Abstract
Individual differences in impulsivity underlie a good deal of the risk taking that is observed during adolescence, and some of the most hazardous forms of this behavior are linked to impulsivity traits that are evident early in development. However, early interventions appear able to reduce the severity and impact of these traits by increasing control over behavior and persistence toward valued goals, such as educational achievement. One form of impulsivity, sensation seeking, rises dramatically during adolescence and increases risks to healthy development. However, a review of the evidence for the hypothesis that limitations in brain development during adolescence restrict the ability to control impulsivity suggests that any such limitations are subtle at best. Instead, it is argued that lack of experience with novel adult behavior poses a much greater risk to adolescents than structural deficits in brain maturation. Continued translational research will help to identify strategies that protect youth as they transition to adulthood.
The dramatic growth of developmental neuroscience in the last decade has produced remarkable findings regarding brain development during childhood and adolescence (Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos, et al., 1999; Sowell, Thompson, Tessner, & Toga, 2001). The perhaps most impressive findings concern the protracted maturation of the prefrontal cortex (PFC) and parietal regions. It appears that around age 11, the PFC and parietal lobes begin a period of prolonged pruning of neuronal axons resulting in thinning of cortical grey matter. At the same time, there appears to be an increase in neuronal myelination. The significance of these maturational changes has yet to be established. However, many researchers have argued that the protracted pruning of the PFC represents growing frontal control over behavior, the absence of which is associated with impulsivity and poor decision-making. Indeed, adolescents have long been described as excessively prone to risk taking and impulsivity as exemplified by drug use, unintentional injuries (especially car accidents), and unprotected sexual activity (Arnett, 1992).
Based on these patterns of brain development and behavior, researchers from different disciplines have proposed two-processes of brain maturation that predispose the adolescent to risk taking and impulsivity. One process that emerges early in adolescence is driven by frontostriatal reward circuits involving the ventral striatum (e.g., the nucleus accumbens) (Casey, Getz, & Galvan, 2008; Chambers, Taylor, & Potenza, 2003; Galvan, Hare, Parra, Penn, Voss, Glover, et al., 2006). These circuits mature relatively early (Fuster, 2002) and encourage the adolescent to venture away from the family and toward increasingly novel and adult-like activities (Spear, 2007). Not surprisingly, many of these activities are fraught with a certain amount of risk (e.g., driving, sex).
At the same time that the adolescent is engaging in novel and risky activities, it is argued that the PFC has not yet matured to the point where risks can be adequately assessed and control over risk taking can be sufficiently exerted to avoid unhealthy outcomes. In particular, the PFC and its connections with other brain regions are thought to be structurally inadequate to provide the control that is optimal for adolescent behavior. This maturational gap in development of PFC-based control relative to more advanced motivational circuitry is said to result in an inevitable period of risk for adolescents (Casey et al., 2008; Nelson, Bloom, Cameron, Amaral, Dahl, & Pine, 2002; Steinberg, 2008). Furthermore, it is suggested that interventions to reduce this period of vulnerability will inevitably have very limited effectiveness (see Steinberg, this issue).
In this paper, I argue that the major sources of adolescent risk taking and impulsive action are of two sorts. One is a pre-existing form of impulsivity that is evident in the early years of life (at least age 3) that persists into adolescence. This source of risk is akin to Moffitt's (1993) “life-course persistent” developmental path and Patterson's (Patterson, Reid, & Dishion, 1992) “early starter” path. A second source of risk is associated with a rise in sensation seeking that results from activation of the ventral striatum (Chambers et al., 2003; Spear, 2009). As already noted, this change encourages experimentation with novel (adult-like) behavior. However, rather than representing a structural deficit in frontal control, these risk-taking tendencies are argued to be more the result of normal development and the inevitable lack of experience associated with engaging in these novel behaviors.
In forging this argument, I first review the evidence regarding early manifestations of impulsivity and how experience during childhood, especially various forms of stress, may predispose some youth to engage in risky activity as they proceed through adolescence. This evidence suggests that a major source of risk taking during adolescence may be a result of impaired impulse control that precedes the adolescent period. As a result, adolescent risk taking is not a uniform phenomenon, and individual differences dominate the emergence of such behavior during adolescence.
Early Manifestations of Adolescent Risk Taking
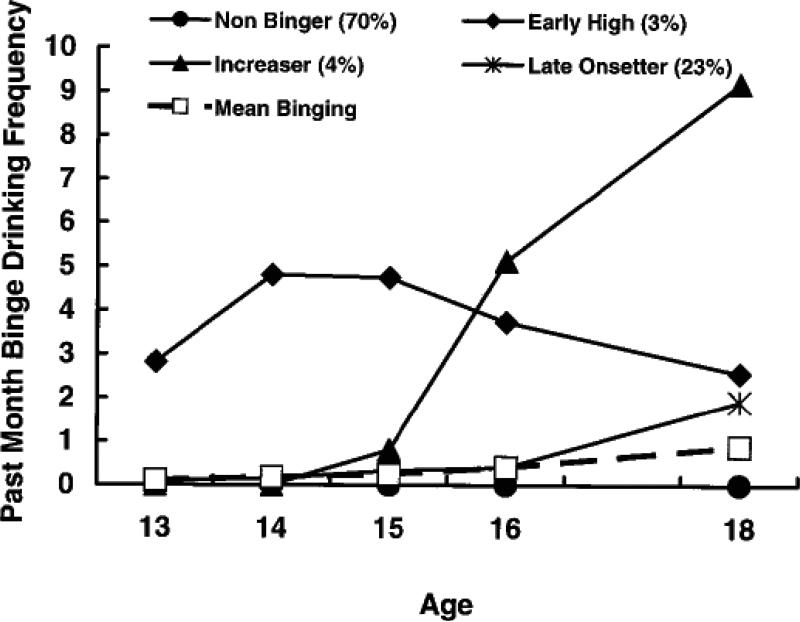
Despite the popular characterization of adolescents as impulsive and lacking cognitive control, the evidence regarding such behavior suggests a more nuanced picture. If we look at recent longitudinal studies of risk behavior trajectories, we see a remarkably consistent pattern. For example, in regard to binge drinking, data from the Seattle Social Development Project (Hill, White, Chung, Hawkins, & Catalano, 2000) shown in Figure 1 indicate that rather than exhibiting a uniform increase across the adolescent period, the dominant pattern for this behavior is not to engage in it. About 70% of the youth in that cohort reported no binge drinking. On the other hand, there was a small group of youth (3%) who exhibited high rates of binge drinking at age 13 and who persisted in this trajectory until age 18. A third group of youth (4%) began to engage in binge drinking during adolescence and a fourth much larger group (23%) began later at age 18.
Figure 1.
Binge drinking trajectories as assessed in the Seattle Social Development Project (reprinted with permission from Hill et al., 2000).
