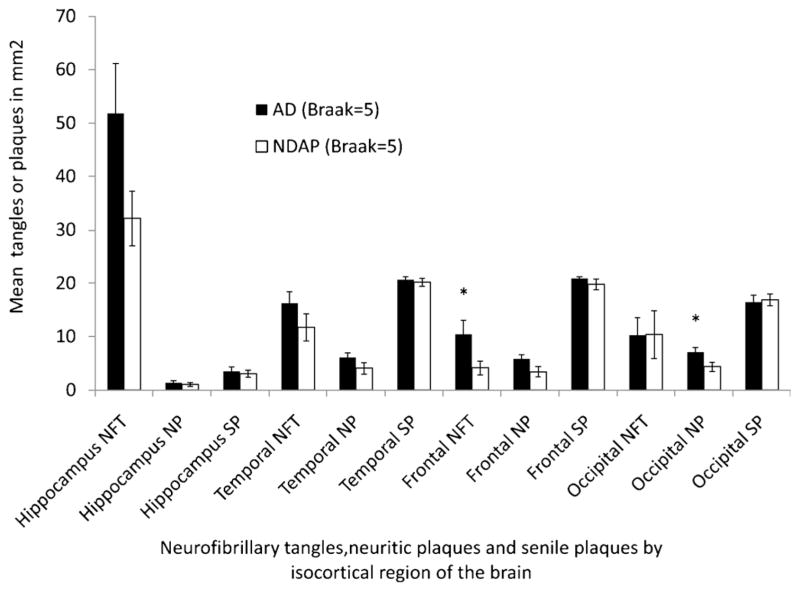
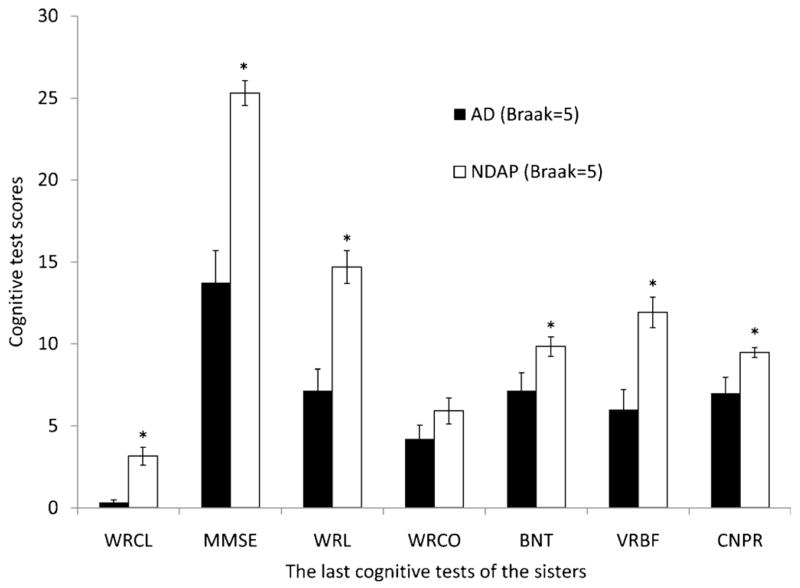
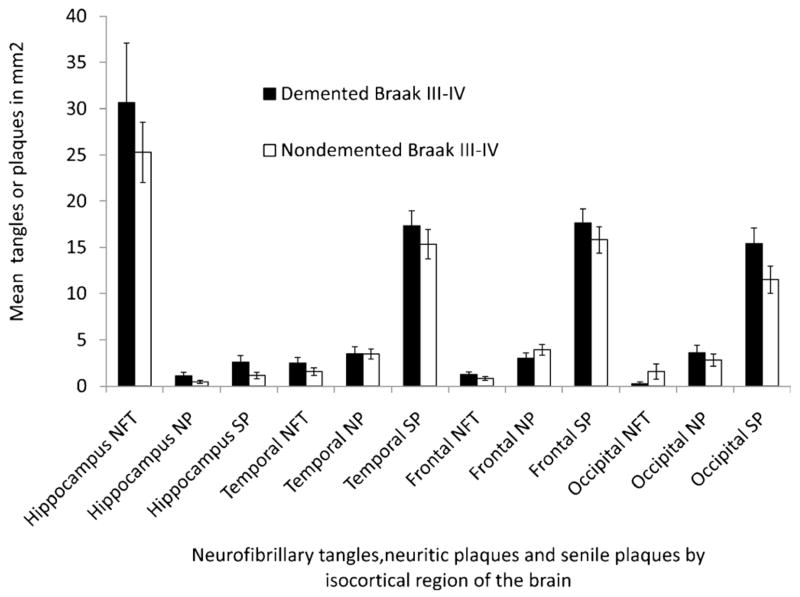
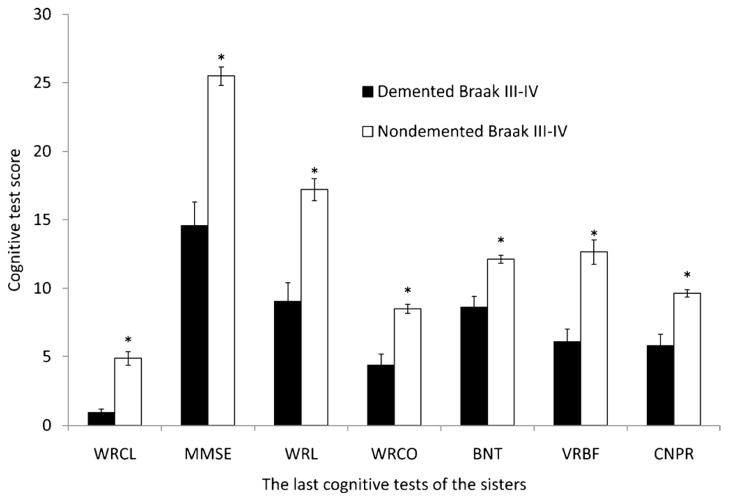
The smaller NFT load observed in both frontal and temporal cortices in the NDAP group may represent a partial explanation for the improved cognitive performance in this group. The 7 NDAP participants with the lowest estimate of overall cognitive function were the same 7 subjects with the highest frontal cortex NFT counts (rank order not shown). It is possible that the NDAP group may perform better than the “typical” AD group with dementia because most (13/15) of the NDAP group are Braak stage V rather than stage VI cases. To assess this possibility, we compared the Braak V cases in our NDAP group group (n = 13) to the 19 Braak stage V participants in the AD group of 46 demented participants without overlapping pathology (Figs. 3 and 4). In this comparison, the number of temporal NFTs was no longer significantly different (range, 2.4–31.7 for NDAP and 5.8–38.9 for AD, p = 0.18), and the difference between frontal NFTs, although still significant, was smaller. The differences in neuritic plaque load also became nonsignificant in all locations except area 18 to 19 of the occipital cortex (range, 0.2–9.6 for NDAP and 0–11.9 for Braak V AD group, p = 0.03). Although these 2 Braak stage V comparison groups were very similar pathologically, the cognitive features that led to the diagnosis of dementia for 19 of these subjects makes them cognitively different than the 13 subjects with similar pathology who were not considered demented. Performance was better in the nondemented group for all cognitive tests except word recognition (Fig. 4) (WRCL range, 0–6 for NDAP and 0–3 for AD, p < 0.001; BNT range, 6–14 for NDAP and 0–14 for AD, p = 0.04; VRBF range, 8–21 for NDAP and 0–17 for AD, p < 0.001; WRL range, 9–20 for NDAP and 0–18 for AD, p < 0.0001; MMSE range, 22–30 for NDAP and 0–24 for AD, p < 0.0001). When participants with Braak stage III–IV pathology who were not demented were compared with those with dementia, no significant differences in NFTs, senile plaques, or neuritic plaques were detected in any brain region examined despite the differences in dementia status and significant differences in performance on all categories of cognitive tests shown (Figs. 5 and 6).
Figure 3.
