Abstract
Individuals who engage in corrupt and immoral behavior are in some ways similar to psychopaths. Normal people refrain from engaging in such behaviors because they tie together the moral value of society and the risk for punishment when they violate social rules. What is it, then, that allows these immoral individuals to behave in this manner, and in some situations to even prosper? When there is a dysfunction of somatic markers, specific disadvantageous impairments in decision-making arise, for example in moral judgment, but paradoxically, under some circumstances, the damage can cause the patient to make optimal financial investment decisions. Interestingly, individuals with psychopathy, a personality disorder, share many of these same behavioral characteristics as those seen in VMPFC and amygdala lesion patients, suggesting that defective somatic markers may serve as a neural framework for explaining immoral and corrupt behaviors. While these sociopathic behaviors of sometimes famous and powerful individuals have long been discussed primarily within the realm of social science and psychology, here we offer a neurocognitive perspective on possible neural roots for immoral and corrupt behaviors.
Keywords: somatic marker hypothesis, psychopathy, corruption, immorality, decision-making
Introduction
We begin by asking about the definition of corruption. According to the Webster English Dictionary, corruption is an impairment of integrity, virtue, or moral principle; inducement to wrong by improper or unlawful means (as bribery; Merriam-Webster Online English Dictionary, 2010). Many people may fit this description, however, psychopaths are one psychiatric group whose social behaviors strongly match this definition. Borrowing from the definition of psychopathy, a psychopath is “…a self-centered, callous, and remorseless person profoundly lacking in empathy,” (Hare, 1999, p.2) and are also considered “social predators who charm, manipulate, and ruthlessly plow their way through life… completely lacking in conscience and in feelings for others” (Hare, 1999, p. xi). They break the moral code of society through various antisocial acts, mostly for personal gain. How does one explain their behavior?
Though the term “psychopath” has long been used colloquially to describe those whose destructive and immoral behaviors do not fit neatly into the social thread of society, in the scientific literature it is considered a personality disorder unto itself (Hare, 1996). What exactly characterizes one as a psychopath? Based on Hervey Cleckley’s clinical observations and interactions with institutionalized psychopaths, he wrote what many consider to be the definitive book on psychopathy, The Mask of Sanity (Cleckley, 1982). He became a pioneer of the field when he provided a clinical description of the disorder, including a list of sixteen behavioral criteria (e.g., having superficial charm, lack of remorse, poor judgment, failure to follow any life plan, failure to learn by experience) that help characterize a person as a psychopath, and which would be later used by Robert Hare in making his own Psychopathy Checklist (PCL-R: Hare, 1991). Cleckley emphasized that although it should be considered a mental illness, there was no delirium or delusion to be observed, and in fact, there seemed to be an absence of “a lesion of the intellect” at all (Cleckley, 1982, p.122). Within psychopathy there has been a theoretical distinction suggested between primary and secondary variants. Karpman’s classic theory is that primary psychopaths are “born” with the core interpersonal and affective features of the disorder; whereas secondary psychopaths develop similar traits in response to such adverse environmental experiences as parental rejection and abuse (Karpman, 1941). Trait anxiety is traditionally used to distinguish between the two subtypes. Many psychopaths can function normally in society and they have been labeled as successful or “functional psychopaths” (Spencer, 2005, p. D1).
In this paper, we would like to offer a somatic marker perspective on corrupt and immoral behavior, using psychopathy as an example. The field of decision-making neuroscience is well developed, and in parallel, the extensive work on psychopathy has also become well established over the decades. The pioneering work of Adrian Raine (Raine, Lee, Yang, & Colletti, 2010), James Blair (Blair, 2008), as well as Michael Koenigs and Joseph Newman (Koenigs, Kruepke, & Newman, 2010), and J. Moll and colleagues (Moll, Zahn, de Oliveira-Souza, Krueger, & Grafman, 2005) have made the link between abnormalities in the prefrontal cortex, amygdala, and the septal region, all of which are neural regions implicated in decision-making, moral judgment and the SMH, with psychopathy. While the literature on psychopathy (especially primary psychopathy) and its neural correlates is relatively rich, the current perspective capitalizes on this existing literature and expands it in order to offer a potential neural road map to investigate and understand the underpinnings of a commonly encountered but generally overlooked social behavior, namely immoral and corrupt behavior, which has been discussed and described in the literature as secondary psychopathy. Therefore, hard empirical evidence that causally ties brain mechanisms on one hand, and psychopathic behavior on the other hand, is naturally lacking. However, the gathering of the available information from a variety of decision-making tasks, a careful analysis of the different types of decisions that may be engaged by different tasks, and the known neural correlates for these mechanisms of decisions provide a compelling rationale for the perspective presented here on the use of the somatic marker framework as a neural guide for future understanding of the complicated behavioral and neural mechanisms associated with psychopathy, moral judgment, and their implications for immoral and corrupt behaviors.
Statement of the Problem
One key question relates to whether there is a neural basis for such a psychopathic behavior, and more specifically a corrupt behavior. We propose that people do not normally engage in immoral and corrupt behavior primarily because they tie together the moral value of society and the risk for punishment when they violate social rules (e.g. accepting bribery). However, there are two possibilities for why some people may engage in corrupt behavior. One is that they have an abnormal VMPFC function. Indeed there are striking similarities between psychopaths and patients who have lesions of the VMPFC with respect to characteristics that include lack of empathy, irresponsibility, poor decision-making, inappropriate social behavior, failure to plan ahead, and diminished sense of guilt (Krajbich, Adolphs, Tranel, Denburg, & Camerer, 2009; Koenigs, Kruepke, & Newman, 2010). Studies examining the neural correlates of moral judgment reveal regions that overlap with the same VMPFC areas implicated in lesion patients (Moll et al., 2005). Like VMPFC patients, psychopaths can know and say “the right thing,” but do “the wrong thing” (Cleckley, 1982).
