Summary
Whilst the co-translational translocation of nascent proteins across the mammalian endoplasmic reticulum (ER) is well defined, the capacity of this organelle for post-translational translocation is poorly delineated. Here we identify two human secretory protein precursors, apelin and statherin, as bona fide substrates for post-translational translocation across the ER membrane. Further studies, in combination with Hyalophora cecropia preprocecropin A (ppcecA), show that all three proteins bind to TRC40 and can utilise this component for their delivery to the ER membrane in a well-established in vitro system. However, ppcecA is not an obligate TRC40 substrate, and it can also be delivered to the ER by an alternative TRC40-independent pathway. Upon arrival at the ER membrane, these short secretory proteins appear to be ubiquitously transported across the ER membrane through the Sec61 translocon, apparently irrespective of their delivery route. We speculate that the post-translational translocation of secretory proteins in higher eukaryotes is more prevalent than previously acknowledged.
Key words: Asna 1, Endoplasmic reticulum, Post-translational translocation, Tail-anchored protein, WRB protein, Eeyarestatin I
Introduction
The compartmentalisation of the eukaryotic cell requires that many proteins synthesised in the cytosol must cross one or more membranes to reach their site of function and various mechanisms exist to ensure the fidelity of this process (Schatz and Dobberstein, 1996). Proteins destined for the secretory pathway are normally targeted to the endoplasmic reticulum (ER) and typically translocated co-translationally into or across its membrane. This process involves the recognition of an N-terminal signal sequence and recruitment of the ribosome/nascent chain complex to the ER membrane by the signal recognition particle (SRP) (for a review, see Cross et al., 2009b). In lower eukaryotes such as Saccharomyces cerevisiae, it is well established that secretory proteins can be post-translationally translocated across the ER membrane (Rapoport et al., 1999). In higher eukaryotes, evidence for the post-translational translocation of secretory proteins has largely been restricted to a limited number of heterologous substrates from insects and amphibians (Zimmermann et al., 1990). These proteins are often precursors for anti-microbial peptides (Brogden, 2005), and they are all unusually short (<70 amino acids). Thus, although they possess an N-terminal signal sequence, it has insufficient time to emerge from the ribosomal exit tunnel and bind SRP before translation is complete (Schlenstedt et al., 1990; Zimmermann et al., 1990). Hence, these precursors do not require SRP or the SRP receptor for their post-translational translocation in vitro (Müller and Zimmermann, 1987; Schlenstedt et al., 1990; Schlenstedt and Zimmermann, 1987; Zimmermann and Mollay, 1986). The alternative pathway(s) by which these proteins are delivered to the ER for post-translational translocation is comparatively poorly defined; although it has been established the process is ATP dependent (Müller and Zimmermann, 1988; Schlenstedt et al., 1990; Schlenstedt and Zimmermann, 1987), and a recent study uncovered a novel role for calmodulin in promoting post-translational translocation (Shao and Hegde, 2011). Once delivered to the membrane, it is believed that these short secretory proteins utilise the classical Sec61 complex in order to translocate into the ER lumen. Specifically, the translocation of ppcecA into ER derived microsomes is inhibited by lanthanum ions, and the small molecule inhibitor eeyarestatin, both of which perturb Sec61 mediated translocation (Cross et al., 2009a; Erdmann et al., 2009).
In contrast to the model substrates outlined above, the ability of validated human secretory proteins to utilise a pathway(s) for post-translational translocation has not been extensively addressed. However, a large class of membrane proteins can be post-translationally inserted into the ER membrane (Kutay et al., 1995). These, so-called, tail-anchored (TA) proteins are targeted by a transmembrane domain at their extreme C-terminus that remains obscured by the ribosomal exit tunnel until translation is complete (Hegde and Keenan, 2011; Kutay et al., 1995; Kutay et al., 1993; Rabu et al., 2009). TA proteins may exploit one or more of several distinct pathways that can mediate their delivery to the ER, with the choice of pathway dictated by specific features including the hydrophobicity of the tail-anchor region (for a review, see Rabu et al., 2009). One of the pathways employed for TA protein delivery to the ER is a novel ATP-dependent route mediated by TRC40 (also known as Asna-1) (Favaloro et al., 2008; Stefanovic and Hegde, 2007). This pathway is conserved in S. cerevisiae (see Rabu et al., 2009), and it also involves both an upstream cytosolic loading complex (Leznicki et al., 2010; Mariappan et al., 2010; Wang et al., 2010) and an ER localised receptor complex (Schuldiner et al., 2008) that in mammals includes WRB, the tryptophan rich basic protein (Vilardi et al., 2011).
In this study we identify two human precursors as bona fide substrates for post-translational translocation across the ER membrane that is comparable to that of H. cecropia ppcecA. All three short secretory proteins are substrates for TRC40 binding, and a dominant-negative form of the TRC40 receptor, WRB, inhibits their efficient delivery to the ER membrane, confirming TRC40-dependent targeting. We conclude that TRC40 plays a key role in the ATP-dependent delivery of short secretory proteins to the ER membrane. We also identify an alternative, TRC40-independent, pathway for the post-translational delivery of short secretory proteins to the ER. Both pathways seem to converge at the Sec61 translocon, underlining its role as the principal route for transferring polypeptides from the cytosol into the ER lumen. We speculate that a significant amount of secretory protein cargo entering the ER may do so via this non-classical post-translational process.
Results
Short human secretory proteins can be post-translationally translocated
Most studies into the post-translational translocation of short secretory proteins in mammalian systems have focussed on heterologous precursors derived from lower metazoans such as insects and amphibians (Schlenstedt et al., 1992; Schlenstedt and Zimmermann, 1987; Zimmermann and Mollay, 1986). We selected two short human preproproteins, statherin (62 aa) and apelin (77 aa), on the basis of their length and the presence of a known or predicted N-terminal signal sequence, and explored their capacity for post-translational translocation. Statherin modulates calcium levels in respiratory mucus, whilst apelin is a hormone implicated in a wide variety of physiological functions, from cardiac contractility to roles in diabetes and obesity (Castan-Laurell et al., 2011; Falcão-Pires and Leite-Moreira, 2005; Schlesinger and Hay, 1977).
Whilst signal sequence cleavage can be used to confirm ppcecA translocation (Cross et al., 2009a), the mobility change after SDS-PAGE is small, and we therefore explored the use of N-glycosylation as an alternative reporter. We added a previously defined opsin tag (cf. Abell et al., 2007) containing one (OPG1) or two (OPG2) N-glycosylation sites to the C-terminus of ppcecA, apelin and statherin (supplementary material Fig. S1). These proteins were synthesised in vitro and used in a post-translational translocation assay where ER derived membranes were added post-synthesis (Fig. 1). The ppcecA, apelin and statherin chimeras with two N-glycosylation (OPG2) sites are efficiently modified in this assay with the isolated membrane fraction containing both singly and doubly N-glycosylated species in each case (Fig. 1, lanes 1, 3 and 5, respectively). These species are all sensitive to enzymatic deglycosylation (Fig. 1, lanes 2, 4 and 6). These data indicate that the precursors of human apelin and statherin are able to translocate post-translationally across the ER membrane, even after the addition of a C-terminal extension. We also confirmed that the efficient translocation of ppcecA, as judged by N-glycosylation, requires nucleotide triphosphates (supplementary material Fig. S2), as previously shown via processing of the N-terminal signal sequence (Schlenstedt et al., 1990).
Fig. 1.
