Abstract
Inflammation cell infiltration and cytokine expression are seen in the vascular walls and intervening stroma of resected brain arteriovenous malformation (bAVM) specimens, even in unruptured and previously untreated lesions. Macrophages may play a critical role in bAVM progression to rupture, and could serve as a marker for rupture risk. We assessed feasibility of imaging macrophages within the bAVM nidus using ferumoxytol-enhanced MRI in four patients with already diagnosed bAVMs using iron-sensitive imaging (ISI; T2*-GE-MRI sequence). Patients were imaged at baseline and at either 1 day (n=2) or 5 days (n=2) after infusion of 5mg/kg of ferumoxytol. Residual intravascular ferumoxytol obscured evaluation for uptake in bAVM vascular walls and stroma at the 1-day time point. The two cases imaged at 5 days showed less intravascular tracer but had signal loss in the nidal region consistent with ferumoxytol localization. One case underwent surgical resection; there was prominent vascular wall CD68 staining. Ferumoxytol-enhanced-MRI for assessing bAVM inflammatory cell burden appears feasible and has the potential to be developed as a biomarker to study lesional inflammatory events.
Keywords: Arteriovenous malformations, Inflammation, Magnetic resonance imaging, Ferumoxytol, USPIO
Introduction
Inflammation is increasingly recognized as a contributing factor in the underlying pathophysiology of brain arteriovenous malformations (bAVM). Examination of resected surgical specimens has demonstrated markedly increased expression of inflammatory cytokines and proteases, including 1000-fold increase in Interleukin-6 and 50-fold increase in MMP-9, compared to the normal brain [1, 2]. MMPs play a role in the pathogenesis of intracranial hemorrhage (ICH) by degrading structural elements of the vascular wall [3]. In pro-angiogenic environments, such as the one that exists in the bAVM nidus [4], macrophages may have a major contribution to MMP-9 activity [5]. In bAVM tissue, macrophages and other inflammatory cells [1, 2, 6] are seen in the vascular walls and intervening stroma, even with no history of rupture and without pre-resection embolization or radiotherapy. Macrophage inhibitory factor [7] and NF-kappaB [8] are both upregulated in lesional endothelial cells and macrophages. Several inflammatory gene promoter polymorphisms associated with increased cytokine elaboration have been associated with increased risk for ICH, e.g., interleukin-6 with index (presenting) ICH [9], and interleukin-1β and TNF-a with natural history ICH, i.e., ICH after diagnosis but before any treatment [10, 11]. Given a strong potential link between inflammation and the risk of bAVM hemorrhage, a non-invasive means to detect inflammation could be of value for identifying bAVM at risk for hemorrhage.
Ferumoxytol (AMAG Pharmaceuticals, Inc., Lexington, Massachusetts), an iron oxide nanoparticle coated by a carbohydrate shell, is gaining recognition for its use as an imaging agent for inflammation and also as an intra-vascular imaging agent [12]. As one of the nanoparticles known as Ultrasmall Superparamagnetic Iron Oxide (USPIO), ferumoxytol was originally developed as a drug to treat iron deficiency anemia in patients with chronic renal failure. The FDA approved this application in 2009 [13].
Ferumoxytol’s clearance by reticuloendothelial system macrophages changes the magnetic properties of the macrophages such that these macrophages can be imaged with MRI, which has led to its proposed use as an inflammation-imaging agent [14]. Ferumoxytol is superparamagnetic, and, as such, appears hypointense on T2*-weighted sequences and hyperintense on T1-weighted sequences. The iron particles can be phagocytized by mononuclear cells, but ferumoxytol accumulation in the interstitial (extracellular) space might also play an important role in signal change in T2*-weighted images. Ferumoxytol uptake into tissue can be visualized within 24 hours. Prior studies have suggested maximum enhancement intensity of ferumoxytol at around 24 hours [14–16]. The intravascular half-life of ferumoxytol is in the range of 10–14 hours [12, 17], and may be dose-dependent. It is less clear how long intravascular tracer persists to an extent which would prevent interpretation of parenchymal or vascular wall localization, particularly in bAVMs. Although USPIO-enhanced MRI shows promise for localizing inflammatory cells in vascular disease states such as atherosclerotic plaques [18], it has not been described for imaging bAVMs. We recently reported a pilot study co-localizing T2* gradient echo MR signal loss at 72 hours after infusion of 5mg/kg of ferumoxytol to Prussian blue and CD68 staining the ferumoxytol nanoparticles within the macrophages in the wall of resected intracranial aneurysm domes [19]. Extrapolating from that study, we hypothesized that ferumoxytol-enhanced MRI could demonstrate macrophage phagocytic activity as an index of inflammation in bAVM tissue.
