Abstract
Background:
Optic neuritis (ON) can be the first presentation of multiple sclerosis (MS) or neuromyelitis optica (NMO). Anti-aquaporin-4 IgG (AQP4 IgG) is a highly specific and moderately sensitive biomarker for NMO. This study was designed to assess the rate of seropositivity for AQP4 IgG, and the short-term outcome of patients presenting with single isolated ON (SION).
Methods:
A cohort of 41 consecutive patients experiencing severe (< 20 / 200) SION (not fulfilling the diagnostic criteria for MS or NMO), was prospectively recruited. Blood sampling was carried out immediately after the diagnosis of ON, and AQP4 IgG was tested qualitatively, using an indirect immunofluorescence kit. After clinical and paraclinical investigations, all the patients were followed up for a short-term period of at least 18 months.
Results:
The seroprevalence among the initial ON patients was 9.7% (4 / 41). The short-term conversion rate to MS and NMO was estimated to be about 7.3 and 4.9%, respectively. The conversion rate to NMO in initially seropositive patients was greater than that for the whole cohort [2 / 4 (50%) vs. 2 / 41 (4.9%); P = 0.035; Odds ratio: 19.5, 95% confidence interval: 1.73 to 219.50].
Conclusion:
AQP4 IgG seropositive SION patients were more likely to develop NMO in comparison to the total SION population. Further studies, with a longer follow-up period and larger sample sizes are warranted to assess the clinical and prognostic value of assessing AQP4 IgG in SION.
Keywords: Anti aquaporin-4 IgG, Iran, Isfahan, multiple sclerosis, neuromyelitis optica, optic neuritis
INTRODUCTION
Optic neuritis (ON) is an acute or subacute inflammatory disorder of the optic nerve(s). Demyelinative ON can be due to Multiple sclerosis (MS), Neuromyelitis optica (NMO), or idiopathic.[1] In recent times, the discovery of anti-aquaporin-4 IgG (AQP4 IgG) as a highly specific, but moderately sensitive biomarker, which has also been shown to be pathogenic, has facilitated in distinguishing MS from NMO.[2–4]
Early differentiation between MS and NMO is of importance, as therapeutic options differ and standard MS therapies exacerbate NMO.[5] On the other hand, the prognosis of NMO is usually worse than MS, and early differentiation could also be of clinical importance in determining the prognosis.[6] To date there are few reports studying AQP4 IgG seropositivity in patients with clinically isolated syndrome (CIS) manifesting as a single isolated ON (SION).[7–9] This prospective study sought to assess the rate of AQP4 IgG seropositivity among patients presenting with unilateral SION, as also to study the short-term outcome of these patients in an Iranian Caucasian population.
METHODS
A cohort of 41 consecutive Iranian CIS patients, presenting with unilateral SION, was prospectively recruited in the two main hospitals of the Isfahan University of Medical Sciences (Feiz Eye Hospital and Al-Zahra Hospital) from December 2008 to August 2009. The study protocol was approved by the Institutional Ethics Board and signed informed consent was obtained from each patient before inclusion in the study.
Patients with acute or subacute vision loss in one eye with unilateral relative afferent pupillary defect and diminished color vision and visual acuity (< 20 / 200) as measured by Ishihara color plates and Snellen chart were included. After the clinical and radiological evaluation patients fulfilling the diagnostic criteria for MS or NMO were excluded.[10,11] We also excluded patients who had any previous neurological event attributable to a demyelinating disease, patients with retinal or macular pathology, and those with accompanying diseases that could cause ON.
The first brain and spinal magnetic resonance imaging (MRI) and blood draw was carried out within a maximum of a week after the diagnosis of ON. Only patients with normal baseline brain and spinal MRI were included. The clinical and demographic data of patients were recorded during the visits by a neurologist and an ophthalmologist. The patients were followed for a short period (at least 18 months), with bimonthly follow-up visits by a neurologist for the onset of new symptoms / signs, to see if they eventually fulfilled the diagnostic criteria for MS or NMO.[10,11] Patients were advised to visit their neurologist when they experienced new symptoms in between the visits.
Brain and spinal MRIs was also performed at month 18 or when a new episode of neurological symptom lasting for at least 24 hours occurred. AQP4–IgG was tested qualitatively and before intravenous corticosteroid therapy, using a commercially available indirect immunofluorescence kit (Euroimmun, Luebeck, Germany).
