Abstract
Objective
Ubiquitin C-terminal hydrolase (UCH-L1), also called neuronal-specific protein gene product (PGP 9.3), is highly abundant in neurons. To assess the reliability of UCH-L1 as a potential biomarker for traumatic brain injury (TBI) this study compared cerebrospinal fluid (CSF) levels of UCH-L1 from adult patients with severe TBI to uninjured controls; and examined the relationship between levels with severity of injury, complications and functional outcome.
Design
This study was designed as prospective case control study.
Patients
This study enrolled 66 patients, 41 with severe TBI, defined by a Glasgow coma scale (GCS) score of ≤8, who underwent intraventricular intracranial pressure monitoring and 25 controls without TBI requiring CSF drainage for other medical reasons.
Setting
Two hospital system level I trauma centers.
Measurements and Main Results
Ventricular CSF was sampled from each patient at 6, 12, 24, 48, 72, 96, 120, 144, and 168 hrs following TBI and analyzed for UCH-L1. Injury severity was assessed by the GCS score, Marshall Classification on computed tomography and a complicated postinjury course. Mortality was assessed at 6 wks and long-term outcome was assessed using the Glasgow outcome score 6 months after injury. TBI patients had significantly elevated CSF levels of UCH-L1 at each time point after injury compared to uninjured controls. Overall mean levels of UCH-L1 in TBI patients was 44.2 ng/mL (±7.9) compared with 2.7 ng/mL (±0.7) in controls (p <.001). There were significantly higher levels of UCH-L1 in patients with a lower GCS score at 24 hrs, in those with postinjury complications, in those with 6-wk mortality, and in those with a poor 6-month dichotomized Glasgow outcome score.
Conclusions
These data suggest that this novel biomarker has the potential to determine injury severity in TBI patients. Further studies are needed to validate these findings in a larger sample.
Keywords: trauma, brain injury, human, biomarkers, proteomics, diagnostic, neuronal death, cerebrospinal fluid
Traumatic brain injury (TBI) is a significant cause of death and disability in the United States (1) with about 52,000 annual deaths and 5.3 million Americans impaired by its effects. Although many advances have been made elucidating the anatomical, cellular and molecular mechanisms of TBI, these advances have not yet yielded significant improvements in treatment. Some of the barriers identified to developing treatments include heterogeneity of the disease, difficulty with stratification of patients by injury severity and lack of markers of injury (2– 4). Unlike other organ-based diseases, such as myocardial ischemia, where rapid diagnosis employing biomarkers from blood tests prove invaluable to guide diagnosis and management, no such rapid, definitive diagnostic tests exist for TBI. Biomarkers measurable in cerebrospinal fluid (CSF) and blood would have important applications in diagnosis, prognosis and clinical research of TBI.
Recent reviews of biomarkers of brain injury have highlighted the need for biomarker development (2, 5–7). Most of the studies on potential biomarkers for brain injury have been conducted over the last 10 yrs and many of the markers are associated with damage and release from cell types and components of brain parenchyma including neurons, astrocytes and axons (7). To date, the most well studied potential biomarkers for TBI and stroke are neuron-specific enolase (NSE) (8 –13), glial protein S-100 beta (β) (13–20), and myelin basic protein (MBP) (11, 21, 22) S100β is the major low affinity calcium binding protein in astrocytes (23) and it is considered a marker of astrocyte injury or death. Neuron-pecific enolase is one of the five isozymes of the gycolytic enzyme enolase found in central and peripheral neurons and it has been shown to be elevated following cell injury (24). Myelin basic protein (MBP), an abundant protein in white matter, has been examined as a marker of axonal damage. Although there are a number of studies published that suggest that these potential biomarkers correlate with degree of injury; studies relating these proteins to pathophysiological parameters of TBI and clinical outcome have produced conflicting results (25–28). Newer markers include astrocyte-specific filament protein glial fibrillary acid protein (GFAP), and the signature breakdown products (SBDPs) of the axonally enriched cytoskeletal protein α-II-spectrin which is proteolyzed by calpain and caspase-3 (29 –31).
Increased sophistication of laboratory techniques and developments in the field of proteomics has led to the discovery and rapid detection of new biomarkers not previously available. Ubiquitin C-terminal hydrolase-L1 (UCH-L1) is also known as neuronal-specific protein gene product 9.5 (PGP9.5) and was previously used as a histologic marker for neurons because of its high abundance and specific expression in neurons [Chemicon]. UCH-L1 is present in almost all neurons and averages 1–5% of total soluble brain protein (32). There are three related enzymes of this class (UCH-L1, UCH-L2 and UCH-L3), but only UCH-L1 is highly enriched in central nervous system. These enzymes are involved in either the addition or removal of ubiquitin from proteins that are destined for metabolism (via the ATP-dependent proteasome pathway) (33). Mutations and polymorphism in UCH-L1 have been associated with familial Parkinson’s disease (34). Furthermore, gracile axonal dystrophy (gad) mice having a deletion within the UCH-L1 gene are characterized by ‘dying-back’ axonal degeneration and formation of spheroid bodies in nerve terminals (35). Proteasome inhibition can arrest neurite outgrowth and cause “dying-back” degeneration in primary culture (36, 37). In focal cerebral ischemia and spinal cord injury, increased protein aggregates and decreased proteasome activity has been observed, respectively (38, 39). Taken together, these data suggest that UCH-L1 plays an important role in the removal of excessive, oxidized or misfolded proteins during both normal and neuropathological conditions (40). Based on the important function of UCH-L1 in neurons and its high specificity and abundance in central nervous system, we selected UCH-L1 as a candidate biomarker for TBI.
This study examined whether UCH-L1 was significantly elevated in human severe TBI patients compared to uninjured controls as well as if levels of UCH-L1 were associated with measures of injury severity, complications and outcome.
MATERIALS AND METHODS
Design and Population
This prospective case control study enrolled patients who presented to the University of Florida Trauma System (Shands Hospital in Gainesville and Jacksonville, Florida) over a 16-month period following a severe TBI, defined by a Glasgow coma scale (GCS) score of ≤8, and requiring a ventricular intracranial pressure (ICP) monitoring as part of their routine clinical care. A parallel prospective study was conducted on severe TBI patients at the Baylor College of Medicine in Houston Texas (Ben Taub General Hospital) between August 2003 and August 2004 as a part of severe TBI protocol requiring ICP monitoring and CSF sample analysis. Clinical data were merged and all CSF samples were banked until biomarker analysis was performed at the University of Florida.
