Abstract
Objectives:
Cisplatin (CP) is used as the commonest drug to treat solid tumors. It is accompanied by a nephrotoxicity side effect. The main objective of this study is to investigate the protective role of magnesium (Mg) supplementation in CP-induced nephrotoxicity in a rat model.
Methods:
Twenty-nine Wistar rats were randomly assigned to four groups (1–4). Groups 1–3 received 20, 80, and 200 mg/kg magnesium sulfate respectively, for 10 days, but on day 3, a single dose of CP (7 mg/kg, i.p.) was also injected. Group 4 (positive control group) received the same regimen of Groups 1–3 except saline instead magnesium sulfate. One week after CP administration, blood samples were obtained and all animals were killed for kidney histopathological investigations.
Results:
All CP-treated animals lost weight, and the percentage of weight loss in Group 1 (low dose Mg sulfate treated) was significantly higher compared with the positive control group (Group 4, P < 0.05). The increase in blood urea nitrogen (BUN) and creatinine (Cr) levels in serum in Group 1 were more than those in other groups (P < 0.05). No statistical differences were observed in serum magnesium, nitrite, and total protein levels among the groups. The kidney tissue damage in Groups 1–3 was not significantly different when compared with Group 4. Moreover, the kidney and testis weights in Group 1 were significantly greater than those in the positive control group (P < 0.05).
Conclusion:
Regarding the BUN and Cr levels in the serum, kidneys weight, and the histopathological study, the low dose of Mg supplementation intensifies kidney toxicity and renal dysfunction in CP-induced nephrotoxicity in the rat model. However, the protective role of Mg with moderate and high doses is not certain.
Keywords: Cisplatin, magnesium, nephrotoxicity, rat
INTRODUCTION
Cisplatin (CP) is recognized as an antitumor drug,[1,2] which is widely used in oncology clinics. While nephrotoxicity is the commonest side effect of CP in clinical and experimental models,[3–8] this drug has become the target of many research studies to determine or to compare its advantages in cancer therapy.[9–15] Nephrotoxicity is manifested by increased serum levels of blood urea nitrogen (BUN) and creatinine (Cr), and various histological aspects of renal tissue. CP-induced nephrotoxicity disturbs tubular reabsorption of magnesium (Mg), and depletion of Mg enhances nephrotoxicity.[16–19] The prevention of CP-induced nephrotoxicity in patients remains a great challenge, because there are no specific nephroprotective therapies.
Although Mg therapy is associated with gastrointestinal (GI) symptoms,[19,20] Mg supplementation is suggested in patients to prevent the induced hypomagnesemia after CP administration.[18,19,21] The other reports indicated that CP-induced hypomagnesemia is not related to the total dose of CP,[22] and in animal models, hypomagnesemia develops from the third week after CP administration.[23] On the other hand, an increase in the serum Mg level after supplemental treatment is not expected.[24] Therefore, it can be summarized that CP-induced hypomagnesemia is controversial, although hypomagnesemia occurs only in 50% of patients treated with CP, and low magnesium diet in a rat enhanced CP-induced nephrotoxicity.[16] However, to prevent the CP-induced hypomagnesemia, Mg supplementation is suggested.[18,19] However, it is not clear completely whether Mg supplementation attenuates CP-induced nephrotoxicity. Hence, to find the nephroprotectant role of Mg supplementation against CP, this study was designed and three different doses of Mg supplementation in CP-induced nephrotoxicity in the rat model was studied.
METHODS
Animals
Twenty-nine adult male (181.8 ± 4.8 g) Wistar rats (Animal Centre, Jundishapur University of Medical Sciences, Ahvaz, Iran) were used. The rats were housed at a temperature of 23–25°C. Rats had free access to water and rat chow. The experimental procedures were approved in advance by the Ethics Committee of Isfahan University of Medical Sciences.