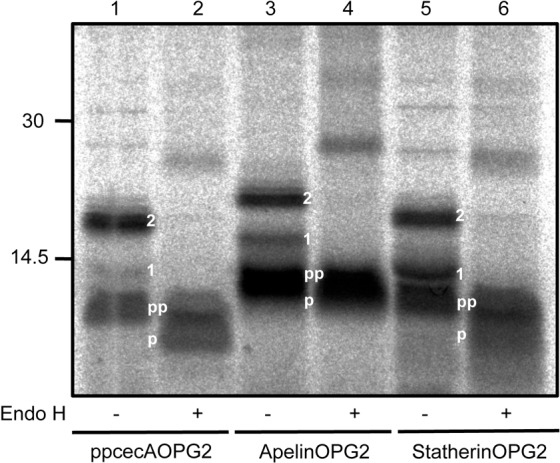
Human proteins can be post-translationally translocated across the ER membrane. Opsin epitope-tagged forms of ppcecA, apelin and statherin (supplementary material Fig. S1) were synthesised in vitro using rabbit reticulocyte lysate and [35S]methionine. Canine pancreatic microsomes were added and samples incubated at 30°C to allow translocation. The membrane fraction was isolated and analysed following Endo H treatment, SDS-PAGE and phosphorimaging. The N-glycosylated species (1, singly N-glycosylated; 2, doubly N-glycosylated), processed (p) and unprocessed forms (pp) of all precursors are indicated. Positions of molecular size markers are indicated on the left in kDa.
TRC40 and Hsp/Hsc70 are present in translocation-competent fractions of the short secretory proteins
Multiple pathways featuring a variety of cytosolic components are implicated in the post-translational targeting and insertion of TA proteins at the ER (Rabu et al., 2009), and we addressed their potential role in the delivery of short secretory proteins. In the first instance we investigated the possibility of an association between known components and newly synthesised short secretory proteins. An in vitro translation of ppcecA was subjected to sucrose gradient centrifugation and individual fractions were collected (Favaloro et al., 2008; Leznicki et al., 2010; Stefanovic and Hegde, 2007). Each fraction was then analysed for the presence of radiolabelled ppcecA and immunoblotted for various candidate cytosolic proteins implicated in TA protein delivery (Fig. 2). We also tested each fraction for its ability to support the post-translational translocation of ppcecA, with the hypothesis that any factors mediating late steps in ER delivery would be present in ‘translocation-competent’ fractions. Although radiolabelled ppcecA is found in almost all fractions, only a discrete population of chains peaking around fractions 4 and 5 was translocation competent (Fig. 2A,B). Whilst both the Hsp/Hsc70 molecular-chaperones and TRC40 are present in these fractions (Fig. 2D,E), neither component appeared to peak with this translocation competent material. In contrast, BAG6 (Bat3/Scythe), a protein acting upstream of TRC40, was only just apparent in fraction 6 (Fig. 2C). When a similar sucrose gradient analysis of apelin and statherin was performed, in each case the peak of translocation-competent material was also located in fractions 4 and 5, consistent with a common delivery mechanism to that of ppcecA (supplementary material Fig. S3). On the basis of this analysis both TRC40 and Hsp/Hsc70 were potential candidates for mediating the ATP-dependent delivery of short secretory proteins to the ER.
Fig. 2.
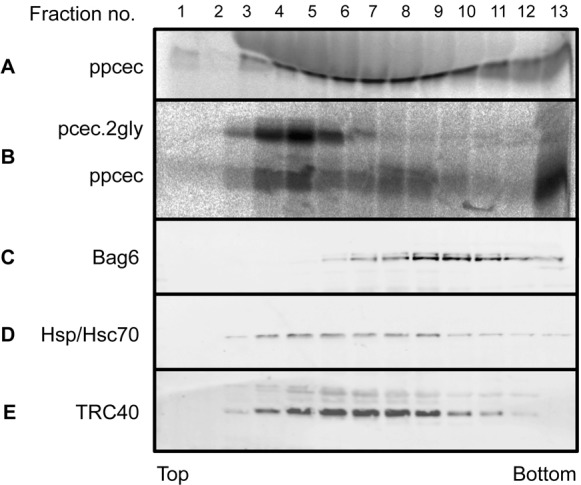
Cytosolic factors potentially involved in ppcecA translocation. Radiolabelled ppcecA was synthesised in vitro and samples puromycin treated as before. The reaction mixture was subjected to centrifugation through a 5–25% sucrose gradient and collected as 13 individual fractions. (A) The location of radiolabelled ppcecA was determined by phosphorimaging. (B) Each fraction was tested for its ability to support ppcecA translocation by incubation with microsomes. Membranes were recovered by centrifugation and translocation assayed by N-glycosylation. (C–E) The fractions were also immunoblotted for specific cytosolic proteins as indicated.
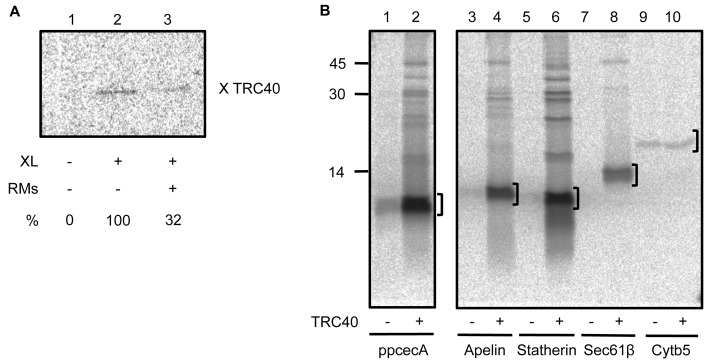
Previous studies have identified both TRC40 (Favaloro et al., 2008; Stefanovic and Hegde, 2007) and Hsp70 (Abell et al., 2007) as cross-linking partners of newly synthesised TA proteins. We therefore used a cross-linking approach to further define the cytosolic components that associate with translocation-competent short secretory proteins, taking ppcecA as our model. When the translocation-competent fractions of ppcecA translation were pooled and treated with the bifunctional reagent, bismaleimidohexane (BMH), a ∼46 kDa product was recovered by antibodies to both the opsin-tagged ppcecA precursor and TRC40 (supplementary material Fig. S4). In contrast, no evidence of adducts with Hsp/Hsc70, Bag6 or the small glutamine-rich tetratricopeptide repeat containing protein α (SGTA), a component previously shown to bind TA proteins (Leznicki et al., 2010; Leznicki et al., 2011), were detected. The in vitro binding of protein precursors to targeting factors on a productive pathway for membrane delivery is normally relieved by the addition of the target membrane, as is the case for the binding of TRC40 to TA proteins (Stefanovic and Hegde, 2007). We therefore investigated the effect of adding ER-derived microsomes to the translocation competent fractions of ppcecA prior to cross-linking. We find that the addition of ER membrane results in a substantial reduction in the formation of ppcecA–TRC40 adducts (Fig. 3A), consistent with TRC40-bound ppcecA representing a bona fide intermediate on a productive delivery pathway to the ER membrane (cf. Stefanovic and Hegde, 2007). On the basis of these cross-linking data we pursued TRC40 as a potential component for the delivery of short secretory proteins to the ER.
Fig. 3.
TRC40 binds short secretory proteins. (A) Translocation-competent fractions of ppcecAOPG2 translations were pooled, and supplemented with ER-derived rough microsomes (RMs) where indicated. Selected samples were then treated with the bifunctional crosslinking reagent BMH, and adducts with TRC40 recovered by immunoprecipitation (cf. supplementary material Fig. S3). The resulting adducts were quantified by phophorimaging, and the value obtained in the presence of RMs was expressed as a percentage of that recovered in their absence. (B) In vitro translations of various precursors were performed as previously described in the presence or absence of recombinant TRC40. After synthesis was complete, the reactions were diluted with buffer and incubated with nickel agarose. The beads were recovered by centrifugation and washed repeatedly before any bound proteins were eluted. Radiolabelled products were analysed by SDS-PAGE and phosphorimaging. Brackets indicate unmodified radiolabelled protein eluted from TRC40.