Neuropathologic comparison of Nun Study participants with Braak stage V pathologic changes and clinical dementia (AD group) to those without dementia (NDAP group). AD indicates Alzheimer disease; NDAP, nondemented with Alzheimer pathology; NFT, neurofibrillary tangles; NP, neuritic plaques; SP, senile plaques. Mean and SEM are shown. *p<0.05, AD versus NDAP group.
Figure 4.
Neuropsychologic profile comparison of Nun Study participants with Braak stage V pathologic changes and clinical dementia (AD group) to those without dementia (NDAP group). AD indicates Alzheimer disease; BNT, Boston naming test; CNPR, constructional praxis score; NDAP, nondemented with Alzheimer pathologic symptom; MMSE, Mini–Mental Status Examination; VRBF, verbal fluency; WRCL, word list recall; WRL, word list memory; WRCO, word recognition. Mean and SEM are shown. *p < 0.05, AD versus NDAP group.
Figure 5.
Neuropathologic comparison of Nun Study participants with Braak stage III–IV pathologic changes and clinical dementia to those without dementia. AD indicates Alzheimer disease; NDAP, nondemented with Alzheimer pathologic symptom; NFT, neurofibrillary tangles; NP, neuritic plaques; SP, senile plaques. Mean and SEM are shown. *p < 0.05, demented versus nondemented groups.
Figure 6.
Neuropsychologic profile comparison of Nun Study participants with Braak stage III–IV pathologic changes and clinical dementia to those without dementia. BNT indicates Boston naming test; CNPR, Constructional Praxis score; MMSE indicates Mini–Mental Status Examination; VRBF, verbal fluency; WRCL, word list recall; WRL, word list memory; WRCO, word recognition. Mean and SEM are shown. *p < 0.05, demented versus nondemented group.
DISCUSSION
Research participants who have been carefully studied with neuropsychologic testing during life and subsequently examined with postmortem neuropathologic assessment provide an important resource for studying the correlation of neuroanatomic changes of AD with cognition (34, 35). These correlations will be of increasing importance as reliable biomarkers are integrated into the diagnostic/prognostic process (36). Although correlation of AD symptoms with biomarkers associated with tau hyperphosphorylation and amyloid deposition is excellent in some settings, participants with markers of high–stage pathologic symptoms who are not demented, even if rare, are important to study because they may provide insights regarding brain reserve. In the future, serial sampling of biomarkers/imaging markers for amyloid plaque and NFT burden may be correlated with cognitive performance over time to define brain reserve in a more precise manner.
There are many explanations for why a small subset of individuals with histopathologic evidence of AD remains nondemented. Neurofibrillary tangles show excellent correlation with dementia status and the fact that very few individuals with high Braak stage pathologic symptoms remain nondemented supports this correlation. However, NFT formation remains an imperfect reflection of disease progression and the system used for staging the distribution of NFTs is also imperfect. Although this is an important limitation to the present study, careful study of this subset of individuals is warranted. It is unlikely that the differences in NFT and neuritic plaque loads in cortex fully explain the differences in cognition. It is always possible to find participants who are well matched with regard to the major histopathologic features of AD but who can be divided into groups with higher or lower levels of cognition. This is best illustrated by the intermediate Braak stage groups (Fig. 5). This disparity strongly suggests that there are additional environmental or genetic factors in higher-performing compared with lower-performing groups that are worthy of study.
An important caveat in studies of this NDAP group is that the comparison group with dementia should be pathologically similar with regard to histologic markers that are considered causative or at least highly relevant to the disease process, in particular the overall distribution of NFTs. Furthermore, cognition in the NDAP group, although significantly better in most respects than in an AD comparison group, is frequently not well preserved. Although a minority of subjects shows severe pathologic changes without all of the classic features of dementia, none of the participants with severe AD pathology were cognitively intact by neuropsychologic testing; most had memory impairment. These participants, however, did perform better on cognitive testing than is typical for individuals with similarly high levels of pathology.
