Abstract
Purpose
To investigate the significance of netrin-1 and vascular endothelial growth factor (VEGF) in the pathogenesis of retinal angiogenesis, the levels of netrin-1 and VEGF in the vitreous fluid and serum of the proliferative diabetic retinopathy (PDR) and non-proliferative diabetic retinopathy (non-PDR) patients were measured. We then determined the netrin-1 and VEGF expression in the oxygen induced retinopathy (OIR) mice retina.
Methods
A total of 18 eyes from 18 patients were included in our study and 10 of them were collected from PDR patients and 8 from non-PDR patients. Undiluted vitreous fluid samples were collected during pars plana vitrectomy. Appropriate blood samples were collected if possible. Netrin-1 and VEGF levels in the vitreous fluid and plasma were determined by Enzyme-linked Immunosorbent Assays. OIR mice models were established, and netrin-1 and VEGF levels were determined by immunohistochemistry analysis.
Results
The levels of netrin-1 and VEGF in the vitreous of PDR patients were significantly higher than those in the controls (Mediannetrin-1=509.94 vs. 85.91 pg/ml, P<0.001 and MedianVEGF=762.60 vs. 77.52 pg/ml, P<0.001). Netrin-1 was mainly expressed in GCL and INL of the retina in mice. Both netrin-1 and VEGF were up-regulated in OIR mice.
Conclusion
Netrin-1 and VEGF levels were elevated in vitreous fluid of the PDR patients and the OIR mice retina. Therefore, netrin-1 may play an important role in pathological retinal angiogenesis.
Keywords: Retinal angiogenesis, Netrin-1, VEGF
Introduction
Pathological growth of new blood vessels is a final characteristic in ocular neovascular diseases, such as proliferative diabetic retinopathy (PDR), age-related macular degeneration, and retinopathy of prematurity, which often leads to catastrophic loss of vision. Relative hypoxia and ischemia are the basic reasons of the pathological growth of neovasculars. For example, long-term hyperglycemia in diabetic retinopathy can trigger inadequate blood supply that eventually leads to blood-retina barrier breakdown, high vascular permeability, and avascularity. Thus, many angiogenic-related cytokines, such as hypoxic-inducible factors (HIFs), vascular endothelial growth factor (VEGF), and erythropoietin are up-regulated to raise blood flow of the ischemic tissue, to increase vascular permeability, and to maintain the perfusion pressure in the tissue1–6. Ultimately, these pathological changes result in fibrosis and hemorrhage in the retina and loss of vision. Many researchers have verified that VEGF, a potent promigratory endothelial growth factor, is up-regulated dramatically in the vitreous of PDR patients and OIR mice retinas. VEGF promotes the proliferative, migrative and tube formation of retinal endothelial cells, and increased the permeability of microvessels in these retinas4,7,8. Besides, VEGF blockade, such as avastin used clinically and other RNAi targeting to VEGF can prevent pathological neovascularization effectively, but not completely. This observation indicates that VEGF plays a significant role in mediating retinal neovascularization in PDR patients, while other more angiogenic factors may be involved in retinal neovascularization.
Netrin-1, a 68-kDa laminin related molecule, was first described in spinal cord as an attractant of commissural axons from dorsal spinal cord to ventral midline floor plate9,10. It has been reported that netrin-1 is bifunctional in axon guidance. Netrin-1 attracts dorsal commissural interneurons when it binds to the deleted in colorectal cancer (DCC) receptor. However, netrin-1 repels certain classes of motor neurons, when it binds with uncoordinated five (UNC5) receptors. During optic fissure closure in embryonic eye development, netrin-1 guide the exit of retinal ganglion cell (RGC) axons from the eye and the extension of these axons into the optic nerve head11,12. In recent years, significant attentions were focused on netrin-1 due to its role in angiogenesis although it was still controversial. For example, Lu et al observed that genetic inactivating netrin-1 receptor UNC5B caused increased angiogenesis, suggesting its potential role as an anti-angiogenic growth factor13. Whereas Wilson and others demonstrated an opposite role of netrin-1 in angiogenesis by inactivating netrin-1, a ligand for UNC5B that played a role in vessel loss during zebrafish development14. In addition, the study by Mehlen et al showed that silencing netrin-1 led to increased cell death in zebrafish embryos, suggesting its potential role in cell survival15–17.