Methods
After institutional approval at UCSF and the University of Iowa, patients gave informed consent between January and September of 2011 to undergo ferumoxytol-enhanced MRI. All patients were ambulatory adults with no history of allergy or hypersensitivity to iron or dextran or iron-polysaccharide preparations; without contraindication to MRI; normal renal and hepatic function; normal iron status; and were not receiving combination antiretroviral therapy. Patients A and B were recruited at the University of Iowa, Patients C and D, at UCSF. Patients underwent baseline imaging followed by immediate injection of ferumoxytol. Delayed imaging was carried out either 1 day or 5 days after injection. MR sequences were the same for each imaging session at each site.
At UCSF, MR imaging was completed on a Philips 1.5T Intera system. Patients completed a baseline MRI consisting of time-of-flight (TOF) angiography, T1-weighted black blood imaging and a T2* GE sequence. The TOF angiographic sequence was collected using a 3D multi-slab technique with the following parameters: TE=6.9ms, TR=23ms, field-of-view=200×200×50mm, matrix=256×256×50, Bandwidth=165Hz/pixel. Two slabs were collected with a 20% overlap. The T1-weighted sequence was sequential single slice 2D Fast Spin Echo sequences with double inversion recovery for blood suppression with the following parameters: TE=23 ms, TR=680 ms, and an echo train length of 7. The T2* weighted sequences were collected using a 2D gradient-echo sequence with the following parameters: TR/TE = 680/23 ms, flip angle = 20, FOV=240×240, matrix=256×256, slice thickness/gap=2.4/1.0mm, Bandwidth=260Hz/pixel, NEX=3
At the University of Iowa, MR imaging was completed on a Siemens 3T TIM Trio system. Patients completed a baseline MRI consisting of time-of-flight (TOF) angiography and T2*GE sequences. The TOF angiographic sequence was collected using a 3D multi-slab technique with the following parameters: TE=3.6ms, TR=20ms, field-of-view=200×200×200mm, matrix=384×384×20, Bandwidth=165Hz/pixel. Four slabs were collected with a 20% overlap. The T2* weighted sequence was collected using a 2D gradient-echo sequence with the following parameters: TE=20ms, TR=500ms, flip angle=20, FOV=220×220, matrix=512×384, slice thickness/gap=3.0/0.3mm, Bandwidth=260Hz/pixel.
One case (Case C) underwent microsurgical resection of the bAVM. We performed Hematoxylin and Eosin, CD 68 and Prussian Blue staining as previously described [6, 19, 20].
Results
The four patients included in the study are summarized in Table 1 and the imaging findings are shown in Table 2. Patient A and B (Iowa) were both imaged at 24 hours and demonstrated substantial residual intravascular tracer which precluded interpretation of nidal uptake (data not shown).
Table 1.
Patient demographics and vascular lesion characteristics
| Pt. No. | Age (Yrs) | Gender | Location | Size (mm) | Clinical History |
|---|---|---|---|---|---|
| A | 78 | Female | Right frontal | 24 × 15 | Severe headache, subacute hemorrhage |
| B | 54 | Male | Pineal region | 35 × 20 | Headaches, dizziness, prior distant hemorrhage |
| C | 62 | Female | Left frontal | 92 × 29 | Visual disturbance consistent with partial seizures in 2002. Underwent Gamma Knife in 2002 and 2007; Surgical resection 2010; 2 weeks after ferumoxytol study. |
| D | 70 | Male | Right temporal | 35 × 30 | Patient presented with seizure in 1974 and underwent embolization. Gamma Knife 2002, with minimal effect. |
Table 2.