Since a psychopath does not show an obvious lesion in the VMPFC, what brings about this putative VMPFC dysfunction? Genetic or environmental factors can lead to abnormal wiring of the prefrontal cortex and alterations in its function. For example, early life stressors are known to cause alterations in the wiring of the frontostriatal neural circuitry, thereby leading to behaviors associated with frontal lobe dysfunction (Braun, Lange, Metzger, & Poeggel, 2000; Hanson et al., 2010). Additionally, variations in the serotonin transporter gene-linked polymorphic region (5-HTTLPR) exert a great influence on decision making under uncertainty (He et al., 2010), as well as affecting functional connectivity between the VMPFC and amygdala (Heinz et al., 2005). In the disorder of psychopathy, these kinds of neurobiological aberrations may have varying degrees of abnormalities that may lead to a wide range of psychopathic behaviors, with crimes and violence on one extreme (primary psychopathy) to antisocial behavior on the other extreme (secondary psychopathy). However, another possibility for both primary (e.g., belonging to a gang and shooting innocent people) and secondary (e.g., just a corrupt behavior) psychopathy is a faulty learning environment (i.e., learning that killing or corrupt behavior is good), which does not necessarily reflect an underlying brain problem as a precursor. The importance of distinguishing between psychopathic behaviors rooted in abnormalities in VMPFC functions (and somatic marker activation), versus psychopathic behaviors that are willful and controlled because they are learned in certain environmental contexts, is very crucial. The reason is that in the former, neurological evidence suggests that individuals with decision-making impairments resulting from VMPFC damage never learn from repeated mistakes (Bechara & Damasio, 2005), and especially when the damage is acquired earlier on in life (i.e., the earlier the damage, the worse the behavioral outcome in adulthood; Anderson, Bechara, Damasio, Tranel, & Damasio, 1999). In contrast, in the latter, the individuals have normal brains, and they will likely adjust their behavior when the social and learning contingencies are changed, such as increasing the risk for negative consequences to their “corrupt” actions. Hence our primary objectives for this article are to show that damaged VMPFC leads to (1) impaired judgment and decision-making, and failure to learn from repeated mistakes, despite high intellect and explicit knowledge of the consequences of their decisions; (2) under certain circumstances, and paradoxically, the damage leads to higher risk taking that results in making optimal financial investment decisions; and (3) impairment in moral judgment. All these behaviors are characteristics of individuals with psychopathic traits, including those who engage in corrupt and immoral conducts. Although the neural circuitry underlying moral behavior has been explored previously by Moll and colleagues (Moll, de Oliveira-Souza, Bramati, & Grafman, 2002; Moll et al., 2002), our perspective relies further on our understanding of the neural basis of these behaviors in neurological patients (e.g., those with VMPFC damage). Taken together, we propose a perspective on the neural basis of corrupt and immoral behavior, using the somatic marker hypothesis (SMH; Damasio, 1994; Bechara & Damasio, 2005) as a theoretical guide.
Impaired judgment and decision-making after VMPFC damage
The Somatic Marker Hypothesis: Overview
One of the first and most famous cases of the so-called “frontal lobe syndrome” was the patient Phineas Gage, described by Harlow (Harlow, 1848, 1868). Interestingly, the case of Phineas Gage, and similar cases that were described after him, received little attention for many years. The revival of interest in this case, and in various aspects of the “frontal lobe syndrome,” came from the patient described by Eslinger and Damasio (Eslinger & Damasio, 1985). Over the years, we have studied numerous patients with VMPFC lesions. Such patients develop severe impairments in personal and social decision-making, in spite of otherwise largely preserved intellectual abilities. These patients were intelligent and creative before their brain damage. After the damage, the actions they elect to pursue, often lead to losses of diverse order, e.g., financial losses, losses in social standing, losses of family and friends. The choices they make are no longer advantageous, and are remarkably different from the kinds of choices they were known to make before their brain damage. These patients often decide against their best interests. They are unable to learn from previous mistakes as reflected by repeated engagement in decisions that lead to negative consequences. In striking contrast to this real-life decision-making impairment, the patients perform normally in most laboratory tests of problem solving. Their intellect remains normal, as measured by conventional clinical neuropsychological tests.
Damasio proposed the SMH (Damasio, 1994), which posits that the neural basis of the decision-making impairment characteristic of patients with VMPFC damage is defective activation of somatic states (emotional signals) that attach value to given options and scenarios. These emotional signals (which are perceived by specific neural regions in the brain) function as covert, or overt, biases for guiding decisions. Deprived of these emotional signals, patients may resort to deciding based on the immediate reward of an option. The failure to enact somatic states, and consequently to decide advantageously, results from dysfunction in a neural system in which the VMPFC is a critical component. However, the VMPFC is not the only region. Other neural regions, including the amygdala, insula and somatosensory cortices, dorsolateral prefrontal cortex and hippocampus, are also components of this same neural system, although the different regions may provide different contributions to the overall process of decision-making (Bechara & Damasio, 2005) (Figure 1). Thus the somatic marker framework articulated elsewhere (e.g., Bechara and Damasio, 2005) does not simply provide a list of brain structures involved in somatic marker activation; rather the framework provides a detailed account of how different neural systems play different roles in the overall process of decision making. [Figure 1 about here].
Figure 1.
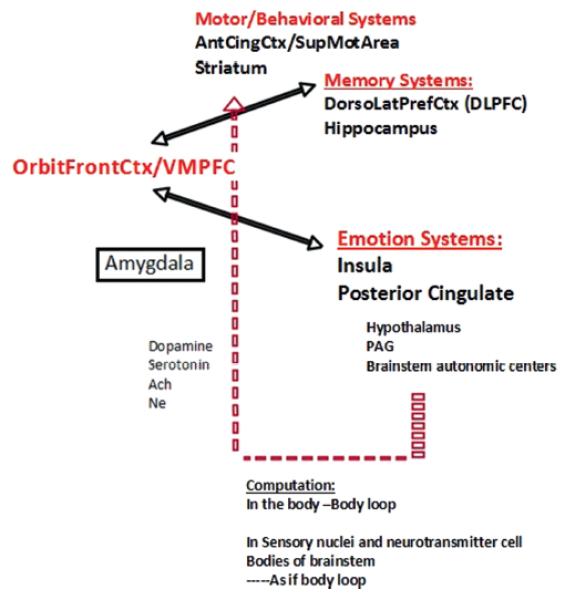
A schematic of all the brain regions involved in decisionmaking according to the somatic marker hypothesis.