TRC40 binds a range of precursors bearing ER-targeting signals
To further address the potential role of TRC40 in ER delivery, the three short secretory protein precursors were synthesised in vitro in the presence of recombinant, 10×His-tagged TRC40. TRC40 was recovered from the reaction using nickel agarose and analysed for any radiolabelled precursors that remained bound. Substantial amounts of all three short secretory protein precursors were specifically associated with TRC40 (Fig. 3B, lanes 1–6, see brackets), as was the TRC40-dependent TA protein, Sec61β (Fig. 3B, lanes 7 and 8, see bracket). In contrast, only background levels of the TRC40-independent TA protein, cytochrome b5 (Cytb5) were recovered (Fig. 3B, lanes 9 and 10, see bracket). This suggests that TRC40 can specifically associate with short secretory proteins in a manner that is comparable to the previously defined binding of TA protein substrates.
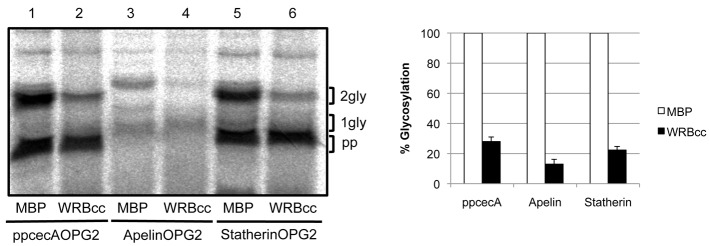
WRBcc inhibits ppcecA translocation
It has recently been shown that a coiled coil fragment of the mammalian TRC40 receptor subunit WRB, denoted WRBcc, can be used to inhibit the TRC40-dependent insertion of TA proteins at the ER (Vilardi et al., 2011). We used this fragment to investigate whether TRC40 plays a functional role in the delivery and translocation of short secretory proteins. To this end, a post-translational translocation assay was performed using ppcecA incubated in the presence of increasing concentrations of WRBcc. Translocation efficiency was measured by quantifying the amount of fully N-glycosylated precursor recovered, relative to an untreated control, and the effect of WRBcc upon the membrane insertion of two well-defined TA proteins, Sec61β and Cytb5, was analysed in parallel (Fig. 4A). The translocation of ppcecA shows a concentration-dependent decrease in response to WRBcc addition that is comparable to that observed for the membrane insertion of the TRC40-dependent TA protein Sec61β (Fig. 4B). In contrast, the membrane insertion of the TRC40-independent TA protein Cytb5 appears to be stimulated at WRBcc concentrations of up to 5 µM, with a modest inhibition only becoming apparent at 10 µM. Taken together, these data suggest that the delivery of ppcecA to the ER membrane is, at least in part, TRC40 dependent.
Fig. 4.
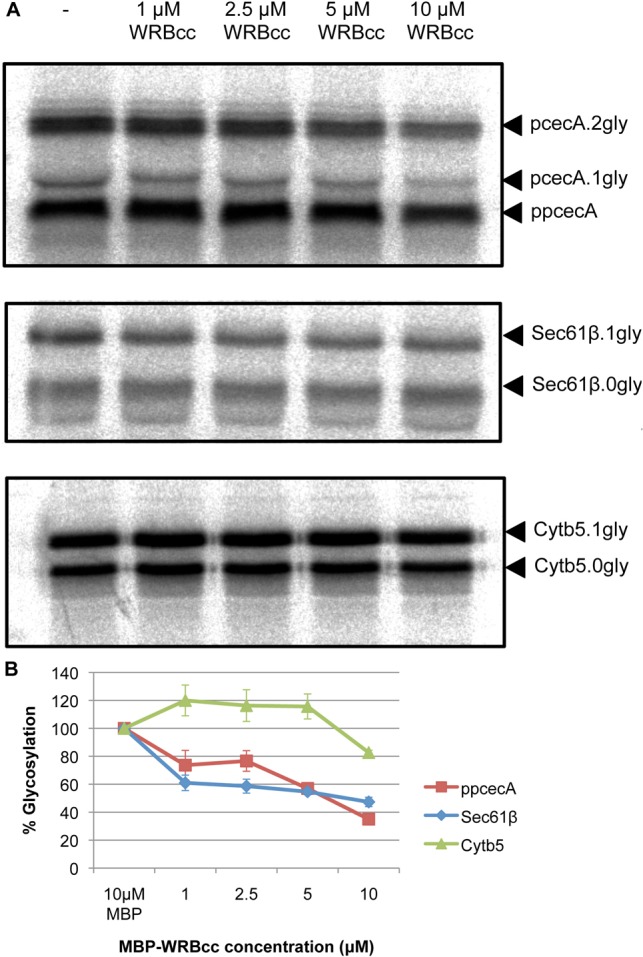
WRBcc inhibits ppcecA translocation. (A) Radiolabelled forms of ppcecA, Sec61β and Cytb5 were synthesised in vitro as before and the resulting reaction mixture then incubated with varying concentrations of MBP-WRBcc as indicated. Canine pancreatic microsomes were added to allow translocation/integration and membranes recovered by centrifugation. Rabiolabelled proteins were detected by phosphorimaging following SDS-PAGE. N-glycosylated forms are indicated and in each case the fully glycosylated forms have been quantified relative to the matched untreated sample. (B) Graphical representation of relative N-glycosylation of proteins in response to increasing concentrations of MBP-WRBcc. Results are means ± s.e.
WRBcc inhibits the translocation-competent fractions of short secretory proteins
In order to distinguish the effect of WRBcc on a TRC40-dependent ER delivery step from a possible perturbation of some upstream event (cf. Mariappan et al., 2010), we repeated our analysis using gradient purified translocation-competent fractions enriched in precursors that are preloaded onto cytosolic delivery factors (cf. Fig. 2). In the case of ppcecA, the level of WRBcc-mediated inhibition was directly comparable for the complete translation reaction and purified translocation-competent fractions (cf. Fig. 5, lanes 1 and 2 with Fig. 4A,B), consistent with a direct effect on TRC40 mediated delivery. When the two human precursors were studied, a substantial inhibition of the translocation-competent fractions was also seen. Hence, statherin translocation was inhibited by ∼80% (Fig. 5, cf. lanes 5 and 6) and apelin was even more sensitive, showing an inhibition of ∼90% (Fig. 5 cf. lanes 3 and 4). These data demonstrate that the translocation-competent fractions of a variety of short secretory protein precursors are strongly inhibited by WRBcc and suggest that a substantial proportion of all of these polypeptides may be delivered to the ER via TRC40.
Fig. 5.
WRBcc inhibits the ER delivery of translocation-competent fractions of short secretory proteins. Translocation-competent fractions of ppcecA, apelin and statherin were each pooled. Equivalent portions of the pooled fractions were incubated with either 10 µM maltose binding protein (MBP) or 10 µM MBP-WRBcc prior to the addition of membranes. The membrane fraction was recovered and proteins visualised by phosphorimaging following SDS-PAGE. N-glycosylated forms are indicated and the doubly modified products have each been quantified relative to their matched MBP control sample (set to 100%). Results in graph are means ± s.e.
In contrast to the work presented here, a recent study by Shao and Hegde (Shao and Hegde, 2011) utilised non-nuclease-treated, and hence EGTA-free, reticulocyte lysate to study the post-translational translocation of short secretory proteins. In order to better compare our results with this study, we also employed a non-nuclease-treated reticulocyte lysate to address the role of TRC40 in short secretory protein targeting. The untreated lysate continues to support the specific binding of all three of our model short secretory proteins to recombinant TRC40 (supplementary material Fig. S5A). Likewise, following synthesis using untreated lysate, the addition of WRBcc reproducibly inhibits ppcecA translocation into ER-derived microsomes by ∼60% as previously established (cf. Fig. 4B; supplementary material Fig. S5B). We conclude that the TRC40-mediated pathway for the in vitro delivery of short secretory proteins we define in this study is not a direct consequence of the precise in vitro translation system employed for its analysis (see also Discussion).