Cerebrospinal fluid (CSF) control samples were obtained from either hydrocephalic patients who had VP shunts placed and who had CSF samples taken intraoperatively or unruptured subarachnoid hemorrhage patients who also had CSF samples taken intraoperatively. Control patients had a normal mental status at the time of enrollment and had no evidence of acute brain injury or hemodynamic instability. Control samples were de-identified and no demographic data were collected on these patients. This study was approved by the respective Institutional Review Boards of the University of Florida and Baylor College of Medicine. Informed consent was obtained from all patients and/or legal authorized representatives from each site.
CSF samples from severe TBI subjects were directly collected from the ventriculostomy catheter at 6, 12, 24, 48, 72, 96, 120, 144, and 168 hrs following TBI. Approximately 3 to 4 mL of CSF was collected from each subject at each sample point (within 60 mins of the expected time point). Samples were immediately centrifuged for 10 mins at 4000 rpm to separate CSF from blood cells and immediately frozen and stored at −70°C until the time of analysis.
Measurement/Biomarker Analysis
Samples were measured using a standard UCH-L1 sandwich enzyme-linked immunosorbent assay (ELISA) protocol as described below. Reaction wells were coated with capture antibody (500 ng/well purified rabbit polyclonal antihuman UCHL1, made inhouse) in 0.1 M sodium bicarbonate, pH 9 and incubated overnight at 4°C. Plates were then emptied and 300 μl/well blocking buffer (Startingblock T20-TBS) was added and incubated for 30 mins at ambient temperature with gentle shaking. This was followed by addition of antigen standard [UCHL1 standard curve: 0.05–50 ng/well) unknown samples (3–10 ul CSF) or assay internal control samples. The plate was incubated for 2 hrs at room temperature, then washed using an automatic plate washer (each well rinsed with 5 × 300 μl with wash buffer (TBST)]. Detection antibody (rabbit polyclonal antihuman UCH-L1-HRP conjugation, made inhouse at 50 μg/mL) in blocking buffer was then added to wells at 100 μl/well, and the plates were further incubated for 1.5 hrs at room temperature. After additional automatic washing, biotinyl-tyramide solution (Perkin Elmer Elast Amplification Kit) was added and the plate was incubated for 15 mins at room temperature followed by automatic washing. Addition of Streptavidin-HRP (1: 500, 100 ul/well) in PBS with 0.02% Tween 20 and 1% BSA for 30 mins incubation at room temperature was followed by automatic washing. Last, the wells were developed with substrate solution: Ultra-TMB ELISA 100ul/well (Pierce# 34028) with incubation for 5–30 mins and read at 652 nm with a 96-well spectrophotometer (Molecular Device Spectramax 190).
Outcome Measures
We compared CSF levels of UCH-L1 in control and TBI patients at each of the specified time points. The relationship between level of UCH-L1 and injury severity acutely was assessed using the GCS score, the initial computed tomographic (CT) findings using the Marshall classification and complicated postinjury course. Long-term outcome was assessed by mortality and the Glasgow outcome score.
The Glasgow Coma Score (GCS) score (41, 42) was assessed immediately after resuscitation (measured after ventriculostomy placement) and within 24 hrs after injury using the commonly used dichotomization of the GCS (GCS 3–5 versus GCS 6 – 8) (43). Admission computed tomographic (CT) findings on the day of injury was determined by the Marshall classification (44). This classification identifies six groups of patients with traumatic brain injury (TBI) based on morphologic abnormalities (Diffuse injury I–IV and mass lesions). Complicated postinjury course included presence of secondary insults (such as hypoxia, hypotension, intracranial hypertension) (45) and delayed, recurrent or evolving traumatic intracranial lesions (any worsening lesion, typically an enlarging contusion). Mortality was determined at 6 wks after injury and encompassed all-cause mortality. The Glasgow Outcome Score (GOS) was assessed at 6 months after injury (46). The GOS was administered via direct patient contact or via telephone interview with the patient and/or a family member. The GOS score was determined using the following standard neurologic parameters: 1) good recovery: resumption of normal life despite minor deficit; 2) moderate disability: person is disabled but independent, travels by public transportation, can work in sheltered setting (exceeds mere ability to perform activities of daily living); 3) severe disability: the person is conscious but disabled, dependent for daily support (may or may not be institutionalized); 4) persistent vegetative state: unresponsive and speechless; and 5) death (47). For the purpose of our analysis, outcome was further classified into two groups using dichotomized GOS categories (good recovery/moderate disability vs. severe disability/vegetative/dead) as previously described (48).
Data Analysis
For statistical analysis, biomarker levels were measured in ng/mL and expressed as mean ± SEM. Data were assessed for equality of variance and distribution and examined using Mann-Whitney U and Kruskall-Wallis tests. Statistical significance was set at 0.05. A receiver operating characteristic (ROC) curve was constructed to explore the diagnostic ability of the biomarker to distinguish between uninjured controls and TBI patients within 6 hrs after injury (when mental status may be confounded by factors such as alcohol, drugs. and sedatives). All analyses were performed using the statistical software package SPSS version 12.0 (SPSS Inc., Chicago, IL).
RESULTS
A total of 66 patients were enrolled in the study and had CSF samples obtained for analysis; 41 TBI patients and 25 controls. There were 41 severe TBI subjects enrolled, including 17 from the University of Florida and 24 from Baylor College of Medicine. Patient characteristics and injury severity for each site are shown in Table 1. There were 25 control patients who had CSF samples taken intraoperatively as part of routine clinical care for either VP shunt placement or unruptured subarachnoid hemorrhage, but no demographic data were obtained for these patients.
Table 1.