Experimental protocol
The animals were randomly assigned to the four groups of experiment. Groups 1–3, respectively, received 20, 80, and 200 mg/kg i.p. magnesium sulfate for 10 days. Moreover, at day 3, a single dose of CP (7 mg/kg body wt, i.p.) was injected. CP (cis-diammineplatinum(II) dichloride, code P4394) was purchased from Sigma (Germany). Group 4 (positive control) received the same regimen except saline instead of magnesium sulfate. All animals were killed at day 10 (1 week after CP administration). Blood samples were obtained from each animal before and 1 week after the CP injection. Serum was collected and stored at -20 °C until measurement. The animals’ body weight was recorded daily. At the end of experiment, the kidneys and testes were removed and weighed rapidly, and then histopathological studies were applied on left kidney.
Measurement
The percentage (%) weight change was calculated using the following formula:
% weight change = (weight at the first day of CP injection – weight at one week after CP injection) *100/ weight at the first day of CP injection.
The levels of serum creatinine (Cr), blood urea nitrogen (BUN), total protein (TP), and Mg were determined using quantitative diagnostic kits (Pars Azmoon, Iran). The serum level of nitrite (stable NO metabolite) was measured using a colorimetric assay kit (Promega Corporation, USA) that involves the Griess reaction. Briefly, after adding sulfanilamide solution and incubation, N-1-naphtyl ethylenediamine dihydrochloride solution was added. Then, absorbance was measured at the wavelength of 540 nm. The nitrite concentration of samples was determined from the nitrite standard reference curve.
Histopathological procedures
The removed kidney was fixed in 10% neutral formalin solution, embedded in paraffin for histopathological staining. The samples were stained by hematoxylin and eosin, and then were evaluated for tubular damage by two independent nephropathologists. This study was totally blind for the pathologists. The presence of hyaline cast, debris, vacuolization, flattening of tubular cells, degeneration of tubular cells, and dilatation of tubular lumen of kidney tissue were considered. On the basis of the intensity of tubular lesions as mentioned above, the lesion intensity was scored from 1 to 4; while the score zero was assigned to normal tubules without damage.
Statistical analysis
Data are expressed as mean ± SEM. One-way ANOVA was applied to compare the parameters between the groups. Due to the qualitative nature of scoring, Kruskal–Wallis tests were applied to compare the pathology damage score between the groups. We also used the Spearman test to determine the correlation between the pathological damage score and the kidney or testis weight. Values of P < 0.05 were considered statistically significant.
RESULTS
Effect of CP on serum BUN and Cr levels
Before CP administration, the levels of BUN and Cr were within the normal range values with no significant differences between the groups (BUN: 22.9 ± 2.7, 21.1 ± 1.0, 18.1 ± 2.6, 19.9 ± 1.5, and Cr: 0.64 ± 0.10, 0.81 ± 0.14, 0.80 ± 0.13, and 1.0 ± 0.23 mg/dl for Groups 1–4, respectively). The CP-induced nephrotoxicity was approved by increasing of BUN and Cr concentrations at the end of the experiment. However, the BUN and Cr levels in Group 1 (low dose of Mg supplementation) were significantly higher than those in other groups (P < 0.05, Figure 1). Furthermore, Groups 2, 3, and 4 were not statistically different with regard to BUN and Cr levels. These results indicate that coadministration of low doses of Mg and CP increased the levels of BUN and Cr when compared with isolated CP administration.
Figure 1.