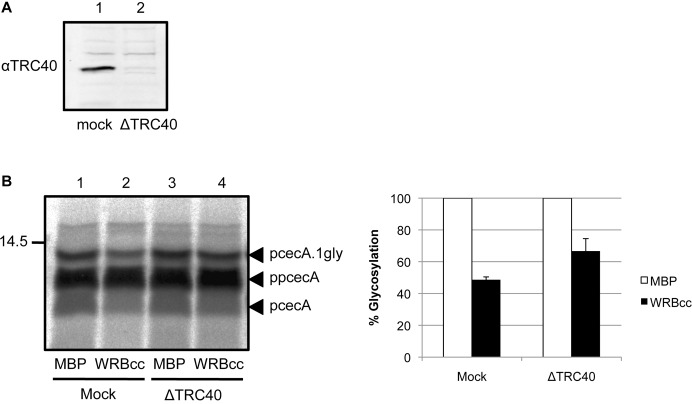
TRC40 is dispensable for ppcecA translocation
In the case of TRC40-dependent TA proteins, immunodepletion of TRC40 results in a strong inhibition of membrane integration (Colombo et al., 2009; Leznicki et al., 2010). In contrast, although we could effectively immunodeplete TRC40 from lysate prior to ppcecA synthesis (Fig. 6A), its removal had no obvious effect on the post-translational translocation of ppcecA (Fig. 6B, lanes 1 and 3). However, when the effect of WRBcc addition was tested using TRC40 immunodepleted lysate, we observe a partial reversal of its inhibitory effect (Fig. 6B, lanes 2 and 4), supporting our hypothesis that WRBcc is acting on the TRC40 pathway as previously defined for TA protein delivery (Vilardi et al., 2011). Interestingly, when ppcecA is synthesised in lysate immunodepleted of TRC40 and subjected to sucrose gradient centrifugation, the peak of translocation-competent fractions appears almost identical to that observed in the presence of TRC40 (supplementary material Fig. S6). Taken together, these data are consistent with multiple yet redundant pathways mediating the delivery of short secretory proteins to the ER that co-fractionate following sucrose gradient centrifugation (see Discussion).
Fig. 6.
WRBcc inhibition is alleviated upon TRC40 immunodepletion. (A) Depletion of TRC40 was confirmed by immunoblotting. (B) Rabbit reticulocyte lysate was immunodepleted for TRC40 or subjected to a mock treatment. Treated lysates were then used as before for in vitro translations and post-translational translocation assays in the presence of 10 µM MBP-WRBcc. N-glycosylated forms are indicated and quantified (means ± s.e.) relative to their matched MBP control samples as before.
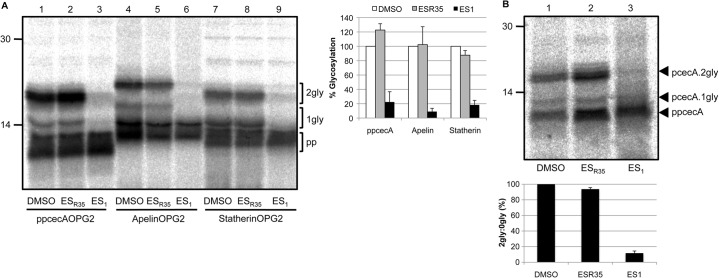
Eeyarestatin I blocks the translocation of short secretory proteins
To date, the TRC40 pathway has only been described in relation to TA proteins that are inserted into the membrane in a process that appears independent of the Sec61 translocon (Rabu et al., 2009; Yabal et al., 2003). However, it has previously been shown that ppcecA translocation can be blocked by inhibitors acting at the Sec61 complex (Cross et al., 2009a; Erdmann et al., 2009). To address the role of the Sec61 complex in the translocation of short human secretory proteins, we utilised the compound eeyarestatin I (ES1), which has previously been shown to block ppcecA translocation by perturbation of the Sec61 translocon (Cross et al., 2009a). ER microsomes were incubated with either solvent alone (DMSO), ES1 or ESR35, an inactive precursor of ES1 (Cross et al., 2009a). The treated microsomes were then used for post-translational translocation assays of ppcecA, apelin and statherin using N-glycosylation as a readout (Fig. 7A) (Cross et al., 2009a). All three precursors showed ∼80% or greater ES1-dependent inhibition of translocation. Interestingly, apelin, which showed the strongest inhibition by recombinant WRBcc (Fig. 5), was also the most sensitive with a ∼90% decrease in translocation (Fig. 7A, lanes 4–6). Importantly, these effects cannot be attributed to the action of ES1 on the ER luminal N-glycosylation machinery, since it has been established that the N-glycosylation of a membrane inserted Cytb5 variant is unaffected by ES1 treatment (Cross et al., 2009a).
Fig. 7.
Eeyarestatin I blocks translocation of short secretory proteins. (A) Canine pancreatic microsomes were incubated with 250 µM ESR35, ES1 or an equal amount of DMSO before being added to a translation reaction performed as previously described. For each precursor, the proportion of doubly N-glycosylated species was expressed relative to their matched DMSO control samples as before. (B) Microsomes treated as above were incubated with pooled translocation-competent fractions of ppcecA. N-glycosylated forms are indicated and, in this case, the ratio of pcecA.2gly to ppcecA is shown to accommodate differences in the amounts of membrane-associated material recovered. Results in graphs are means ± s.e.
As for WRBcc-mediated inhibition (cf. Fig. 5), we wished to confirm that the effect of ES1 reflected the perturbation of a late stage in short secretory protein translocation by studying translocation-competent fractions (Fig. 2). Furthermore, this approach should enrich for precursors directly committed to both TRC40-dependent and TRC40-independent pathways (cf. supplementary material Fig. S6). When the effect of ES1 treatment is analysed using the previously defined translocation-competent fractions of ppcecA (Figs 2, 5), we observe an even more substantial decrease in ER translocation than seen with an unfractionated translation reaction (cf. Fig. 7A,B). This level of inhibition suggests that both pathways are inhibited by ES1 and we therefore conclude that TRC40, and an as yet unidentified alternative route for ppcecA targeting, both facilitate the delivery of precursors to the Sec61 translocon (see also Fig. 8).
Fig. 8.
