Abstract
A new megastigmane derivative, (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside 1 and two known compounds, the biflavone agathisflavone 2 and the carboxylic acid derivative 4-hydroxibenzoic acid 3 were isolated and purified from leaves and stems of Ouratea polyantha Engl. Agathisflavone was isolated in a single high-speed counter-current chromatography run, meanwhile the megastigmane and the carboxylic acid derivatives were purified in two steps, a combination of high-speed countercurrent chromatography and analytical column chromatography. All structures were elucidated on the basis of spectral evidences and comparisons with literatures. Compound 1 was characterized by [α]D20, UV-Vis, IR, MS, 1H-NMR, 13C-NMR, HMQC, HMBC, COSY and NOESY. Compounds 1 and 2 were tested as hepatic glucose-6-phosphatase inhibitors.
Keywords: Ouratea polyantha; counter-current chromatography; terpenes; megastigmanes; (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside; agathisflavone; glucose-6-phosphatase
1. Introduction
Diabetes mellitus is the commonest endocrine disorder [1] and it is characterized by hyperglycaemia resulting from insufficient insulin production and/or insulin resistance [2]. In Venezuela It is commonly treated with herbal extracts. Although the utility of plant materials is well known in folk medicines based on the empirical knowledge, it needs to be scientifically confirmed because there are many risks on plant material ingestion by decoction, infusion, beverage, etc. [3].
The genus Ouratea belongs to the Ochnaceae family. It is widely represented in tropical areas and comprises approximately 35 genera and 600 species [4]. Several studies of Ouratea species describe the isolation of chloroisoflavonoids [5], biflavonoids [6,7], terpenes like megastigmane [8], kauranes [9], depside [10], among other metabolites. Rodriguez et al. [11] reported that MeOH extract from the aerial parts of Ouratea polyantha Engl. (Op) had a strong inhibition on glucose-6-phosphatase, (G-6-Pasa) EC 3.1.3.9.
The enzyme G-6-Pase is a multi-component system constituted by a catalytic subunit and three transporters: T1 for glucose-6-phosphate (G-6-P), T2 for phosphate/pyrophosphate, and T3 for glucose [12] and both, enzyme and its transporters, are potential targets for anti-diabetic therapy [13–14]. Fractions from methanol extract of O. polyantha showed inhibitory effects on hepatic microsomal G-6-Pase. Therefore, an effective separation and isolation technique is required for the identification of the bioactive fractions.
Countercurrent chromatography (CCC) is a technique used to separate mixtures into their individual components that was first developed in late 1970s and refined to High Speed Countercurrent chromatography (HSCCC) in 1980s when it overshadowed other chromatography methods with its superior capacity to achieve rapid and efficient separation. This chromatographic system is now employed in a wide range of applications, most notably for extracting bioactive compounds from medicinal plants [15].
In the present paper, we report the isolation and structural elucidation of three compounds from O. polyantha aerial parts (leaves and stems) that inhibit rat liver microsomal G-6-Pase.
2. Experimental
2.1. General
Phlorizin, G-6-P and histones II-A were acquired from Sigma-Aldrich (Milwaukee, USA). All other chemicals used were analytical grade.
High-speed counter-current chromatography were performed using two chromatograph, one was an Ito Multi-Layer Coil Separator-Extractor (P.C. Inc. Potomac, MD, USA) with a single column of 325 mL and 1.6 mm internal diameter with a β between 0.5 and 0.85, and the other one was a CCC-1000 High-speed Counter-Current Chromatograph (Pharma-Tech Research, Baltimore, MD, USA) equipped with three coils connected in series (inner diameter of tubing 1.6 mm) with a β between 0.5 and 0.75. The total capacity of the column is 325 mL.
The NMR was carried out in a JEOL spectrometer model Eclipse with an application camp of 270 MHz for 1H and 67.5 MHz for 13C, and Bruker spectrometer with an application camp of 500 MHz for 1H and 125 MHz for 13C.
2.2. Plant material
Aerial parts (leaves and stems) of O. polyantha Engl. were collected in the Sipapo River: near to Cerro Pelota, southern Laja de Garza, between Autana and Guayapola Rivers at the Amazon forest, Amazon State, Venezuela in 1992, and identified by Dr. Anibal Castillo from the Biology School, Science Faculty, Venezuela Central University. A voucher specimen (N° 3308AC) was deposited in the Venezuela National Herbarium, (VEN).