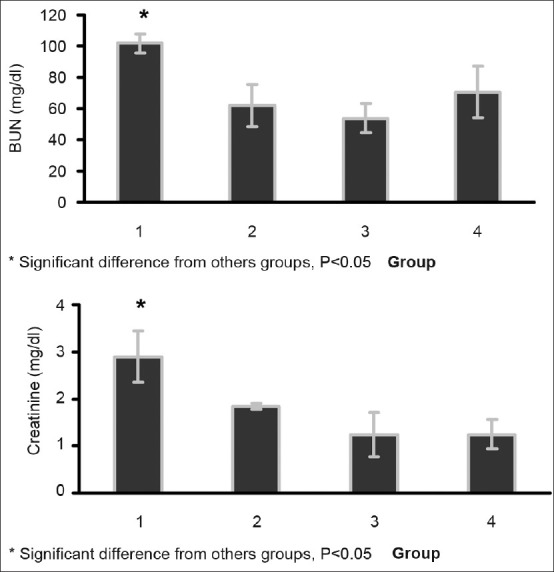
Serum levels of BUN (above) and Cr (below) in the four experimental groups 1 week after CP administration. Significant difference was observed between Group 1 and others groups (P< 0.05). Groups 1–4, respectively, received 20, 80, 200 mg/kg magnesium sulfate, and saline for 10 days plus a single dose of CP (7 mg/kg, i.p.) in day 3. One-way ANOVA was applied to compare the parameters between the groups
Effect of CP on Mg, TP, and nitrite levels
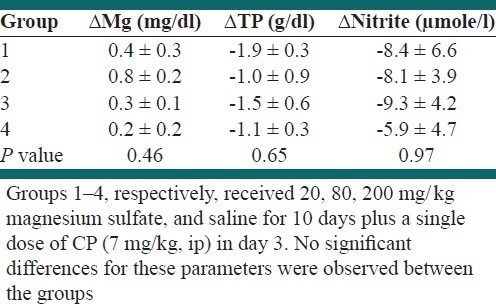
CP may disturb protein excretion or Mg concentration, or may lead to endothelial dysfunction. To investigate the effect of Mg supplementation on serum levels of Mg, TP, and nitrite, the serum concentration difference (after - before, Δ) of these parameters were determined [Table 1]. An increasing in Mg level and a decrease in TP and nitrite levels were observed, but no significant difference was detected between the groups.
Table 1.
The serum concentration difference (after - before, Δ) of Mg, TP, and nitrite levels
Effect of CP on body, kidney, and testis weight
Before CP administration, the animals’ weight was recorded. No significant differences were detected between the groups (weight: 184.5 ± 10.5, 175.1 ± 7.4, 182.5 ± 12.8, 185.4 ± 10.5 g for Groups 1–4, respectively). After the experiment, the percentage of weight loss in Group 1 treated with low dose of magnesium sulfate was significantly greater than that in the positive control group (P < 0.05, Figure 2). However, the percentage of weight loss in other groups was not significantly different. Similarly, the normalized kidney and testis weights (tissue weight per 100 g of body weight) of group 1 were significantly different from those in the positive control group (P < 0.05, Figure 2).
Figure 2.
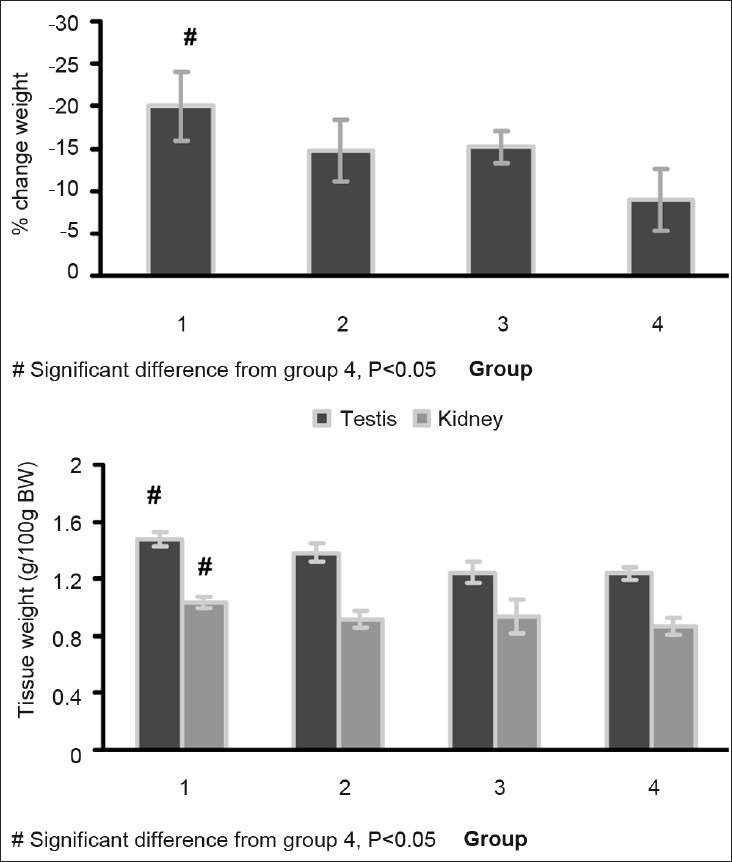
The percentage weight change, total kidney weight/100 g of body weight, and total testis weight/100 g of body weight of animals in the four groups 1 week after CP administration. One-way ANOVA was applied to compare groups with regard to the parameters. Significant difference was observed between Groups 1 and 4 (P< 0.05). Groups 1–4, respectively, received 20, 80, 200 mg/kg magnesium sulfate, and saline for 10 days plus a single dose of CP (7 mg/kg, i.p.) in day 3