Arteriovenous malformation findings
| AVM No. | Timing of delayed imaging | MR Comments* | Histopathology |
|---|---|---|---|
| A | 24 h | Relative signal loss on post-infusion and difference images attributed to substantial residual ferumoxytol within lumen of vascular lesion | N/A |
| B | 24 h | Moderate amount of uptake visualized within arteriovenous malformation wall; substantial residual intraluminal ferumoxytol | N/A |
| C | 5 days | Moderate amount of uptake visualized within arteriovenous malformation nidus. Residual intravascular tracer is minimal. | Prominent macrophage staining seen in vascular walls. Prussian blue not informative due to long interval between ferumoxytol injection and resection. |
| D | 5 days | Small amount of signal loss visualized that corresponds to the arteriovenous malformation vessel walls. There is some signal loss apparent in post-ferumoxytol images in normal vasculature distant from bAVM, although no signal loss is seen in sagittal sinus. | N/A |
Images for Patient C, who had a large left frontal AVM, are shown in Figures 1 and 2. The angiogram at the time of diagnosis in 2002 is shown in Figure 1A and 1B. Figure 1C shows the angiogram in 2010 after two courses of gamma knife in 2002 and 2007. In 2010, Patient C underwent microsurgical resection, 19 days after receiving the ferumoxytol injection. A paraffin block was available for examination. Figure 1D and 1E show Hematoxylin and Eosin staining of the lesion; the thickened vessels are consistent with prior radiosurgery. There were rare hemosiderin clusters noted (not shown). There was a prominent infiltration of inflammatory cells of all types. Figures 1F, G, and H show CD68 staining consistent with macrophage infiltration into the vascular walls. There were only sporadic Prussian Blue positive clusters (Figure 1I and 1J). In Figure 2, Panels A and B show the baseline and post-ferumoxytol images. Panels C and D correspond to magnified panels E and F, respectively. In the post-ferumoxytol delayed imaging (Panels D and F), there is minimal intravascular signal loss in normal brain areas. In the magnified Panel F view, post-ferumoxytol signal loss is seen within the nidus, consistent with the CD68 pattern seen in Figure 1C. However, the extent of blooming obscures the detailed distribution of the signal loss between vascular walls and intervening stromal tissue.
Figure 1. Case C.

A & B. Lateral and A-P carotid angiograms. Large left frontal AVM at diagnosis, prior to gamma knife treatment (2002). AVM is fed predominantly by left dysplastic ACA with small flow-related aneurysms. The deep drainage is via enlarged left basal vein of Rosenthal to Vein of Galen. There is a coil-excluded basilar tip aneurysm.
C. Pre-surgical angiogram (2010) shows a markedly decreased but still present nidus, now with superficial-only drainage.
D. Intermediate magnification of Hematoxylin and Eosin stain showing vascular structures and intervening parenchyma in AVM. There are intraluminal foamy macrophages and a mixed lymphoplasmacytic inflammatory infiltrate surrounding the vascular structures containing numerous macrophages and neutrophils. The surrounding neuropil demonstrates reactive gliosis. E. High magnification Hematoxylin and Eosin stain of AVM with markedly thickened vessels and intervening parenchymal and perivascular inflammatory infiltrates that include lymphocytes, neutrophils and macrophages.
F. 10x magnification of paraffin tissue section of resection surgical specimen; stained for CD68. Macrophage infiltration is prominent is vessels walls.
G. 20x magnification. Scattered intraluminal positive cells are seen, but the macrophage density is clearly seen in walls of vascular structures and intervening gliotic tissue.
H. 20x magnification. Similar to G, with more extensive macrophage infiltration into vascular wall.
I. Low magnification H/E stain showing localization of scattered Prussian Blue positive staining
J. High magnification H/E stain showing localization of scattered Prussian Blue positive staining (arrows).
Figure 2. Case C.