To evaluate whether netrin-1 plays a role in retinal angiogenesis, we collected undiluted vitreous fluids and serum during the intraocular vitrectomy from 18 patients with or without PDR. These patients were diagnosed by fundus examination and fluorescein angiography. We then detected the vitreous or plasma netrin-1 and VEGF levels simultaneously by enzyme-linked immunosorbent assays (ELISA). Besides, we built OIR mice neovascular models, measured the netrin-1 and VEGF expression in the retina to evaluate their roles in the retinal angiogenesis. This report summarizes our study on the role of netrin-1 as well as VEGF in the pathological retinal angiogenesis.
Materials and Methods
Collection of human vitreous and serum
A total of 18 patients with PDR or non-PDR, who have undergone monocular or bi-ocular vitrectomy, were selected in our study with informed consents (shown in Table 1). All patients were diagnosed by fundus examinations, fundus fluorescein angiography. The results of these findings were confirmed by operative findings. Their vitreous fluids were collected into sterile microfuge tube and blood samples were drawn if possible. The vitreous fluids were centrifuged at 14,000 rpm for 15 min at 4°C and the supernatants were collected and stored at −70°C for ELISA. The blood was centrifuged at 3,000 rpm for 15 min and the supernatant was stored at −70°C for ELISA.
Enzyme-linked immunosorbent assays
The concentration of human netrin-1 in vitreous and blood serum was measured using an ELISA kit from USCNLIFE™ (Wuhan EIAab Science Co., Ltd. China) and VEGF was measured using an ELISA kit from R&D systems. All the reagents were detected according to manufacturer’s instructions.
Animal models establishment
Animal experiments were conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Murine OIR models were described previously. Briefly, C57BL/6J mice (purchased from Shanghai laboratory animal center, Chinese Academy of Sciences) of postnatal day 7 (P7) were exposed to 75±2% oxygen for 5 days with the nursing mothers. On P12, the mice were removed from hyperoxia and maintained in room air until P17. Age-matched C57BL/6J mice kept in room air were used as controls.
Netrin-1 and VEGF immunocytochemistry
At P17, mice of each group were sacrificed and the eyes were enucleated and fixed in 4% paraformaldehyde for 15–30 min, then steyed in 30 g/L sucrose overnight. embedded in the OCT and freezed it at −70°C. Serial sections (10 µm) of whole eyes were cut sagittally through the cornea and parallel to the optic nerve. Ten nonserial sections were analyzed per eye. Sections including the optic nerve were excluded and the nuclei of new vessels extending from the retina into the vitreous were counted in 360 sections. Immunocytochemical analysis for netrin-1 and VEGF was performed on the slides prepared as previously introduced. Slides were incubated overnight at 37°C with chicken anti-mouse netrin-1 polyclonal antibody (1:100; Neuromics Biotechnology) or rabbit polyclonal anti-human VEGF antibody (1:100; Santa Cruz Biotechnology). Appropriate second antibodies were added correspondingly. Slides were photographed by microscopy.
Statistical analysis
Data were presented as means±SEM. Mann-Whitney U test was used for the data of non-normal distribution in ELISA detections.
Results
Up-regulation of netrin-1 in the vitreous of PDR patients
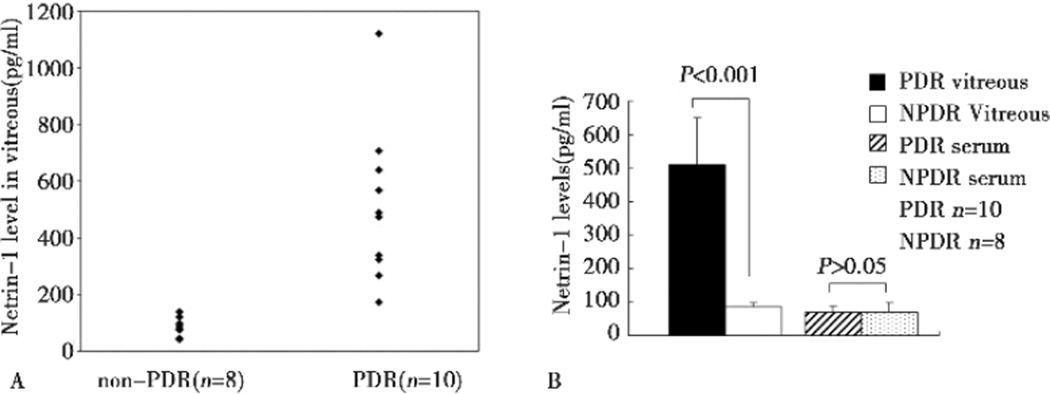
Samples of undiluted vitreous fluid were harvested from the eyes of 18 patients with PDR or nondiabetic ocular diseases, including those with idiopathic epiretinal membrane, idiopathic macular hole, rhegmatogenous retinal detachment. These patients served as controls. The average level of netrin-1 in the vitreous of PDR patients was significantly higher than that of control patients (509.94±138.85 vs. 85.92±11.72 pg/ml, P<0.001). The serum level of netrin-1 was marginally increased PDR patients, with no statistical significance(70.26±16.92 vs. 69.77±29.93 pg/ml, P>0.05), as shown in Figure 1.