The kidney damage induced by CP was evaluated and scored (pathology damage score: 1.7 ± 0.3, 1.4 ± 0.3, 1.3 ± 0.3, 1.8 ± 0.4 for Groups 1–4, respectively). No significant difference was detected between Mg-treated and positive control groups. This pathological data indicated no preventive effect for Mg against CP-induced nephrotoxicity [Figure 3]. A statistically significant correlation between the normalized kidney or testis weights and the pathology damage score was detected (kidney, r = 0.534, P < 0.05; testis, r = 0.335, P < 0.05).
Figure 3.
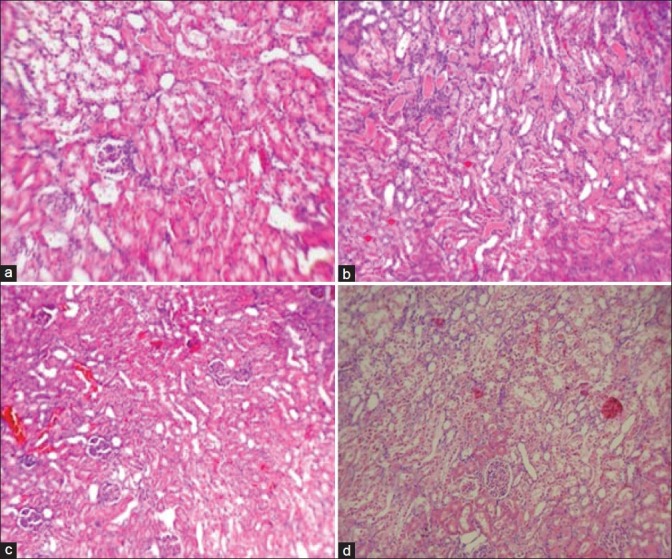
The images (100×) of kidney tissue. (a) Group 1; low dose of magnesium + cisplatin, (b) Group 2; moderate dose of magnesium + cisplatin, (c) Group 3; high dose of magnesium + cisplatin, (d) Group 4; saline + cisplatin. Although the higher scores of damage were obtained in Groups 1 (a) and 4 (d), but no significant differences were detected between the groups
DISCUSSION
The main objective of this study was to determine the role of Mg supplementation in CP-induced nephrotoxicity. CP caused weight loss, which may be related to GI disturbance. This finding is in accordance with those reported in previous studies.[25,26] Mg itself affects the GI system, and Mg therapy is associated with GI disturbance symptoms.[19,20,27–29] Similarly, weight loss in our model may be related to both CP and Mg supplementation.
The groups were not significantly different in concentration differences (Δ) of Mg, TP, and nitrite. Mg depletion is a side effect of CP.[16] In the rat model, this may occur 2 weeks after CP administration.[23] We did not observed Mg depletion in the positive control group because of the duration of our study (1 week). The serum ΔMg was not different between the Mg supplementation groups and the positive control group. This is in agreement with the findings of other study, in which an increase in the Mg serum level after supplementation treatment is not expected.[24] NO is generally involved in CP-induced nephrotoxicity,[30] and NO blockade aggravates the toxicity induced.[31] In contrast to this finding, Wink et al. found that NO may enhance the CP toxicity.[32] It seems that it is not easy to find the relationship between CP and NO.[33] There is also a relationship between Mg and NO; such that Mg deficiency can result in a rise in plasma NO in rats.[34] In our study, CP decreased the serum levels of NO in all groups and no significant difference between groups was seen. This reduction in NO level may be related to endothelial damage induced by CP,[35] and Mg supplementation could not improve this disturbance.