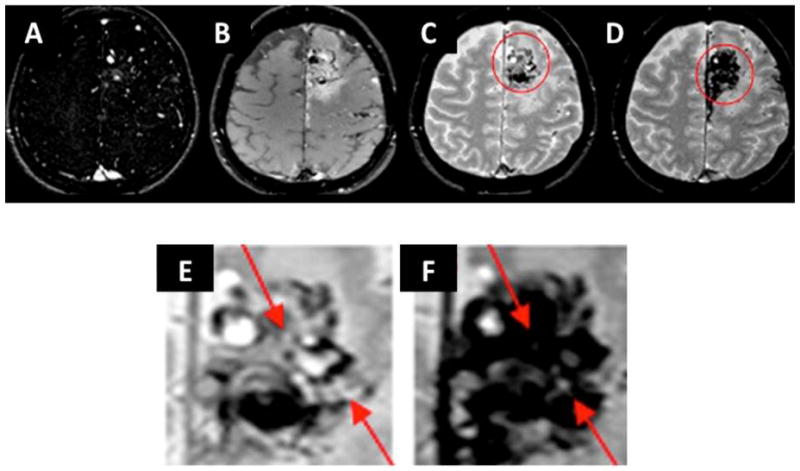
A. Select axial image MR angiography, baseline prior to injection
B. Baseline T1-weighted MRI
C. 3D T2*-GE MRI
D. Five days post-ferumoxytol injection T2*-GE MR. Note signal loss and blooming consistent with uptake of USPIO into lesional tissue.
E. Magnified view of baseline T2*-GE MRI
F. Magnified view of post-ferumoxytol injection T2*-GE MRI. Arrows indicate areas of nidus with signal loss and blooming, consistent with uptake of USPIO. Blooming effect precludes discerning discrete pattern of uptake into vascular wall.
Case D was diagnosed in 1973 with a right temporal bAVM, remained untreated until 2002, and then underwent gamma knife therapy in 2002. No diagnostic or pre-treatment imaging was available for this patient. He was seen in a follow-up consultation in 2010 and the images in Figure 3A (CT angiography) and Figure 3B (MRA) were obtained. The bAVM was still present but the patient elected not to undergo further interventional therapy. A catheter angiogram was not performed. At this time the patient underwent ferumoxytol-enhanced MRI. In Figure 4, Panels B and C correspond to magnified Panels E and F, respectively. On the 5-day post-ferumoxytol images, there appears to be greater signal loss in the normal cortical vasculature, although signal loss in the sagittal sinus is not apparent. This case is remarkable, however, for what appears to be a clear pattern of signal loss conforming to the vascular walls of the intranidal vessels.
Figure 3. Case D.
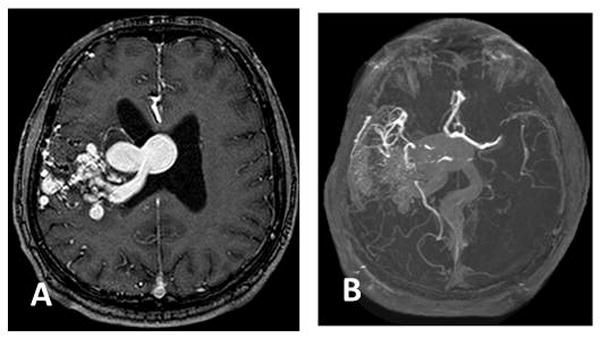
A. Select axial image MR angiography showing large right temporal AVM with deep draining vein and varix along lateral ventricle wall.
B. Contrast-enhanced maximum intensity projection (MIP)collapsed image demonstrating the extent of abnormality
Figure 4. Case D.