Figure 1.
Comparison of netrin-1 levels between PDR and non-PDR patients. A: vitreous netrin-1 levels between PDR and non-PDR patients. B: average netrin-1 levels in the vitreous and the serum of PDR and non-PDR patients. Vitreous netrin-1 level was significantly higher in PDR patients (P<0.001). However, no significant elevation in serum netrin-1 level in PDR patients was observed (P>0.05).
Up-regulation of VEGF in the vitreous of PDR patients
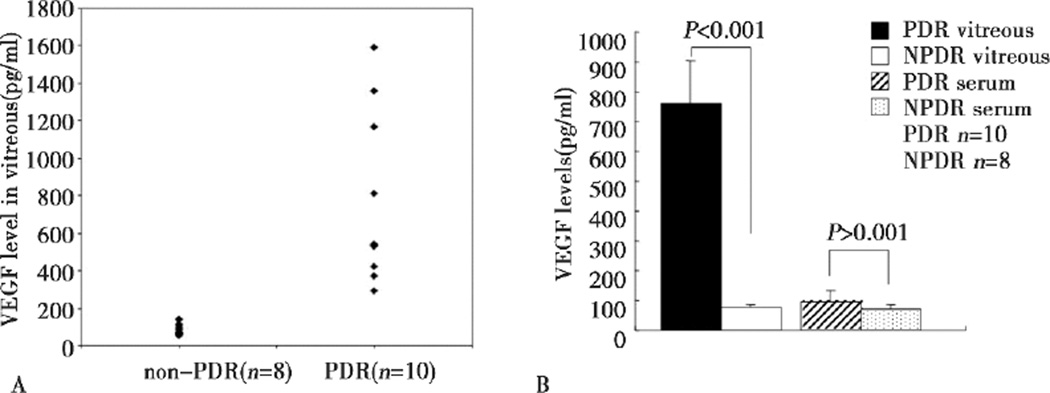
VEGF expression was examined in the same samples of undiluted vitreous fluid from the 18 patients. The average VEGF concentration in the vitreous of the PDR patients was strikingly higher than that of the control patients (762.60±143.14 vs. 77.52±7.75 pg/ml, P<0.001). The VEGF level in serum was increased in PDR patients (Figure 2), compared with controls, however, the increase was not statistically significant (95.07±38.79 vs. 69.24±17.40 pg/ml, P>0.05).
Figure 2.
Comparison of VEGF levels between PDR and non-PDR patients. A: vitreous VEGF levels between PDR and non-PDR patients. B: average VEGF levels in the vitreous and the serum of PDR and non-PDR patients. Vitreous VEGF level was significantly higher in PDR patients (P<0.001). However, no significant difference in serum VEGF level between two groups (P>0.05).
