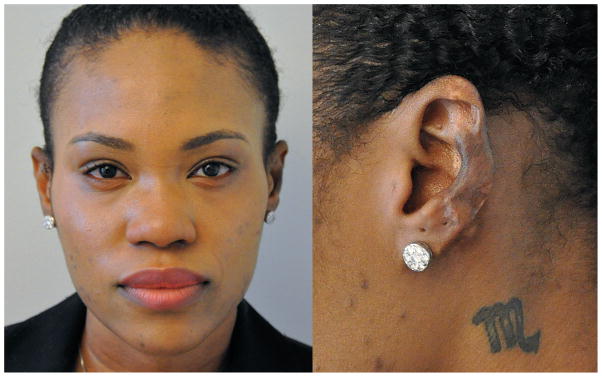Abstract
Keloids present a formidable clinical challenge. Surgical excision in conjunction with radiation therapy may decrease the chance of keloid recurrence. Split-thickness skin grafts, however, are more prone to failure in the setting of radiation. In this report, we present a patient with a recurrent auricular keloid who underwent excision and immediate Integra® application, followed by high dose rate brachytherapy and interval split-thickness skin graft placement. A 23-year old female with a history of a recurrent auricular keloid after previous surgical excision, corticosteroid injection, and radiation, underwent re- excision of her keloid. Integra® was used to cover the resultant exposed auricular perichondrium. The patient then received high dose rate brachytherapy (1500 cGy) on post-operative days 1 and 2, followed by definitive split-thickness skin graft placement 3 weeks following her initial surgery. The patient recovered from all interventions without complication. There was no evidence of keloid formation 27 months following interval split- thickness skin graft placement at either the auricular recipient or thigh donor sites. We report the first case of two-staged reconstruction of a recurrent auricular keloid (comprised of keloid excision and placement of Integra® in conjunction with high dose rate brachytherapy, followed by subsequent interval split-thickness skin grafting), resulting in an acceptable cosmetic result without evidence of recurrence at long-term follow-up.
Keywords: High dose rate brachytherapy, keloid, split-thickness skin graft
INTRODUCTION
Despite decades of research, keloids remain a formidable clinical challenge. While surgical excision alone may result in temporary cosmetic and symptomatic relief, recurrence rates are between 40% and 100% (1–3). Adjuvant therapies intended to complement surgical excision have been utilized, including occlusive dressings, compression therapy, intralesional corticosteroid injections, laser treatment, pharmacologic agents (such as bleomycin, 5-FU, and interferon), and radiation (2). Of these, radiation therapy in conjunction with surgical excision provides the best long-term outcome and has been reported to decrease post-excision recurrence rates to as low as approximately 33% (4, 5). Amongst the different radiation therapy regimens, high dose rate brachytherapy (HDRBT) has been demonstrated to be effective without incurring significant morbidity (6).
Although the use of the dermal regeneration template Integra® (Integra LifeSciences, Plainsboro, NJ) is well established in the management of acute burns, reports of other clinical applications are limited (7). In this report, we describe the first use of Integra® for coverage of exposed auricular cartilage and perichondrium after keloid resection, thereby allowing for immediate HDRBT without risking graft failure, followed by interval split-thickness skin graft (STSG) coverage.
PATIENTS AND METHODS
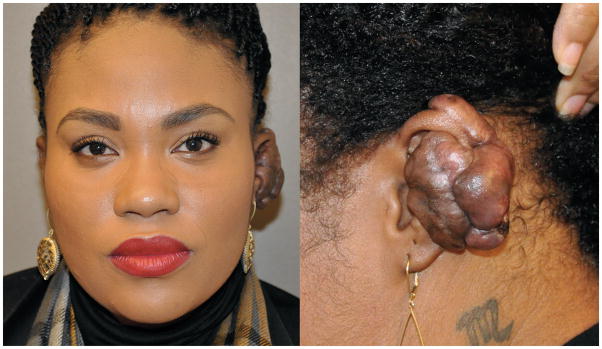
A 23-year-old African-American female presented with a large, recurrent keloid on her left pinna that developed following an upper-ear piercing 5 years earlier. Previous unsuccessful therapeutic interventions performed at other institutions included 2 excisions followed by steroid injections, CO2 laser ablation, and excision followed by postoperative radiation (1500cGy). After each excision, the keloid recurred to larger than its original size within several months. At the time of presentation, the mass was 4×5cm, extending along the midportion of the helix/antihelix, anteriorly into the lateral aspect of the conchal bowl, and posteriorly to the postauricular sulcus (Figure 1).