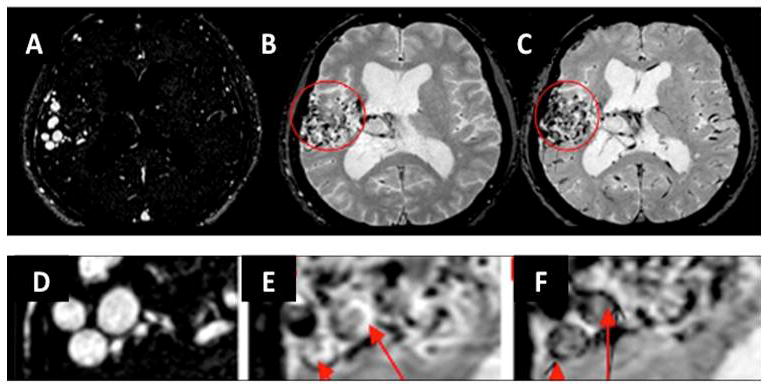
A. Select axial image MR angiography, baseline prior to injection
B. Baseline 3D T2*-GE MRI
C. Five days post-ferumoxytol injection T2*-GE MR. Residual intravascular signal loss is present in cortical vessels, although not in sagittal sinus.
D. Magnified view of baseline MRA
E. Magnified view of baseline 3D T2*-GE MRI
F. Magnified view of 5-day post-ferumoxytol injection 3D T2*-GE MRI. Arrows indicate areas of nidus with signal loss within discrete areas of the vascular walls, consistent with uptake of USPIO.
Discussion
This report provides proof-of-principle that macrophage infiltration in the bAVM nidus appears amenable to clinical imaging using the USPIO agent, ferumoxytol. Having an in vivo, non-invasive means of assessing macrophages presents several opportunities for study. The multiple lines of evidence that inflammation is an important component of the lesional phenotype of bAVM may indicate that inflammation may play a role in the pathogenesis or natural history of the disease. Understanding the prevalence and time course of inflammation, as assessed by macrophage infiltration, could provide a means to better understand the biology of the disease and may provide information on the risk of spontaneous rupture. Further, macrophage burden could present a potential target for therapeutic manipulation.
Several useful points come from this preliminary pilot study that can help guide future studies. Because there appears to be some unpredictable difference among patients in how fast intravascular tracer is cleared, it may be useful to scan patients immediately after ferumoxytol injection, to test how the intravascular compartment appears without the anticipated parenchymal or vascular wall signal loss that will be seen at the delayed imaging time-point. For this purpose, probably a small portion of the total injected ferumoxytol could be given first for this purpose to avoid extensive blooming artifacts. Another point that will need to be validated is that, even with persistent intravascular tracer, it may still be possible to discern vascular wall localization such as that seen in Case D. A comparison to histological examination will be needed for this conclusion, however.
We were able to demonstrate CD68 positive cells consistent with macrophage infiltration in case C. Additional studies will need to compare macrophage infiltration in resected surgical specimens with the MR imaging patterns to determine the sensitivity and specificity of ferumoxytol imaging. If tissue can be examined relatively quickly after imaging, it is possible to use Prussian Blue to document the extent of ferumoxytol uptake and assess its co-localization with CD68 staining if the biopsy is within several days after injection [14, 19]. Our resected case was imaged 19 days post-injection, by which time the inert dextran-coated USPIO particles were no longer resident at the site of inflammation.
Imaging subtle changes in the bAVM may be challenging because of the high blood volume of the nidus with its dense vascular network. Residual intravascular ferumoxytol signal loss may confound interpretation by making it difficult to differentiate USPIO particles that are still in the intravascular compartment from those that have been phagocytized by cells in the vascular wall or intervening stromal tissue. Our study suggests that 24 hours is insufficient time for clearance of tracer from the intravascular compartment. Both cases imaged at 5 days demonstrated signal loss in nidal structures consistent with ferumoxytol localization, but only one had minimal signal loss in uninvolved cerebral vessels distant from the nidus. It is not clear why there was still apparent signal loss in the normal vasculature distant from the lesion in Case D, considering that sagittal sinus did not show increased signal loss. Given the 10–14-hour half-life of ferumoxytol [12], sufficient time for clearance of intravascular tracer should have elapsed. Although speculative, some degree of increased leak or inflammatory involvement of distant vessels might explain the observed pattern. Subsequent experimental protocols will likely employ at least a five-day delay after injection of ferumoxytol before attempting to image tissue uptake.