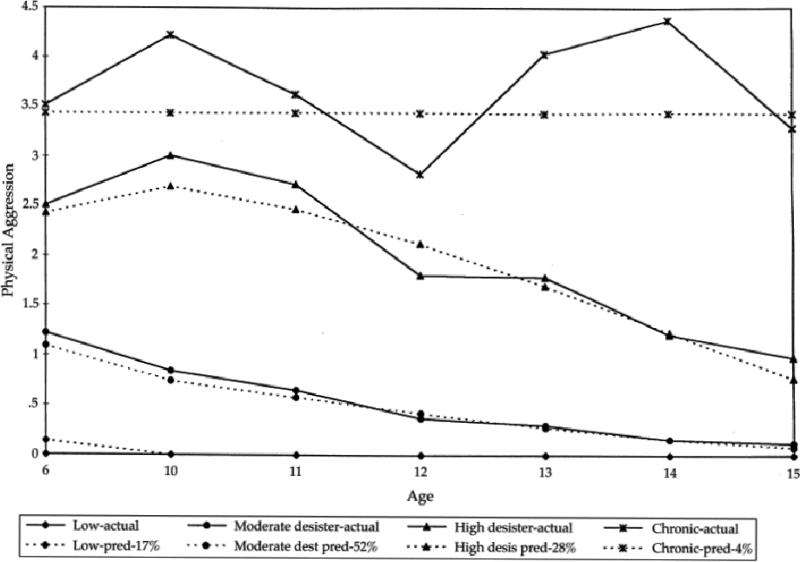
A perhaps more worrisome behavior, physical aggression, was studied by Nagin and Tremblay (1999) in their cohort of male youth in high-risk neighborhoods of Montreal. As seen in Figure 2, even in this high-risk cohort, a large proportion of youth (17%) never engaged in aggressive behavior. However, many youth who did so at an early age (80%) exhibited declining rates of aggression as they aged. These patterns are hardly evidence of weak cognitive control during adolescence. However, as with binge drinking, a small group of youth (4%) exhibited high and persistent rates of aggression early in childhood and continued on this trajectory into adolescence.
Figure 2.
Aggressive behavior trajectories as assessed in high risk neighborhoods of Montreal (reprinted with permission from Nagin & Tremblay, 1999). Four trajectories were identified: Low (17%), moderate desisters (52%), high desisters (28%), and chronically aggressive (4%).
These patterns are consistent with both Moffitt's and Patterson's proposals that many forms of risky maladaptive behavior have their origins in the early years prior to adolescence. Indeed, these age trends suggest that adolescents do not uniformly engage in high-risk behaviors and that a major source of adolescent risk taking is present prior to the adolescent period. It is not surprising therefore given the large individual differences in adolescent risk taking that a small proportion of adolescent account for a large share of the serious forms of risk taking that cause concerns about adolescents. For example, Biglan and Cody (2003) found that 18% of youth ages 12 to 20 accounted for about two thirds of drunk driving and 88% of criminal arrests.
The role of Impulsivity in Early Adolescent Risk Taking
Considerable evidence suggests that youth who engage in early risk taking, such as drug use and aggressive behavior, exhibit higher levels of impulsive behavior as early as age 3 (Caspi & Silva, 1995; Caspi, Henry, McGee, Moffitt, & Silva, 1995; Caspi, Moffitt, Newman, & Silva, 1996; Masse & Tremblay, 1997; Raine, Reynolds, Venables, Mednick, & Farrington, 1998). Indeed, the entire spectrum of externalizing behavior appears to be related to a core set of impulsive traits (Kreuger et al., 2002) that is evident early in development (McGue, Iacono, & Kreuger, 2006). This evidence is again supportive of the idea that a good deal of the problematic behavior observed in adolescents is clustered in a small percentage of youth (cf. Biglan and Cody, 2003).
In studying the role of impulsivity, however, it is important to recognize that the tendency is multidimensional and does not manifest as a single trait. Instead, it is evident in at least three potentially independent forms. One such trait, which can be called acting without thinking, is characterized by hyperactivity without evidence of deliberation or attention to the environment. It is assessed by at least two self-report scales: The Barratt Impulsivity Scale's motor impulsivity subscale (Patton, Stanford, & Barratt, 1995) and the Eysenck I7 scale (Eysenck & Eysenck, 1985). When assessed by observer report, it is characterized by uncontrolled and hyperactive temperament, such as displayed in children with attention deficit hyperactivity disorder (ADHD) (Barkley, 1997).
Acting without thinking is the focus of neurobehavioral theories of early risk for substance use problems (Tarter et al., 2003; Zucker, 2006). Researchers who use tests of executive function to characterize this temperament focus on measures of response inhibition, such as stop signal tasks (Williams, Ponesse, Shachar, Logan, & Tannock, 1999). These tasks assess the ability to monitor conflicting cues to action and inhibit prepotent responses when they are no longer adaptive. In young children, a simpler task involves monitoring cues that flank a dominant focus of attention (the flanker task). Children with ADHD do less well on such tasks (Vaidya, Bunge, Dudukoric, Zalecki, Elliot, Gabrieli, 2005).
A second form of impulsivity is characterized by the tendency to exhibit impatience when given a choice between an immediate small reward versus a larger but delayed reward. It is often assessed using a delay discounting paradigm that can measure differences in preference for delayed rewards (Ainslie, 1975; Rachlin, 2000). Mischel and colleagues (1988) used a simpler task in which children as young as age 4 were given the task of waiting to receive a tempting treat such as a pair of marshmallows. Those children who could deny themselves one marshmallow in order to receive two at a later time were scored as exhibiting patience. Furthermore, children who scored well on this task continued to exhibit patience on such indicators as higher academic performance during adolescence. Other research indicates that adolescents who lack patience are also more likely to experiment with and use drugs (B. Reynolds, 2006; Romer, Duckworth, Sznitman, & Park, 2010).
Just as acting without thinking is associated with deficits in executive function, differences in delay discounting are correlated with variation in working memory capacity and IQ (Shamosh, DeYoung, Green, Reis, Johnson, Conway, et al., 2008). This association suggests that individuals with weaker ability to maintain distant goals in working memory when choosing between immediate and delayed rewards are more prone to discount delayed rewards. The association between weaker executive function and each of these forms of impulsivity is not surprising given that impulsive behavior is often defined as lacking cognitive control over behavior.
Despite the fact that weak executive function underlies both impatience and acting without thinking, evidence from both animal and human models indicates that these forms of impulsivity are independent (Pattij & Vanderschuren, 2008; B. Reynolds, Penfold, & Patak, 2008). That is, individuals who exhibit one type of impulsivity are no more or less likely to exhibit the other. In addition, there is a third type of impulsivity that is independent of the other two (Whiteside & Lynam, 2001). The tendency to approach novel and exciting experiences, known as sensation (Zuckerman, 1994) or novelty (Cloninger, Sigvardsson, & Bohman, 1988) seeking, is characterized by exploration of novel stimuli and the tendency to experiment with exciting activities despite the risks associated with them. It has been found to be greater in children who exhibit early forms of aggressive and other forms of externalizing behavior (Raine et al., 1998).