Now, one major question is whether Mg supplementation is protective against CP-induced nephrotoxicity or not? The levels of BUN and Cr, tissue damage scores, testis and kidney weights, as well as the significant correlation between kidney or testis weight and kidney damage score all together indicate that intensity of kidney toxicity in group 1 (low dose of Mg) is higher than those in other groups. At this time, we cannot interpret any documented mechanism for this finding, but the human organic cation transporter 2 (OCT2) is responsible for the uptake of organic cations across the basolateral membrane in kidneys.[36] Moreover, OCT2 is Mg-dependent and hypomagnesemia causes upregulation of OCT2, which increases the accumulation of CP in the kidney.[37,38] Therefore, low dose of Mg may act as a stimulator to promote CP-induced nephrotoxicity. Moderate and high doses of Mg supplementation did not provide a significantly better result when compared with the positive control group, although it may not be confirmed by various clinical and experimental data.[16,39–44] In addition, the high dose of Mg supplementation used in this study may be toxic.[45] Nevertheless, our data (serum levels of BUN and Cr, pathological data, and weight loss) did not confirm such toxicity. Therefore, it could be concluded that Mg supplementation is not nephroprotective against CP-induced nephrotoxicity. However, under some conditions, supplementation may promote kidney toxicity, and therefore further studies are required to determine the exact mechanisms.
ACKNOWLEDGMENTS
This study was supported by Isfahan University of Medical Sciences (grant # 290021).
Footnotes
Source of Support: Isfahan University of Medical Sciences (grant # 290021)
Conflict of Interest: None declared
REFERENCES
- 1.Lee YC, Saijo N, Sasaki Y, Takahashi H, Sakurai M, Ishihara J, et al. Antitumor effect of two-drug simultaneous or sequential use of cisplatin, vindesine or etoposide on human pulmonary adenocarcinoma cell lines in tumor clonogenic assay. Jpn J Cancer Res. 1986;77:312–8. [PubMed] [Google Scholar]
- 2.Pinto AL, Lippard SJ. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta. 1985;780:167–80. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- 3.Shord SS, Thompson DM, Krempl GA, Hanigan MH. Effect of concurrent medications on cisplatin-induced nephrotoxicity in patients with head and neck cancer. Anticancer Drugs. 2006;17:207–15. doi: 10.1097/00001813-200602000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Erdlenbruch B, Nier M, Kern W, Hiddemann W, Pekrun A, Lakomek M. Pharmacokinetics of cisplatin and relation to nephrotoxicity in paediatric patients. Eur J Clin Pharmacol. 2001;57:393–402. doi: 10.1007/s002280100319. [DOI] [PubMed] [Google Scholar]
- 5.Nagai N, Kinoshita M, Ogata H, Tsujino D, Wada Y, Someya K, et al. Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol. 1996;39:131–7. doi: 10.1007/s002800050548. [DOI] [PubMed] [Google Scholar]
- 6.Donadio C, Lucchesi A, Gadducci A. Minimization of cisplatin nephrotoxicity in high-risk patients. Nephron. 1996;73:325–6. doi: 10.1159/000189066. [DOI] [PubMed] [Google Scholar]