A wide variety of markers of disease progression can be used to select a population with features suggestive of cognitive reserve. Not only Braak stage, but NFT burden, neuritic plaque load, and neuropil thread density are all relevant to disease progression. Rather than proposing a single pathologic group that is most reflective of cognitive reserve, we describe a logical method for selecting groups using highly correlated disease markers without ignoring the effect of other important histopathologic (and environmental and genetic) markers of disease progression. Study population selection will ultimately depend on the research question. For a study comparing a group that performs better than expected for a given neuritic plaque load, CERAD plaque scores are of primary interest, but Braak stage cannot be ignored. Our purpose is to provide a framework for making appropriate comparisons; study groups will need to be well matched with regard to a wide variety of established pathologic markers before examining the contribution of less well-established environmental and genetic factors to cognitive status.
The participants who we feel are most representative of a population exhibiting brain reserve are those who are not demented but have Braak stage V–VI pathology and do not have other neurologically impairing diseases (Tables 3 and 4). This NDAP group only represents 10% to 11% of nondemented Nun Study and ACT participants. It has been shown previously that, among Nun Study participants who met the neuropathologic criteria for AD, those with brain infarcts had poorer cognitive function and a higher prevalence of dementia than those without infarcts. In particular, participants with lacunar infarcts in the basal ganglia, thalamus, or deep white matter (vs those without infarcts) had an especially high prevalence of dementia (16). Information regarding other relevant clinical factors, such as treatment with anti-inflammatory or antidepressant medication, or cognitive issues related to polypharmacy, may also be important to consider, but these data were not available for assessment in this study.
Although the presence of other neurologically impairing conditions is an important consideration relevant to brain reserve in participants with pure AD pathology, eliminating participants with overlapping disease may not always be necessary. Expanding the study group to include nondemented participants with overlapping disease results in a sample of 31 of 252 Nun Study participants and 18 of 215 ACT participants (Tables, Supplemental Digital Content 1 and 2, http://links.lww.com/NEN/A267 and http://links.lww.com/NEN/A268). For NDAP versus typical AD comparisons (Table 1; Figs. 1 and 2), cases were included without regard to CERAD plaque score. Because neuritic plaques correlate with dementia status (37), it would be necessary to consider plaque load; strategies include removing cases with low CERAD scores, matching CERAD scores within the group, or adjusting statistically for the effect of cortical neuritic plaques on cognition.
For studies examining subtle differences in genetic or environmental factors and their impact on cognition, still larger group sizes may be necessary. To achieve larger group sizes, demented and nondemented participants with intermediate Braak stage pathologic symptoms can be compared. In the absence of alternative explanations for dementia, Braak stages III and IV are considered to contribute to dementia. Therefore, nondemented participants (especially those participants with high cognitive function) could be compared with demented participants within these less definitive groups. For the Nun Study and ACT Study this substantially increased group sizes to 89 of 252 and 104 of 215, respectively. The caveat is that it becomes increasingly important to match the dementia comparison group (e.g. to stratify by or statistically adjust for pathologic differences between the groups) in an appropriate manner.
Our data indicate that the NDAP group has less pathology than typical Braak stage V–VI AD cases and, most importantly, that almost all of these participants have Braak stage V rather than stage VI pathology in isocortical regions. There are differences in cognition between nondemented participants with Braak stage V pathology, even when compared with demented participants with only Braak stage V pathology (Fig. 4). In the Nun Study, participants with high-stage tangle pathologic symptoms but relatively less severe cortical neuritic plaque pathology seem to be less impaired (i.e. not demented) versus participants with combined high-stage tangle and plaque pathology, suggesting that both plaque and tangle pathology should be considered when comparing cognitive function in participants with similar pathology. The correlation of both Braak stage and neuritic plaque load with age further complicates the correlation of disease markers with dementia. A recent study suggested that the ability to predict dementia using Braak stage and CERAD plaque scores is more difficult in an older population (38). In the Nun Study, however, where all participants are 75 years or older (mean age at death, 90 years; SD, 5), both CERAD plaque scores (56% sensitivity/73% specificity) and Braak stages (57% sensitivity/89% specificity) correlate well with dementia status. CERAD and Braak grading schemes are reliable for predicting dementia (39), allowing for a pathologic definition of brain reserve, although additional factors such as age, education, and ApoE status may need to be considered when matching NDAP participants to a more typical AD group. In the Nun Study, the percentage of participants with at least one ApoE-ε4 allele in AD (45%) and NDAP (21%) groups is similar to that of Erten-Lyon et al, who reported 42% in an AD group and 17% in a comparable cognitively intact group (40). One explanation for a lack of similar findings in the ACT study is the small group sizes used in this comparison. Although there is a trend for less cognitively impaired participants to have higher brain weights, group differences within each study are not significant and comparison of Nun Study data to ACT study data is complicated by the fact that all Nun Study participants are female and brain weights were based on fresh rather than fixed tissue.