More specifically, the amygdala as well as the VMPFC are critical structures for triggering somatic states, but the amygdala seems more important for triggering somatic states from emotional events that occur in the environment (that is, primary inducers), whereas the VMPFC region seems more important for triggering somatic states from memories, knowledge, and cognition (that is, secondary inducers; Bechara & Damasio, 2005). Decision-making is a complex process that relies on the integrity of at least two sets of neural systems: (1) one set is important for memory (e.g., the hippocampus), and especially working memory (e.g., the dorsolateral prefrontal cortex), in order to bring online knowledge and information used during the deliberation of a decision; (2) another set is important for triggering emotional responses. This set includes effector structures such as the hypothalamus and autonomic brainstem nuclei that produce changes in internal milieu and visceral structures along with other effector structures such as the ventral striatum, periacqueductal gray, and other brainstem nuclei, which produce changes in facial expression and specific approach or withdrawal behaviors. It also includes cortical structures that receive afferent input from the viscera and internal milieu, such as the insular cortex and the posterior cingulate gyrus, retrosplenial cortex, and cuneus region.
During the process of pondering decisions, the immediate prospects of an option may be driven by more subcortical mechanisms (e.g., via the amygdala) that do not require a prefrontal cortex. However, weighing the future consequences requires a prefrontal cortex for triggering somatic responses about possible future consequences. Specifically, when pondering the decision, the immediate and future prospects of an option may trigger numerous somatic responses that conflict with each other (i.e., positive and negative somatic responses). The end result, though, is that an overall positive or negative signal emerges (a “go” or “stop” signal). There is a debate as to where this overall somatic state may be computed. We have argued that this computation occurs in the body proper (via the so-called body loop), but it can also occur in the brain itself, in areas that represent “body” states such as the dorsal tegmentum of the midbrain, or areas such as the insula and posterior cingulate (via the so-called as-if-body loop). The controversy surrounding the hypothesis has largely been in relation to the body loop, with certain investigators arguing that decision-making is not necessarily dependent on “somatic markers” expressed in the body (e.g., Dunn, Dalgleish, & Lawrence, 2006; Maia & McClelland, 2004), which we admit is the weakest link of the theory,—but also see Bechara, Damasio, Tranel, & Damasio, 2005 and Persaud, McLeod, & Cowey, 2007 for counter arguments. Irrespective of whether this computation occurs in the body itself, or within the brain, we have proposed that the emergence of this overall somatic state is consistent with the principles of natural selection. In other words, numerous and conflicting signals may be triggered simultaneously, but stronger ones gain selective advantage over weaker ones, until a winner takes all emerges, a positive or negative somatic state that emerges, and consequently bias the decision one way or the other (Bechara et al., 2005).
In order for somatic signals to influence cognition and behavior, they must act on the appropriate neural systems. One target for somatic state action is the striatum. A large number of channels convey body information (that is, somatic signals) to the central nervous system (e.g., spinal cord, vagus nerve, and humoral signals). Evidence suggests that the vagal route is especially critical for relaying somatic signals (Martin, Denburg, Tranel, Granner, & Bechara, 2004). Further, it was proposed that the next link in this body-brain channel involves neurotransmitter systems (Bechara & Damasio, 2005; Damasio, 1996). Indeed, the cell bodies of the neurotransmitter dopamine, serotonin, noradrenaline, and acetylcholine are located in the brainstem; the axon terminals of these neurotransmitter neurons synapse on cells and/or terminals all over the cortex and striatum (Blessing, 1997). When somatic state signals are transmitted to the cell bodies of dopamine or serotonin neurons, for example, the signaling influences the pattern of dopamine or serotonin release at the terminals. In turn, changes in dopamine or serotonin release will modulate synaptic activities of neurons sub-serving behavior and cognition within the cortex. Preliminary pharmacological studies indicate that learning to perform advantageously on the IGT is influenced by at least two neurotransmitter systems: dopamine and serotonin (Bechara, 2003; Sevy et al., 2007). This chain of neural mechanisms provides a way for somatic states to exert a biasing effect on decisions. At the cellular, and more recently the functional neuroimaging level, the pioneering work of Schultz et al. (1997) has emphasized the role of dopamine in reward processing and error prediction (Schultz, Dayan, & Montague, 1997). While the cellular work of Schultz and colleagues focused solely on dopamine, and while the functional neuroimaging work cannot speak directly as to whether dopamine or serotonin is involved, our work and that of several others (Fineberg, 2010) suggest that both dopamine and serotonin contribute to the ability to learn from previous mistakes (e.g., improved learning on the IGT), a behavioral process that has been termed in more recent literature as reward prediction error. Thus, while our work with the IGT clearly demonstrates a reward prediction error curve (i.e., the subject adjusts their next response based on the outcome of the previous trial), we did not use the same term. Given the fact that the dopamine mechanism addresses only one specific component of a larger neural network that is important for implementing decisions, it is quite possible that the terms “somatic marker” and “dopamine reward prediction error signal” are two different terms that describe the same behavior.
Empirical Tests of the Somatic Marker Hypothesis
For many years, VMPFC patients presented a puzzling defect, because it was difficult to explain their disturbance in terms of defects in knowledge pertinent to the situation or deficient general intellectual ability. Although the decision-making impairment was obvious in the real-world behavior life of these patients, there was no effective laboratory probe to detect and measure this impairment. Bechara’s development of what became known as the Iowa Gambling Task (IGT; Bechara, 1994) has enabled researchers, for the first time, to detect the decision-making impairment characteristic of patients with VMPFC lesions and investigate its possible causes. Such work using the IGT has provided the key empirical support for the proposal that somatic markers significantly influence decision-making (Bechara & Damasio, 2005). Why was the IGT successful in detecting the decision-making impairment in VMPFC patients, and why is it important for the study of the neurology of decision-making? Perhaps this is because the IGT mimics real-life decisions so closely. The task is carried out in real-time and it resembles real-world contingencies. It factors reward and punishment (i.e. winning and losing money) in such a way that it creates a conflict between an immediate, luring reward and a delayed, probabilistic punishment. Therefore, the task engages the subject in a quest to make advantageous choices. Each choice is full of uncertainty because a precise calculation or prediction of the outcome of a given choice is not possible.