Data were entered into PASW-v.18.00 and MedCalc-v.10 for further analysis. Results have been reported as a mean ± 1SD. The Fisher's exact test was used to compare the conversion rate to NMO between the total cohort and those who were initially seropositive. The same analysis was employed comparing the conversion rate to MS between the total cohort and those who were initially seronegative. All tests were two-tailed and a P value of < 0.05 was regarded as statistically significant.
RESULTS
Forty-one patients (33 women and 8 men) with a mean age of 30.12 ± 8.36 (Range: 18 – 49) years were studied. ON was right-sided in 19 and left-sided in 22 patients. None of the patients had any evidence of sarcoidosis, vasculitis, clinically manifest systemic lupus erythematosus, or Sjogren's syndrome, or other diseases causing ON. The patients were followed for a mean duration of 19.10 ± 0.75 months (Range: 18 – 21 months).
At the end of the follow-up period, the patients were classified into the following diagnostic categories: (i) SION: Seronegative patients without any other relapses and not fulfilling the diagnostic criteria for MS or NMO; (ii) NMO spectrum disorders: Seropositive patients without any other relapses and not fulfilling the diagnostic criteria for MS or NMO;[12] (iii) Patients who converted to MS; (iv) Patients who converted to NMO; (v) Recurrent isolated ON (RION): Patients who experienced isolated recurrence of ON not fulfilling the diagnostic criteria for MS or NMO; (vi) CIS: Patients who experienced further relapses (other than ON), but did not fulfill the diagnostic criteria for MS or NMO. Table 1 summarizes the clinical and demographic findings in our patients.
Table 1.
AQP4 IgG seroprevalence among different subgroups of patients
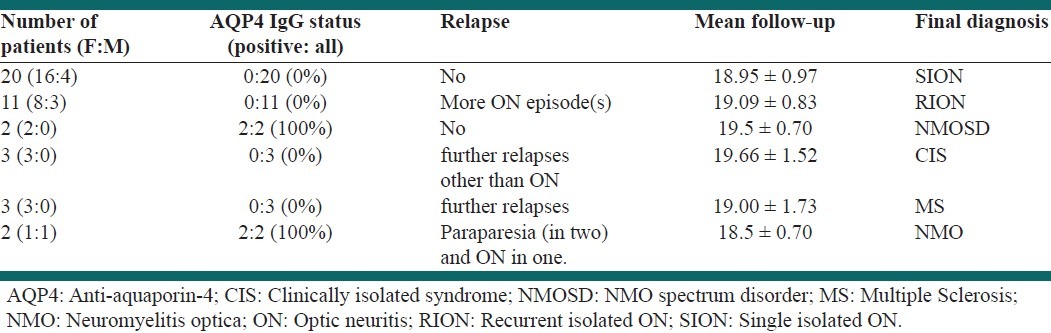
Among the RION patients, eight patients had a single relapse and three had two. Two CIS patients experienced a single episode of paresthesia and one developed an episode of diplopia. Among MS patients one experienced relapses of ON and hemiparesis, one had paraparesis and the last one developed paresthesia.
After the end of the study, the MRI studies of the brain and spinal cord were normal in all RION patients, except one female patient who had a single callosal lesion. Among patients with a diagnosis of SION, only one had a brain stem lesion and two others had cervical and lumbar lesions with a length of < 3 vertebrae segments. The two NMO patients had normal brain MRI and developed a longitudinally extensive spinal cord lesion (LESCL) extending over three vertebrae. However, one of them experienced another episode of ON prior to the occurrence of LESCL. Among patients with NMO spectrum disorder, except one female patient, who had a lesion extending over two cervical vertebrae, the other scans were normal. MS patients had multiple periventricular lesions and juxtacortical lesions at the second clinical episode. Among patients classified as CIS, one had normal scans, one had a brainstem lesion, and the other had only small periventricular lesions.
Four patients were initially seropositive for AQP4-IgG, thus, the seroprevalence among the initial SION cohort was 9.7% (4/41). Among the 22 cases who did not have any further neurological relapses (NMO spectrum disorder or SION) and did not fulfill the diagnostic criteria for MS or NMO (mean follow-up of 19 ± 0.83), AQP4 IgG was positive in two, giving a seropositivity rate of 9.1%.