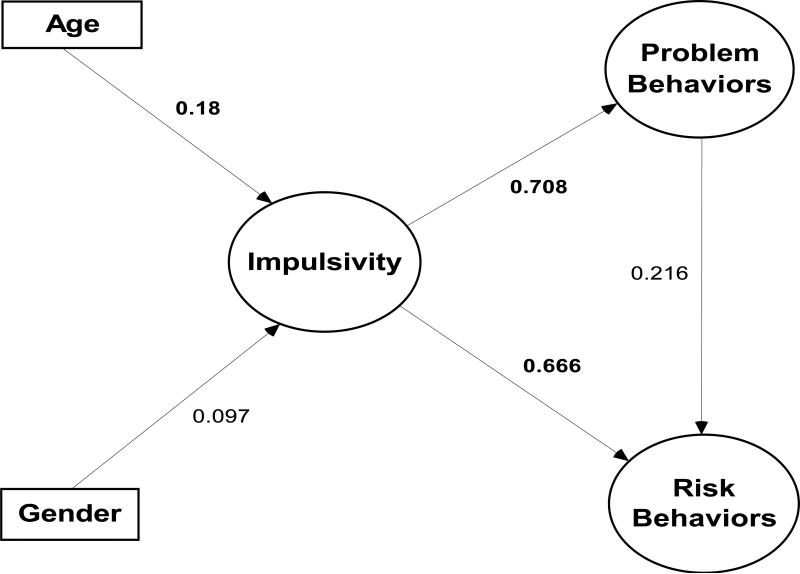
In a study conducted in Philadelphia with a community sample of 387 youth ages 10 to 12, I and several colleagues found that impulsivity as assessed by acting without thinking and sensation seeking was a powerful correlate of early forms of problem and risky behaviors (Romer, Betancourt, Giannetta, Brodsky, Farah, & Hurt, 2009). As seen in Figure 3, a causal model with the two measures of impulsivity (they were somewhat correlated in this young sample, r = .30) was able to completely explain the relation between problem behaviors (such as oppositional behavior and symptoms of ADHD) and risk taking (such as drinking alcohol, gambling for money, fighting, and cigarette smoking) with no significant residual relation between the two. This study confirms the importance of two forms of impulsivity for early manifestations of risky behavior and is consistent with theories that place emphasis on childhood trajectories of disinhibition as predictive of early adolescent problem and risky behavior (Tarter et al., 2003; Zucker, 2006).
Figure 3.
Results of causal model showing that impulsivity explains covariation in risk and problem behaviors in a community sample of Philadelphia preadolescents (ages 10 to 12) (from Romer, et al., 2009). Path from problem behaviors to risk behaviors was not significant.
The Role of Early Stressors in Predisposing Children to Adolescent Risk Taking
Rapidly accumulating evidence from neuroscience and behavioral genetics underscores the importance of early exposure to severe stressors for later health. There is considerable evidence that severe stressors, those that are persistent and not under the individual's control, have “toxic” effects on a wide range of health outcomes (Shonkoff, Boyce, & McEwen, 2009). With regard to adolescent risk taking, the Adverse Childhood Experiences (ACE) Study conducted by the CDC (Anda et al., 2006; Middlebrooks & Audage, 2008), shows how exposure to various forms of stress during childhood predicts later adverse forms of risk taking. In particular, such early stressors as physical and emotional abuse, emotional neglect, parental substance use, and exposure to violence in the household were linked to later adverse adolescent outcomes including drug use, addiction, and suicide. In female youth, experience of sexual abuse was highly related to exposure to other sources of stress and was linked to earlier age at first intercourse, and unintended pregnancy. In general, the more ACEs experienced, the greater the emergence of risky behavior in adolescence and later life.
Research on primates and rodents provides some understanding of how early adverse experiences can produce long-term effects on behavior that can emerge in adolescence. The research of Meaney and colleagues with rats indicates that variation in early maternal care can produce epigenetic effects on offspring. In their model, genes that control stress responses in the hypothalamic-pituitary-adrenal axis (HPA) are “silenced” leading to greater reactivity to stress (Meaney, 2001). In the rat, mothers who are less nurturing in their care of newborns are more likely to produce these effects. These effects appear to be mediated in part by reduced levels of serotonin functioning in the hippocampus. There also appear to be adverse effects on spatial ability and memory mediated by hippocampal functioning. This also leads to less than optimal responses to stressful experiences in offspring (Meaney, 2007).
The perhaps most remarkable consequence of these epigenetic processes is that female offspring of less nurturant mothers are more likely to behave in a similar manner with their offspring. Using cross-fostering designs, it is possible to determine that this results from intergenerational transmission of experience rather than genes. That is, it is the experience of maternal behavior that produces the effect rather than genetic transmission from parent to offspring.
Early experience in primates produces similar effects. Suomi's research with rhesus monkeys that are either reared by their mothers or by much less nurturant peers finds that peer-reared males exhibit greater externalizing behavior in adolescence (Suomi, 1997). In research with rhesus macaque monkeys, Maestripieri and colleagues have examined neurobehavioral effects of maternal abuse and neglect on offspring (Maestripieri, 2008). They also find that maternal maltreatment is transmitted by behavior rather than genetics. In addition, they find a particular role for serontonergic mediation that appears to increase impulsivity in offspring. That is, abused offspring exhibit lower levels of serotonin in cerebral spinal fluid, an indicator that has been linked to increased impulsivity (McCormack, Newman, Higley, Maestripieri, & Sanchez, 2009). One interesting aspect of this research is that the short allele of the serotonin transporter gene enhances the effects of maternal abuse, a finding consistent with research in humans who experience abuse during childhood (Caspi, Sugden, Moffitt, Taylor, Craig, Harrington, et al., 2003).
Research with humans also suggests that early maltreatment by parents is associated with later conduct problems. In a longitudinal study of high risk children from ages 2 to 8 (Kotch et al., 2008), parental neglect prior to age 2 was predictive of aggressive behavior at age 8. Later neglect did not predict aggressive behavior at this early age. Other research has identified abnormal reactivity to stress mediated by the HPA axis as a consequence of early abuse (Tarullo & Gunnar, 2006).
One difficulty in testing the epigenetic explanation for increased HPA axis reactivity in humans is the need to examine brain tissue. In a recent study, McGowan and colleagues (2009) examined hippocampal tissue in deceased persons who committed suicide or died by other means. In addition, those who died by suicide were distinguished as to whether they had experienced abuse or neglect as children or not. According to the epigenetic explanation, persons who suffered child maltreatment should have exhibited greater evidence of gene silencing in regions related to the stress response, including the hippocampus. Their study indeed identified such effects, thus providing the first evidence of similar epigenetic effects in humans.
Meaney's research suggests that maternal behavior toward offspring is a function of the stress experienced by the mother. Mothers who experience heightened stress treat their newborns with less nurturance, a process that is attributed to a defensive reaction to the environment. Although this may confer some advantage to offspring in the form of increased impulsivity, it can be a detrimental characteristic in humans especially when it results in conduct disorder and other externalizing conditions that increase risk for injury and incarceration. Needless to say, heightened stress experienced by mothers is more likely to occur in low socio-economic environments in which uncertainties surrounding food and other supports can be particularly challenging (Evans & Kim, 2007).
Changes in Impulsivity during Adolescence
Studies of risk behavior trajectories during childhood and adolescence indicate that in addition to an early onset trajectory that persists throughout adolescence, there are often one or more trajectories that develop during adolescence and late adulthood. Moffitt referred to these as adolescent-limited trajectories because they tend to decline as youth enter adulthood. One of the largest sources of these trajectories is a rise in sensation seeking that appears to characterize a majority of youth during the adolescent period. The rise in sensation seeking is linked to an increase in the release of dopamine to the ventral striatum (Chambers et al., 2003). Spear (2007) has identified this as a biological universal in mammals that appears to encourage the adolescent animal to leave the family and to venture forth with peers to explore new territory and select mates.