Because high Braak stage was the pathologic finding most predictive of dementia in both the Nun Study and the ACT study, we propose that relevant aspects of brain reserve are best studied in individuals showing highly diagnostic Braak categories of AD changes who have relatively less impairment than a typical AD comparison group. This group of individuals is quite small in the 2 disparate populations (12% in the Nun Study and 8% in the ACT study), suggesting that this group may also be rare in the general population. In addition, as a cross-sectional analysis, this study cannot determine whether the NDAP group is on a different (i.e. slower) slope of cognitive decline, suggesting true cognitive reserve, or simply on a different part of the same slope during which changes in cognitive decline are occurring more rapidly than the accumulation of pathologic markers. Dynamic studies would be necessary to answer this question. The NDAP cohort, however, may still be an important group to study elucidating genetic or environmental factors associated with relative retention of cognition in the face of neuroanatomic evidence of brain damage. Even a small relative preservation of cognition may be enough to allow a person to remain more independent. When comparing participants with high-stage pathologic changes who are not demented to those who are demented, it is important to consider matching the group with regard to severity of isocortical plaque and tangle involvement, age, and possibly educational level and ApoE allele status.
We propose a pathologic definition of brain reserve based only on Braak stage V–VI and CERAD B/C categories; however, nondemented individuals meeting these criteria are very rare. Therefore, to obtain a larger study group, selection criteria could be relaxed to include NDAP participants with stage IV changes who are matched to participants with similar pathology who are demented. For example, a group of subjects with stage IV–VI pathology who did not have antemortem memory impairment could be compared with a group with matched pathologic diagnosis who did have memory impairment. Such a comparison would allow the study of predictors of functional resistance to specific neuroanatomic markers of AD pathology.
Both neuritic plaques and NFTs are of utmost biologic importance with regard to dementia status and increasingly important with regard to the development and implementation of new diagnostic tools. Treatments that fail to alter pathways leading to the abnormal intracellular accumulation of tau and to the formation of neuritic plaques may have only modest effects on preservation of cognition. Consideration of both of these lesions is critical to a good pathologic definition of brain reserve. We believe our definition of an NDAP group provides a foundation for asking important questions regarding the cognitive profile of these people who are relatively protected from the devastating cognitive effects of AD-associated lesions.
Supplementary Material
Acknowledgments
This project was funded by AG06781, AG023801, AG05136, and the University of Minnesota. The authors thank Dr. David Snowdon, Founding Director of the Nun Study, who entrusted a core of researchers at the University of Minnesota to continue the study with the support of the School Sisters of Notre Dame. The authors acknowledge the late Dr. William Markesbery for his dedication to the Nun Study and for the collection of all of the neuropathologic data analyzed in the article. The authors also acknowledge Dr. Kathy Riley for directing the collection of neurobehavioral data and for interpreting categories of cognitive impairment used in the analysis and Dr. Mike Kuskowski for statistical comparisons. The authors would also like to acknowledge Dr Eric B Larson for his dedication to the Adult Changes in Thought study.