- 7.Santos NA, Catao CS, Martins NM, Curti C, Bianchi ML, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 8.Sayed-Ahmed MM, Eissa MA, Kenawy SA, Mostafa N, Calvani M, Osman AM. Progression of cisplatin-induced nephrotoxicity in a carnitine-depleted rat model. Chemotherapy. 2004;50:162–70. doi: 10.1159/000080689. [DOI] [PubMed] [Google Scholar]
- 9.Gupta V, Singh SM. Sex dimorphism in antitumor response of chemotherapeutic drug cisplatin in a murine host-bearing a T-cell lymphoma. Anticancer Drugs. 2008;19:583–92. doi: 10.1097/CAD.0b013e3282fb97bf. [DOI] [PubMed] [Google Scholar]
- 10.Babu DM, Satish CS, Shah AP, Shivakumar HG. Preparation and characterization of poly(2-hydroxyethyl methacrylateco- methyl methacrylate) hydrogels for sustained delivery of antitumor drug. Curr Drug Deliv. 2011 [In press] [PubMed] [Google Scholar]
- 11.Verma AK, Sachin K. Novel hydrophilic drug polymer nano-conjugates of Cisplatin showing long blood retention profile: Its release kinetics, cellular uptake and bio-distribution. Curr Drug Deliv. 2008;5:120–6. doi: 10.2174/156720108783954806. [DOI] [PubMed] [Google Scholar]
- 12.Taratula O, Garbuzenko O, Savla R, Wang YA, He H, Minko T. Multifunctional nanomedicine platform for cancer specific delivery of siRNA by superparamagnetic iron oxide nanoparticles-dendrimer complexes. Curr Drug Deliv. 2011;8:59–69. doi: 10.2174/156720111793663642. [DOI] [PubMed] [Google Scholar]
- 13.Fuertes MA, Alonso C, Perez JM. Biochemical modulation of Cisplatin mechanisms of action: Enhancement of antitumor activity and circumvention of drug resistance. Chem Rev. 2003;103:645–62. doi: 10.1021/cr020010d. [DOI] [PubMed] [Google Scholar]
- 14.N’Soukpoe-Kossi CN, Descoteaux C, Asselin E, Tajmir-Riahi HA, Berube G. DNA interaction with novel antitumor estradiol-platinum(II) hybrid molecule: A comparative study with cisplatin drug. DNA Cell Biol. 2008;27:101–7. doi: 10.1089/dna.2007.0669. [DOI] [PubMed] [Google Scholar]
- 15.Tardi PG, Dos Santos N, Harasym TO, Johnstone SA, Zisman N, Tsang AW, et al. Drug ratio-dependent antitumor activity of irinotecan and cisplatin combinations in vitro and in vivo. Mol Cancer Ther. 2009;8:2266–75. doi: 10.1158/1535-7163.MCT-09-0243. [DOI] [PubMed] [Google Scholar]
- 16.Lajer H, Kristensen M, Hansen HH, Nielsen S, Frokiaer J, Ostergaard LF, et al. Magnesium depletion enhances cisplatin-induced nephrotoxicity. Cancer Chemother Pharmacol. 2005;56:535–42. doi: 10.1007/s00280-005-1010-7. [DOI] [PubMed] [Google Scholar]
- 17.Goren MP. Cisplatin nephrotoxicity affects magnesium and calcium metabolism. Med Pediatr Oncol. 2003;41:186–9. doi: 10.1002/mpo.10335. [DOI] [PubMed] [Google Scholar]
- 18.Hodgkinson E, Neville-Webbe HL, Coleman RE. Magnesium depletion in patients receiving cisplatin-based chemotherapy. Clin Oncol (R Coll Radiol) 2006;8:710–8. doi: 10.1016/j.clon.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Lajer H, Kristensen M, Hansen HH, Christensen S, Jonassen T, Daugaard G. Magnesium and potassium homeostasis during cisplatin treatment. Cancer Chemother Pharmacol. 2005;55:231–6. doi: 10.1007/s00280-004-0899-6. [DOI] [PubMed] [Google Scholar]