The conversion rate to MS, among the total cohort of unilateral SION patients (3/41, 7.3%) and those patients who were initially seronegative for AQP4 IgG (3/37, 8.1%) was not significantly different (p-value = 0.61). The conversion rate to NMO was significantly higher among the AQP4 IgG-positive patients than the total cohort [2/4 (50%) vs. 2/41 (4.9%); P = 0.035; Odds ratio: 19.5, 95% confidence interval: 1.73 to 219.50] denoting an increased conversion rate to NMO among the seropositive patients.
DISCUSSION
In our series, during a short follow-up, the conversion rate to MS and NMO was estimated to be 7.3 and 4.9%, respectively, in a SION cohort, which is comparable with another report from the Mayo Clinic, in which the one-year conversion rate to MS and NMO was estimated to be about 2.8 and 5.6%, respectively.[13]
In our cohort, the rate of seropositivity (9.7%, 4/41) in patients experiencing their first demyelinating event as ON, was similar to the previous studies, reporting a seropositivity rate of 5–10%.[7–9] In line with the previous observations, our results have shown a higher conversion rate among those positive for AQP4 IgG. Identification of patients at higher risk of developing NMO is of clinical importance, as early diagnosis and early initiation of treatment may benefit these patients.[14] Further studies are warranted to assess the rationale safety, and efficacy of early treatment in AQP4 IgG-positive SION patients, who are at a high risk of developing NMO.
Different studies have found that 10–20% of RION patients have tested positive for AQP4 IgG.[8,9] However, in our series none of those patients who were categorized as RION at the end of the follow-up period were positive for AQP4 IgG. These differences might be due to different patient populations, different assays, or different inclusion criteria. In this study none of patients who converted to MS were positive for AQP4 IgG, which was in line with the previous observations.[7,8]
To sum up, the conversion rate to NMO was increased in initially seropositive ON patients, hence, it could be concluded that seropositive SION / CIS patients were more likely to develop NMO. Further follow-up of this CIS cohort is needed to better clarify the value of an AQP4 IgG test in evaluating the long-term outcome of initially seronegative and seropositive patients. More prospective studies are warranted, to examine the value of AQP4 IgG in the prediction of the clinical outcome of CIS.
ACKNOWLEDGMENT
We are very grateful to all the individuals who made this project possible, for their patience, and for their valuable contribution. We particularly thank Dr. Peyman Roomizadeh for the technical and scientific assistance throughout the process of this project. Results of this study are dedicated to Dr. Afsane Khandan (Internist) who devoted her precious life to health development and medical research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Hickman SJ, Dalton CM, Miller DH, Plant GT. Management of acute optic neuritis. Lancet. 2002;360:1953–62. doi: 10.1016/s0140-6736(02)11919-2. [DOI] [PubMed] [Google Scholar]
- 2.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet. 2004;364:2106–12. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 3.Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–31. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–43. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu J, Hatanaka Y, Hasegawa M, Iwata A, Sugimoto I, Date H, et al. IFNβ-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology. 2010;75:1423–7. doi: 10.1212/WNL.0b013e3181f8832e. [DOI] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–14. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 7.Jarius S, Frederikson J, Waters P, Paul F, Akman-Demir G, Marignier R, et al. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. J Neurol Sci. 2010;298:158–62. doi: 10.1016/j.jns.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Petzold A, Pittock SJ, Lennon VA, Maggiore C, Weinshenker BG, Plant GT. Neuromyelitis optica-IgG (aquaporin-4) autoantibodies in immune mediated optic neuritis. J Neurol Neurosurg Psychiatry. 2010;81:109–11. doi: 10.1136/jnnp.2008.146894. [DOI] [PubMed] [Google Scholar]
- 9.Matiello M, Lennon VA, Jacob A, Pittock SJ, Lucchinetti CF, Wingerchuk DM, et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology. 2008;70:2197–200. doi: 10.1212/01.wnl.0000303817.82134.da. [DOI] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 11.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 12.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 13.Pirko I, Blauwet Lk, Lesnick TG, Weinshenker BG. The Natural History of Recurrent Optic Neuritis. Arch Neurol. 2004;61:1401–5. doi: 10.1001/archneur.61.9.1401. [DOI] [PubMed] [Google Scholar]
- 14.Etemadifar M, Abtahi SH, Dehghani A, Abtahi MA, Akbari M, Tabrizi N, et al. Myasthenia Gravis during the Course of Neuromyelitis Optica. Case Rep Neurol. 2011;3:268–73. doi: 10.1159/000334128. [DOI] [PMC free article] [PubMed] [Google Scholar]