Results of studies using the IGT revealed that the performance profile of patients with VMPFC lesions is comparable to their real-life inability to decide advantageously. This is especially true in personal and social matters, a domain for which in life, as in the task, an exact calculation of the future outcomes is not possible and choices must be based on hunches and gut feelings. Further studies addressed the question of whether the behavioral decision-making impairment in VMPFC lesion patients is linked to a failure in somatic (emotional) signaling (Bechara, Tranel, Damasio, & Damasio, 1996). We studied IGT performance of two groups, normal subjects and VMPFC lesion patients, while we recorded their electrodermal activity (skin conductance responses; SCRs), which provides an indirect measure of the emotion experienced by the subject. Both normal subjects and VMPFC patients generated SCRs after they had picked a card and were told that they won or lost money. The most important difference, however, was that normal subjects, as they became experienced with the task, began to generate SCRs prior to the selection of any cards, i.e., during the time when they were pondering from which deck to choose. These anticipatory SCRs were more pronounced before picking a card from the disadvantageous choices (risky decks), when compared to the advantageous choices (the safe decks). In other words, these anticipatory SCRs were like “gut feelings” that warned the subject against picking from the bad decks. VMPFC patients failed to generate such SCRs before picking a card. This failure to generate anticipatory SCRs before picking cards from the bad decks correlates with their failure to avoid these bad decks and choose advantageously in this task (Figure 2). These results provide strong support for the notion that decision-making is guided by emotional signals (gut feelings) that are generated in anticipation of future events. [Figure 2 about here]
Figure 2.
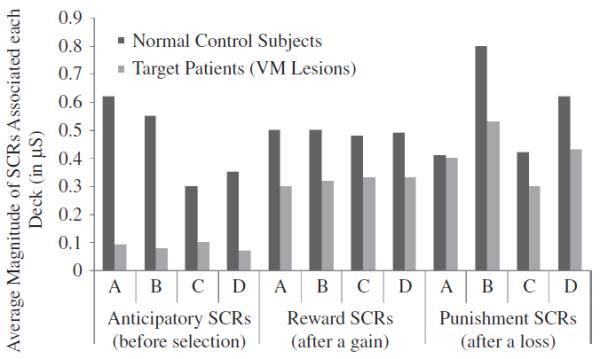
Results of skin conductance responses (SCRs) after selecting a card (Reward or Punishment SCRs) or before selecting a card (Anticipatory SCRs) in the Iowa Gambling Task from normal subjects and a group of patients with bilateral lesions of the VMPFC.
Further experiments revealed that these biasing somatic signals (gut feelings) do not need to be perceived consciously. We carried out an experiment similar to the previous one, in which we tested normal subjects and VMPFC patients on the gambling task while recording their SCRs. However, every time the subject picked 10 cards from the decks, we would stop the game briefly, and ask the subject to declare whatever they knew about what was going on in the game (Bechara, Damasio, Tranel, & Damasio, 1997). From the answers to the questions, we were able to distinguish four periods as subjects went from the first to the last trial in the task. The first was a pre-punishment period, when subjects sampled the decks, and before they had yet encountered any punishment. The second was a pre-hunch period, when subjects began to encounter punishment, but when asked about what was going on in the game, they had no clue. The third was a hunch period, when subjects began to express a hunch about which decks were riskier, but were not sure. The fourth was a conceptual period, when subjects knew very well the contingencies in the task, and which decks were the good ones, and which decks were the bad ones, and why this was so. When examining the anticipatory SCRs from each period, we found that, as expected, there was no significant activity during the pre-punishment period because, at this stage, the subjects have not encountered any losses yet. Then there was a substantial rise in anticipatory responses during the pre-hunch period, i.e., after encountering some money losses, but still before the subject had any clue about what was going on in the game. This SCR activity was sustained for the remaining periods, i.e., during the hunch and then during the conceptual period. When examining the behavior during each period, we found that there was a preference for the high paying decks (A and B) during the pre-punishment period. Then there was a hint of a shift in the pattern of card selection, away from the bad decks, even in the pre-hunch period. This shift in preference for the good decks became more pronounced during the hunch and conceptual periods. The VMPFC patients on the other hand, never reported a hunch about which of the decks were good or bad. Furthermore, they never developed anticipatory SCRs, and they continued to choose more cards from the bad decks relative to the good decks. An especially intriguing observation was that not all the normal control subjects were able to figure out the task, explicitly, in the sense that they did not reach the conceptual period. Only 70% of them were able to do so. Although 30% of controls did not reach the conceptual period, they still performed advantageously. On the other hand, 50% of the VMPFC patients were able to reach the conceptual period and state explicitly which decks were good and which ones were bad and why. Although 50% of the VMPFC patients did reach the conceptual period, they still performed disadvantageously. After the experiment, when these VMPFC patients were confronted with the question: why did you continue to pick from the decks you thought were bad? These patients would resort to excuses such as “…I knew that my luck was going to change and win …”.
These results show that VMPFC patients continue to choose disadvantageously in the gambling task, even after realizing explicitly the consequences of their action. This suggests that the anticipatory SCRs represent unconscious biases derived from prior experiences with reward and punishment. These biases help deter the normal subject from pursuing a course of action that is disadvantageous in the future. This occurs even before the subject becomes aware of the goodness or badness of the choice s/he is about to make. Without these biases, the knowledge of what is right and what is wrong may still become available. However, by itself, this knowledge is not sufficient to ensure an advantageous behavior. Therefore, although the VMPFC patient may manifest declarative knowledge of what is right and what is wrong, s/he fails to act accordingly and may “say” the right thing, but they “do” the wrong thing. Thus “knowledge” without “emotion/somatic signaling” leads to dissociation between what one knows or says, and how one decides to act. This dissociation is not restricted to neurological patients, but it also applies to neuropsychiatric conditions with suspected pathology in the VMPFC or other components of the neural circuitry that process emotion. Psychopathy is one such an example, where the psychopath can be fully aware of the consequences of their action, but they may fail to inhibit that action.