This study is limited by its small size, its use of two different protocols, and its use of two different magnet strengths. Further, two of the cases (Patients C and D) with informative imaging findings had undergone prior treatment. Accordingly, inflammatory changes in these lesions cannot be uniquely attributed to the natural history of the bAVM. Nonetheless, the extent of macrophage infiltration seen in the CD68 staining in Figure 1 is grossly similar to the extent of inflammatory cell infiltration that we noted in unruptured lesions coming to surgery without prior embolization or radiosurgery [1, 6]. Our results are descriptive; methods to quantify the degree of USPIO uptake in AVM will need to be developed and validated. Nonetheless, this study demonstrates the ability to image what probably represents USPIO phagocytized by macrophages, relative to normal, uninvolved, and uninjured tissues using a standard clinical dose of ferumoxytol. We also could not address safety issues in this pilot study, both in terms of possible adverse effects of iron overload or the potential for residual tissue iron confounding subsequent clinical MR examinations.
It is currently not precisely understood how USPIOs move out of the intravascular space. The USPIOs could be engulfed by monocytes while still in the intravascular compartment or in the process of moving through the capillary wall, or there could be direct transfer of the virus-sized particles through the endothelium by transcytosis or some paracellular route, followed by phagocytosis by macrophages already present in the tissue [14, 21]. Also, a defective blood-brain barrier might allow direct passage of particles into the tissue, as has been observed for brain tumors [22].
We recently reported that there is a high prevalence of silent intralesional microhemorrhage (SIM) in bAVM, roughly 30% in unruptured and 50% in ruptured cases, based on histopathological examination of resected tissue to determine microscopic evidence of hemosiderin [20]. In that study, there was a strong correlation between macrophage infiltration and presence of hemosiderin. Both microhemorrhage and macrophage infiltration appeared in most cases to be immediately perivascular, and in many instances within the walls of the bAVM vessels, similar to Figure 1F–H. The ferumoxytol-associated signal loss seen in Figure 4F from Patient D corresponds to this pattern, and appears to reside within the wall itself, although we could not independently confirm the localization with histology. There is the possibility that the signal loss could be due to marginated monocytes at the endothelial surface, as we could not verify that the observed signal loss corresponds to histological evidence of macrophage infiltration, but this seems less likely. However, since the signal loss was only present after ferumoxytol administration, it represents new iron that was not present at baseline. Since it is relatively unlikely that a new hemorrhage occurred within the 5 days between ferumoxytol and delayed imaging, this signal loss most probably represents ferumoxytol localization in the vascular wall rather than a prior microhemorrhage.
Summary
This pilot, proof-of-principle study suggests that a dose of 5mg/kg of ferumoxytol and imaging at 5 days post injection using T2* GE MRI may be an appropriate starting point to develop this imaging method in the setting of bAVM to image macrophage infiltration of the vascular structures of the nidus. However, additional studies are necessary to demonstrate co-localization of USPIOs within macrophages and to optimize dose and timing parameters. This method shows excellent promise for developing an empirical marker of lesional inflammation to study the natural course of the disease and possibly bAVM rupture risk. In addition, it may be possible to use macrophage burden as a surrogate endpoint in the development of pharmacological agents to lower spontaneous rupture risk.
Acknowledgments
This work was supported in part by NIH R01 NS034949, R01 NS027713 (WLY), P01 NS044155 (WLY, HS), and R01 NS059944 (DS). The authors gratefully acknowledge the contributions of Anne Fedoroff, RN, Elizabeth Gardner, BS, Nancy J. Quinine, RN, Philippe Jolivalt, and Voltaire Gungab for assistance in data collection and preparation of the manuscript.