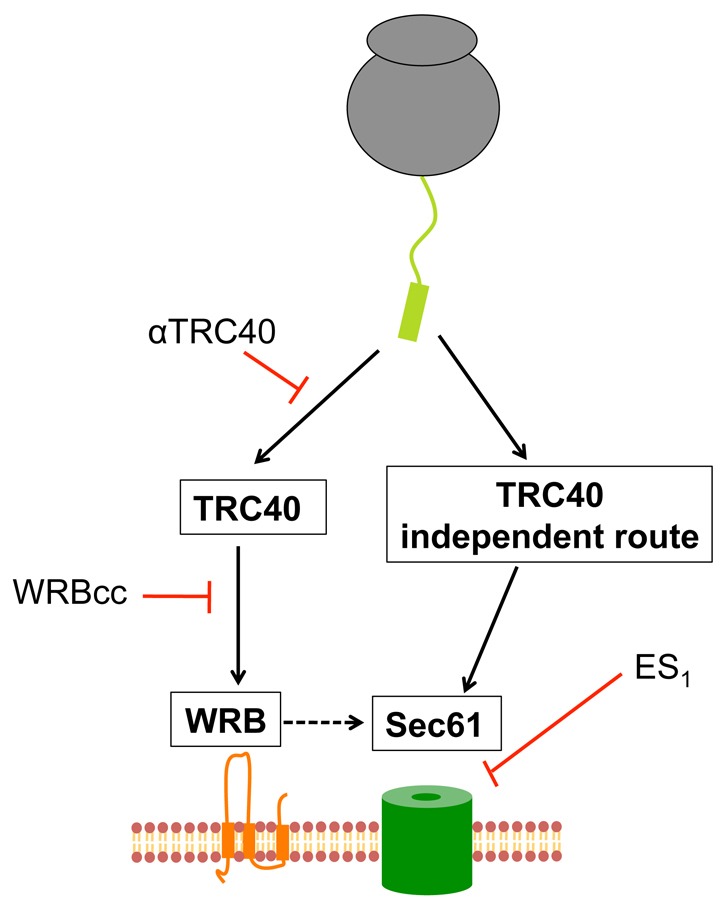
A model for the TRC40-mediated delivery of short secretory proteins to the Sec61 translocon via WRB. Short secretory proteins are bound by TRC40 and targeted to the WRB receptor complex at the ER membrane. After release, the protein is transferred directly, or via unidentified components (dashed line), to the Sec61 complex, which is responsible for the translocation of the protein into the ER lumen. At least one alternative pathway exists that is independent of TRC40 and WRB but also appears to deliver precursors to the Sec61 translocon. We note that post-translational transport of ppcecA into the ER of semi-permeabilised cells is inhibited by siRNA-mediated depletion of Sec61 complex, Sec62 and Sec63 (Lang et al., 2012). In the absence of TRC40 (i.e. after immunodepletion), proteins use the alternative pathway(s). However, when TRC40 is present, WRBcc has a dominant-negative effect because proteins have committed to the TRC40-dependent route.
Discussion
Most previous in vitro studies of the post-translational translocation of short secretory proteins have exploited heterologous precursors from amphibians and insects as convenient models to delineate the process (Zimmermann et al., 1990). We now show that the fundamental aspects of this pathway are equally applicable to short secretory protein precursors of human origin. Specifically, both apelin and statherin are post-translationally translocated in a manner that mirrors that of the well-studied H. cecropia ppcecA.
It seems likely that all classes of short proteins are underrepresented in most databases, largely because many bioinformatic approaches impose an arbitrary cut-off for the minimum size of an open reading frame (Frith et al., 2006). Even so, there are >200 entries for human proteins of between 40 and 100 amino acids with known or predicted N-terminal signal sequences (see www.signalpeptide.de). We conclude that post-translational translocation at the mammalian ER reflects a fundamental biological process occurring in most, if not all, eukaryotes (Zimmermann et al., 2011); and postulate that this route may prove to be responsible for a significant, and previously unappreciated, proportion of secretory protein synthesis in humans (Shao and Hegde, 2011).
The identity of the cytosolic components that facilitate post-translational translocation in higher eukaryotes has been elusive, and indeed chemically synthesised ppcecA can be translocated into ER-derived microsomes in the absence of lysate, albeit less efficiently (Klappa et al., 1991). Equally, studies of other short secretory protein precursors identified a strong nucleotide dependence for the post-translational translocation step (Schlenstedt and Zimmermann, 1987), and identified Hsc70 as one part of a two-component system that can stimulate this process (Dierks et al., 1993; Wiech et al., 1987; Zimmermann et al., 1988). We now show that a second ATP-dependent component, TRC40, can deliver short secretory proteins to the ER, and define a process that involves at least two distinct, but apparently redundant, pathways. This model bears a striking similarity to the biogenesis of TA membrane proteins where TRC40 acts as one of two or more partially redundant routes for ER delivery (Brodsky, 2010; Rabu et al., 2009). TRC40 binds all three short secretory proteins studied and is cross-linked to the signal sequence of ppcecA via a unique cysteine at this location (cf. supplementary material Figs S1, S4). We conclude that the hydrophobic core of such N-terminal signal sequences will most likely occupy the same binding site on TRC40 that was previously shown to accommodate TA regions (see Simpson et al., 2010). Given the overlap that we demonstrate here between the biogenesis of short secretory and tail-anchored proteins, we speculate that the recently identified role for the BAG6 complex in promoting the ubiquitination and degradation of mislocalised proteins (Hessa et al., 2011) may also extend to short secretory proteins that fail to be post-translationally delivered to the ER (cf. Shao and Hegde, 2011).
The interaction of short secretory proteins with TRC40 appears to be functionally relevant since the translocation of these precursors is inhibited by a dominant-negative fragment of WRB, a component of the mammalian TRC40 receptor on the ER membrane (Vilardi et al., 2011). Furthermore, the inhibitory effect of the WRBcc fragment strongly supports our model for multiple pathways that co-fractionate upon sucrose gradient centrifugation and can independently deliver short secretory proteins to the ER (Fig. 8). Hence, we never observe complete inhibition with WRBcc using either a complete translation system or fractions enriched in translocation-competent species. Likewise, the effect of TRC40 immuno-depletion upon ppcecA translocation is also consistent with multiple delivery routes. Thus, whilst the depletion of TRC40 has a marked effect on TA protein insertion at the ER (Colombo et al., 2009; Leznicki et al., 2010), we see no obvious decrease in ppcecA translocation using the same procedure. This suggests that when an interaction with TRC40 is prevented, ppcecA can efficiently utilise an alternative TRC40-independent, delivery route (Fig. 8). Conversely, in the presence of both TRC40 and WRBcc, a higher proportion of the precursor appears to enter a non-productive/dead-end pathway that most likely represents either substrate binding to TRC40 where release is prevented, or by analogy with the yeast Get pathway, competition of WRBcc for the substrate binding site of TRC40 (Fig. 8) (cf. Mariappan et al., 2011; Stefer et al., 2011). The level of WRBcc mediated inhibition we observe suggests that a sizable proportion of short secretory proteins can employ a TRC40-mediated route in our in vitro system. Whilst we have not addressed the nature of the cytosolic component(s) that mediate the TRC40-independent route we observe (cf. Fig. 8), the recent work of Shao and Hegde (Shao and Hegde, 2011) identifies calmodulin as a compelling candidate for this role, both in vitro and in vivo. Paradoxically, the addition of Ca2+-calmodulin inhibits TA protein insertion into the ER membrane in vitro (Hassdenteufel et al., 2011), and the binding and release of calmodulin to both short secretory and TA proteins is modulated by Ca2+ levels, suggesting that there may be a previously unappreciated level of regulation during post-translational protein targeting to the ER (Hassdenteufel et al., 2011; Shao and Hegde, 2011). We speculate that for short secretory proteins pathway selection/preference will most likely vary from substrate to substrate, perhaps reflecting specific properties of the precursor such as signal sequence hydrophobicity (Ng et al., 1996; Rabu et al., 2008; Shao and Hegde, 2011).