The SMH, working memory, and psychopathy
The SMH describes working memory (and other executive processes of working memory such as response inhibition and reversal learning) as a key process in decision-making. Consequently, damage to neural structures that impair working memory, such as the dorsolateral prefrontal cortex (DLPFC), also lead to impaired decision-making. Nonetheless, some criticisms of the theory were made on the basis that deficits in decision-making as measured by the IGT may not be specific to the VMPFC (Manes et al., 2002) or it may be explained by deficits in other processes, such as reversal learning (Fellows & Farah, 2003). However, research has demonstrated that the relationship between decision-making on one hand and working memory or reversal learning on the other hand are asymmetrical in nature (Bechara & Damasio, 2005). In other words, working memory and/or reversal learning are not dependent on the intactness of decision-making (that is, subjects can have normal working memory and normal reversal learning in the presence or absence of deficits in decision-making). Some patients with VMPFC lesions who were severely impaired in decision-making on the IGT had superior working memory, and are perfectly normal on simple reversal learning tasks. In contrast, decision-making seems to be influenced by the intactness or impairment of working memory and/or reversal learning. That is, decision-making is worse in the presence of abnormal working memory and/or poor reversal learning. Patients with right DLPFC lesions and severe working memory impairments showed low normal results on the IGT (Bechara, Damasio, Tranel, & Anderson, 1998). Patients with damage to the more posterior sector of the VMPFC (which includes the basal forebrain), such as the patients who were included in the study by Fellows & Farah (2003), showed impairments on reversal learning tasks, but similar patients with similar lesions also showed poor performance on the IGT (Bechara et al, 1998). Consistent with these neurological findings, psychopaths have been shown to have impaired response reversal (Budhani, Richell, & Blair, 2006). Therefore, perhaps the dysfunction in the VMPFC in these individuals extends to include this posterior region involved in response reversal, in addition to the somatic marker impairments, which may contribute to their disadvantageous decision-making. This possibility is corroborated by the work of Raine and colleagues, which confirm that the abnormalities in psychopaths lie far more posteriorly to include the septal nuclei (Raine, et al., 2010).
Frontal lobe dysfunction as an advantage under certain circumstances: relevance to psychopathy
As indicated earlier, one of the peculiar aspects of psychopathy is that some individuals, the functional psychopaths specifically, can actually excel in certain domains of their life, such as the financial markets. For example, while people tend to be risk averse in a losing financial market, individuals who lack the emotion (somatic) signal that fears risk (or at least individuals who can control their emotions) may fair better in such an environment, where risk taking is the rational thing to do. One study in neurological patients supported this exact point (Shiv, Loewenstein, Bechara, Damasio, & Damasio, 2005) where we investigated how normal participants, patients with stable focal lesions in brain regions related to emotion (target patients), and patients with stable focal lesions in brain regions unrelated to emotion, such as orbital/VMPFC, insula, and amygdala (patient-controls) made 20 rounds of investment decisions. We used a “risky decision-making task” closely modeled on a paradigm developed in previous economic research to demonstrate “myopic loss aversion”. In each round, participants decide to invest $1 or not invest. A coin is flipped, when they win $2.50 for a heads outcome and lose $1 for a tails outcome. It is clear that the most rational strategy is to keep investing in this task. The most intriguing results of this study were that target patients made more advantageous decisions and ultimately earned more money from their investments than the normal-controls and patient-controls. When normal-controls and patient-controls either won or lost money on an investment round, they adopted a conservative strategy and became more reluctant to invest on the subsequent round, suggesting that they were more affected than target patients by the outcomes of decisions made in the previous rounds (Shiv et al., 2005). This is an example where the lack of a brain mechanism to trigger emotions (or somatic markers) can be advantageous in this particular context. Remember that on the whole, VMPFC damage leads to impaired and disadvantageous decisions, but there are specific circumstances where this deficiency can be helpful. It may be these particular contexts that allow some psychopaths, not being hindered by emotional signals, to perform superiorly in certain financial situations and to lead successful lives (albeit this successful life can suddenly end up in disaster, such as the B. Murdoff case).
Impairment in moral judgment
Several studies have shown that VMPFC damage is associated with impairments in making moral judgments. For instance, patients with frontotemporal dementia, a disorder characterized by abnormal social behavior and potential sociopathy, have been shown to be impaired in making emotional moral judgments (Mendez, Anderson, & Shapira, 2005) and that these impairments may be related to impaired affective Theory of Mind (ToM), the ability to attribute one’s own and others’ mental states (Gleichgerrcht, Torralva, Roca, Pose, & Manes, 2011). This overlaps with the suggestion that the VMPFC is believed to be involved in empathy, but specifically when using abstract visual information about another’s affective state (Lamm, Decety, & Singer, 2011). Another example of patients having moral judgment impairments are VMPFC patients who exhibited abnormal judgments when judging moral situations, using a more utilitarian approach than control subjects (Koenigs et al., 2007). As a result, they are more likely than control subjects and other brain damaged patients to approve of harmful actions in situations they deem as appropriate or reasonable (Young et al., 2010). Consequently, it was surmised that the VMPFC adds an emotional component to the decision making process involved in moral judgments. When this component is absent, the person is left making a more pragmatic decision based on the facts of the situation, with a special emphasis on the outcome of the situation and less so on the inferred or abstract events (intentions) that came before it. To test this, VMPFC patients, other brain damaged patients, and normal participants were given 24 scenarios where the task was to judge harmful intent (Young et al., 2010). The study used a 2×2 design: (1) the protagonist either intended to cause harm to another person (negative intent) or intended to cause no harm (neutral intent), and (2) the protagonist either caused harm to another person (negative outcome) or caused no harm (neutral outcome; Young et al., 2010). The results were as predicted. Patients with VMPFC lesions judged attempted harms, including attempted murder, as more morally permissible, relative to controls. VMPFC patients showed neglect of negative intentions in moral judgment and instead focused on the action’s neutral outcome. It may be that the way an individual judges an attempted harm as immoral, and therefore forbidden, is for the intent behind the attempted harm to elicit an emotional response (Valdesolo & DeSteno, 2006). It appears that VMPFC patients do not enjoy the benefits of this guiding emotional response and therefore, instead focus on the outcome of a situation rather than the intent (Koenigs et al., 2007). Further, a review investigating the role of emotion in morality concluded that emotion is involved in moral judgments, particularly those mediated by the VMPFC (Young & Koenigs, 2007), and hence when it is damaged, impairments of this type of judgment arise.