2.3. Animals
Male Sprague–Dawley rats of 180–220 g were used after an overnight fast period.
2.4. Purification of Microsomes and Glucose-6-phosphatase Assay
The microsomal fraction was purified following the method described Marcucci et al. [16], in brief: the rat livers were homogenized in 3 volumes of 0.32 M sucrose, 3 mM MgCl2, centrifuged at 20000g for 20 min. at 4° C, the pellet was discarded and the supernatant was centrifuged at 105000g for 1 h at 4° C, and the pellet constituted the microsomal fraction. The microsomal fraction was resuspended in 0.25 mM sucrose, 1 mM MgCl2, 5 mM HEPES (pH 6.5) to give a final protein concentration of 20 mg/mL and frozen at −80°C until use. Protein concentration was estimated using the Lowry method [17] modified by Markwell et al. [18].
G-6-Pase assays were performed by the method described by Burchell et al. [19] with intact and disrupted (histone treated) microsomes. In brief, the G-6-Pase hydrolyze glucose-6-phosphate (G-6-P) and produce glucose an inorganic phosphate ion, the last one was converted to the blue complex with ammonium heptamolypdate in acidic medium and measured at 820 nm using a Novaspec II spectrophotometer (Pharmacia).
2.5. Metabolites Isolation
Dried and powdered vegetal material, leaves and stems (944.04 g), was macerate and extracted with MeOH at room temperature to produce methanol extract, MeOHrt. After that, the residual material was extracted with MeOH using a soxhlet extractor to obtain the hot methanolic extract (MeOHΔ). Both extracts were concentrated in vacuo yielding 174.82 g (18.52%) and 8.14 g (0.86%) for MeOHrt and MeOHΔ, respectively. They were tested as G-6-Pase inhibitors (Table 1).
Table 1.
Effects of O. polyantha MeOH extracts, fractions and metabolites on the hepatic microsomal glucose-6-phosphatase.
| ppm | n | Intact microsomes (%) | Disupted microsomes (%) | |
|---|---|---|---|---|
| Control | 4 | --- | --- | |
| MeOHrt | 10.0 | 3 | 67.5 | 13.6 |
| MeOHΔ | 10.0 | 3 | 38.6 | 6.7 |
| CH2Cl2a | 10.0 | 3 | 41.5 | 12.8 |
| AcOEta | 10.0 | 3 | 55.6 | 7.5 |
| OpA | 10.0 | 4 | 90.6 | 94.3 |
| OpB | 10.0 | 4 | 67.9 | 42.8 |
| OpC | 10.0 | 4 | 87.6 | 71.2 |
| OpD | 10.0 | 4 | 92.5 | 45.2 |
| Agathisflavone | 2.5 | 3 | 63.6 | 63.1 |
| (6R,9S)-Roseosideb | 20.0 | 3 | 58.5 | 12.2c |
| (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside | 100.0 | 8 | 13.7 | 2.2c |
| Phlorizin | 20.0 | 3 | 27.8 | 0 |
Twenty grams of the MeOHrt extract were suspended in a mixture of methanol-water (1:1; v/v) yielding two fractions, soluble (Op-1) and insoluble (Op-2). Op-1 was concentrated in vacuo, while Op-2 was air-dried. Both fractions were treated with acetone separately, yielding four new fractions named OpA (5.19 g, 25.95%) and OpB (1.36 g, 6.80%) from Op-1 and OpC (5.96 g, 29.80%) and OpD (1.72 g, 8.60%) from Op-2. OpA and OpC were acetone non-soluble while OpB and OpD were acetone soluble.
Fractions OpA, OpB and OpC were fractionated using a CCC-1000 HSCCC from Pharma-Tech Research (Unplublished results), and OpD was analyzed using an HSCCC instrument from P.C. Inc.
OpB and OpD fractions had a common biflavonoid aglycone as the major compound determined by the iodine reagent. In order to obtain this biflavonoid as pure compound the sample solution was prepared by dissolving the crude fraction, OpD, in a solution composed of the upper and lower phases (1:1, v/v) of the solvent system used in HSCCC separation. The selected solvent system (CHCl3-MeOH-H2O, 4:3.5:2, v/v/v) was thoroughly equilibrated in a separation funnel by repeated vigorous shaking at room temperature. The two phases were separated shortly before use. The separation was initiated by filling the entire column with the stationary phase (upper phase) and this was immediately followed by sample inyection dissolved in a mixture of stationary and mobile phases. The mobile phase was then eluted through the column at 2 mL/min while the column was rotated at 800 rpm in the combined head to tail elution mode.