Up-regulation of netrin-1 and VEGF in OIR mice retinas
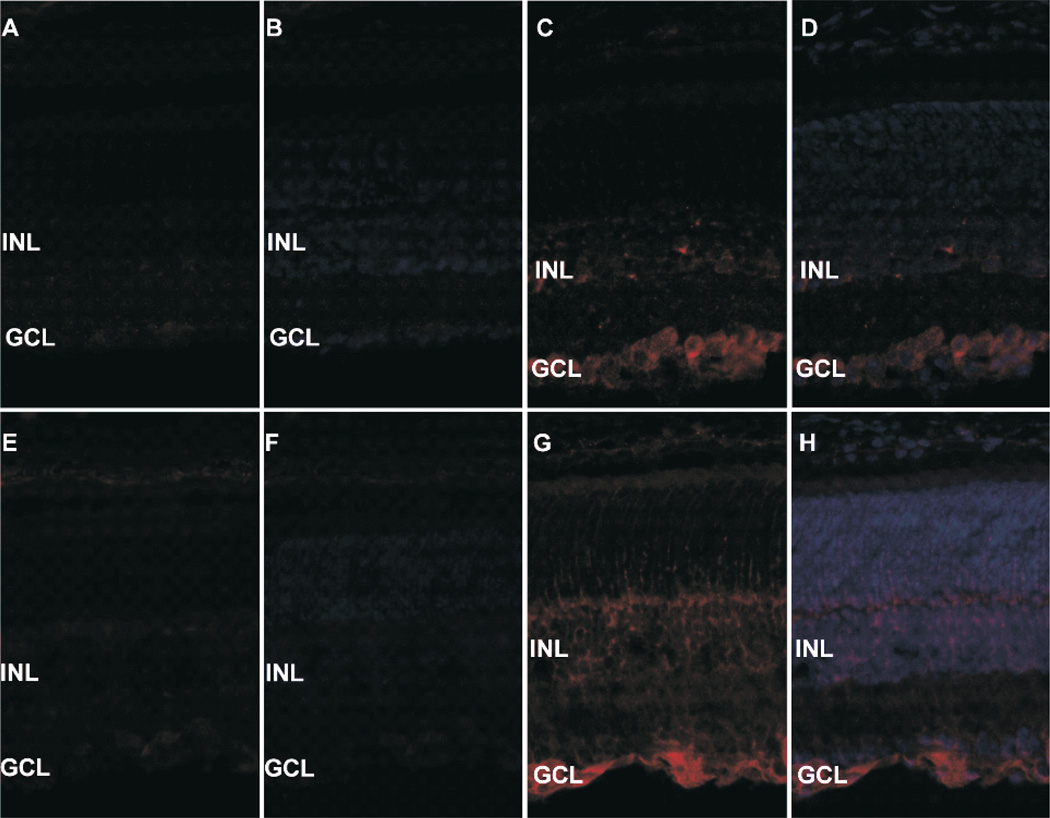
Netrin-1 was mainly located in the retinal ganglion cell layer (GCL) and the inner nucleic layer (INL) of normal mice, and GCL. Additionally, netrin-1 expressed in the outgrowing neovascular cells of the inner limit membrane (ILM) of OIR mice. Compared to normal controls, both netrin-1 and VEGF expressed in the P17 retinas of OIR mice elevated apparently. (As shown in Figure 3).
Figure 3.
A.B show netrin-1 in normal mice, C.D show netrin-1 in OIR mice, E.F indicate VEGF in normal mice, E.F show VEGF in OIR mice. Netrin-1 is mainly expressed in GCL and INL of the retina. Both netrin-1 and VEGF are up-regulated in OIR mice.
Discussion
Netrin-1 is considered as an angiogenic factor, which can induce sprout angiogenesis in corneal limbus with or without the augment of VEGF. It can also promote the migratory and mitogenic activity of primary endothelial cells and vascular smooth muscle cells16. It may promote angiogenesis by maintaining endothelial cell’s survival activity on one hand, and reduce their apoptosis on the other hand18,19. Gene silencing of Netrin-1α results in the disappearance of parachordal vessel, a longitudinal vessel adjacent to the notochord along zabrafish trunk13. Our results showed that netrin-1, similar to VEGF, was significantly up-regulated in the vitreous of PDR patients, compared with that in non-PDR controls. These data suggest that overexpression of netrin-1 in the vitreous may play a role in developing the proliferative pathology of diabetic retinopathy. There are two possible mechanisms. First, netrin-1 may promote angiogenesis by activating DCC-dependent ERK1/2-eNOS feed-forward pathway, which induces NO production subsequently. NO production eventually leads to endothelial cell proliferation, as it can promote endothelial survival20. Second, it may also play a role in anti-apoptotic signaling cascades in endothelial cells by inhibiting Death Associated Protein kinase (DAP kinase), which inhibits the apoptosis of endothelial cells consequently18.