Demographic and clinical data for all subjects included in the study (data from patients enrolled overall and at each center)
| Characteristics | Total, n = 41 | University of Florida Site, n = 17 | Baylor College of Medicine Site, n = 24 |
|---|---|---|---|
| Mean age, yrs | 38 | 38 | 37 |
| Range | 18–67 | 18–65 | 18–67 |
| Sex, male/female | 33/8 | 10/7 | 23/1 |
| GCS score, postresuscitation | |||
| GCS 3–5 | 22 | 10 | 12 |
| GCS 6–8 | 19 | 7 | 12 |
| Marshall classification | |||
| Diffuse injury class I | 2 | 2 | 0 |
| Diffuse injury class II | 16 | 10 | 6 |
| Diffuse injury class III | 5 | 0 | 5 |
| Diffuse injury class IV | 0 | 0 | 0 |
| Evacuated mass lesion | 15 | 3 | 12 |
| Nonevacuated mass lesion | 3 | 2 | 1 |
| Pre-ICU hypoxia | |||
| Yes | 3 | 2 | 1 |
| No | 39 | 15 | 24 |
| Pre-ICU hypotension | |||
| Yes | 11 | 8 | 3 |
| No | 30 | 9 | 21 |
| Intoxicated (alcohol or drugs) (n = 21) | |||
| Yes | 12 | 2 | 10 |
| No | 9 | 3 | 6 |
| Lesion types | |||
| Epidural hematoma | 5 | 0 | 5 |
| Subdural hematoma | 12 | 4 | 8 |
| Subarachnoid hemorrhage (traumatic) | 5 | 3 | 2 |
| Contusion | 7 | 2 | 5 |
| Diffuse injury | 28 | 15 | 13 |
| Combination | 6 | 3 | 3 |
GCS, Glasgow Coma Scale; ICU, intensive care unit.
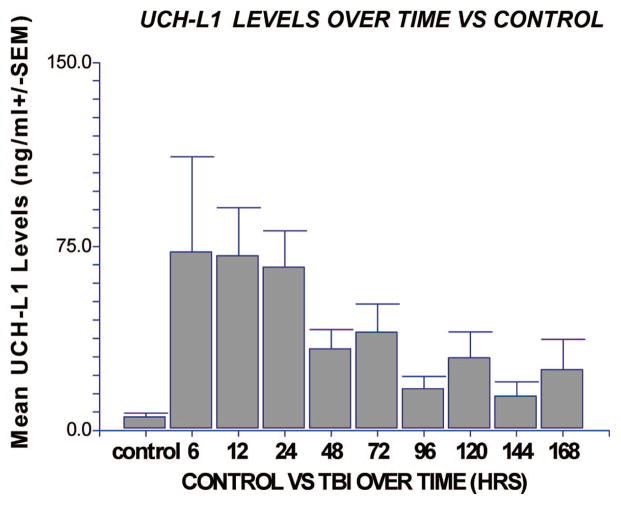
TBI patients had significantly elevated CSF levels of UCH-L1 at each time point after injury compared with uninjured controls (Fig. 1). Overall mean levels of UCH-L1 in TBI patients was 44.2 ng/mL (±7.9) (range 0 –218.4) compared with 2.7 ng/mL (±0.7) (range 0.7–15.9) in controls (p < .001). UCH-L1 was detectable at the earliest time point measured in the CSF (i.e., within 6 hrs of injury) and peaked within the first 24 hrs. Using the earliest CSF sample obtained (to assess value of UCH-L1 when mental status is confounded) an ROC Curve measuring 6-hr levels of UCH-L1 vs. control had an area under the curve of 0.88 (95% CI 0.68 –1.00) (p = .001).
Figure 1.
Comparison of cerebrospinal fluid levels of ubiquitin C-terminal hydrolase (UCH-L1) in patients with traumatic brain injury (TBI) vs. control at all time points. Levels of UCH-L1 were predominantly elevated in the first 24 hrs after injury compared with control patients for each time point (n = 9, 10, 20, 26, 35, 32, 15, 22, 12, 18, respectively) (*p ≤ .05 compared with control). Values represent means (ng/mL ± SEM).
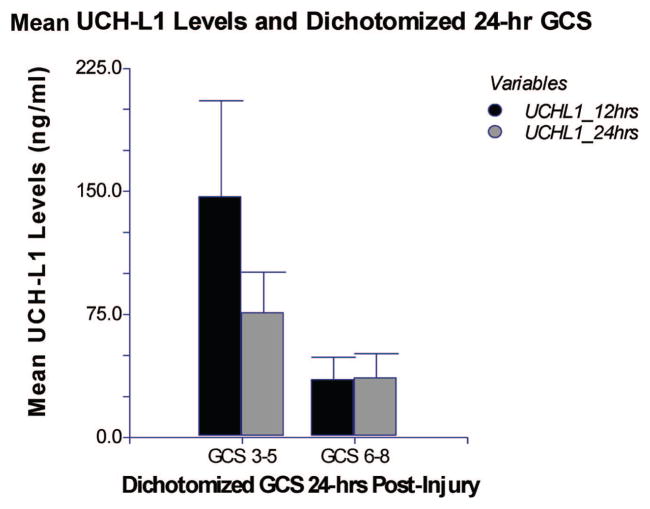
Patients with a GCS 3–5 on day 1 had significantly higher levels of UCH-L1 measured at the 12-hr (p = .007) and 24-hr (p = .045) time points compared with patients with a GCS 6 – 8 (Fig. 2). We also assessed GCS in the postresuscitation period after ventriculostomy placement. In this postresuscitation period, levels of UCH-L1 measured at 6 and 12 hrs after injury were not significantly elevated in patients with a GCS 3–5 compared with patients with a GCS 6 – 8 (p = .72 and p = .25, respectively) (Table 2).
Figure 2.
Comparison of levels of ubiquitin C-terminal hydrolase (UCH-L1) in patients with a Glasgow Coma Scale (GCS) score 3–5 and GCS score 6 – 8 in the first 24 hrs after injury: Mean levels of UCH-L1 (ng/mL ± SEM). Mean 12- and 24-hr levels of UCH-L1 in cerebrospinal fluid are significantly elevated in patients with GCS 3–5 (n = 5, 8) compared with those with a GCS 6 – 8 (n = 20, 21) at day 1 after injury.
Table 2.