- 20.Martin M, Diaz-Rubio E, Casado A, Lopez Vega JM, Sastre J, Almenarez J. Intravenous and oral magnesium supplementations in the prophylaxis of cisplatin-induced hypomagnesemia.Results of a controlled trial. Am J Clin Oncol. 1992;15:348–51. doi: 10.1097/00000421-199208000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Bodnar L, Wcislo G, Gasowska-Bodnar A, Synowiec A, Szarlej-Wcislo K, Szczylik C. Renal protection with magnesium subcarbonate and magnesium sulphate in patients with epithelial ovarian cancer after cisplatin and paclitaxel chemotherapy: A randomised phase II study. Eur J Cancer. 2008;44:2608–14. doi: 10.1016/j.ejca.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Ariceta G, Rodriguez-Soriano J, Vallo A, Navajas A. Acute and chronic effects of cisplatin therapy on renal magnesium homeostasis. Med Pediatr Oncol. 1997;28:35–40. doi: 10.1002/(sici)1096-911x(199701)28:1<35::aid-mpo7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Mavichak V, Wong NL, Quamme GA, Magil AB, Sutton RA, Dirks JH. Studies on the pathogenesis of cisplatin-induced hypomagnesemia in rats. Kidney Int. 1985;28:914–21. doi: 10.1038/ki.1985.217. [DOI] [PubMed] [Google Scholar]
- 24.Virag V, May Z, Kocsis I, Blazovics A, Szentmihalyi K. Effects of magnesium supplementation on calcium and magnesium levels, and redox homeostasis in normolipidemic and food-induced hyperlipidemic rats. Orv Hetil. 2011;152:1075–81. doi: 10.1556/OH.2011.29152. [DOI] [PubMed] [Google Scholar]
- 25.Ammer U, Natochin Y, David C, Rumrich G, Ullrich KJ. Cisplatin nephrotoxicity: Site of functional disturbance and correlation to loss of body weight. Ren Physiol Biochem. 1993;16:131–45. doi: 10.1159/000173759. [DOI] [PubMed] [Google Scholar]
- 26.Ohno T, Kato S, Wakatsuki M, Noda SE, Murakami C, Nakamura M, et al. Incidence and temporal pattern of anorexia, diarrhea, weight loss, and leukopenia in patients with cervical cancer treated with concurrent radiation therapy and weekly cisplatin: Comparison with radiation therapy alone. Gynecol Oncol. 2006;103:94–9. doi: 10.1016/j.ygyno.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Ikarashi N, Ushiki T, Mochizuki T, Toda T, Kudo T, Baba K, et al. Effects of magnesium sulphate administration on aquaporin 3 in rat gastrointestinal tract. Biol Pharm Bull. 2011;34:238–42. doi: 10.1248/bpb.34.238. [DOI] [PubMed] [Google Scholar]
- 28.Hemmings FJ, Edbury SM, Davie RJ, Birch NJ. Magnesium and lithium antagonism of carbachol- and electrically-induced smooth muscle contractions in the rat gastrointestinal tract. Magnes Res. 1994;7:179–86. [PubMed] [Google Scholar]
- 29.Hemmings FJ, Rai J, Padgham C, Birch NJ. Magnesium inhibition of carbachol-induced contractions of the rat uterus and gastrointestinal tract: Ex vivo effect of long term streptozotocin-induced diabetes. Magnes Res. 1993;6:343–8. [PubMed] [Google Scholar]
- 30.Srivastava RC, Farookh A, Ahmad N, Misra M, Hasan SK, Husain MM. Evidence for the involvement of nitric oxide in cisplatin-induced toxicity in rats. Biometals. 1996;9:139–42. doi: 10.1007/BF00144618. [DOI] [PubMed] [Google Scholar]
- 31.Saleh S, El-Demerdash E. Protective effects of L-arginine against cisplatin-induced renal oxidative stress and toxicity: Role of nitric oxide. Basic Clin Pharmacol Toxicol. 2005;97:91–7. doi: 10.1111/j.1742-7843.2005.pto_114.x. [DOI] [PubMed] [Google Scholar]