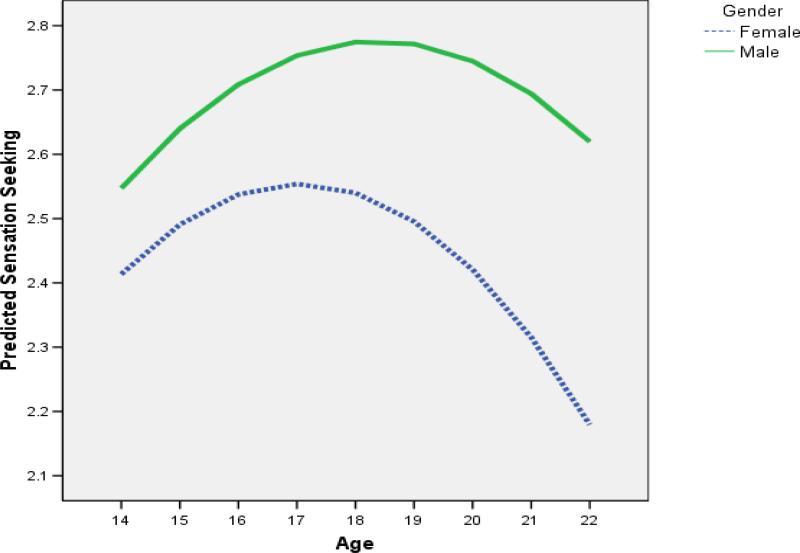
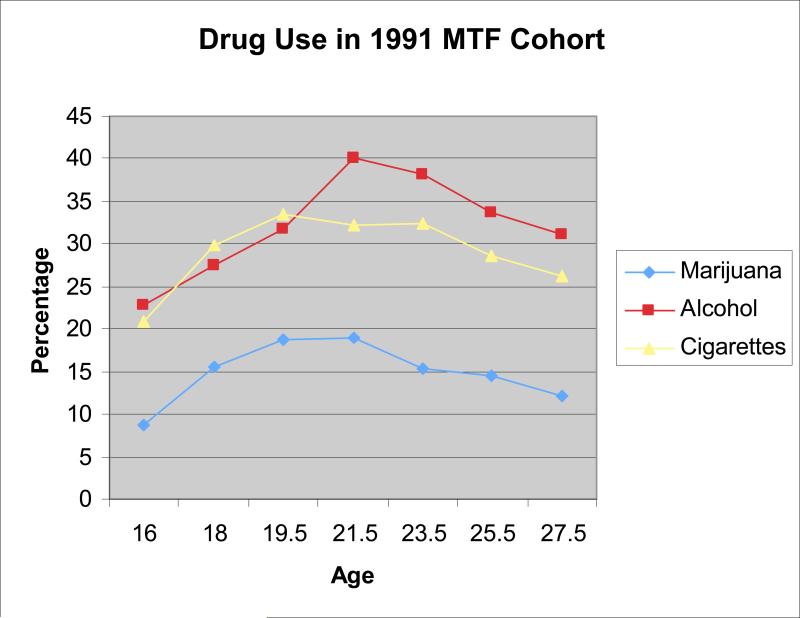
We have observed this rise in sensation seeking in national samples of youth ages 14 to 22 (Romer & Hennessy, 2007)(see Figure 4). The overall level of sensation seeking is greater in males than in females, and males exhibit a prolonged period of change in this trait. Whereas female youth peak around age 16, male youth do not reach their peak until about age 19. This rise in sensation seeking is one manifestation of dopaminergic activation of the nucleus accumbens, a process that peaks during adolescence. This rise in sensation seeking is remarkably congruent with other age gradients in risk taking, such as arrests for criminal behavior and drug use (see Figure 5) as assessed by the Monitoring the Future Study (Johnston, O'Malley, Bachman, & Schulenberg, 2006). Furthermore, individual differences in this trait have been linked to a host of risky behavior tendencies in both adolescents and adults (Roberti, 2004; Zuckerman, 1994).
Figure 4.
Trends in sensation seeking by age in National Annenberg Survey of Youth (taken from Romer & Hennessy, 2007, with permission).
Figure 5.
Longitudinal trends in use of alcohol, marijuana, and cigarettes as reported in the Monitoring the Future Study.
One important question related to the rise in sensation seeking during adolescence is whether it is associated with a lack of executive control over behavior as the other forms of impulsivity manifest. Evidence is sparse on this question, but given the small but significant positive correlation between sensation seeking and IQ (Zuckerman, 1994), it would seem that persons who exhibit stronger sensation seeking drives are no less able to exert executive control over their behavior. Indeed, in the Philadelphia trajectory study, we are finding that differences in sensation seeking are positively correlated with working memory performance (Romer, Betancourt, Brodsky, Giannetta, Yang, & Hurt, 2009). Thus, it seems that one of the more powerful sources of risk taking in adolescence is not associated with deficits in executive function.
A recent study by Raine and colleagues (Raine, Moffitt, Caspi, Loeber, Stouthamer-Loeber, & Lynam, 2005) examined neurocognitive function in a community sample of persistently anti-social youth as well as more adolescent-limited and non-offending youth. They found spatial and long-term memory deficits in anti-social youth that are consistent with deficient hyppocampal function brought on by childhood abuse. However, youth who merely exhibited a small rise in anti-social behavior during adolescence were no different from non-offending youth on most measures of cognitive function.
The Role of Sensation Seeking in Adolescent Risk Taking
Given the powerful role of sensation seeking in adolescent risk taking, it is of interest to determine whether its effects on decision-making involve different processes from those that are used by adults. In a recently proposed model of adolescent risk taking, Romer and Hennessy (2007) suggested that the influence of sensation seeking is mediated by the same processes that underlie adult decision making, namely the use of affect as the basis for evaluating behavioral alternatives. In particular, as suggested by Slovic and colleagues (Finucan, Alhakami, Slovic, & Johnson, 2000; Slovic, Finucane, Peters, & MacGregor, 2002), the affect heuristic is a robust and simple decision rule that relies on the dominant affective reaction to a response option as the criterion for evaluating its reward potential. Furthermore, use of the heuristic introduces a reciprocal relation between perceptions of risk and reward. That is, the more favorable the affect attached to an option, the less risk is associated with it.
The inverse relation between risk and reward is a deviation from rational choice models of decision-making in which risks and rewards are evaluated independently. Indeed, risks and rewards are generally not correlated in the world of uncertain consequences (Slovic et al., 2002). However, it appears to be a characteristic of our decision making to impose an inverse relation between these two dimensions of choice. This decision calculus makes us subject to certain biases of judgment controlled by dominant affective reactions to behavioral options. Those activities we enjoy tend to be seen as less risky than those that are actually safer but less affectively pleasant. Hence, we prefer to drive cars rather than take trains even though, all else constant, trains are far safer than cars. Nevertheless, the heuristic renders decision making simpler than a careful consideration of both risks and rewards would require.
From the perspective of developmental neuroscience, the use of the affect heuristic is an interesting phenomenon. Because it requires very little deliberation, it can guide behavior without the need for extensive cognitive control. As a result, there is little reason to believe that it should depend on extensive maturation of cognitive control mechanisms during adolescence. Indeed, the ventral PFC regions that underlie affect evaluation mature earlier than dorsal and lateral regions (Fuster, 2002) that are critical for many executive functions (Miller & Cohen, 2001). Not surprisingly, when we examine the risk taking behavior of adolescents, we find that the affect heuristic is alive and well in this decision making realm. Furthermore, its use does not seem to vary with age from mid-adolescence (age 14) to early adulthood (age 22) (Romer & Hennessy, 2007). For example, in evaluating the affect attached to smoking, drinking alcohol, and smoking marijuana, judgments of favorable affect and risk are strongly inversely related to each other and form one factor that is strongly related to use of each drug. Indeed, risk judgments add no significant prediction of drug use beyond the positive affect attached to each drug.