Levels of ubiquitin C-terminal hydrolase-L1 at all time points for each clinical outcome measure
| Clinical Variable | Levels of Ubiquitin C-Terminal Hydrolase-L1 (ng/mL); SEM
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 6 hrs, n = 10 | 12 hrs, n = 20 | 24 hrs, n = 26 | 48 hrs, n = 35 | 72 hrs, n = 32 | 96 hrs, n = 15 | 120 hrs, n = 22 | 168 hrs, n = 18 | |
| Postresuscitation | ||||||||
| Glasgow Coma Scale | ||||||||
| GCS 3–5 | 88.3; 8.6 | 72.4; 29.9 | 45.8; 15.5 | 44.5; 12.5 | 57.7; 25.0 | 46.2; 25.9 | 21.3; 9.3 | 50.6; 35.9 |
| GCS 6–8 | 128.4; 94.9 | 43.9; 20.6 | 49.1; 21.5 | 40.1; 12.4 | 20.0; 7.2 | 32.8; 20.5 | 15.2; 10.3 | 11.7; 4.8 |
| Best day 1 GCS | ||||||||
| GCS 3–5 | 96.7; 3.3 | 146.8; 58.6 | 76.1; 24.7 | 55.3; 20.1 | 72.1; 40.2 | 69.9; 39.1 | 24.8; 10.2 | 56.5; 43.4 |
| GCS 6–8 | 117.0; 74.4 | 35.3; 13.6 | 36.4; 14.8 | 37.3; 9.4 | 23.4; 6.1 | 27.9; 16.0 | 14.2; 9.6 | 12.8; 4.4 |
| Marshall class | ||||||||
| I | — | — | — | 0 | 0 | — | — | — |
| II | 200.7; 102.5 | 82.5; 28.1 | 56.9; 18.3 | 43.0; 13.3 | 53.0; 25.3 | 48.9; 44.8 | 11.9; 6.8 | 52.9; 44.4 |
| III | — | 18.6; 15.0 | 129.1; 84.1 | 67.5; 46.7 | 18.1; 11.7 | 8.1; 5.4 | 1.8; 1.0 | 11.7; 5.0 |
| IV | — | — | — | — | — | — | — | — |
| Evacuated mass | 55.0; 28.2 | 21.0; 11.0 | 11.5; 3.5 | 40.1; 9.8 | 30.7; 10.9 | 42.5; 20.3 | 27.8; 15.2 | 16.4; 5.7 |
| Nonevacuated | 11.5; 51.5 | 173.6; 163.3 | 60.3; 39.7 | 0 | 1.6 | — | 8.7 | — |
| Intracranial computed tomography lesions | ||||||||
| Evolving | — | 8.4; 2.7 | 7.3; 5.0 | 12.2; 5.8 | 5.0; 1.9 | 6.3; 1.4 | 1.8; 0 | 1.8; 0 |
| Nonevolving | — | 44.3; 27.6 | 12.1; 4.5 | 61.0; 15.3 | 45.7; 11.2 | 28.5; 14.9 | 39.5; 33.1 | 12.8; 6.5 |
| Mortality at 6 wks | ||||||||
| Deceased | 53.9; 42.4 | 119.6; 63.6 | 98.5; 31.5 | 44.1; 27.8 | 56.2; 35.7 | — | 3.8; 2.2 | — |
| Alive | 134.2; 70.2 | 42.1; 14.9 | 34.1; 13.0 | 41.8; 9.0 | 31.8; 12.5 | — | 20.8; 9.0 | — |
| Glasgow Outcome Scale at 6 mos | ||||||||
| Poor | 135.7; 69.2 | 70.7; 26.4 | 58.4; 18.6 | 43.2; 11.9 | 29.5; 12.2 | 44.1; 19.8 | 19.6; 9.3 | 18.1; 4.1 |
| Good | 50.0; 50.0 | 41.3; 21.9 | 26.1; 11.7 | 38.2; 11.3 | 54.5; 33.5 | 4.2; 2.3 | 0.6; 0.6 | 0.6; 0.6 |
GCS, Glasgow Coma Scale.
Levels of ubiquitin C-terminal hydrolase-L1 at each time point are presented for each clinical outcome. Values represent means (ng/mL) ± SEM.
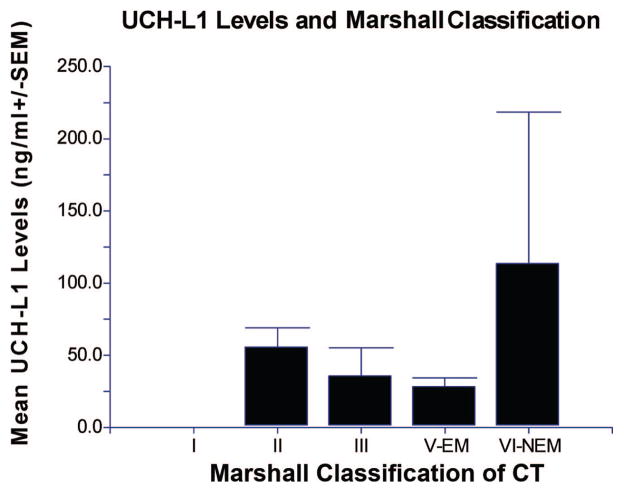
In Figure 3 the overall mean levels of UCH-L1 are plotted in each of the categories of the Marshall classification. There were no significant differences in levels of UCH-L1 between the Marshall classes at any time point (Table 2). The overall mean level of UCH-L1 in the evacuated mass lesion (V-EM) group vs. the nonevacuated mass lesion (VI-NEM) group was 28.3 (±6.1) and 113.6 (±104.8), respectively (p = .43). Upon examination of patients who underwent neurosurgery at any time during their hospitalization compared with those who did not, overall mean UCH-L1 levels were 34.7 (±7.9) versus 22.3 (±11.3) respectively (p = 0.17).
Figure 3.
Overall mean levels of ubiquitin C-terminal hydrolase (UCH-L1) and the Marshall classification on admission computed tomography (CT) scan. There were no significant differences in levels of UCH-L1 between the Marshall classes (n = 1, 15, 4, 14, 2, respectively). There were no patients in the class IV group. The overall mean level of UCH-L1 in the evacuated mass lesion (V-EM) group vs. the nonevacuated mass lesion (VI-NEM) group was 28.3 (±6.1) and 113.6 (±104.8), respectively (p = .43).