In order to highlight the distinction between some of the behaviors of VMPFC lesion patients and sociopaths, the term “acquired sociopathy” has been previously used to describe these patients (Damasio, Tranel, & Damasio, 1990), thus reflecting the fact that the VMPFC lesion patients may not engage in extreme immoral or corrupt acts (including criminal) that characterize developmental sociopaths and/or psychopaths. This distinction (acquired versus developmental) may help explain some of the abilities of VMPFC lesion patients to use their lifetime’s worth of social learning to avoid engaging in extreme immoral or corrupt acts. However these behavioral differences do not warrant fundamentally different brain mechanisms that mediate them. Indeed some of our VMPFC patients did engage in financial decisions that involved unscrupulous people (e.g., (Damasio, Tranel, & Damasio, 1990), but the handicap associated with their stroke (or tumor) may have helped curb the extent of their societal engagement that could have led to more severe acts of immoral and corrupt behaviors. Furthermore, patients who acquire VMPFC damage earlier on in life tend to grow up to commit more severe antisocial behavioral acts (Anderson, Barrash, Bechara, & Tranel, 2006), thus suggesting that if the onset of brain damage were at an earlier age, then the line between acquired and developmental sociopathy becomes more blurred.
Who among us is the Sociopath?: A Neurocognitive Perspective
The neural origin of psychopathy has been long debated (Blair, 2008; Glenn & Raine, 2008; Kiehl, 2006), with the amygdala perhaps being implicated in the severe emotional aspects of psychopathy (e.g., Blair, 2008), and the VMPFC in the less severe aspects, albeit that a very posterior structure of this region, namely the septal region, has been linked to primary psychopathy as well (Raine, et al., 2010) A recent study even highlights the possible structural differences between successful and unsuccessful psychopaths (based on criminal convictions, not cognitive or social functioning) in the greater prefrontal cortex and the amygdala, with unsuccessful psychopaths having reduced grey matter volume in these regions (Yang, Raine, Colletti, Toga, & Narr, 2010). In addition to the neuroanatomical evidence, behavioral data suggests that psychopaths show deficits in fear conditioning (Birbaumer et al., 2005), fearful facial expression recognition and processing (Blair, Colledge, Murray, & Mitchell, 2001), augmentation of startle reflex by visual threat primes (Levenston, Patrick, Bradley, & Lang, 2000), and show less interference by emotional distracters (Mitchell, Richell, Leonard, & Blair, 2006) – all of which are also symptoms of amygdala lesions and dysfunction. As indicated earlier, although the IGT has been used largely to detect impaired decision-making in patients with VMPFC lesions, the task is not specific to this region, and impaired performance can result from damage in other areas of the somatic marker circuitry, including the amygdala (Bechara and Damasio, 2005). However, this lack of specificity arises when other cognitive deficits are present, including deficits such as poor memory, and impaired conditioning learning. The poor IGT performance becomes specific to VMPFC damage when, and only when, all other cognitive deficits are ruled out. As such when normal individuals perform disadvantageously on the IGT, although this poor performance may implicate the VMPFC, abnormalities of other neural structures cannot be ruled out.
It is intriguing that when we test a large sample of the “normal” population on the IGT, there is a small subgroup that achieves scores that are comparable to those of patients with VMPFC lesions (Figure 3). Why does this small percentage of “normal subjects” perform like VMPFC patients on the IGT? As we indicated earlier, we suspect that poor performance arises from a potentially dysfunctional prefrontal cortex due to genetic and/or environmentally induced reasons. However, the key question is whether individuals with such low IGT scores also show signs of psychopathic behavior. [Figure 3 about here] How do psychopaths fare on the IGT? Mitchell (2002) found poor performance on the IGT in psychopathic inmates as compared to non-psychopathic inmates (Mitchell, Colledge, Leonard, & Blair, 2002). Another study found that criminal psychopaths with low attention scores exhibited the expected disadvantageous decision making performance on the IGT (Lösel & Schmucker, 2004). However, in another study that also used criminal psychopaths, Schmitt and colleagues administered the IGT and the Welsh Anxiety Scale (Schmitt, Brinkley, & Newman, 1999). The control group in this study was also a group of prisoners. However, this study was the only one that divided the psychopathy group by trait anxiety into: primary (low-anxious) and secondary (high-anxious) psychopathy. They found that all groups performed poorly on the IGT, but levels of anxiety, and not psychopathy, predicted whether the subjects would learn to choose more advantageously (or become more risk-averse) over time. Also this study did not find significant differences between the psychopathy groups and the control group, which also involved incarcerated individuals, but who were considered as non-psychopaths. The fact that all the psychopathy groups in this study performed poorly on the IGT is consistent with the somatic marker perspective argued here. The fact that the control group also performed poorly on the IGT is inconsistent with the earlier study of non-psychopathic inmates showing relatively more advantageous performance on the IGT (Mitchell, Colledge, Leonard, & Blair, 2002). While it is clear that these non-psychopathic prisoners do not meet the criteria of primary psychopathy, it is not clear whether these individuals meet the criteria for secondary psychopathy, especially since the psychopathy instruments used to evaluate prisoners are more sensitive to detecting and measuring signs of primary psychopathy. Indeed evidence suggests that an incarcerated population differs from a non-incarcerated population in important ways (e.g. risk aversion; Raine, 1993). These differences in the ways of assessing psychopathy in incarcerated and non-incarcerated populations may explain these apparent inconsistencies. In support, other studies that looked at non-institutionalized populations of psychopaths found in undergraduates that high psychopathy traits (based on Levenson’s Self-Report Psychopathy Scale, or LSRP; Levenson, Kiehl, & Fitzpatrick, 1995) performed significantly worse on the IGT (Mahmut, Homewood, & Stevenson, 2008). Another study found that boys with psychopathic tendencies also showed impaired performance on the IGT (Blair, Colledge, & Mitchell, 2001). Together these results are consistent with our perspective that psychopathic behaviors (primary and to a milder extent secondary) are associated with poor performance on the IGT, thus reflecting potential abnormalities in the activation of the somatic marker circuitry.
Figure 3.
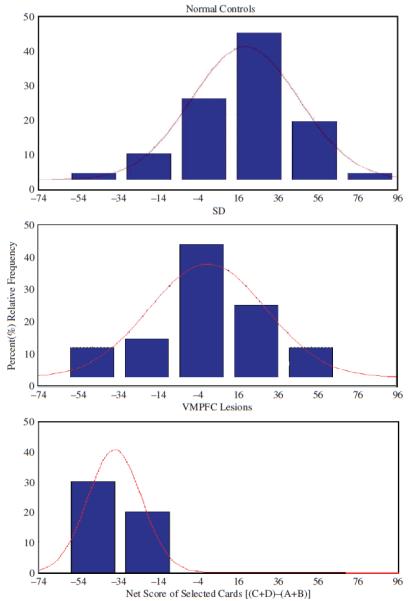
Distribution of IGT scores from 3 samples of the population: normal controls, substance dependent individuals (SD), and patients with VMPFC lesions. Negative scores reflect disadvantageous decisions, while positive scores reflect advantageous decisions. The Y-axis represents the percentage of the sample that achieves a particular score.