Abbreviations
- bAVM
Brain arteriovenous malformation
- GE
Gradient Echo
- ICH
Intracranial hemorrhage
- ISI
Iron-sensitive imaging
- MMP
Matrix Metalloproteinase
- TE
Echo Time
- TR
Relaxation Time
- MRI
Magnetic Resonance Imaging
- SIM
Silent intralesional microhemorrhage
- TNF-α
Tumor Necrosis Factor alpha
- TOF
Time-of-flight
- UCSF
University of California, San Francisco
- USPIO
Ultrasmall Superparamagnetic Iron Oxide
References
- 1.Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, et al. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–8. doi: 10.2741/2037. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, et al. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59(1):72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- 3.Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18(11):1163–72. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl. 2011;111:83–92. doi: 10.1007/978-3-7091-0693-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao Q, Su H, Palmer D, Sun B, Gao P, Yang GY, et al. Bone marrow-derived cells contribute to vascular endothelial growth factor-induced angiogenesis in the adult mouse brain by supplying matrix metalloproteinase-9. Stroke. 2011;42(2):453–8. doi: 10.1161/STROKEAHA.110.596452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, et al. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008;62(6):1340–9. doi: 10.1227/01.neu.0000333306.64683.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Zheng M, Shu H, Zhan S, Wang H, Zhou D, et al. Macrophage migration inhibitory factor reduces apoptosis in cerebral arteriovenous malformations. Neurosci Lett. 2012;508(2):84–8. doi: 10.1016/j.neulet.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Aziz MM, Takagi Y, Hashimoto N, Miyamoto S. Activation of nuclear factor kappaB in cerebral arteriovenous malformations. Neurosurgery. 2010;67(6):1669–80. doi: 10.1227/NEU.0b013e3181fa00f1. [DOI] [PubMed] [Google Scholar]
- 9.Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35(10):2294–300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Hysi PG, Pawlikowska L, Poon A, Burchard EG, Zaroff JG, et al. Common variants in interleukin-1-beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc Dis. 2009;27(2):176–82. doi: 10.1159/000185609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achrol AS, Pawlikowska L, McCulloch CE, Poon KY, Ha C, Zaroff JG, et al. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke. 2006;37(1):231–4. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 12.Stabi KL, Bendz LM. Ferumoxytol use as an intravenous contrast agent for magnetic resonance angiography. Ann Pharmacother. 2011;45(12):1571–5. doi: 10.1345/aph.1Q431. [DOI] [PubMed] [Google Scholar]
- 13.Rosner MH, Auerbach M. Ferumoxytol for the treatment of iron deficiency. Expert Rev Hematol. 2011;4(4):399–406. doi: 10.1586/ehm.11.31. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab. 2010;30(1):15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dosa E, Tuladhar S, Muldoon LL, Hamilton BE, Rooney WD, Neuwelt EA. MRI using ferumoxytol improves the visualization of central nervous system vascular malformations. Stroke. 2011;42(6):1581–8. doi: 10.1161/STROKEAHA.110.607994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton BE, Nesbit GM, Dosa E, Gahramanov S, Rooney B, Nesbit EG, et al. Comparative analysis of ferumoxytol and gadoteridol enhancement using t1- and t2-weighted MRI in neuroimaging. AJR Am J Roentgenol. 2011;197(4):981–8. doi: 10.2214/AJR.10.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuwelt EA, Hamilton BE, Varallyay CG, Rooney WR, Edelman RD, Jacobs PM, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009;75(5):465–74. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivedi RA, JMUK-I, Graves MJ, Cross JJ, Horsley J, Goddard MJ, et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35(7):1631–5. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 19.Hasan DM, Mahaney KB, Magnotta VA, Kung DK, Lawton MT, Hashimoto T, et al. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler Thromb Vasc Biol. 2012;32(4):1032–8. doi: 10.1161/ATVBAHA.111.239871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y, Saunders T, Su H, Kim H, Akkoc D, Saloner DA, et al. Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke. 2012 doi: 10.1161/STROKEAHA.111.647263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manninger SP, Muldoon LL, Nesbit G, Murillo T, Jacobs PM, Neuwelt EA. An exploratory study of ferumoxtran-10 nanoparticles as a blood-brain barrier imaging agent targeting phagocytic cells in CNS inflammatory lesions. AJNR Am J Neuroradiol. 2005;26(9):2290–300. [PMC free article] [PubMed] [Google Scholar]
- 22.Neuwelt EA, Varallyay CG, Manninger S, Solymosi D, Haluska M, Hunt MA, et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60(4):601–11. doi: 10.1227/01.NEU.0000255350.71700.37. [DOI] [PubMed] [Google Scholar]