The fractions were monitored and combined by TLC similarity yielding 16 collective fractions (Fig. 2). From OpD-Fr5 (57.00 mg) a pure biflavonoid, agathisflavone was isolated. OpD-Fr9 was purified on a silice gel column chromatography using the organic phase of the solvent system CH2Cl2-n-BuOH-MeOH-H2O (4:0.1:1.5:2, v/v) as eluent to afford 4.91 mg of (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside, while OpD-Fr10–11 was purified on a silice gel column chromatography using the mixture CH2Cl2-MeOH (94:6, v/v) as an eluent to obtain 2.27 mg of 4-hydroxybenzoic acid. In brief, three pure compounds were identified: agathisflavone, (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside and 4-hydroxybenzoic acid and their structure are shown in Fig. 2.
Figure 2.
Structures of compounds 1, 2 and 4.
2.5.1. (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside
Amorphus solid, Rf value: 0.68 (AcOEt-MeOH-H2O; 100:13.5:10; v/v/v), UV-Vis (MeOH) λmax: 229, 298 nm; IR (films) υmax: 3410.5, 2970.9, 2929.7, 1692.0, 1652.6, 1589.5, 1500.7, 1442.7, 1374.5, 1277.5, 1163.9, 1067.6, 854.0 cm−1; 1H-NMR (500 MHz, CD3OD) and 13C-NMR (125 MHz, CD3OD), given in Table 2; TSI-MS m/z (rel. Int.); 506.88 (100), 488.88 (10), 282.96 (97), 264.96 (75), 207.00 (92), 189.00 (34), 149.04 (52), 138.96 (75), 120.96 (45), scheme 1.
Table 2.
1H (500 MHz) and 13C-NMR (125 MHz) Chemical Shifts of (6R,9S)-6′-(4″-hydroxybenzoil)-Roseoside.
| H/C | δH (ppm) | δC (ppm) | DEPTa | HMQC | HMBC | COSY | NOESY |
|---|---|---|---|---|---|---|---|
| 1 | 40.93 | C | H-11; H-12 | ||||
| 2 | 2.44 | 49.28 | CH2 | H-2 | H-11; H-12 | H′-2 | H′-2;H-11;H-7 |
| 2.15 | H′-2 | H-2 | H-2;H-11;H-12 | ||||
|
| |||||||
| 3 | 199.93 | C | |||||
| 4 | 5.85 | 125.70 | CH | H-4 | H-10 | H-13 | H-13 |
|
| |||||||
| 5 | 165.53 | C | H-10 | ||||
| 6 | 78.46 | C | H-10;H-11;H-12;H-13 | ||||
|
| |||||||
| 7 | 5.76 | 132.44 | CH | H-7 | H-2; H-12 | ||
| 8 | 5.69 | 132.25 | CH | H-8 | H-10 | H-9 | H-2; H-10 |
|
| |||||||
| 9 | 4.39 | 73.49 | CH | H-9 | H-10; H-1′ | H-8; H-10 | H-10; H-2′ |
| 10 | 1.25 | 20.78 | CH3 | H-10 | H-8 | H-9 | H-8; H-9 |
|
| |||||||
| 11 | 0.94 | 23.31 | CH3 | H-11 | C-1; C-2; C-6; C-12 | H-2; H′-2 | |
| 12 | 1.00 | 22.03 | CH3 | H-12 | C-1; C-2; C-6; C-11 | H′-2; H-7 | |
|
| |||||||
| 13 | 1.91 | 18.21 | CH3 | H-13 | C-4; C-5; C-6 | H-4 | H-4 |
| 1′ | 4.29 | 99.79 | CH | H-1′ | C-9; H-10 | H-2′ | H-5′ |
|
| |||||||
| 2′ | 3.22 | 73.49 | CH | H-2′ | C-4′ | H-1′; H-3′ | H-9; H-10 |
| 3′ | 3.29–3.32 | 76.81 | CH | H-3′ | H-2′ | H-5′;H-3″;H-5″ | |
|