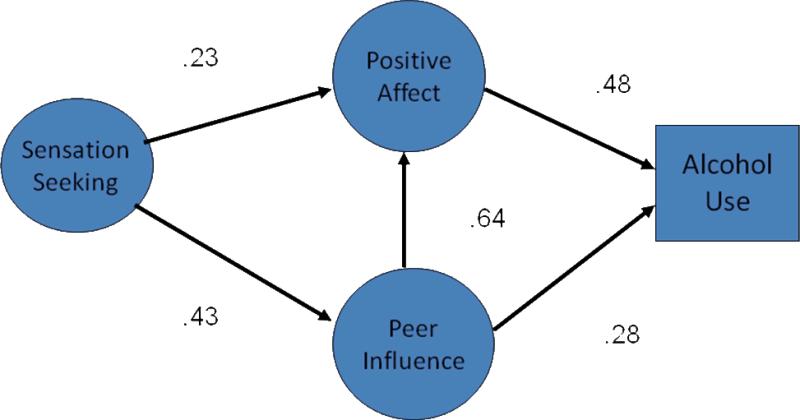
Another important characteristic of adolescent risk taking is the influence of peers. As seen in Figure 6, sensation seekers not only attach favorable affect to novel and exciting experiences, they also seek out peers who have the same interests. This selection process creates a social environment that not only encourages risk taking, but that also enhances the favorable affect that is attached to novel experiences. Because youth who differ in sensation seeking essentially congregate with similar peers, the effects of their own sensation seeking levels are reinforced by exposure to others through a process of affect transfer. Given that youth of a similar age simultaneously experience the same rise in sensation seeking, this peer effect magnifies the affective attraction to novel and exciting behavior such as drug use. As a result, the effects of affect on behavior are enhanced by peer influences.
Figure 6.
Results of causal model showing how affect evaluation and peer influence mediate the relation between sensation seeking and alcohol use in youth ages 14 to 22 (adapted from Romer & Hennessy, 2007).
As seen in Figure 6, the path weights linking the factors in the model suggest that both sensation seeking and peer influence converge on affect evaluation and produce more change in behavior through this path than through peer influence alone. In total, affect evaluation and peer influences account for over half of the variation in the use tobacco, alcohol, and marijuana. This influence in not limited to effects on drugs. In a study of failure to use seat belts when adolescents travel in cars, Dunlop and Romer (2009) found that about half of the variation in this behavior was related to affect evaluation and peer influence. In that case however, the influence of peers was somewhat stronger than affect alone.
Our findings regarding the effects of sensation seeking on adolescent risk taking suggest that it is possible to explain a great deal of the rise in risky behavior during adolescence to the increase in this form of impulsivity. Furthermore, the decision processes that are influenced by sensation seeking are the same as those that are used by adults. Indeed, the affect heuristic requires little deliberation and would appear to be available for use by the beginning of adolescence if not earlier. Finally, sensation seeking does not appear to reflect a deficit in executive functioning as is the case with other forms of impulsivity. Thus, there is little evidence to suggest that the risk taking associated with sensation seeking reflects a deficit in PFC brain maturation.
Is There Evidence Regarding Brain Structure and Adolescent Risk Taking?
The evidence we have reviewed suggests that adolescent risk taking is not a universal phenomenon and that individual differences related to at least three types of impulsivity underlie such behavior in adolescents. Furthermore, at least two forms of impulsivity are associated with weak executive function as assessed by working memory and response inhibition tasks. However, sensation seeking does not appear to be inversely related to either of these executive functions and may actually be somewhat positively related to working memory ability. Nevertheless, it is also the case that cognitive control as assessed by working memory and response inhibition tasks continues to improve during adolescence (Bunge & Crone, 2009; Spear, 2009; Williams, Ponesse, Shachar, Logan, & Tannock, 1999). Could these maturational changes reflect alterations in brain structure that place limits on adolescent cognitive control over risk taking?
There is virtually no direct evidence to support a relation between natural maturation in brain structure during adolescence and impulsive behavior. This is partly due to the fact that it is difficult to observe changes in brain structure that could be implicated in impulsive behavior. As noted by Galvan et al., 2006:
Neuroimaging studies cannot definitively characterize the mechanism of such developmental change (e.g., synaptic pruning, myelination). However, these volume and structural changes may reflect refinement and fine-tuning of reciprocal projections from these brain regions (PFC and striatum) during maturation. Thus, this interpretation is only speculative. (6885)
Lu and Sowell (2009) reviewed what is known about the relation between changes in brain structure during development and performance on cognitive and motor skills. Their summary does not provide much evidence for the hypothesis that cortical thinning reflective of synaptic pruning leads to improved cognitive performance. For example, holding IQ constant, Sowell and colleagues (2004) found that cortical thinning from ages 5 to 11 was associated with greater improvement in vocabulary, an effect that would seem to be driven by learning rather than brain maturation. In a study examining changes in cortical thickness from ages 7 to 19 as a function of different levels of IQ, Shaw and colleagues (2006) found that individuals with superior IQ began the thinning process later than those with normal IQ. If cortical thinning facilitates the development of cognitive skills, then one would expect it to occur earlier for those with higher IQ. Finally, in regions related to language skills (the peri-Sylvan left hemisphere), cortical thickening rather than thinning has been associated with increased language skill development (Lu, Leonard, & Thompson, 2007). Hence, cortical thinning does not even characterize skill development across all regions of the cortex.
With regard to changes in white matter, Berns, Moore, & Capra (2009) examined the relation between myelination in the PFC and risk taking in youth ages 12 to 18. Holding constant age, they found that risk taking tendencies were positively correlated with white matter development. Consistent with this finding, DeBellis and colleagues (2008) found that myelination of the corpus callosum was more advanced in youth with alcohol disorders than in control youth without such conditions. Thus, evidence in support of delay in PFC myelination as a risk factor for problem behavior in youth is not only absent but also contrary to what would be expected.
In summarizing this research, Lu and Sowell (2009) noted that:
Correlations between morphological and skill maturation, although instructive, reveal only associations and cannot elucidate causality. Neuroscience must still rely on animal studies using controlled experimental designs to learn whether morphological maturation enables the acquisition of skills or if skill acquisition drives morphological change. (19)
Some researchers have attempted to observe differences in brain function while engaging in risky decision-making that could help to identify age-related differences in brain development. These studies have used functional magnetic imaging (fMRI) of individuals varying in age from childhood to adulthood while engaging in a variety of tasks. However, the results regarding differential activation of the PFC have not yielded a clear picture of how PFC activation relates to risky decision-making.
Consistent with theories that attribute increased risk taking during adolescence to sensation seeking (Chambers et al., 2003), Galvan et al. (2006) found that adolescents (ages 13 to 17) exhibited greater peak activation of the nucleus accumbens than either younger (ages 7 to 11) or older individuals (ages 23 to 29) when anticipating a reward. However, adolescents did not differ from adults on the same measure in regard to activation of orbital frontal cortex (OFC), a ventral area of the PFC. Children exhibited a stronger response than either adolescents or adults. These results were somewhat difficult to interpret, however, given the use of a reward cue that could easily differ in excitement value and interest as a function of age (a picture of a cute pirate in different poses).
In a comprehensive study of brain activation, Eshel, Nelson, Blair, Pine, & Ernst (2007) examined various brain regions in pre- to late adolescents (ages from 9 to 17) and young to older adults (ages 20 to 40) while making choices between options that varied in risk. The critical comparisons were between choices that had high probabilities of reward for small monetary outcomes versus those that had low probabilities of reward for larger outcomes. In an interesting design decision, the researchers did not keep the expected values of the two types of options constant. Choosing the risky alternative was always disadvantageous compared to the less risky alternative. They found that older individuals activated lateral OFC more strongly than younger ones when they selected the risky disadvantageous option. This finding was taken as evidence of greater PFC activation in older individuals. An alternative interpretation is that older individuals exhibit greater PFC activation than younger ones when making ill-advised decisions. Clearly, this study does little to confirm superior frontal control in adults.