Lastly, we addressed the components that mediate the translocation of short secretory proteins across the ER membrane. Whilst previous studies had convincingly implicated the Sec61 complex in this process (Cross et al., 2009a; Erdmann et al., 2009; Klappa et al., 1994), we could not exclude the possibility that this reflected delivery via a TRC40-independent route. We observe a specific, ES1-dependent, reduction in the translocation of all three short secretory protein substrates, varying from 60–90% inhibition consistent with the ability of this small molecule to perturb Sec61 function (Cross et al., 2009a). In the case of ppcecA, when we repeated our analysis using the peak translocation-competent fractions enriched for both TRC40- dependent and TRC40-independent activities, the reduction in translocation was even more striking. On this basis, we conclude that both the TRC40-dependent and TRC40-independent pathways ultimately facilitate the delivery of short secretory proteins to the Sec61 translocon. Whilst ES1 inhibits Sec61 mediated translocation into and across the ER membrane, both in vitro and in vivo, it also perturbs other cellular components and processes (Aletrari et al., 2011; Cross et al., 2009a; McKibbin et al., 2011; Richards et al., 2011). However, our model that both ER delivery routes for ppcecA converge at the canonical Sec61 complex (cf. Fig. 8) is entirely consistent with the effect of siRNA-mediated knockdown of Sec61α, Sec62 and Sec63. In each case, a substantial inhibition of the ppcecA translocation into the ER of semi-permeabilised mammalian cells depleted of these components is observed, strongly supporting a role for the Sec61 complex and associated components in the post-translational translocation of short secretory proteins (Lang et al., 2012). Furthermore, the calmodulin-dependent translocation of ppcecA also appears to exploit the Sec61 translocon (Shao and Hegde, 2011) (cf. Fig. 8). Thus, pathways for both the co- and post-translational translocation of secretory proteins across the ER membrane most likely converge at the well-defined ER translocation complex (Cross et al., 2009b).
Materials and Methods
Materials
Apelin and Statherin clones were obtained from Genscript. The monoclonal anti-opsin tag antibody (Adamus et al., 1991) was provided by Paul Hargrave (Department of Ophthalmology, University of Florida, FL). Commercial antibodies were used to detect Hsp/Hsc70 (Stressgen) and BAG6 (Abcam). Nuclease-treated rabbit reticulocyte lysate for in vitro translation was from Promega. ESR35 and ES1 were prepared as previously described (Cross et al., 2009a; McKibbin et al., 2011).
cDNAs and transcription
PpcecA with two methionine residues in the mature region (Erdmann et al., 2009), was subcloned into the pTNT vector (Promega) in-frame with a fragment of bovine opsin containing either one or two N-glycosylation sites (OPG1/OPG2) (MGPNFYVPFSNKTG/MNGTEGPNFYVPFSNKTG). Wild-type apelin and statherin were also subcloned into pTNT with the OPG2 tag whilst Cytb5OPG and Sec61βOPG are as previously described (Rabu et al., 2008). Transcripts were synthesised from PCR derived templates using T7 RNA polymerase.
In vitro translation and membrane translocation
Proteins were synthesised from RNA transcripts in 20 µl reactions using either nuclease-treated rabbit reticulocyte lysate (Promega) unless otherwise stated, in which case untreated lysate (Promega) that had not been supplemented with EGTA was used. In both cases, translations were performed in the presence of [35S]methionine (0.615 MBq per 20 µl reaction, specific activity 43.48 TBq/mmol) for 15 minutes at 30°C. Puromycin was added to 1 mM and the reaction was incubated for a further 5 minutes at 30°C to ensure the complete release of all polypeptide chains from the ribosome. 10% v/v of an ER microsome suspension (40 A280/ml) was added to the translation reaction and samples incubated for 20 minutes at 30°C.
Membranes were recovered by centrifugation through 120 µl HSC [750 mM sucrose, 500 mM KOAc, 5 mM Mg(OAc)2, 50 mM HEPES-KOH, pH 7.9] at 100,000 g for 10 minutes. The membrane pellet was resuspended in 20 µl LSC [100 mM sucrose, 100 mM KOAc, 5 mM Mg(OAc)2, 50 mM HEPES-KOH pH 7.9, 1 mM DTT] and treated with 250 µg/ml RNaseA at 37°C for 10 minutes to remove any residual peptidyl-tRNA species. The resulting samples were analysed by SDS-PAGE and phosphorimaging. Comparisons of the relative efficiency of protein translocation or membrane insertion are based on the quantification of N-glycosylated species. Unless otherwise stated, the major glycoform of a model protein was quantified for each of the experimental conditions indicated, and the resulting values expressed as a percentage of the relevant matched control (set to 100%). For the data shown in Figs 4,7; supplementary material Fig. S2, Fig. S5B, values represent the mean of three independent experiments and show the s.e. All quantification was performed using Aida software.
WRBcc inhibition assay
Proteins were synthesised using nuclease treated or untreated rabbit reticulocyte lysate in the presence of [35S]methionine for 15 minutes at 30°C as before. The reaction was treated with 1 mM puromycin, immediately split, and MBP (Creative Biomart) or MBP-WRBcc (Vilardi et al., 2011) added to 10 µM. The reaction was incubated for 10 minutes at 30°C prior to addition of microsomes. Alternatively, pooled translocation-competent fractions from a translation reaction carried out using nuclease treated rabbit reticulocyte lysate were incubated with 10 µM MBP or MBP-WRBcc for 10 minutes at 30°C prior to the addition of microsomes. In both cases membranes were recovered and analysed as previously described.
Recombinant TRC40 binding assay
The cDNA encoding TRC40 was cloned into the pET-16B vector (Novagen). A VSV-G tag was added in frame at the 5′ end by PCR resulting in the expression of recombinant TRC40 featuring sequential N-terminal fusions of 10×His and VSV-G tags (His-VSV-TRC40). His-VSV-TRC40 was expressed in Escherichia coli BL21 Gold (DE3) pLysS and purified using Ni-NTA agarose according to standard protocols. A 20 µl translation reaction was performed using nuclease treated lysate (Fig. 3), or untreated lysate (supplementary material Fig. S5A), in the presence of 2 µM TRC40 or buffer (50 mM HEPES-KOH, pH 7.5, 300 mM NaCl, 20 mM imidazole, 10% glycerol) and incubated for 15 minutes at 30°C. Puromycin was added to 1 mM and the reaction was incubated for a further 5 minutes at 30°C. The reaction was diluted with 132 µl of buffer containing 50 mM imidazole, 10 µl (bead volume) Ni-NTA agarose (Qiagen) added, and then incubated for 2 hours at 4°C with shaking. The agarose was pelleted and washed three times with 1 ml buffer containing 50 mM imidazole before elution in 30 µl buffer containing 500 mM imidazole. The resulting samples were analysed by SDS-PAGE and phosphorimaging.
Eeyarestatin treatment
Microsomes were incubated with 250 µM ESR35, ES1 or an equal volume of DMSO for 1 hour on ice as previously described (Cross et al., 2009a). Microsomes were then added to translations for translocation reactions as before.
Sucrose gradient centrifugation
Sucrose gradient centrifugation was performed as previously described (Leznicki et al., 2010). Briefly, gradients were prepared with 5–25% sucrose in physiological salt buffer [100 mM KOAc, 50 mM HEPES, pH 7.4, 2 mM Mg(OAc)2]. 90 µl translations were layered on top and subjected to sedimentation at 100,000 g for 5 hours at 4°C using a TLS-55 swing out rotor (Beckman). Individual fractions of 76 µl (1–13) were collected from the top and either analysed directly or used in further experiments. The ability of individual fractions to support translocation into ER microsomes was tested as previously described (Leznicki et al., 2010). In subsequent experiments, translocation-competent fractions were pooled and 20 µl incubated with 15% v/v ER microsomes for 50 minutes at 30°C. Membranes were isolated and analysed as described for the membrane translocation reaction.
Crosslinking and immunoprecipitation
Translocation competent fractions prepared by sucrose gradient centrifugation were pooled and incubated for 10 minutes on ice with 1 mM bismaleimidohexane, diluted from a 20 mM stock in DMSO. The reaction was quenched by incubation with 20 mM β-mercaptoethanol on ice for 5 minutes. Denaturing immunoprecipitations were carried out as described previously (Abell et al., 2003).