| |||||||
| 4′ | 3.36 | 70.59 | CH | H-4′ | H-2′ | H-5′ | |
| 5′ | 3.40–3.47 | 74.18 | CH | H-5′ | H′-6′ | H-6′; H′-6′ | H-3′ |
|
| |||||||
| 6′ | 4.61 | 63.53 | CH2 | H-6′ | C-5′; C7″ | H′-6′ | H′-6′;H-5′ |
| 4.37 | H′-6′ | H-5′; H-6′ | H-6′ | ||||
| 1″ | 120.73 | C | H-3″; H-5″ | ||||
|
| |||||||
| 2″ | 7.87 | 114.88 | CH | H-2″ | C-7″ | H-3″ | H-3″ |
| 3″ | 6.81 | 131.42 | CH | H-3″ | C-1′ | H-2″ | H-3′;H-2″ |
|
| |||||||
| 4″ | 162.22 | C | |||||
| 5″ | 6.81 | 131.42 | CH | H-5″ | C-7″ | H-6″ | H-3′;H-6″ |
|
| |||||||
| 6″ | 7.87 | 114.88 | CH | H-6″ | C-1′ | H-5″ | H-5″ |
| 7″ | 166.58 | C | H-2″; H-6′; H-6″ | ||||
at 270 MHz
2.5.2. Agathisflavone (6→8″-biapigenin)
Yellow solid, Rf value: 0.93 (AcOEt-MeOH-H2O; 100:13.5:10; v/v/v), UV-Vis (MeOH) λmax: 229, 298 nm; 1H-NMR (500 MHz, CD3OD): δ 13.30 (1H, bs, 5-OH), 13.04 (1H, bs, 5″-OH), 8.01 (2H, d, J=8.75 Hz, H-2′, H-6′), 7.58 (2H, d, J=8.75 Hz, H-2′, H-6′), 6.97 (2H, d, J=8.75 Hz, H-3′, H-5′), 6.77 (2H, d, J=8.75 Hz, H-3′, H-5′), 6.88 (1H, s, H-3), 6.79 (1H, s, H-3″), 6.70 (1H, s, H-8), 6.38 (1H, s, H-6″); 13C-NMR (125 MHz, CD3OD), δ 182.51 (C-4″), 182.30 (C-4), 164.16 (C-2″), 163.99 (C-2), 163.06 (C-7″), 162.95 (C-7), 161.65 (C-4‴), 161.48 (C-4′, C-5), 161.06 (C-5″), 157.14 (C-9″), 155.23 (C-9), 129.02 (C-2‴, C-6‴), 128.50 (C-2′, C-6′), 121.83 (C-1′), 121.66 (C-1‴), 116.45 (C-3′, C-5′), 116.33 (C-3‴, C-5‴), 104.11 (C-10), 103.95 (C-10″), 103.68 (C-3″), 103.31 (C-3), 103.05 (C-6″), 99.54 (C-8), 99.00 (C-6), 93.85 (C-8″).
3. Results and discussion
(6R,9S)-6′-(4″-hydroxybenzoil)-roseoside 1 was isolated as an amorphous solid ([α]D20 – 57). The UV spectrum of 1 (λmax 229 nm) and the IR absorption (νmax 1652.6 cm−1) indicated the presence of an α,β-unsaturated ketone. The molecular formula of 1 was established as C26H34O10 from the TSI-MS [m/z 507.88 (M)+, 529.40 (M+Na)+ and 1035.40 (2M-H+Na)+] and NMR data. The mass fragmentation of the ion peak at 506.88 gave fragments for water loss at 488.88 (rel. Int., 10), sugar fragmentations at 282.96 (97) and 264.96 (75), alkene fragmentations at 207.00 (92) and 149.04 (52), ester fragmentation at 120.96 (45) and ester fragmentation throught McLafferty rearrangement (Figure 3). The IR spectrum showed the presence of hydroxyl (broad band 3410.5 cm−1), a conjugated ester (1692.0, 1277.5, 1067.6 cm−1) and a trans olefin (1589.5 cm−1). The 1H and 13C-NMR spectra (Table 2), which were assigned by 2D experiments (COSY, HMQC, HMBC and NOESY) of 1 showed the presence of a 4-hydroxybenzoil and β-glucopyranosyl units and an aglycone moiety.