In a recent review of these and several other studies using fMRI to detect differences in brain activation across age groups, Ernst and Hardin (2009) noted that:
The goal of delineating the trajectory of ontogenetic development increases the complexity of this research and requires theoretical models to constrain hypotheses and guide the development of experimental paradigms for a step-wise systematic approach. (69-70)
The concern about constraining hypotheses is particularly critical when comparing different age groups that not only differ in brain development but also in experience. Given the concerns raised by Lu and Sowell (2009), it would seem difficult to disentangle the effects of experience on brain structure from those of morphologic maturation that do not depend on learning.
Another approach suggested by Bunge and Crone (2009) is to differentially expose adolescents to cognitive training exercises. If appropriate training could produce better decision making in adolescents, it would argue against the maturation hypothesis, which would predict that training would be inadequate in the absence of adequate brain maturation. Because research on the effects of experience will undoubtedly add to our understanding of the role of morphological maturation versus experience, it is to such research that we now turn.
Evidence for Effects of Experience on Impulsivity
In view of the very strong predictions based on limitations in brain maturation during adolescence, it is of interest to determine whether experience can overcome such limitations. In particular, given the important role that impulsivity plays in adolescent risk taking, is there any evidence that experience can alter any form of impulsivity? Here the evidence is quite clear: There are numerous examples of interventions that can change brain function to the effect that impulsivity and associated risk taking is reduced. In reviewing these interventions, it is helpful to distinguish between those that are delivered in childhood versus those that have been successful later during adolescence. Childhood interventions should help to prevent the early forms of impulsivity that continue into adolescence if left untreated. Adolescent interventions should be able to counteract the rise in sensation seeking and potentially other forms of impulsivity that emerge during the second decade of life.
Early Interventions
There are two forms of early intervention that have been tested with success. One involves intervening with parents who are at risk for maltreating their children and thereby preventing adverse outcomes of such treatment on offspring. The other is to intervene later with families and children either together or just with the children in school settings.
One of the most successful early intervention with parents is the nurse visitation program designed by David Olds and colleagues (1998). This program involves visiting the expectant parent prior to birth and providing training to cope with stressors that might otherwise lead to a less than optimal natal experience for the child. As expected by research summarized above, parents experiencing stress are likely to pass this experience onto their children in the form of less nurturing care. This treatment is then likely to produce non-optimal brain development in children, leading to poor adaption in school and later in adolescence. However, parental support during visitation with high-risk parents enables them to cope better with stressors and to reduce the tendency to pass on stress reactions to children. Evaluations of the program indicate that children perform better in school and experience fewer psychiatric symptoms, including lower rates of conduct disorder. In addition, parents exhibit healthier behavior as their children age into adolescence (Izzo, Eckenrode, Smith, Henderson, Cole, Kitzman, et al., 2005). This program has been targeted for federal support given its success in preventing adverse outcomes for children and for reducing later costs in schooling, incarceration, and welfare supports.
In addition to intervening with parents early in a child's life, there is growing evidence that certain forms of early training can have lasting effects on behavior, especially on academic outcomes and various forms of externalizing behavior. For example, reviews of intensive preschool programs (A. Reynolds & Temple, 2008), such as the High/Scope Perry Preschool project and the Chicago Child-Parent Preschool Program indicate that such interventions improve academic performance, keep children in school, and reduce adolescent problem behaviors that risk incarceration. These programs appear to influence cognitive and behavioral skills, such as greater persistence and self-regulation that are inversely related to impulsivity.
In a recent study by Diamond and colleagues (Diamond, Barnett, Thomas, & Munro, 2007), researchers were able to train skills in preschoolers that affect executive functions highly related to academic performance and to impulse disorders, such as ADHD and conduct problems. These skills have been found to be associated with various PFC functions that underlie behavior control, such as the ability to operate on thoughts in working memory and to reduce interference from distracters.
Other research with children in the elementary years indicates that impulse control strategies can be trained that improve executive function and reduce impulsivity (Barry, & Welsh, 2007; Riggs, Greenberg, Kusche, & Pentz, 2006). One program that has long-term follow-up data is the good behavior game (Petras, Kellam, Brown, Muthen, Ialongo, & Poduska, 2008). Kellam and colleagues tested this program in low-income first and second grade classes in which teachers were trained to administer incentives for good behavior to entire classrooms. Rewards were delivered on a consistent basis to reduce disruptive behavior, increase cooperation, and enhance attention to schoolwork. Follow-up data at ages 19 to 21 revealed remarkably long-lasting effects on those who exhibited the highest rates of aggressive and uncontrolled behavior prior to intervention. In particular, rates of anti-social personality disorder remained lower in the highest risk youth at the follow-up.
It should also not be forgotten that medication has been found to be very helpful in reducing impulsive symptoms in children with ADHD. Klingberg (2009) suggests that moderate doses of stimulants can improve executive functioning in general and working memory in particular in children suffering from ADHD and thereby improve their academic performance. There is even evidence that use of these medications can reduce the likelihood of later drug use during adolescence (Wilens, Faraone, Biederman, & Gunawardene, 2003). Klingberg and colleagues (2005) have also developed a protocol for children with ADHD that can improve working memory and reduce symptoms of ADHD using computer-based training. Posner and colleagues (Rueda, Rothbart, McCandliss, Saccamanno, & Posner, 2005) have proposed and tested similar strategies for children with attention problems.
In summary, research on early interventions indicates that intensive training focused on executive functioning and self-regulation skills can reduce impulsive tendencies that might otherwise impede performance in school and lead to maladaptive outcomes in adolescence. These strategies would be unlikely to be successful if brain maturation processes during adolescence prevented successful adaptation to rises in sensation seeking or other risk taking impulses.
Later Interventions
Space limitations preclude a detailed examination of interventions in the adolescent years. However, there is considerable evidence that adolescents can learn to avoid maladaptive behaviors, especially if they are given information that is linked to affective reactions to those behaviors. For example, extensive tracking of drug use since 1974 in the Monitoring the Future Study indicates that one of the best predictors of individual and aggregate drug use is the perception that drugs are dangerous to one's health (Bachman, Johnston, & O'Malley, 1998). Media campaigns do not always succeed in transmitting this information effectively however. For example, some media interventions sponsored by the government have inadvertently transmitted the message that many youth are using drugs, a message that can increase the perception that peers find drugs exciting (Fishbein, Hall-Jamieson, Zimmer, von Haeften, & Nabi, 2002; Hornik, Jacobsohn, Orwin, Piesse, & Kalton, 2008). As noted above, such perceptions can enhance favorable affective reactions to the prospect of drug use.