Our results also demonstrated that VEGF was more significantly up-regulated in the vitreous fluid of PDR patients than that of non-PDR controls. VEGF, a potent and crucial pro-migratory endothelial growth factor, was highly up-regulated in the vitreous of many neovascular retinopathies, such as PDR. This phenomenon indicates that VEGF plays a significant role in mediating retinal neovascularization. It can promote migration, proliferation, and tube formation of retinal endothelial cells, and can increase the permeability of microvessels in these neovascular diseases such as PDR4,7,8. Anti-VEGF therapies have shown the efficacy in preventing retinal angiogenesis and reducing retinal ischemia partially in a number of retinopathies with neovascular complications. As netrin-1 was highly expressed in the vitreous of PDR patients parallel to VEGF changes, it may be a potentially novel therapeutic target in diabetic neovascular retinopathies.
Many pro-angiogenic growth factors have been elevated in the vitreous of PDR patients, such as VEGF, EPO, and HIF-11,2. Increased levels of these angiogenic factors in the vitreous of diabetic patients could be attributed to three mechanisms21. Firstly, it is hypothesized that the breakdown of the blood-retina barrier leads to increased levels of angiogenic factors in the vitreous of diabetics22. Secondly, angiogenic factors may be expressed by cells within the vitreous fluid, such as macrophages, monocytes, glial cells, and new vessels23. Thirdly, these factors are expressed by the hypoxic retina as shown in the retinal ischemia-reperfusion model24. Our data demonstrated that there were no significant increases in the levels of netrin-1 and VEGF in the serum of these two groups, although there was strikingly difference in the up-regulation of these proteins in the vitreous fluid of PDR patients, indicating that the increased netrin-1 and VEGF levels in the vitreous fluid may be due to an increased local production in the retina. While the areas of ischemia and avascularity increase in diabetic retinopathy, the synthesis and secretion of netrin-1 and VEGF were stimulated and increased in the retina, which resulted in higher levels of netrin-1 and VEGF in the vitreous. Consequently more retinal angiogenesis were stimulated. A previous study has identified that netrin-1 is an angiogenic factor that can induce sprout angiogenesis in corneal limbus independently.16 When combined with the VEGF, netrin-1 can augment more sprout angiogenesis in corneal limbus.16 Thus, we speculate that netrin-1 is capable of stimulating new retinal vessel sprouts alone. When combined with VEGF, netrin-1 may augment more retinal sprout angiogenesis.
Besides, our immunohistchemical examination showed a much stronger expression of netrin-1 in OIR retina, mainly located in GCL, INL, and the areas of neovascular vessels broking through the ILM in the retina. These observations let us ponder if netrin-1, a laminin protein expressed highly in GCL and INL in the retina, secreted to epi-retina and further to vitreous cavity to exert its role in retinal neovascularization. Therefore, netrin-1 may be an important participant in pathological angiogenesis in the retina which may act as VEGF does.
Although netrin-1 has dual functions in angiogenesis, it can promote migration, proliferation, and tube formation in human umbilical vein endothelial cells (HUVEC) at a concentration lower than 100 ng/ml25. Netrin-1 is also a potent mitogen and chemoattractant for vascular smooth muscle cells (VSMC) and other endothelial cells13,16. Wilson and colleagues observed a maximal activity at 50 ng/ml in their migratory assays using gradient doses of netrin-1. When netrin-1 concentration was up to 1 µg/ml, the increase of endothelial cell migration was no longer significant13,16. Other researchers oppositely believe that netrin-1 works as an anti-angiogenic factor, as it can result in filopodial retraction, especially at the tip cells in the Transwell migration systems and wound healing model14,15. However, the lowest concentration used in their studies was 1 µg/ml. The detected netrin-1 level in the vitreous of our PDR patients was strikingly higher than that in non-PDR patients, but was lower than 1 µg/ml. These data suggest that netrin-1 may act as a pro-angiogenic factor in retinal neovascularization. It may evoke angiogenesis by promoting the migration, proliferation, and tube formation of endothelial cells in the retina.