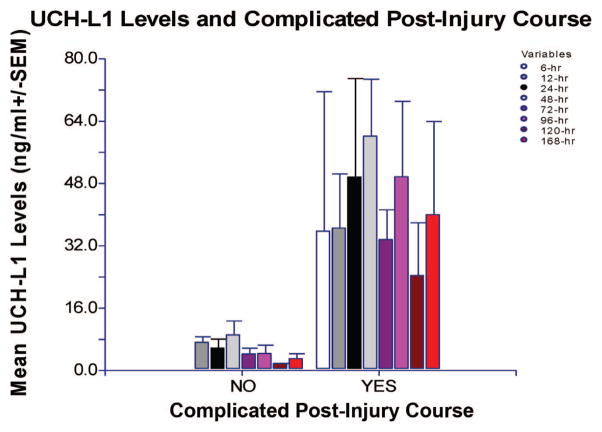
In the Baylor cohort (n = 24) there were 16 (67%) patients who experienced a complicated postinjury course: 4 (17%) with secondary insults; 5 (21%) with recurrent/delayed lesions; and 7 (8%) with evolving lesions. The overall mean level of UCH-L1 in patients with complications postinjury was 43.1 (±8.4) ng/mL (range 3.6 –107.7) compared with 6.1 (±1.0) ng/mL (range 2.0 –10.8) in those without complications (p = .002). In patients with a complicated postinjury course, levels of UCH-L1 were significantly elevated at 24, 48, and 72 hrs after injury (p = .033, p = .004 and p = .045, respectively) compared with those without a complicated course (Fig. 4). These data were only collected per the Baylor protocol and were not available for the University of Florida cohort.
Figure 4.
Comparison of levels of ubiquitin C-terminal hydrolase (UCH-L1) measured at all time points after injury in patients with and without a complicated postinjury course. In patients with a complicated postinjury course, levels of UCH-L1 were significantly elevated at 24, 48, and 72 hrs after injury (p = .033, p = .004 and p = .045, respectively) compared with those without a complicated course. The number of patients without a complicated postinjury course at each time point was n = 0, 8, 7, 7, 5, 3, 2, 2, and those with a complicated course n = 2, 8, 11, 14, 12, 9, 10, 9, respectively.
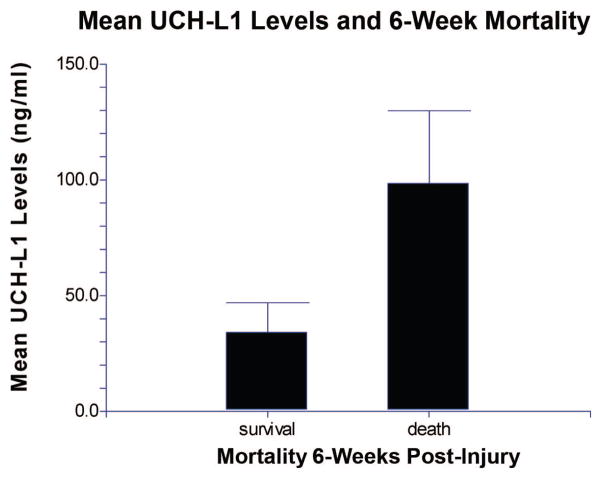
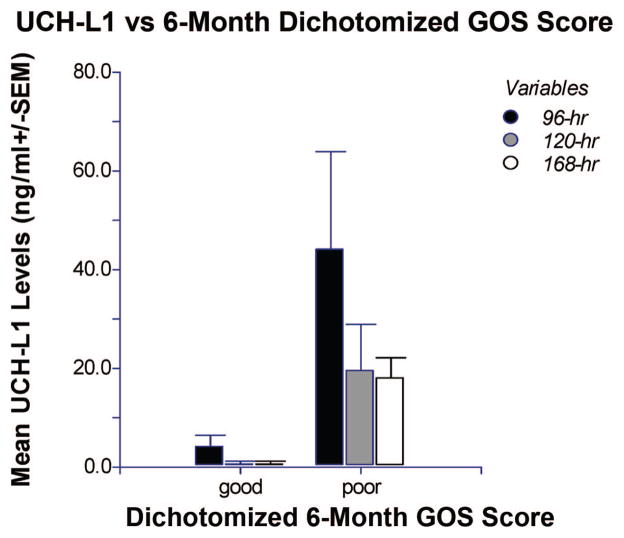
Eight of the 41 (20%) subjects died within 6 weeks of injury. Mean UCH-L1 levels quantified in CSF of these subjects 24 hrs after injury were significantly higher than in those who survived (p = .010) (Fig. 5). At 6 months after injury, 3 (7%) of the 41 patients enrolled were lost to follow-up. Of the remaining 38, 11 (29%) had a good outcome and 27 (71%) had a poor outcome. Mean UCH-L1 levels drawn at 96 and 168 hrs after injury were significantly higher in those with poor outcome compared with those with good outcome (p = .05 and p = .032, respectively). Levels drawn at 120 hrs were also higher in those with poor outcome, but the association was not statistically significant (p = .08) (Fig. 6).
Figure 5.
Comparison of levels of ubiquitin C-terminal hydrolase (UCH-L1) in cerebrospinal fluid 24 hrs after injury in patients with who survived to 6 wks vs. nonsurvivors. Patients who did not survive for 6 wks had significantly higher values (mean ± SEM) of UCH-L1 in cerebrospinal fluid at 24 hrs after injury when compared with patients who survived (p = .010) (n = 6, 23, respectively).
Figure 6.
Comparison of levels of ubiquitin C-terminal hydrolase (UCH-L1) in cerebrospinal fluid at 96, 120, and 168 hrs after injury in patients with poor outcome vs. good outcome at 6 months. Patients with poor outcome had significantly higher values (mean ± SEM) of UCH-L1 at 96 and 168 hrs after injury when compared with patients with good outcome (p = .05 and p = .032, respectively). UCH-L1 levels drawn at 120 hrs showed a similar trend, but values were not statistically significant (p = .08). The number of patients with good outcome at the respective time points was n = 3, 3, 3 and those with a poor outcome n = 7, 15, 9. GOS, Glasgow Outcome Scale.