Immunodepletion of TRC40
TRC40 was immunodepleted from the rabbit reticulocyte lysate as previously described (Leznicki et al., 2010). Briefly, 20 µl (bead volume) of protein-A Sepharose was incubated with 15 µl antibody raised against TRC40 for 1 hour at 4°C. The beads were washed once with 1 ml PBS and then incubated with 120 µl rabbit reticulocyte lysate for 1 hour at 4°C. The beads were then pelleted and the immunodepleted lysate removed and used in translation reactions as normal. Mock immunodepletions were carried out in parallel, replacing the antibody with an equal volume of water.
Western blotting
Proteins were separated by SDS-PAGE, and transferred to Immobilon-FL PVDF membrane (Millipore, Watford, UK) for 1.5 hours at 300 mA. Membranes were blocked for 1 hour with 1× blocking buffer (Sigma, Poole, UK) in TBS (10 mM Tris-HCl, pH 8.0, 150 mM NaCl), then incubated with primary antibodies overnight at 4°C in 1× TBS/Sigma blocking buffer supplemented with 0.1% Tween-20. Membranes were washed three times for 10 minutes each with TBS containing 0.1% Tween-20 (TBST), then incubated in the dark for 1 hour at room temperature with IRDye 800CW goat anti-mouse/rabbit/chicken IgG (LI-COR Biosciences, Cambridge, UK) diluted 1:5000 in 1× TBST/Sigma blocking buffer containing 0.01% SDS. After three further washes with TBST, proteins were visualised with the Odyssey Infrared Imaging System (LI-COR Biosciences).
Supplementary Material
Acknowledgments
We are indebted to Katja Kapp for her assistance in the identification of candidate short secretory proteins, and Martin Pool and Rostislav Veselinov for their comments during preparation of the manuscript. Special thanks go to Bernhard Dobberstein for his support and encouragement throughout the course of the project.
Footnotes
Funding
This work was supported by a PhD studentship from the BBSRC (N.J.), and project grant funding from the Wellcome Trust [grant number WT092107] (P.L. and S.H.). Deposited in PMC for immediate release.
Note added in proof
Lakkaraju and colleagues (Lakkaraju et al., 2012) recently identified a role for mammalian Sec62 during the post-translational translocation of secretory protein precursors of up to 160 amino acids long.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.102608/-/DC1
References
- Abell B. M., Jung M., Oliver J. D., Knight B. C., Tyedmers J., Zimmermann R., High S. (2003). Tail-anchored and signal-anchored proteins utilize overlapping pathways during membrane insertion. J. Biol. Chem. 278, 5669–5678 10.1074/jbc.M209968200 [DOI] [PubMed] [Google Scholar]
- Abell B. M., Rabu C., Leznicki P., Young J. C., High S. (2007). Post-translational integration of tail-anchored proteins is facilitated by defined molecular chaperones. J. Cell Sci. 120, 1743–1751 10.1242/jcs.002410 [DOI] [PubMed] [Google Scholar]
- Adamus G., Zam Z. S., Arendt A., Palczewski K., McDowell J. H., Hargrave P. A. (1991). Anti-rhodopsin monoclonal antibodies of defined specificity: characterization and application. Vision Res. 31, 17–31 10.1016/0042-6989(91)90069-H [DOI] [PubMed] [Google Scholar]
- Aletrari M. O., McKibbin C., Williams H., Pawar V., Pietroni P., Lord J. M., Flitsch S. L., Whitehead R., Swanton E., High S., et al. (2011). Eeyarestatin 1 interferes with both retrograde and anterograde intracellular trafficking pathways. PLoS ONE 6, e22713 10.1371/journal.pone.0022713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J. L. (2010). The special delivery of a tail-anchored protein: why it pays to use a dedicated courier. Mol. Cell 40, 5–7 10.1016/j.molcel.2010.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden K. A. (2005). Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250 10.1038/nrmicro1098 [DOI] [PubMed] [Google Scholar]
- Castan–Laurell I., Dray C., Attané C., Duparc T., Knauf C., Valet P. (2011). Apelin, diabetes, and obesity. Endocrine 40, 1–9 10.1007/s12020-011-9507-9 [DOI] [PubMed] [Google Scholar]
- Colombo S. F., Longhi R., Borgese N. (2009). The role of cytosolic proteins in the insertion of tail-anchored proteins into phospholipid bilayers. J. Cell Sci. 122, 2383–2392 10.1242/jcs.049460 [DOI] [PubMed] [Google Scholar]
- Cross B. C., McKibbin C., Callan A. C., Roboti P., Piacenti M., Rabu C., Wilson C. M., Whitehead R., Flitsch S. L., Pool M. R., et al. (2009a). Eeyarestatin I inhibits Sec61-mediated protein translocation at the endoplasmic reticulum. J. Cell Sci. 122, 4393–4400 10.1242/jcs.054494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B. C., Sinning I., Luirink J., High S. (2009b). Delivering proteins for export from the cytosol. Nat. Rev. Mol. Cell Biol. 10, 255–264 10.1038/nrm2657 [DOI] [PubMed] [Google Scholar]
- Dierks T., Klappa P., Wiech H., Zimmermann R., Lindquist S., Welch W. J., Viitanen P., Lorimer G. H. (1993). The role of molecular chaperones in protein transport into the endoplasmic reticulum. Philos. Trans. R. Soc. Lond. B Biol. Sci. 339, 335–341 10.1098/rstb.1993.0032 [DOI] [PubMed] [Google Scholar]
- Erdmann F., Jung M., Eyrisch S., Lang S., Helms V., Wagner R., Zimmermann R. (2009). Lanthanum ions inhibit the mammalian Sec61 complex in its channel dynamics and protein transport activity. FEBS Lett. 583, 2359–2364 10.1016/j.febslet.2009.06.032 [DOI] [PubMed] [Google Scholar]
- Falcão–Pires I., Leite–Moreira A. F. (2005). Apelin: a novel neurohumoral modulator of the cardiovascular system. Pathophysiologic importance and potential use as a therapeutic target. Rev. Port. Cardiol. 24, 1263–1276 [PubMed] [Google Scholar]
- Favaloro V., Spasic M., Schwappach B., Dobberstein B. (2008). Distinct targeting pathways for the membrane insertion of tail-anchored (TA) proteins. J. Cell Sci. 121, 1832–1840 10.1242/jcs.020321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith M. C., Forrest A. R., Nourbakhsh E., Pang K. C., Kai C., Kawai J., Carninci P., Hayashizaki Y., Bailey T. L., Grimmond S. M. (2006). The abundance of short proteins in the mammalian proteome. PLoS Genet. 2, e52 10.1371/journal.pgen.0020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassdenteufel S., Schäuble N., Cassella P., Leznicki P., Müller A., High S., Jung M., Zimmermann R. (2011). Ca2+-calmodulin inhibits tail-anchored protein insertion into the mammalian endoplasmic reticulum membrane. FEBS Lett. 585, 3485–3490 10.1016/j.febslet.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde R. S., Keenan R. J. (2011). Tail-anchored membrane protein insertion into the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 12, 787–798 10.1038/nrm3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessa T., Sharma A., Mariappan M., Eshleman H. D., Gutierrez E., Hegde R. S. (2011). Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature 475, 394–397 10.1038/nature10181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappa P., Mayinger P., Pipkorn R., Zimmermann M., Zimmermann R. (1991). A microsomal protein is involved in ATP-dependent transport of presecretory proteins into mammalian microsomes. EMBO J. 10, 2795–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappa P., Zimmermann M., Zimmermann R. (1994). The membrane proteins TRAMp and sec61 alpha p may be involved in post-translational transport of presecretory proteins into mammalian microsomes. FEBS Lett. 341, 281–287 10.1016/0014-5793(94)80473-7 [DOI] [PubMed] [Google Scholar]
- Kutay U., Hartmann E., Rapoport T. A. (1993). A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 3, 72–75 10.1016/0962-8924(93)90066-A [DOI] [PubMed] [Google Scholar]