Although the exact role of netrin-1 in angiogenesis is still uncertain, accumulated evidence suggests that netrin-1 is a pro-angiogenic factor. Its exact role may differ due to its concentrations and distributions in various tissue. However, it is clear that netrin-1 may play an important role in promoting angiogenesis in diabetic retinopathy, either independently or synergistically with VEGF. More works are needed to determine its exact role and signaling cascades. Nevertheless, netrin-1 may be a potentially new therapeutic target in angiogenesis in PDR.
Acknowledgement
This research was supported by the National Natural Science Foundation of China (grant 30872822) and NIH grant R01EY20900.
References
- 1.Watanabe D, Suzuma K, Matsui S, et al. Erythropoietin as a Retinal Angiogenic Factor in Proliferative Diabetic Retinopathy. N Engl J Med. 2005;353(8):782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Wang G, Wang Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2009;148(6):883–889. doi: 10.1016/j.ajo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Aiello LP, Northrup JM, Keyt BA, et al. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch. Ophthalmol. 1995;113(12):1538–1544. doi: 10.1001/archopht.1995.01100120068012. [DOI] [PubMed] [Google Scholar]
- 4.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12(12):4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 5.Bai Y, Ma JX, Guo J, et al. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219(4):446–454. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Xu X, Elliott MH, et al. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59(9):2297–3305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchill AJ, Carter JG, Ramsden C, et al. VEGF polymorphisms are associated with severity of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(8):3611–3616. doi: 10.1167/iovs.07-1383. [DOI] [PubMed] [Google Scholar]
- 8.Kakehashi A, Inoda S, Mameuda C, et al. Relationship among VEGF, VEGF receptor, AGEs, and macrophages in proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2008;79(3):438–445. doi: 10.1016/j.diabres.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78(3):425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 10.Serafini T, Kennedy TE, Galko MJ, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans unc-6. Cell. 1994;78(3):409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 11.Deiner MS, Kennedy TE, Fazeli A, et al. Netrin-1 and dcc mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19(3):575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 12.Oster SF, Deiner M, Birgbauer E, Sretavan DW. Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol. 2004;15(1):125–136. doi: 10.1016/j.semcdb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Lu X, Le Noble F, Yuan L, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432(7014):179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 14.Wilson BD, li M, Park KW, et al. Netrins Promote Developmental and Therapeutic Angiogenesis. Science. 2006;313(5787):640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larrivée B, Freitas C, Trombe M, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21(19):2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park KW, Crouse D, Lee M, et al. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci USA. 2004;101(46):16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cristofaro B, Emanueli C. Possible novel targets for therapeutic angiogenesis. Curr Opin Pharmacol. 2009;9(2):102–108. doi: 10.1016/j.coph.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castests M, Coissieux MM, Delloye-Bourgeois C, et al. Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis. Dev Cell. 2009;16(4):614–620. doi: 10.1016/j.devcel.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Castets M, Mehlen P. Netrin-1 role in angiogenesis: To be or not to be a pro-angiogenic factor? Cell Cycle. 2010;9(8):1466–1471. doi: 10.4161/cc.9.8.11197. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci USA. 2006;103(17):6530–6535. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier R, Weqer M, Haller-Schober EM, et al. Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis. 2008;14:637–643. [PMC free article] [PubMed] [Google Scholar]
- 22.Vinores SA, Derevjanik NL, Mahlow J, et al. Electron microscopic evidence for the mechanism of blood-retinal barrier breakdown in diabetic rabbits: comparison with magnetic resonance imaging. Pathol Res Pract. 1998;194(7):497–505. doi: 10.1016/s0344-0338(98)80118-0. [DOI] [PubMed] [Google Scholar]
- 23.Malecaze F, Clamens S, Simorre-Pinatel V, et al. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994;112(11):1476–1482. doi: 10.1001/archopht.1994.01090230090028. [DOI] [PubMed] [Google Scholar]
- 24.Jo N, Wu GS, Rao NA. Upregulation of chemokine expression in the retinal vasculature in ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2003;44(9):4054–4060. doi: 10.1167/iovs.02-1308. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Zou L, Wang Y, et al. Axon guidance cue netrin-1 has dual function in angiogenesis. Cancel Biol Ther. 2007;6(5):743–748. doi: 10.4161/cbt.6.5.3976. [DOI] [PubMed] [Google Scholar]