DISCUSSION
Increased sophistication of laboratory techniques and developments in the field of proteomics has led to the discovery and rapid detection of new biomarkers not previously available (21). UCH-L1 is a highly enriched protein in neurons and alterations in its activity has been associated with neurodegenerative diseases (49), including disorders such as Parkinson’s, Huntington’s and Alzheimer’s diseases (40). Through our previous neuroproteomic work, UCH-L1 was identified as a protein with a twofold increase in abundance in the injured cortex 48 hrs after controlled cortical impact in a rat model of TBI (50). Studies assessing UCH-L1 in human TBI are limited at this time, and this study is among the first to systematically assess UCH-L1 in human CSF following TBI. Using ELISA analysis, we confirmed that the UCH-L1 protein is significantly elevated in human CSF following a severe TBI and is detectable very soon after injury and remains significantly elevated for 168 hrs after injury. Rapid appearance of a biomarker in biologic material is essential to its clinical utility, and preliminary ROC curves for samples of CSF suggest that UCH-L1 was able to distinguish between TBI and uninjured controls at 6-hrs when mental status can be confounded by drugs, alcohol and/or other pathology. Additionally, UCH-L1 remained elevated in patients who experienced postinjury complications and may have added value in the management of these patients in the intensive care unit.
The postresuscitation GCS score was not significantly associated with UCH-L1 levels after injury. One potential explanation for this is that the timing of ventriculostomy placement postinjury was variable among patients. Another explanation is that the GCS score may be significantly influenced in the first hours after injury by intoxicants, sedatives, neuromuscular blockade and hemodynamic instability. Nevertheless, we did find an association between levels of UCH-L1 and the best 24-hr GCS score. The best 24-hr GCS was likely to be the most consistent and unaltered documented GCS score available in the medical record. It has been shown that the accuracy of the GCS score improves with repeated evaluations (51). Additionally, the best 24-hr GCS score is less likely to be influenced by confounding factors, and it has been found to be a significant predictor of outcome compared to the postresuscitation GCS score (29, 52).
There were no statistically significant associations between UCH-L1 levels and the Marshall classification of the admission CT. These findings may be explained by the relatively small number of patients in each category and the lack of class IV patients in this cohort. Also, the Marshall classification is based on morphologic abnormalities on initial CT, and perhaps may not reflect the biochemical changes that occur after injury. In 2006, Maas et al (53) examined the predictive value of the Marshall CT classification in comparison with alternative CT models and determined that it is preferable to use combinations of individual CT predictors rather than the Marshall CT classification for prognostic purposes in TBI. The significance of our findings will require further study using a larger cohort.
Secondary insults after TBI are known to influence clinical outcome and the biochemical response to TBI (54 –56). We found significant elevations in patients who experienced a complicated postinjury course including secondary insults and worsening traumatic intracranial lesions, particularly in the first 72 hrs after injury. The potential of UCH-L1 as an indicator of progressive and ongoing injury would be very valuable for clinicians to gauge management and for assessing new therapies. Further analysis on the biokinetic properties of UCH-L1 are being conducted by our group. The temporal profile of changes in biomarker levels will give insight into delayed release of UCH-L1 under specific clinical circumstances as well as defining optimal frequency of sampling after injury.
In 2005, Majetschak et al (57) measured CSF ubiquitin over 7 days in six patients with TBI and found that ubiquitin levels progressively recovered in survivors, whereas levels continued to increase in until death in nonsurvivors. Although Majetschak’s study assessed the parent compound ubiquitin and not UCH-L1, it emphasizes how elevations in this protein family are associated with poorer outcome. In our study, not only was UCH-L1 measured at 24 hrs a significant predictor of 6-week mortality, but UCH-L1 measured later in the postinjury hospital course was associated with 6-month GOS. This suggests that elevated levels of UCH-L1 soon after injury could potentially predict early mortality and that elevations later in the course of hospitalization may be useful for identifying patients with a poor functional outcome at 6 months. These findings suggest that biomarker changes, both early and late in the postinjury course, are important predictors of clinical outcome. Equally important, these data indicate that there may be critical periods following injury during which these markers predict injury severity, mortality, and functionality.
The direct medical costs for treatment of TBI in the United States have been estimated at more than $4 billion annually (58). If the costs of lost productivity that result from TBI are added to this, then the overall estimated cost is closer to $56.3 billion. There are major opportunities to assess how biomarkers could be used to reduce healthcare spending.
UCH-L1 is appealing as a candidate biomarker for several reasons. First, UCH-L1 is highly expressed in neurons (32) with tissue distribution almost exclusively in the brain. Second, UCH-L1 is a small protein with a molecular weight of about 24 kDa and has a compact and almost globular shape (59). Western blots of CSF fluids show that it remains as an intact protein with no detectable breakdown product. These features would facilitate its crossing of the brain-blood barrier and stability in biofluid allowing for its detection. Third, the role of UCH-L1 in removing conjugated ubiquitin from degradation-targeted proteins further implicates its importance in protein turnover in neurons. Often, misfolded proteins, if not appropriately catabolized, could result in protein aggregation that is neurotoxic. Elevated protein aggregate formation has been documented following cerebral ischemia (60, 61). This suggests that UCH-L1 could provide us with information on mechanism of injury at a molecular level for acute brain injury.
Limitations
While these data are encouraging, the authors recognize there are limitations to this study. The current study was performed in a limited cohort of patients with severe TBI, a disease that tends to be heterogeneous in nature. At this time, we cannot confirm that UCH-L1 is entirely central nervous system specific and not released from other organs. Current studies of patients with multitrauma will help address the effect of extracranial injuries on UCH-L1 values.
Since this study was noninterventional, the availability of samples was dependent on the management decisions by the treating physicians. As a result, patients had a variable number of samples available for analysis at the different time points, particularly later in the course of hospitalization because of early discontinuation of the ventriculostomy catheter in patients who improved (making it medically unnecessary) or patient death. Since our study was not designed to obtain samples sooner than 6 hrs, we were unable to plot the time course of UCH-L1 release in CSF closer to the time of injury.
It is arguable that patients with hydrocephalus or unruptured subarachnoid hemorrhage patients are not true normals, but this has been acceptable in prior publications and do show differences with TBI patients (29). The results are actually more favorable because differences were detected between the groups. Practically, it is more difficult to obtain early CSF samples than serum samples, so ongoing studies by our group are focused on refining a serum-based assay sensitive enough to accurately detect UCH-L1 in blood of patients with all severities of TBI. Blood-based assays will also facilitate the development of appropriate normative data for calculations of sensitivity and specificity.