- Kutay U., Ahnert–Hilger G., Hartmann E., Wiedenmann B., Rapoport T. A. (1995). Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane. EMBO J. 14, 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A K K., Thankappan R., Mary C., Garrison J L., Taunton J., Strub K. (2012). Efficient secretion of small proteins in mammalian cells relies on Sec62-dependent posttranslational translocation. Mol. Biol. Cell. 23, 2712–2722 10.1091/mbc.E12-03-0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S., Benedix J., Fedeles S. V., Schorr S., Schirra C., Schäuble N., Jalal C., Greiner M., Haßdenteufel S., Tatzelt J., et al. (2012). Different effects of Sec61α, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. J. Cell Sci. 125, 1958–1969 10.1242/jcs.096727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leznicki P., Clancy A., Schwappach B., High S. (2010). Bat3 promotes the membrane integration of tail-anchored proteins. J. Cell Sci. 123, 2170–2178 10.1242/jcs.066738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leznicki P., Warwicker J., High S. (2011). A biochemical analysis of the constraints of tail-anchored protein biogenesis. Biochem. J. 436, 719–727 10.1042/BJ20101737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan M., Li X., Stefanovic S., Sharma A., Mateja A., Keenan R. J., Hegde R. S. (2010). A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature 466, 1120–1124 10.1038/nature09296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan M., Mateja A., Dobosz M., Bove E., Hegde R. S., Keenan R. J. (2011). The mechanism of membrane-associated steps in tail-anchored protein insertion. Nature 477, 61–66 10.1038/nature10362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin C., Mares A., Piacenti M., Williams H., Roboti P., Puumalainen M., Callan A. C., Lesiak–Mieczkowska K., Linder S., Harant H., et al. (2011). Inhibition of protein translocation at the endoplasmic reticulum promotes activation of the unfolded protein response. Biochem. J. 442, 639–648 10.1042/BJ20111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Zimmermann R. (1987). Import of honeybee prepromelittin into the endoplasmic reticulum: structural basis for independence of SRP and docking protein. EMBO J. 6, 2099–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Zimmermann R. (1988). Import of honeybee prepromelittin into the endoplasmic reticulum: energy requirements for membrane insertion. EMBO J. 7, 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D. T., Brown J. D., Walter P. (1996). Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J. Cell Biol. 134, 269–278 10.1083/jcb.134.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabu C., Wipf P., Brodsky J. L., High S. (2008). A precursor-specific role for Hsp40/Hsc70 during tail-anchored protein integration at the endoplasmic reticulum. J. Biol. Chem. 283, 27504–27513 10.1074/jbc.M804591200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabu C., Schmid V., Schwappach B., High S. (2009). Biogenesis of tail-anchored proteins: the beginning for the end? J. Cell Sci. 122, 3605–3612 10.1242/jcs.041210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T. A., Matlack K. E., Plath K., Misselwitz B., Staeck O. (1999). Posttranslational protein translocation across the membrane of the endoplasmic reticulum. Biol. Chem. 380, 1143–1150 10.1515/BC.1999.145 [DOI] [PubMed] [Google Scholar]
- Richards R., Scholz I., Powers C., Skach W. R., Früh K. (2011). The cytoplasmic domain of rhesus cytomegalovirus Rh178 interrupts translation of major histocompatibility class I leader peptide-containing proteins prior to translocation. J. Virol. 85, 8766–8776 10.1128/JVI.05021-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G., Dobberstein B. (1996). Common principles of protein translocation across membranes. Science 271, 1519–1526 10.1126/science.271.5255.1519 [DOI] [PubMed] [Google Scholar]
- Schlenstedt G., Zimmermann R. (1987). Import of frog prepropeptide GLa into microsomes requires ATP but does not involve docking protein or ribosomes. EMBO J. 6, 699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt G., Gudmundsson G. H., Boman H. G., Zimmermann R. (1990). A large presecretory protein translocates both cotranslationally, using signal recognition particle and ribosome, and post-translationally, without these ribonucleoparticles, when synthesized in the presence of mammalian microsomes. J. Biol. Chem. 265, 13960–13968 [PubMed] [Google Scholar]
- Schlenstedt G., Gudmundsson G. H., Boman H. G., Zimmermann R. (1992). Structural requirements for transport of preprocecropinA and related presecretory proteins into mammalian microsomes. J. Biol. Chem. 267, 24328–24332 [PubMed] [Google Scholar]
- Schlesinger D. H., Hay D. I. (1977). Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J. Biol. Chem. 252, 1689–1695 [PubMed] [Google Scholar]
- Schuldiner M., Metz J., Schmid V., Denic V., Rakwalska M., Schmitt H. D., Schwappach B., Weissman J. S. (2008). The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 134, 634–645 10.1016/j.cell.2008.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S., Hegde R. S. (2011). A calmodulin-dependent translocation pathway for small secretory proteins. Cell 147, 1576–1588 10.1016/j.cell.2011.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. J., Schwappach B., Dohlman H. G., Isaacson R. L. (2010). Structures of Get3, Get4, and Get5 provide new models for TA membrane protein targeting. Structure 18, 897–902 10.1016/j.str.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic S., Hegde R. S. (2007). Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell 128, 1147–1159 10.1016/j.cell.2007.01.036 [DOI] [PubMed] [Google Scholar]
- Stefer S., Reitz S., Wang F., Wild K., Pang Y. Y., Schwarz D., Bomke J., Hein C., Löhr F., Bernhard F., et al. (2011). Structural basis for tail-anchored membrane protein biogenesis by the Get3-receptor complex. Science 333, 758–762 10.1126/science.1207125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardi F., Lorenz H., Dobberstein B. (2011). WRB is the receptor for TRC40/Asna1-mediated insertion of tail-anchored proteins into the ER membrane. J. Cell Sci. 124, 1301–1307 10.1242/jcs.084277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Brown E. C., Mak G., Zhuang J., Denic V. (2010). A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol. Cell 40, 159–171 10.1016/j.molcel.2010.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech H., Sagstetter M., Müller G., Zimmermann R. (1987). The ATP requiring step in assembly of M13 procoat protein into microsomes is related to preservation of transport competence of the precursor protein. EMBO J. 6, 1011–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabal M., Brambillasca S., Soffientini P., Pedrazzini E., Borgese N., Makarow M. (2003). Translocation of the C terminus of a tail-anchored protein across the endoplasmic reticulum membrane in yeast mutants defective in signal peptide-driven translocation. J. Biol. Chem. 278, 3489–3496 10.1074/jbc.M210253200 [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Mollay C. (1986). Import of honeybee prepromelittin into the endoplasmic reticulum. Requirements for membrane insertion, processing, and sequestration. J. Biol. Chem. 261, 12889–12895 [PubMed] [Google Scholar]
- Zimmermann R., Sagstetter M., Lewis M. J., Pelham H. R. (1988). Seventy-kilodalton heat shock proteins and an additional component from reticulocyte lysate stimulate import of M13 procoat protein into microsomes. EMBO J. 7, 2875–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Zimmermann M., Wiech H., Schlenstedt G., Müller G., Morel F., Klappa P., Jung C., Cobet W. W. (1990). Ribonucleoparticle-independent transport of proteins into mammalian microsomes. J. Bioenerg. Biomembr. 22, 711–723 [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Eyrisch S., Ahmad M., Helms V. (2011). Protein translocation across the ER membrane. Biochim. Biophys. Acta 1808, 912–924 10.1016/j.bbamem.2010.06.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.