Figure 3.
Proposed fragmentation mechanism of (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside.
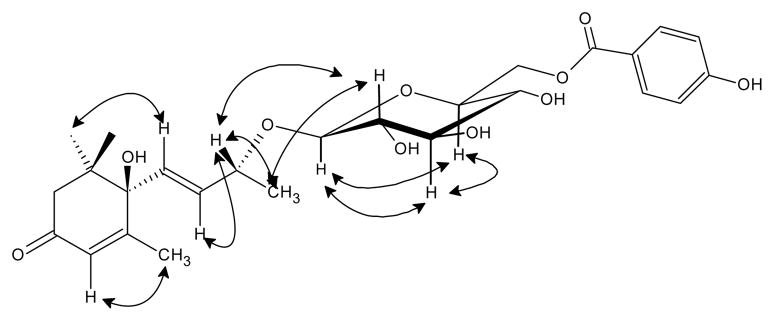
The 1H-NMR spectrum showed two doblet signals at δ 7.87 (2H, d, J=8.75, H-2″ and H-6″) and 6.82 (2H, d, J=8.75, H-3″ and H-5″), attributed to the AA′BB′ system in a 1,4- substituted benzene ring, assigned to the 4-hydroxybenzoil. In the HMBC spectrum, the proton signal at δ 7.87 showed a cross peak with the carbon signal at δ 166.58 (C-7″) which exhibited a long range coupling with the proton signal at δ 4.61 (1H, bs, H-6′) assignable to one proton of methylene of sugar unit, thus the location of 4-hydroxybenzoil group in the pyranosyl moiety was established at C-6′ (Fig. 4). In the 1H-NMR spectrum, a signal at δ 4.29 (1H, d, J=7.8 Hz, H-1′) with a large coupling constant (7.8 Hz) suggested an anomeric proton with β-configuration. Furthermore, the presence of the COSY cross peak between H-6′ and the signal at δ 3.40–3.47 (1H, m, H-5′) and NOESY correlation between H-5′ and the signals at δ 4.29 (1H, d, J=7.8 Hz, H-1′) and δ 3.29–3.32 (1H, m, H-3′) demonstrated the presence of glucopyranosyl moiety (Figs. 5 and 6).
Figure 4.
COSY (bold bonds) and HMBC (→) correlation of (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside.
Figure 5.
NOESY correlation of (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside.
The position of the glucosydic linkage was deducted from the 13C-NMR, chemical shift of C-9 (δC 73.49), and this was confirmed by long correlation between H-1′ and C-9. In addition to the signals of the 4-hydroxybenzoil and glucopyranosyl groups, the remaining proton signals, corresponding to 13 carbons, were assignable to the aglycone, which include two trans-coupled olefinic protons at δ 5.76 (1H, d, J=15.6 Hz, H-7) and δ 5.69 (1H, dd, J=15.6, 6.8 Hz, H-8), and a olefinic proton at δ 5.85 (1H, s, H-4) located on α-position of an α,β-unsaturated ketone and four methyl groups at 0.94, 1.00, 1.25 and 1,91. The deshielded methyl signal at δ 1.91 (3H, d, J=0.95 Hz, H-13) and the olefinic proton H-4 indicated that the methyl is directly attached to an olefinic carbon, this was supported by the HMBC correlation between δH 1.91 (H-13) and δC 125.70 (C-4). The COSY and HMBC experiments showed a cross peak between a doublet at δ 1.25 (3H, d, J=6.4 Hz, H-10) an a oxygenated methine at δ 4.39 (1H, dd, J=14.9, 6.2 Hz, H-9). In addition, gem-dimethyl groups at δ 0.94 (3H, s, H-11) and δ 1.00 (3H, s, H-12) were observed. Geminally coupled signals at δ 2.44 (1H, d, J=16.8 Hz, H-2a) and δ 2.15 (1H, d, J=16.8 Hz, H-2b) suggested that a carbonyl group was linked to this methylene. Finally, the absolute configuration of C-6 proved to be R by the negative value of its optical rotation as determine Yamano and Ito in the total synthesis of four stereoisomers of roseoside [20].