A more direct link between the VMPFC damage and psychopathy came from a recent study, by Koenigs and colleagues (2010), who studied the responses of primary and secondary psychopathic prisoners on two different economic decision making tasks (Ultimatum Game and the Dictator Game), which were then analyzed and compared to those of VMPFC patients (Koenigs, Kruepke, & Newman, 2010). The psychopathic subtypes were divided based on trait anxiety scores and subsequently compared to each other, to criminal non-psychopaths, as well as to the VMPFC lesion patients. Primary psychopathy was associated with significantly lower acceptance rates of unfair Ultimatum offers and lower offer amounts in the Dictator Game when compared to secondary psychopaths and non-psychopaths (Koenigs, Kruepke, & Newman, 2010). In addition, primary psychopaths were quantitatively more similar to the VMPFC lesion patients in their response patterns.
While the Ultimatum and Dictator games were not developed and discussed in the context of the somatic marker hypothesis, studies have shown that performance on these tasks do indeed engage the “body loop” of the SMH circuitry (Hewig et al., 2011). Furthermore, patients with lesions to the VMPFC show abnormal performance on these tasks (i.e. higher rejection rates to unfair offers on the Ultimatum game and lower offers on the Dictator game; Koenigs and Tranel, 2007; Krajbich, Adolphs, Tranel, Denburg, Camerer 2009) that are consistent with the type of “somatic marker” impairment described in these patients. For instance, it has been shown that while they appear apathetic in many social situations, their tendency to express anger and violent behavior is relatively more spared (Bechara, Damasio, & Damasio, 2003; Bechara, Tranel, & Damasio, 2002), thus explaining their intense reaction to “injustice” as reflected by their Ultimatum Game behavior. The recent study by Koenigs et al. (2010) provides an additional support for a link between performance on these games and psychopathy. This supports further our perspective regarding a common neural link between psychopathy and the neural circuitry for somatic marker activation.
Another line of evidence that may link the poor performance of psychopaths on the Ultimatum and Dictator games to poor performance on the IGT and the somatic marker framework is the following: the IGT may be considered a measure of decisions under ambiguity (i.e., the outcome of the choice is completely unknown), whereas the ultimatum and dictator games, in which the contingencies are already known to the participant, may be considered as measures of decisions under risk. Lesion studies that examined the neural substrates underlying decisions under ambiguity versus risk (Weller, Levin, Shiv, & Bechara, 2007; Xue et al., 2009) did not reveal dissociations or fundamental differences, albeit that a few functional neuroimaging studies have suggested partially separate neural substrates (Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005; Huettel, Stowe, Gordon, Warner, & Platt, 2006). Taken together, there is a considerable overlap in the neural circuits that sub-serve the different types of decision-making (ambiguity versus risk), which are taxed by these different tasks.
Conclusion
The media has a penchant of sensational stories, and therefore when the general public hears the word “psychopath” it is usually the image of a killer or convict that comes to mind, though these are social stereotypes. Psychopaths, especially the functional or secondary type, can be successful individuals having careers as entrepreneurs, politicians, CEOs, or other respectable positions (Hare, 1999). Many psychopaths can function seemingly normally in society where they don’t have official criminal records. These individuals commit crimes of another nature by using, manipulating, and hurting the people which surround them in order to enrich themselves. In the workforce they are perfidious employees and untrustworthy businessmen who victimize the people who surround them (Hare, 1999). Perhaps it is time to use a neuroscientific perspective to revisit the underlying brain causes that lead to corruption and psychopathic behaviors, especially the non-criminal type, the “functional” one that is a part of our social realm.
Acknowledgement
This research was supported by research NIDA grants R01 DA16708 and R01 DA022549 and NINDS grant P01 NS19632.
References
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of International Neuropsychological Society. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2(11):1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio AR. The somatic-marker hypothesis and decision making. In: Boller F, Grafman J, Rizzolatti G, editors. Handbook of Neuropsychology. 2nd ed Vol. 7. Elsevier; Amsterdam: 2002. pp. 117–143. [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation Of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998;18(1):428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9(4):159–162. doi: 10.1016/j.tics.2005.02.002. discussion 162-154. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6(2):215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2005;62(7):799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc Lond B Biol Sci. 2008;363(1503):2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Mitchell DG. Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? J Abnorm Child Psychol. 2001;29(6):499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol. 2001;29(6):491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blessing WW. Inadequate frameworks for understanding bodily homeostasis. Trends Neurosci. 1997;20(6):235–239. doi: 10.1016/s0166-2236(96)01029-6. [DOI] [PubMed] [Google Scholar]
- Blumer D, Benson DF. Personality changes with frontal and temporal lobe lesions. In: Benson DF, Blumer D, editors. Psychiatric aspects of neurological disease. Grune and Stratton; New York: 1975. pp. 151–170. [Google Scholar]
- Braun K, Lange E, Metzger M, Poeggel G. Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience. 2000;95(1):309–318. doi: 10.1016/s0306-4522(99)00420-0. [DOI] [PubMed] [Google Scholar]
- Budhani S, Richell RA, Blair RJ. Impaired reversal but intact acquisition: probabilistic response reversal deficits in adult individuals with psychopathy. J Abnorm Psychol. 2006;115(3):552–558. doi: 10.1037/0021-843X.115.3.552. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Wood RL. Neuropsychology of behaviour disorders following brain injury. In: Wood RL, editor. Neurobehavioural sequelae of traumatic brain injury. Taylor and Francis; New York: 1990. pp. 110–133. [Google Scholar]
- Cleckley HM. The Mask of Sanity. New American Library; Mosby; New York: 1982. [Google Scholar]
- Corruption Merriam-Webster online. 2010 Retrieved from: http://www.merriam-webster.com/dictionary/corruption.