Figure 1.
A 23-year-old African-American female presented with a large, recurrent keloid on her left pinna. At the time of presentation, the mass was 4×5cm, extending along the midportion of the helix/antihelix as well as anteriorly into the lateral aspect of the conchal bowl, and posteriorly to the postauricular sulcus.
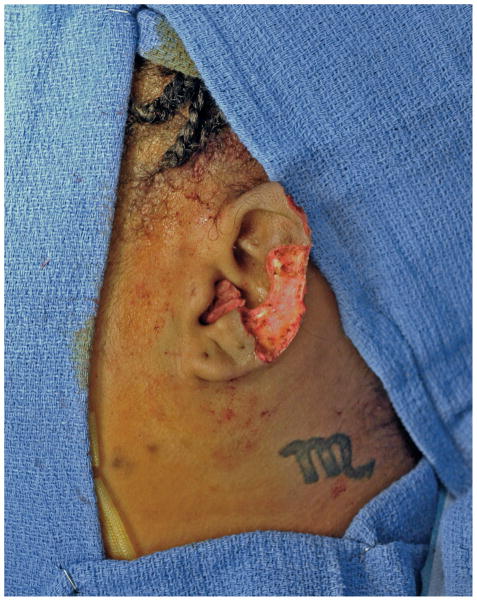
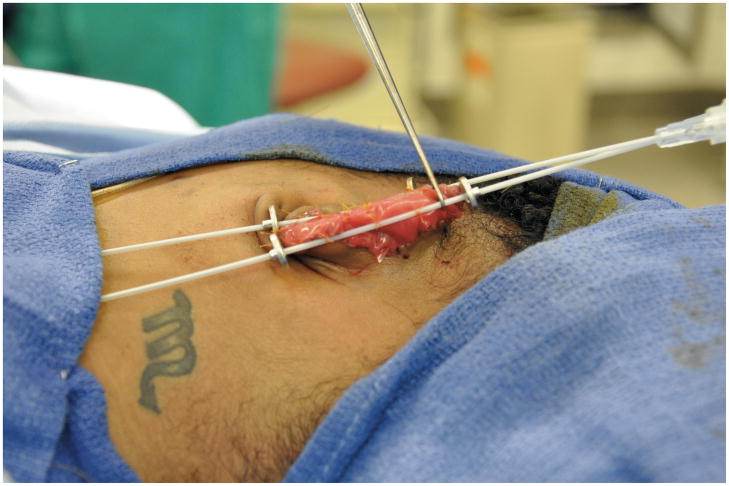
Following a detailed discussion with the patient, the keloid was excised under general anesthesia, leaving exposed auricular perichondrium and cartilage (Figure 2). Integra® was applied to the exposed auricular scaffold. Brachytherapy catheters were secured anteriorly and posteriorly via sutures through the Integra® (Figure 3). On postoperative days 1 and 2, a total of 1500cGy was delivered via the brachytherapy catheters in 3 equal doses. The catheters were removed on postoperative day 5 at the time of the first dressing change. Three weeks after the initial operation, the silicone membrane of the Integra® was removed in the operating room, revealing a healthy, vascularized matrix. Due to her concerns regarding the potential development of additional head and neck keloids, the patient refused both scalp and post-auricular donor sites, and instead elected transfer of a 12/1000” STSG harvested from the proximal left thigh.
Figure 2.
The keloid was excised under general anesthesia, leaving exposed auricular perichondrium.
Figure 3.
Brachytherapy catheters were secured to the anterior and posterior aspects of the patient’s pinna via plain-gut sutures through the Integra® graft.
RESULTS
Three weeks following the procedure, the STSG had 95% epithelialized. At 27-months follow-up, there was no evidence of keloid recurrence (Figure 4). Although the STSG demonstrated mild hyperpigmentation due to color differences between the donor site and auricular skin, the patient was extremely satisfied with her outcome.