Because of the small sample size, multivariate analysis incorporating other predictors of TBI outcome was not performed; nor were we able to directly measure the impact of factors affecting mental status initially such as intoxicants. Prospective studies are ongoing that will allow these type of analyses to be performed with adequate power.
CONCLUSIONS
Studies assessing UCH-L1 in human TBI are limited at this time, and this study is among the first to systematically assess UCH-L1 in human CSF following TBI. We confirmed that UCH-L1 protein is present in human CSF and that its levels were significantly elevated after severe TBI using enzyme-linked immunosorbent assay (ELISA) analysis. UCH-L1 was detectable in CSF very early after injury and was associated with measures of injury severity and outcome. This work extends findings collected in animal models following a severe head injury and supports the hypothesis that UCH-L1 is a potential marker of injury magnitude in severe TBI patients.
Footnotes
Drs. Papa, Hayes, and Wang are consultants for Banyan Biomarkers. The remaining authors have not disclosed any potential conflicts of interest.
For information regarding this article, lpstat@aol.com
References
- 1.Consensus conference. Rehabilitation of persons with traumatic brain injury. NIH Consensus Development Panel on Rehabilitation of Persons With Traumatic Brain Injury. JAMA. 1999;282:974–983. [PubMed] [Google Scholar]
- 2.Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doppenberg EM, Choi SC, Bullock R. Clinical trials in traumatic brain injury: Lessons for the future. J Neurosurg Anesthesiol. 2004;16:87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Pineda JA, Wang KK, Hayes RL. Biomarkers of proteolytic damage following traumatic brain injury. Brain Pathol. 2004;14:202–209. doi: 10.1111/j.1750-3639.2004.tb00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papa L, Robinson G, Oli M, et al. Use of biomarkers for diagnosis and management of traumatic brain injury patients. Expert Opin Med Diagn. 2008;2:937–945. doi: 10.1517/17530059.2.8.937. [DOI] [PubMed] [Google Scholar]
- 7.Kochanek PM, Berger RP, Bayr H, et al. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: Diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr Opin Crit Care. 2008;14:135–141. doi: 10.1097/MCC.0b013e3282f57564. [DOI] [PubMed] [Google Scholar]
- 8.Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- 9.Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- 10.Cooper E. Neuron-specific enolase. Int J Biol Markers. 1994:205–210. doi: 10.1177/172460089400900401. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki Y, Yada K, Morii S, et al. Diagnostic significance of serum neuron-specific enolase and myelin basic protein assay in patients with acute head injury. Surg Neurol. 1995;43:267–270. doi: 10.1007/978-4-431-68231-8_86. discussion 270 –261. [DOI] [PubMed] [Google Scholar]
- 12.Ross SA, Cunningham RT, Johnston CF, et al. Neuron-specific enolase as an aid to outcome prediction in head injury. Br J Neurosurg. 1996;10:471– 476. doi: 10.1080/02688699647104. [DOI] [PubMed] [Google Scholar]
- 13.Missler U. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956–1960. doi: 10.1161/01.str.28.10.1956. [DOI] [PubMed] [Google Scholar]
- 14.Woertgen C, Rothoerl RD, Holzschuh M, et al. Comparison of serial S-100 and NSE serum measurements after severe head injury. Acta Neurochir (Wien) 1997;139:1161–1164. doi: 10.1007/BF01410977. discussion 1165. [DOI] [PubMed] [Google Scholar]
- 15.Ytrebø LMNG, Korvald C, et al. Renal elimination of protein S-100beta in picgs with acute encephalopathy. Scand J Clin Lab Invest. 2001;61:217–225. doi: 10.1080/003655101300133658. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson HJP, Hoglund P, Alling C, et al. The elimination of S-100b and renal function after cardiac surgery. J Cardiothorac Vasc Aneth. 2000;14:698–701. doi: 10.1053/jcan.2000.18444. [DOI] [PubMed] [Google Scholar]
- 17.Usui AKK, Abe T, Murase M, et al. S-100ao protein in blood and urine during open-heart surgery. Clin Chem. 1989;35:1942–1944. [PubMed] [Google Scholar]
- 18.Raabe A, Grolms C, Seifert V. Serum markers of brain damage and outcome prediction in patients after severe head injury. Br J Neurosurg. 1999;13:56–59. doi: 10.1080/02688699944195. [DOI] [PubMed] [Google Scholar]
- 19.Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-a and S100-b proteins in normal nonnervous human tissues. Lab Invest. 1987;57:489– 498. [PubMed] [Google Scholar]
- 20.Romner B, Ingebrigtsen T, Kongstad P, et al. Traumatic brain damage: Serum S-100 protein measurements related to neuroradiological findings. J Neurotrauma. 2000;17:641– 647. doi: 10.1089/089771500415391. [DOI] [PubMed] [Google Scholar]
- 21.Wang KK, Ottens AK, Liu MC, et al. Proteomic identification of biomarkers of traumatic brain injury. Expert Rev Proteomics. 2005;2:603– 614. doi: 10.1586/14789450.2.4.603. [DOI] [PubMed] [Google Scholar]
- 22.Berger RP, Beers SR, Richichi R, et al. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J Neurotrauma. 2007;24:1793–1801. doi: 10.1089/neu.2007.0316. [DOI] [PubMed] [Google Scholar]
- 23.Xiong H, Liang WL, Wu XR. Pathophysiological alterations in cultured astrocytes exposed to hypoxia/reoxygenation. Sheng Li Ke Xue Jin Zhan. 2000;31:217–221. [PubMed] [Google Scholar]
- 24.Skogseid IM, Nordby HK, Urdal P, et al. Increased serum creatine kinase BB and neuron specific enolase following head injury indicates brain damage. Acta Neurochir (Wien) 1992;115:106–111. doi: 10.1007/BF01406367. [DOI] [PubMed] [Google Scholar]
- 25.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 26.Laskowitz EA. Serum markers of cerebral ischemia. J Stroke Cerebro Dis. 1998;7:234–241. doi: 10.1016/s1052-3057(98)80032-3. [DOI] [PubMed] [Google Scholar]
- 27.Roine EA. Neurological outcome after out-of-hospital cardiac arrest. Prediction by cerebrospinal fluid enzyme analysis. Arch Neurol. 1989;46:753–756. doi: 10.1001/archneur.1989.00520430047015. [DOI] [PubMed] [Google Scholar]