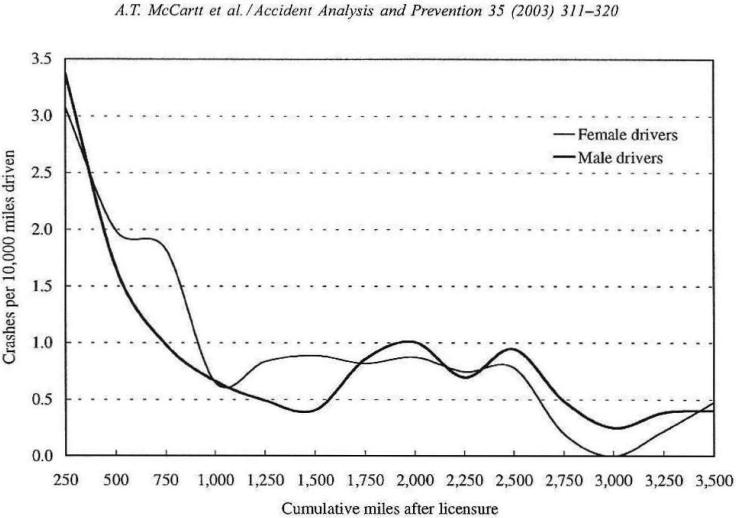
A good example of a strategy that can help to prevent adverse outcomes when engaging in novel behavior is the graduated driver program that has been adopted by many states in the U. S. This strategy is based on the idea that driving is a complex behavior that takes experience to master. As seen in Figure 7, adolescent drivers experience a significant reduction in crashes after driving about 1000 miles (six months on average) (McCartt, Shabanova, & Leaf, 2003). If such early learning experience could be accomplished under lower-risk supervised conditions, it might reduce the chances for hazardous outcomes until greater mastery over the behavior has been achieved. The strategy of graduated licensing has been adopted by a many states. In this procedure, adolescents are not given full licenses until they have passed a trial period during which they cannot drive at night and must drive with an adult. Evidence of the effectiveness of this strategy indicates that it reduces crash rates and serious injuries and does so in a manner responsive to the number of restrictions in place in a state (Morrissey, Grabowski, Dee, & Campbell, 2006).
Figure 7.
Trends in reported car crashes among adolescent drivers as a function of miles driven indicate that crashes decline dramatically after about 1000 miles of driving experience (reprinted with permission from McCartt et al., 2003).
In a recent study of the effects of sensation seeking during the adolescent and early adult years (14 to 22), my colleagues and I found that experience with risk taking leads to a reduction in impatience as assessed with a delay discounting task (Romer et al., 2010). High sensation seeking youth who use drugs more than other youth exhibit a decline in impatience as they age. This reduction also carries over to less drug use. Other youth tend not to exhibit changes in discounting during adolescence. This finding suggests that experience gained from excessive risk taking enables high sensation seekers to develop greater patience, a factor that reduces risk taking. Research with conduct-disordered youth also suggests that impatience declines more for such youth than for others (Turner & Piquero, 2002). Hence, despite their greater risk taking, high-sensation seeking youth can learn from the consequences of their behavior and ultimately become less impatient than their less risky peers. The challenge for future translational research is to identify interventions that can provide the experience that adolescents need to transition to adulthood while also protecting them from the adverse consequences that can endanger their long-term health and development.
As noted by Spear (2009),
Experiences occurring during adolescence may serve to customize the maturing brain in a way commensurate with those experiences. Depending on the nature of those experiences, their timing, and hence their consequences, this customizing of the brain can be viewed as an opportunity, as well as a vulnerability. (308).
Future research should help to disentangle the interacting effects of experience and brain maturation. As noted earlier, studies that examine structural brain maturation and function in combination with training programs that improve cognitive and behavioral control skills (e.g., working memory) should be able to identify the role of experience at different levels of structural maturation. This research should help in developing training exercises that can provide adolescents with the experience they seek while simultaneously reducing the risks they encounter if left to their own devices.
References
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Anda RA, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Reckless behavior in adolescence: A developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- Bachman J,G, Johnston LD, O'Malley PM. Explaining recent increases in students’ marijuana use: Impacts of perceived risks and disapproval, 1976 through 1996. American Journal of Public Health. 1998;88(6):887–892. doi: 10.2105/ajph.88.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. Public Library of Science, One. 2009;4(8):1–12. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglan A, Cody C. Preventing multiple problem behaviors in adolescence. In: Romer D, editor. Reducing adolescent risk: Toward an integrated approach. Sage Publications; Thousand Oaks, CA: 2003. pp. 125–131. [Google Scholar]
- Bunge SA, Crone EA. Neural correlates of the development of cognitive control. In: Rumsey JM, Ernst M, editors. Neuroimaging in developmental clinical neuroscience. Cambridge University Press; New York: 2009. pp. 22–37. [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Neuropsychology. 2008;28(11):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Henry B, McGee RO, Moffitt TE, Silva PA. Temperamental origins of child and adolescent behavior problems: From age three to age fifteen. Child Development. 1995;66(1):55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Archives of General Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Caspi A, Silva P. Temperamental qualities at age three predict personality traits in young adulthood: Longitudinal evidence from a birth cohort. Child Development. 1995;66:486–498. doi: 10.1111/j.1467-8624.1995.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Bohman M. Childhood personality predicts alcohol abuse in young adults. Alcoholism: Clinical and Experimental Research. 1988;121(4):494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Van Vorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, et al. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32(3):395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop S, Romer D. An integrative model of youth seatbelt non-use: The roles of sensation seeking, affective evaluations, and media use. Annenberg Public Policy Center, University of Pennsylvania; Philadelphia, PA: 2009. [Google Scholar]
- Ernst M, Hardin MG. Goal-directed behavior: Evolution and ontogeny. In: Rumsey JM, Ernst M, editors. Neuroimaging in developmental clinical neuroscience. Cambridge University Press; New York: 2009. pp. 53–72. [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychological Science. 2007;18(11):953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ. Age norms for impulsiveness, venturesomeness and empathy in adults. Personality and Individual Differences. 1985;6:613–619. [Google Scholar]
- Finucan ML, Alhakami AS, Slovic P, Johnson SM. The affect heuristic in judgments of risks and benefits. Journal of Behavioral Decision Making. 2000;13:109–17. [Google Scholar]
- Fishbein M, Hall-Jamieson K, Zimmer E, von Haeften I, Nabi R. Avoiding the boomerang: Testing the relative effectiveness of antidrug public service announcements before a national campaign. American Journal of Public Health. 2002;92(22):238–245. doi: 10.2105/ajph.92.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. Journal of Neurocytology. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Hill KG, White HR, Chung I, Hawkins JD, Catalano RF. Early adult outcomes of adolescent binge drinking: Person- and variable-centered analyses of binge drinking trajectories. Alcoholism: Clinical and Experimental Research. 2000;24(6):892–901. [PMC free article] [PubMed] [Google Scholar]
- Hornik R, Jacobsohn L, Orwin R, Piesse A, Kalton G. Effects of the national youth anti-drug media campaign on youths. American Journal of Public Health. 2008;98(1238):2229–2236. doi: 10.2105/AJPH.2007.125849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo CV, Eckenrode JJ, Smith EG, Henderson CR, Cole RE, Kitzman HJ, et al. Reducing the impact of uncontrollable stressful life events through a program of nurse home visitation for new parents. Prevention Science. 2005;6(4):269–274. doi: 10.1007/s11121-005-0010-5. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman J, Schulenberg JE. Monitoring the future: National survey results on drug use, 1975-2005, vol. II, college students and adults 19-45. National Institutes of Health; Bethesda, MD: 2006. [Google Scholar]
- Klingberg T. The overflowing brain: Information overload and the limits of working memory. Oxford University Press; New York: 2009. [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD: A randomized, controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kotch JB, Lewis T, Hussey JM, English D, Thompson R, Litrownik AJ, et al. Importance of early neglect for childhood aggression. Pediatrics. 2008;121(4):725–731. doi: 10.1542/peds.2006-3622. [DOI] [PubMed] [Google Scholar]
- Kreuger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111(3):411–424. [PubMed] [Google Scholar]