References
- 1.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 3.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: A clinicopathologic study. Arch Neurol. 2005;62:758–65. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris JC, Storandt M, McKeel DW, Jr, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–19. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 7.Davis DG, Schmitt FA, Wekstein DR, et al. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–88. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–15. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henneman WJ, Vrenken H, Barnes J, et al. Baseline CSF p-tau levels independently predict progression of hippocampal atrophy in Alzheimer disease. Neurology. 2009;73:935–40. doi: 10.1212/WNL.0b013e3181b879ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G, Sokal I, Quinn JF, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: A follow-up study. Neurology. 2007;69:631–39. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 11.Ewers M, Buerger K, Teipel SJ, et al. Multicenter assessment of CSFphosphorylated tau for the prediction of conversion of MCI. Neurology. 2007;69:2205–12. doi: 10.1212/01.wnl.0000286944.22262.ff. [DOI] [PubMed] [Google Scholar]
- 12.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–22. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–36. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. [PubMed] [Google Scholar]
- 16.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–17. [PubMed] [Google Scholar]
- 17.SantaCruz KS, Tasaki CS, Kim RC, et al. Brainstem and cortical Lewy bodies in patients presenting clinically with Alzheimer’s disease. J Alzheimers Dis. 2002;4:11–17. doi: 10.3233/jad-2002-4102. [DOI] [PubMed] [Google Scholar]
- 18.Nelson PT, Kukull WA, Frosch MP. Thinking outside the box: Alzheimertype neuropathology that does not map directly onto current consensus recommendations. J Neuropathol Exp Neurol. 2010;69:449–54. doi: 10.1097/NEN.0b013e3181d8db07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alafuzoff I, Arzberger T, Al-Sarraj S, et al. Staging of neurofibrillary pathology in Alzheimer’s disease: A study of the BrainNet Europe Consortium. Brain Pathol. 2008;18:484–96. doi: 10.1111/j.1750-3639.2008.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snowdon DA, Kemper SJ, Mortimer JA, et al. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–32. [PubMed] [Google Scholar]
- 21.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 22.Consensus Recommendations for the Postmortem Diagnosis of Alzheimer’s Disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 23.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease- associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snowdon DA. Aging and Alzheimer’s disease: Lessons from the Nun Study. Gerontologist. 1997;37:150–56. doi: 10.1093/geront/37.2.150. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 27.Chandler MJ, Lacritz LH, Hynan LS, et al. A total score for the CERAD neuropsychological battery. Neurology. 2005;65:102–6. doi: 10.1212/01.wnl.0000167607.63000.38. [DOI] [PubMed] [Google Scholar]
- 28.Kruiansky J, Gurland B. The performance test of activities of daily living. Int J Aging Hum Dev. 1976;7:343–52. doi: 10.2190/x45l-tww7-wxxy-ka6k. [DOI] [PubMed] [Google Scholar]
- 29.Potvin AR, Tourtellotte WW, Dailey JS, et al. Simulated activities of daily living examination. Arch Phys Med Rehabil. 1972;53:476–86. [PubMed] [Google Scholar]
- 30.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: Findings from the Nun Study. Ann Neurol. 2002;51:567–77. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Revised) 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 32.Riley KP, Snowdon DA, Saunders AM, et al. Cognitive function and apolipoprotein E in very old adults: Findings from the Nun Study. J Gerontol B Psychol Sci Soc Sci. 2000;55:S69–75. doi: 10.1093/geronb/55.2.s69. [DOI] [PubMed] [Google Scholar]
- 33.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol. 2002;59:1737–46. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 34.Tyas SL, Snowdon DA, Desrosiers MF, et al. Healthy ageing in the Nun Study: Definition and neuropathologic correlates. Age Ageing. 2007;36:650–55. doi: 10.1093/ageing/afm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonnen JA, Larson EB, Haneuse S, et al. Neuropathology in the adult changes in thought study: A review. J Alzheimers Dis. 2009;18:703–11. doi: 10.3233/JAD-2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 37.Bancher C, Jellinger K, Lassmann H, et al. Correlations between mental state and quantitative neuropathology in the Vienna Longitudinal Study on Dementia. Eur Arch Psychiatry Clin Neurosci. 1996;246:137–46. doi: 10.1007/BF02189115. [DOI] [PubMed] [Google Scholar]
- 38.Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 39.Geddes JW, Tekirian TL, Soultanian NS, et al. Comparison of neuropathologic criteria for the diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S99–105. doi: 10.1016/s0197-4580(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 40.Erten-Lyons D, Woltjer RL, Dodge H, et al. Factors associated with resistance to dementia despite high Alzheimer disease pathology. Neurology. 2009;72:354–60. doi: 10.1212/01.wnl.0000341273.18141.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.