- 28.Martens P. Serum neuron-specific enolase as a prognostic marker for irreversible brain damage in comatose cardiac arrest surviviors. Acad Emerg Med. 1996;3:126–131. doi: 10.1111/j.1553-2712.1996.tb03399.x. [DOI] [PubMed] [Google Scholar]
- 29.Pineda JA, Lewis SB, Valadka AB, et al. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- 30.Ringger NC, O’Steen BE, Brabham JG, et al. A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. J Neurotrauma. 2004;21:1443–1456. doi: 10.1089/neu.2004.21.1443. [DOI] [PubMed] [Google Scholar]
- 31.Pike BR, Flint J, Dave JR, et al. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2004;24:98–106. doi: 10.1097/01.WCB.0000098520.11962.37. [DOI] [PubMed] [Google Scholar]
- 32.Jackson P, Thompson RJ. The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J Neurol Sci. 1981;49:429– 438. doi: 10.1016/0022-510x(81)90032-0. [DOI] [PubMed] [Google Scholar]
- 33.Tongaonkar P, Chen L, Lambertson D, et al. Evidence for an interaction between ubiquitin-conjugating enzymes and the 26S proteasome. Mol Cell Biol. 2000;20:4691– 4698. doi: 10.1128/mcb.20.13.4691-4698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lincoln S, Vaughan J, Wood N, et al. Low frequency of pathogenic mutations in the ubiquitin carboxy-terminal hydrolase gene in familial Parkinson’s disease. Neuroreport. 1999;10:427– 429. doi: 10.1097/00001756-199902050-00040. [DOI] [PubMed] [Google Scholar]
- 35.Saigoh K, Wang YL, Suh JG, et al. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat Genet. 1999;23:47–51. doi: 10.1038/12647. [DOI] [PubMed] [Google Scholar]
- 36.Laser H, Mack TG, Wagner D, et al. Proteasome inhibition arrests neurite outgrowth and causes “dying-back” degeneration in primary culture. J Neurosci Res. 2003;74:906–916. doi: 10.1002/jnr.10806. [DOI] [PubMed] [Google Scholar]
- 37.Coleman MP, Ribchester RR. Programmed axon death, synaptic dysfunction and the ubiquitin proteasome system. Curr Drug Targets CNS Neurol Disord. 2004;3:227–238. doi: 10.2174/1568007043337436. [DOI] [PubMed] [Google Scholar]
- 38.Hu BR, Liu CL, Ouyang Y, et al. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Keller JN, Huang FF, Zhu H, et al. Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2000;20:1467–1473. doi: 10.1097/00004647-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Gong B, Leznik E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 2007;20:365–370. doi: 10.1358/dnp.2007.20.6.1138160. [DOI] [PubMed] [Google Scholar]
- 41.Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien) 1976;34:45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- 42.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81– 84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 43.Narayan RK, Greenberg RP, Miller JD, et al. Improved confidence of outcome prediction in severe head injury. A comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg. 1981;54:751–762. doi: 10.3171/jns.1981.54.6.0751. [DOI] [PubMed] [Google Scholar]
- 44.Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9 (Suppl 1):S287–S292. [PubMed] [Google Scholar]
- 45.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Initial management. J Neurotrauma. 2000;17:463– 469. doi: 10.1089/neu.2000.17.463. [DOI] [PubMed] [Google Scholar]
- 46.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 47.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480– 484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 48.Choi SC, Clifton GL, Marmarou A, et al. Misclassification and treatment effect on primary outcome measures in clinical trials of severe neurotrauma. J Neurotrauma. 2002;19:17–22. doi: 10.1089/089771502753460204. [DOI] [PubMed] [Google Scholar]
- 49.Willis D, Li KW, Zheng JQ, et al. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25(4):778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobeissy FH, Ottens AK, Zhang Z, et al. Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol Cell Proteomics. 2006;5:1887–1898. doi: 10.1074/mcp.M600157-MCP200. [DOI] [PubMed] [Google Scholar]
- 51.Stocchetti N, Pagan F, Calappi E, et al. Inaccurate early assessment of neurological severity in head injury. J Neurotrauma. 2004;21:1131–1140. doi: 10.1089/neu.2004.21.1131. [DOI] [PubMed] [Google Scholar]
- 52.Phuenpathom N, Choomuang M, Ratanalert S. Outcome and outcome prediction in acute subdural hematoma. Surg Neurol. 1993;40:22–25. doi: 10.1016/0090-3019(93)90164-v. [DOI] [PubMed] [Google Scholar]
- 53.Maas AI, Hukkelhoven CW, Marshall LF, et al. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–1182. doi: 10.1227/01.neu.0000186013.63046.6b. discussion 1173–1182. [DOI] [PubMed] [Google Scholar]
- 54.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 55.Hukkelhoven CW, Steyerberg EW, Rampen AJ, et al. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99:666– 673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- 56.Campbell CG, Kuehn SM, Richards PM, et al. Medical and cognitive outcome in children with traumatic brain injury. Can J Neurol Sci. 2004;31:213–219. doi: 10.1017/s0317167100053853. [DOI] [PubMed] [Google Scholar]
- 57.Majetschak M, King DR, Krehmeier U, et al. Ubiquitin immunoreactivity in cerebrospinal fluid after traumatic brain injury: clinical and experimental findings. Crit Care Med. 2005;33:1589–1594. doi: 10.1097/01.ccm.0000169883.41245.23. [DOI] [PubMed] [Google Scholar]
- 58.TBI state demonstration grants. J Head Trauma Rehabil. 2000;15:750–760. doi: 10.1097/00001199-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Johnston SC, Riddle SM, Cohen RE, et al. Structural basis for the specificity of ubiquitin C-terminal hydrolases. EMBO J. 1999;18:3877–3887. doi: 10.1093/emboj/18.14.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu BR, Janelidze S, Ginsberg MD, Busto R, et al. Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metab. 2001;21:865– 875. doi: 10.1097/00004647-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Ge P, Luo Y, Liu CL, et al. Protein aggregation and proteasome dysfunction after brain ischemia. Stroke. 2007;38:3230–3236. doi: 10.1161/STROKEAHA.107.487108. [DOI] [PMC free article] [PubMed] [Google Scholar]