The stereochemistry of C-9 was determined by comparison with the chemical data for roseoside [20]. Therefore, the absolute configuration of the C-9 (δ 73.49) in 1 was assigned as S. On the basis of this data, 1 turned out to be (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside. To the best of our knowledge, this is the first report of the isolation and characterization of (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside from nature.
Agathisflavone (C30H18O10) was isolated as a yellow amorphous solid and exhibited a (M-1)+ molecular ion peak at m/z 537.2813. In the 13C-NMR displayed 30 carbons including two carbonyl carbons at δ 182.3 and 182.5, 16 quaternary sp2 carbons with eight linked to an oxygen atom and 12 tertiary sp2 carbons. The 1H-NMR spectrum showed two singlets at δ 13.32 and 13.06 attributed to hydroxyl groups, two sets of A2B2-type doublets, one set at δ 8.01 and 6.97 and the other at δ 7.58 and 6.77 attributed to two AA′BB′ systems in two para-substituted aromatic rings, and four singlets at δ 6.88, 6.79, 6.38 and 6.37 attributed to four hydrogen attached to sp2 carbon atoms. The complete assignment of the proton and carbon was made by the comparison with literature data [21, 22]. 4-Hydroxybenzoic acid was isolated as a white solid and was identified on the basis of its 1H-NMR and 13C-NMR data and a comparative TLC with an authentic sample.
As shown in table 1, (6R,9S)-6′-(4′-hydroxybenzoil)-roseoside showed a statistically significant (p<0.02)inhibition of 13.7% of the G-6-Pase in intact microsomes without affecting the enzyme activity of the disrupted system, the effect which is lower in comparison to 27.8% of inhibition of the G-6-Pase in intact microsomes obtained with phlorizin, a known inhibitor of T1 transporter [23]. These results allowed to suggest that 1 inhibits the G-6-P transporter (T1) without affecting the catalytic subunit or phosphate/pyrophosphate transporter (T2) of the G-6-Pase system. The (6R,9S)-roseoside, isolated by Avila et al. [24], exerted an inhibition of 58.5% of the enzyme in intact microsomes, suggesting that 4-hydroxybenzoil moiety exerts a negative effect in the biological activity.
Figure 1.
Purification of agathisflavone, (6R,9S)-6′-(4″-hydroxybenzoil)-roseoside and 4-hydroxybenzoic acid from OpD fraction of O. polyantha Engl.
Acknowledgments
This work was supported by Grant PG.03.7345.2008 and PI-09-7645-09/1 from the Consejo de Desarrollo Científico y Humanístico de la Universidad Central de Venezuela.
References
- 1.WHO: World Health Organization. 2004 [Google Scholar]
- 2.Kuo-Chen C. Molecular Therapeutic Target for Type-2 Diabetes. J Proteome Res. 2004;3:1284–1288. doi: 10.1021/pr049849v. [DOI] [PubMed] [Google Scholar]
- 3.Bruneton J. Plantas tóxicas: Vegetales peligrosos para el hombre y los animales, Acribia S. A. 2001 [Google Scholar]
- 4.Watson L, Dallwitz MJ. The Families of Flowering Plants. 1992 (available on http://biodiversity.uno.edu.delta)
- 5.Velandia J, Carvalho My, Braz-Filho R. Novel trichloro- and tetrachloroisoflavones isolated fromOuratea semiserrata. Nat Prod Res. 1998;12:191–198. [Google Scholar]
- 6.Ramos L. Doctoral Thesis. Universidade Federal Rural do Rio de Janeiro; 2007. Considerações sobre oe gêneros Ouratea e Luxemburgia, estudo químico de duas espécies de ochnaceae: Ouratea hexasperma St Hil e Ouratea cuspidata St. Hil e Actividades Biologicas. (artícle in Portugues) [Google Scholar]
- 7.De Souza J, Fernandes C, Grivicich I, Da Rocha Ay, De Carvalho M. Antitumor activity of biflavonoids from Ouratea and Luxemburgia on human cancer cells lines. Indian J Ethnopharm. 2007;39:184–189. [Google Scholar]