- Lu LH, Leonard CM, Thompson PM. Normal developmental changes in inferior gray matter are associated with improvements in phonological processing: A longitudinal analysis. Cerebral Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Lu LH, Sowell ER. Morphological development of the brain: What has imaging told us? In: Rumsey JM, Ernst M, editors. Neuroimaging in developmental clinical neuroscience. Cambridge University Press; New York: 2009. pp. 5–21. [Google Scholar]
- Maestripieri D. Neuroendocrine mechanisms underlying the intergenerational transmission of maternal behavior and infant abuse in rhesus macaques. In: Pfaff D, Kordon C, Chanson P, Christen Y, editors. Hormones and Social Behavior. Springer-Verlag; Berlin: 2008. pp. 121–130. [Google Scholar]
- Masse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Archives of General Psychiatry. 1997;54:62–68. doi: 10.1001/archpsyc.1997.01830130068014. [DOI] [PubMed] [Google Scholar]
- McCartt AT, Shabanova VI, Leaf WA. Driving experience, crashes and traffic citations of teenage beginning drivers. Accident Analysis and Prevention. 2003;35:311–320. doi: 10.1016/s0001-4575(02)00006-4. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyt M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;128(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Kreuger RF. The association of early adolescent problem behavior and adult psychopathology: A multivariate behavioral genetic perspective. Behavior Genetics. 2006;36(4):591–602. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal programming of defensive response through sustained effects on gene expression. In: Romer D, Walker EF, editors. Adolescent psychopathology and the developing brain: Integrating brain and prevention science. Oxford University Press; New York: 2007. pp. 148–172. [Google Scholar]
- Middlebrooks JS, Audage NC. The effects of childhood stress on health across the lifespan. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2008. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Peake PK. The nature of adolescent competencies predicted by preschool delay of gratification. Journal of Personality and Social Psychology. 1988;54(4):687–696. doi: 10.1037//0022-3514.54.4.687. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Morrissey MA, Grabowski DC, Dee T,S, Campbell C. The strength of graduated drivers license programs and fatalities among teens and passengers. Accident Analysis and Prevention. 2006;38:135–141. doi: 10.1016/j.aap.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Nagin D, Tremblay RE. Trajectories of boys’ physical aggression, opposition, and hyperactivity on the path to physically violent and nonviolent juvenile delinquency. Child Development. 1999;70(5):1181–1196. doi: 10.1111/1467-8624.00086. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Development and Psychopathology. 2002;14(3):499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- Olds D, Henderson CRJ, Cole R, Eckenrode J, Kitzman H, Luckey D, et al. Long-term effects of nurse home visitation on children's criminal and antisocial behavior: 15-year follow-up of a randomized controlled trial. The Journal of the American Medical Association. 1998;(1238):1244. doi: 10.1001/jama.280.14.1238. [DOI] [PubMed] [Google Scholar]
- Patterson GR, Reid J,B, Dishion TJ. Antisocial boys. Castalia; Eugene, OR: 1992. [Google Scholar]
- Pattij T, Vanderschuren LJMJ. The neuropharmacology of impulsive behavior. Trends in Pharmacological Sciences. 2008;29(4):192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petras H, Kellam SG, Brown HC, Muthen BO, Ialongo NS, Poduska JM. Developmental epidemiological courses leading to antisocial personality disorder and violent and criminal behavior: Effects by young adulthood of a universal preventive intervention in first- and second-grade classrooms. Drug and Alcohol Dependence. 2008;95S:S45–S59. doi: 10.1016/j.drugalcdep.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H. The science of self-control. Harvard University Press; Cambridge, MA: 2000. [Google Scholar]
- Raine A, Moffitt TE, Caspi A, Loeber R, Stouthamer-Loeber M, Lyman D. Neurocognitive impairments in boys on the life-course persistent antisocial path. Journal of Abnormal Psychology. 2005;114(11):38–49. doi: 10.1037/0021-843X.114.1.38. [DOI] [PubMed] [Google Scholar]
- Raine A, Reynolds C, Venables PH, Mednick SA, Farrington DF. Fearlessness, stimulation-seeking, and large body size at age 3 years as early predispositions to childhood aggression at age 11 years. Archives of General Psychiatry. 1998;55:745–751. doi: 10.1001/archpsyc.55.8.745. [DOI] [PubMed] [Google Scholar]
- Reynolds A,J, Temple JA. Cost-effective early childhood development programs from preschool to third grade. Annual Review of Clinical Psychology. 2008;4:109–139. doi: 10.1146/annurev.clinpsy.3.022806.091411. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behavioural Pharmacology. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Penfold RB, Patak M. Dimensions of impulsive behavior in adolescents: Laboratory behavioral assessments. Experimental and Clinical Psychopharmacology. 2008;16(2):124–131. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- Riggs NR, Greenberg MT, Kusche CA, Pentz MA. The mediational role of neurocognition in the behavioral outcomes of a social-emotional prevention program in elementary school students. Prevention Science. 2006;70:91–102. doi: 10.1007/s11121-005-0022-1. [DOI] [PubMed] [Google Scholar]
- Roberti JW. A review of behavioral and biological correlates of sensation seeking. Journal of Research in Personality. 2004;38:256–279. [Google Scholar]
- Romer D, Betancourt L, Brodsky NL, Giannetta JM, Yang W, Hurt Does adolescent risk taking imply weak executive function? A prospective study of relations between working performance, impulsivity, and risk taking in early adolescents. Developmental Science. 2011;14(5):1119–1133. doi: 10.1111/j.1467-7687.2011.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47:2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Duckworth AL, Sznitman S, Park S. Can adolescents learn selfcontrol? Delay of gratification in the development of control over risk taking. Prevention Science. 2010;11(3):319–330. doi: 10.1007/s11121-010-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Hennessy M. A biosocial-affect model of adolescent sensation seeking: The role of affect evaluation and peer-group influence in adolescent drug use. Prevention Science. 2007;8:89–101. doi: 10.1007/s11121-007-0064-7. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccamanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, et al. Individual differences in delay discounting: Relation to intelligence, working memory, and anterior prefrontal cortex. Psychological Science. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Slovic P, Finucane M, Peters E, MacGregor DG. The affect heuristic. In: Gilovich T, Griffin D, Kahneman D, editors. Intuitive judgment: Heuristics and biases () Cambridge University Press; New York: 2002. [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. The Journal of Neuroscience. 2001;20(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. In: The developing brain and adolescent-typical behavior patterns: An evolutionary approach. Adolescent psychopathology and the developing brain: Integrating brain and prevention science. Romer D, Walker EF, editors. Oxford University Press; New York: 2007. pp. 9–30. [Google Scholar]
- Spear L,P. The behavioral neuroscience of adolescence. W. W. Norton & Co.; New York: 2009. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behavior: Evidence from primate studies. British Medical Bulletin. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, et al. Neurobehavioral disinhibition in childhood predicts early age of onset of substance use disorder. The American Journal of Psychiatry. 2003;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Turner MG, Piquero AR. The stability of self-control. Journal of Criminal Justice. 2002;30:457–471. [Google Scholar]
- Vaidya CJ, Bunge SA, Dudukoric NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: Evidence from functional magnetic imaging. American Journal of Psychiatry. 2005;162(9):1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five-factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differeneces. 2001;30:669–689. [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of atttention-deficit/hyperactivity disorder beget later substance abuse? Pediatrics. 2003;111(1):179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Shachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Zucker RA. Alcohol use and the alcohol use disorders: A developmentalbiopsychosocial systems formulation covering the life course. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Volume three: Risk, disorder, and adaptation. 2nd ed. John Wiley; Hoboken, NJ: 2006. pp. 620–656. [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge University Press; New York: 1994. [Google Scholar]