Figure 4.
At the patient’s 27-month follow-up visit, there was no evidence of keloid recurrence. A color difference exists due to the location of the donor site.
DISCUSSION
There is no standardized regimen for the treatment of keloids. Most therapeutic options yield high recurrence rates. For example, steroid injections incur at least a 50% recurrence, while laser therapies result in only transient improvement (1, 2, 8). Even surgical excision combined with radiotherapy result in recurrence in at least 33% of patients (4, 5).Although there is a theoretical risk of a radiation-induced secondary tumor, a direct link between radiotherapy and malignancy in the treatment of keloids has not been established (3, 9).
Due to the risk of STSG failure incurred by irradiating a newly-placed graft, most reports that discuss excision in combination with radiotherapy advocate primary closure (6, 8). However, in the aforementioned case, the size and anatomic location of the resultant defect precluded primary closure. By placing Integra® over the exposed perichondrium, we accomplished the short-term goal of wound coverage and provided a durable means of securing the brachytherapy catheters in place. Upon completion of HDRBT, the catheters were removed and the Integra® was allowed to vascularize. Such management allowed for immediate HDRBT and successful interval STSG placement without jeopardizing skin graft survival.
The requirement of a two-staged procedure is typically considered a disadvantage associated with the use of dermal regeneration templates. In this case, however, obligatory delay was advantageous as it allowed for immediate brachytherapy. In addition, although skin graft harvest in a patient prone to keloids is problematic due to the risk of inciting new lesions, an alternative for epithelial coverage of the large exposed auricular cartilaginous framework did not exist in this instance. While a supraclavicular graft would have provided a superior color match, the patient was concerned about the potential for formation of a second keloid in an anatomically exposed area, and thus the concealed proximal thigh was selected as the donor site.
Two-staged reconstruction (comprised of keloid excision and placement of Integra® in conjunction with high dose rate brachytherapy, followed by subsequent interval split- thickness skin grafting) allows for durable reconstruction and appropriate cosmesis in patients with recurrent keloids, and should be considered as part of the treatment algorithm for patients with recurrent keloids who undergo brachytherapy.
Acknowledgments
A portion of this research was supported by a National Institutes of Health Institutional Research Training Grant (NIH T32 HL083824-05), the Weill Cornell Medical College Clinical and Translational Science Center (TL1RR024998), and the New York State Empire Clinical Research Investigator Program.
References
- 1.Al-Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286–300. doi: 10.1097/01.prs.0000195073.73580.46. [DOI] [PubMed] [Google Scholar]
- 2.Butler PD, Longaker MT, Yang GP. Current progress in keloid research and treatment. J Am Coll Surg. 2008;206:731–741. doi: 10.1016/j.jamcollsurg.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Robles DT, Berg D. Abnormal wound healing: keloids. Clin Dermatol. 2007;25:26–32. doi: 10.1016/j.clindermatol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557–568. doi: 10.1097/PRS.0b013e3181c82dd5. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa R, Mitsuhashi K, Hyakusoku H, et al. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg. 2003;111:547–553. doi: 10.1097/01.PRS.0000040466.55214.35. discussion 554–545. [DOI] [PubMed] [Google Scholar]
- 6.Guix B, Henriquez I, Andres A, et al. Treatment of keloids by high-dose-rate brachytherapy: A seven-year study. Int J Radiat Oncol Biol Phys. 2001;50:167–172. doi: 10.1016/s0360-3016(00)01563-7. [DOI] [PubMed] [Google Scholar]
- 7.Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns. A multi- center randomized clinical trial. Ann Surg. 1988;208:313–320. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stahl S, Barnea Y, Weiss J, et al. Treatment of earlobe keloids by extralesional excision combined with preoperative and postoperative “sandwich” radiotherapy. Plast Reconstr Surg. 2010;125:135–141. doi: 10.1097/PRS.0b013e3181c2a46e. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124:1196–1201. doi: 10.1097/PRS.0b013e3181b5a3ae. [DOI] [PubMed] [Google Scholar]