- 8.Velandia J, De Carvalho M, Braz-Filho Ry, Werle A. Biflavonoids and glucopyranoside derivate from Ouratea semiserrata. Phytochem Anal. 2002;13:283–292. doi: 10.1002/pca.656. [DOI] [PubMed] [Google Scholar]
- 9.Velandia J, Carvalho M, Braz-Filho R. Ácido ent-16α, 17-diidroxicauran-19-óico isolado de Ouratea semiserrata e os desafios estereoquímicos dos carbonos quirais C-4 e C-16. Química Nova. 1998;21:397–404. (Article in Portugues) [Google Scholar]
- 10.Carvalho M, Carvalho Gy, Braz-Filho R. Chemical constituents from Ouratea floribunda: Complete 1H13C NMR assignments of Atranorin and its new acetyl derivate. Journal Brazilian Chemical Society. 2000;11:143–147. [Google Scholar]
- 11.Rodríguez D, Hasegawa M, González-Mújica F. Biflavona de Ouratea polyantha (ochnaceae) con actividad inhibitoria sobre la enzima glucosa-6-fosfatasa. Sociedad Venezolana de Química Medicinal. 2007 (Article in Spanish) [Google Scholar]
- 12.Burchell A, Waddell ID. The Molecular Basis of the Hepatic Microsomal Glucose-6-Phosphatase. Biochim Biophys Acta-Molecular Cell Research. 1991;1092:129–137. doi: 10.1016/0167-4889(91)90146-o. [DOI] [PubMed] [Google Scholar]
- 13.McCormack JG, Westergaard N, Kristiansen M, Brand CL, Lau J. Pharmacological Aproaches to Inhibit Endogenous Glucose Production as a Mean of Anti-Diabetic Therapy. Curr Pharm Design. 2001;7:1451–1474. doi: 10.2174/1381612013397393. [DOI] [PubMed] [Google Scholar]
- 14.Estrada O, Hasegawa M, González-Mujica F, Motta N, Perdomo E, Solórzano A, Méndez J, Méndez B, Zea E. Evaluation of Flavonoids from Bauhinia megalandra Leaves as Inhibitor of Glucose-6-Phosphatase System. Phytother Res. 2006;19:859–863. doi: 10.1002/ptr.1703. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Conway W. High-Speed Countercurrent Chromatography. Wiley-Interscience; New York: 1996. [Google Scholar]
- 16.Marcucci O, González-Mujica Fy, Pérez-Ayuso E. Alterations of Liver nuclear envelopes accompanying thioacetamide administration in rats. Acta Cient Venezolana. 1983;34:109–117. [PubMed] [Google Scholar]
- 17.Lowry O, Rosebrough H, Farr Ay, Randall R. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:209–220. [PubMed] [Google Scholar]
- 18.Markwell M, Hass S, Bieber Ly, Tolbert N. A modification of the Lowry procedure to simplify protein determination in membrane lipoprotein sample. Analytical Biochemistry. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 19.Burchell A, Hume R, Burchell B. A new microtechnique for the analysis of the human hepatic microsomal glucose-6-phosphatase system. Clin Chim Acta. 1988;173:183–192. doi: 10.1016/0009-8981(88)90256-2. [DOI] [PubMed] [Google Scholar]
- 20.Yamano Y, Ito M. Synthesis of Optically active Vomifoliol and Roseoside Stereoisomers. Chem Phar Bull. 2005;55:541–546. doi: 10.1248/cpb.53.541. [DOI] [PubMed] [Google Scholar]
- 21.Bahia M, dos Santos J, David J, David J. Biflavonoids and other phenolics from Caesalpinia pyramidalis (Fabaceae) J Braz Chem Soc. 2005;16:1402–1405. [Google Scholar]
- 22.Suenningsen A, Madsen K, Liljefors T, Stafford G, van Steden J, Tager A. Biflavones from Rhus species with affinity for the GABAA 1 benzodiazepine receptor. J of Ethnopharmacology. 2006;103:2276–2280. doi: 10.1016/j.jep.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Arion WJ, Lange AJ, Walls HE. Microsomal membrane integrity and the interaction of phlorizin with the glucose-6-phosphatase system. J Biol Chem. 1980;255:10387–10395. [PubMed] [Google Scholar]
- 24.Avila L, Hasegawa M, González-Mujica F, Rodríguez M, Tillet S. Aislamiento de los metabolitos de Bauhinia variegata L., biodirigido por ensayos de inhibición sobre la enzima glucosa-6-fosfatasa. XVII Jornadas Científicas “Dr. Francisco Venazi”; Caracas, Venezuela. 2008. (Article in Spanish) [Google Scholar]