- 32.Wink DA, Cook JA, Christodoulou D, Krishna MC, Pacelli R, Kim S, et al. Nitric oxide and some nitric oxide donor compounds enhance the cytotoxicity of cisplatin. Nitric Oxide. 1997;1:88–94. doi: 10.1006/niox.1996.0108. [DOI] [PubMed] [Google Scholar]
- 33.Turchi JJ. Nitric oxide and cisplatin resistance: NO easy answers. Proc Natl Acad Sci U S A. 2006;103:4337–8. doi: 10.1073/pnas.0601001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rock E, Astier C, Lab C, Malpuech C, Nowacki W, Gueux E, et al. Magnesium deficiency in rats induces a rise in plasma nitric oxide. Magnes Res. 1995;8:237–42. [PubMed] [Google Scholar]
- 35.Kohn S, Fradis M, Podoshin L, Ben-David J, Zidan J, Robinson E. Endothelial injury of capillaries in the stria vascularis of guinea pigs treated with cisplatin and gentamicin. Ultrastruct Pathol. 1997;21:289–99. doi: 10.3109/01913129709021925. [DOI] [PubMed] [Google Scholar]
- 36.Fujita T, Urban TJ, Leabman MK, Fujita K, Giacomini KM. Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. J Pharm Sci. 2006;95:25–36. doi: 10.1002/jps.20536. [DOI] [PubMed] [Google Scholar]
- 37.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoo K, Murakami R, Matsuzaki T, Yoshitome K, Hamada A, Saito H. Enhanced renal accumulation of cisplatin via renal organic cation transporter deteriorates acute kidney injury in hypomagnesemic rats. Clin Exp Nephrol. 2009;13:578–84. doi: 10.1007/s10157-009-0215-1. [DOI] [PubMed] [Google Scholar]
- 39.Yuan J, Zhou J, Chen BC, Zhang X, Zhou HM, Du DF, et al. Magnesium supplementation prevents chronic cyclosporine nephrotoxicity via adjusting nitric oxide synthase activity. Transplant Proc. 2005;37:1892–5. doi: 10.1016/j.transproceed.2005.02.098. [DOI] [PubMed] [Google Scholar]
- 40.Holzmacher R, Kendziorski C, Michael Hofman R, Jaffery J, Becker B, Djamali A. Low serum magnesium is associated with decreased graft survival in patients with chronic cyclosporin nephrotoxicity. Nephrol Dial Transplant. 2005;20:1456–62. doi: 10.1093/ndt/gfh831. [DOI] [PubMed] [Google Scholar]
- 41.Asai T, Nakatani T, Yamanaka S, Tamada S, Kishimoto T, Tashiro K, et al. Magnesium supplementation prevents experimental chronic cyclosporine a nephrotoxicity via renin-angiotensin system independent mechanism. Transplantation. 2002;74:784–91. doi: 10.1097/00007890-200209270-00009. [DOI] [PubMed] [Google Scholar]
- 42.Pere AK, Lindgren L, Tuomainen P, Krogerus L, Rauhala P, Laakso J, et al. Dietary potassium and magnesium supplementation in cyclosporine-induced hypertension and nephrotoxicity. Kidney Int. 2000;58:2462–72. doi: 10.1046/j.1523-1755.2000.00429.x. [DOI] [PubMed] [Google Scholar]
- 43.Mervaala EM, Pere AK, Lindgren L, Laakso J, Teravainen TL, Karjala K, et al. Effects of dietary sodium and magnesium on cyclosporin A-induced hypertension and nephrotoxicity in spontaneously hypertensive rats. Hypertension. 1997;29:822–7. doi: 10.1161/01.hyp.29.3.822. [DOI] [PubMed] [Google Scholar]
- 44.Rankin LI, Krous H, Fryer AW, Whang R. Enhancement of gentamicin nephrotoxicity by magnesium depletion in the rat. Miner Electrolyte Metab. 1984;10:199–203. [PubMed] [Google Scholar]
- 45.Mochizuki M, Akagi K, Inoue K, Shimamura K. A single dose toxicity study of magnesium sulfate in rats and dogs. J Toxicol Sci. 1998;23(Suppl 1):31–5. doi: 10.2131/jts.23.supplementi_31. [DOI] [PubMed] [Google Scholar]