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error : emotion, reason, and the human brain. G.P. Putnam; New York: 1994. [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: a critical evaluation. Neurosci Biobehav Rev. 2006;30(2):239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35(12):1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35(3):591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E, Torralva T, Roca M, Pose M, Manes F. The role of social cognition in moral judgment in frontotemporal dementia. Soc Neurosci. 2011;6(2):113–122. doi: 10.1080/17470919.2010.506751. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A. The neurobiology of psychopathy. Psychiatr Clin North Am. 2008;31(3):463–475. vii. doi: 10.1016/j.psc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. Psychopathy Checklist: Revised. Multi-Health Systems; New York: 1991. [Google Scholar]
- Hare RD. Psychopathy and Antisocial Personality Disorder: A Case of Diagnostic Confusion. Psychiatric Times. 1996:13. [Google Scholar]
- Hare RD. Without conscience : the disturbing world of the psychopaths among us. Guilford Press; New York: 1999. [Google Scholar]
- Harlow JM. Passage of an iron bar through the head. Boston Medical and Surgical Journal. 1848;39:389–393. [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society. 1868;2:327–347. [Google Scholar]
- He Q, Xue G, Chen C, Lu Z, Dong Q, Lei X, Bechara A. Serotonin transporter gene-linked polymorphic region (5-HTTLPR) influences decision making under ambiguity and risk in a large Chinese sample. Neuropharmacology. 2010;59(6):518–526. doi: 10.1016/j.neuropharm.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Büchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8(1):20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Hewig J, Kretschmer N, Trippe RH, Hecht H, Coles MG, Holroyd CB, Miltner WH. Why humans deviate from rational choice. Psychophysiology. 2011;48(4):507–514. doi: 10.1111/j.1469-8986.2010.01081.x. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49(5):765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Karpman B. On the need of separating psychopathy into two distinct clinical types: the symptomatic and the idiopathic. Journal of Criminal Psychopathology. 1941;3:112–137. [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142(2-3):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Kruepke M, Newman JP. Economic decision-making in psychopathy: a comparison with ventromedial prefrontal lesion patients. Neuropsychologia. 2010;48(7):2198–2204. doi: 10.1016/j.neuropsychologia.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J Neurosci. 2007;27(4):951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446(7138):908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J Neurosci. 2009;29(7):2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. J Pers Soc Psychol. 1995;68(1):151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: emotion and attention in picture processing. J Abnorm Psychol. 2000;109(3):373–385. [PubMed] [Google Scholar]
- Lösel F, Schmucker M. Psychopathy, risk taking, and attention: a differentiated test of the somatic marker hypothesis. J Abnorm Psychol. 2004;113(4):522–529. doi: 10.1037/0021-843X.113.4.522. [DOI] [PubMed] [Google Scholar]
- Mahmut MK, Homewood J, Stevenson RJ. The characteristics of non-criminals with high psychopathy traits: Are they similar to criminal psychopaths? Journal of Research in Personality. 2008;42:679–692. [Google Scholar]
- Maia TV, McClelland JL. A reexamination of the evidence for the somatic marker hypothesis: what participants really know in the Iowa gambling task. Proc Natl Acad Sci. 2004;101(45):16075–16080. doi: 10.1073/pnas.0406666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Martin CO, Denburg NL, Tranel D, Granner MA, Bechara A. The effects of vagus nerve stimulation on decision-making. Cortex. 2004;40(4-5):605–612. doi: 10.1016/s0010-9452(08)70156-4. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Anderson E, Shapira JS. An investigation of moral judgement in frontotemporal dementia. Cogn Behav Neurol. 2005;18(4):193–197. doi: 10.1097/01.wnn.0000191292.17964.bb. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Colledge E, Leonard A, Blair RJ. Risky decisions and response reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologia. 2002;40(12):2013–2022. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Richell RA, Leonard A, Blair RJ. Emotion at the expense of cognition: psychopathic individuals outperform controls on an operant response task. J Abnorm Psychol. 2006;115(3):559–566. doi: 10.1037/0021-843X.115.3.559. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Bramati IE, Grafman J. Functional networks in emotional moral and nonmoral social judgments. Neuroimage. 2002;16(3 Pt 1):696–703. doi: 10.1006/nimg.2002.1118. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, Bramati IE, Mourão-Miranda J, Andreiuolo PA, Pessoa L. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. J Neurosci. 2002;22(7):2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: the neural basis of human moral cognition. Nat Rev Neurosci. 2005;6(10):799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Persaud N, McLeod P, Cowey A. Post-decision wagering objectively measures awareness. Nat Neurosci. 2007;10(2):257–261. doi: 10.1038/nn1840. [DOI] [PubMed] [Google Scholar]
- Raine A. The psychopathology of crime. Academic Press; New York: 1993. [Google Scholar]
- Raine A, Lee L, Yang Y, Colletti P. Neurodevelopmental marker for limbic maldevelopment in antisocial personality disorder and psychopathy. Br J Psychiatry. 2010;197(3):186–192. doi: 10.1192/bjp.bp.110.078485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt WA, Brinkley CA, Newman JP. Testing Damasio’s somatic marker hypothesis with psychopathic individuals: risk takers or risk averse? J Abnorm Psychol. 1999;108(3):538–543. doi: 10.1037//0021-843x.108.3.538. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, Bechara A. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr Res. 2007;92(1-3):74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiv B, Loewenstein G, Bechara A, Damasio H, Damasio AR. Investment behavior and the negative side of emotion. Psychol Sci. 2005;16(6):435–439. doi: 10.1111/j.0956-7976.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- Spencer J. Wall Street Journal. Jul 21, 2005. Lessons from the brain-damaged investor; Unusual study explores links between emotion and results; ‘Neuroeconomics’ on wall street; p. D.1. eastern ed. [Google Scholar]
- Valdesolo P, DeSteno D. Manipulations of emotional context shape moral judgment. Psychol Sci. 2006;17(6):476–477. doi: 10.1111/j.1467-9280.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Shiv B, Bechara A. Neural correlates of adaptive decision making for risky gains and losses. Psychol Sci. 2007;18(11):958–964. doi: 10.1111/j.1467-9280.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex. 2009;19(5):1019–1027. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. J Abnorm Psychol. 2010;119(3):546–554. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- Young L, Bechara A, Tranel D, Damasio H, Hauser M, Damasio A. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron. 2010;65(6):845–851. doi: 10.1016/j.neuron.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Koenigs M. Investigating emotion in moral cognition: a review of evidence from functional neuroimaging and neuropsychology. Br Med Bull. 2007;84:69–79. doi: 10.1093/bmb/ldm031. [DOI] [PubMed] [Google Scholar]


