Abstract
OBJECTIVES
Spray cryotherapy (SCT) delivers a liquid nitrogen spray via a catheter to produce cellular death. This study seeks to determine the histological changes after bronchoscopic, endoscopic and open SCT on tissues in the thoracic cavity.
METHODS
Yorkshire pigs underwent flexible bronchoscopy, endoscopy and thoracotomy for SCT of the airway, oesophagus and other intrathoracic structures, respectively. Variations in the duration and number of spray cycles for the same dosimetry were compared.
RESULTS
Bronchoscopic SCT of the airway resulted in cellular death up to the cartilage layer. Endoscopic SCT of the oesophagus led to cell death up to the adventitial layer. Tissue necrosis was severe in the lung, of full thickness in the pleura, but very superficial in the great vessels. The extracellular matrix (ECM) of treated tissues remained well-preserved. Having shorter but more cycles of SCT decreased the depth of the cellular necrosis. One pig developed ventricular fibrillation during the surgery and expired.
CONCLUSIONS
SCT causes reproducible tissue injury with the preserved ECM of most tissues within the thoracic cavity, making it enticing for ablation around vital structures like the great vessels with a decreased long-term risk. Further study is warranted to investigate the adverse events during SCT.
Keywords: Bronchoscopes, Cryosurgery, Cryoablation, Lung cancer, Minimally invasive surgery, Pleural disease
INTRODUCTION
Recent advances in technology have led to the ability to deliver liquid nitrogen at −196°C in a spray form via a flexible catheter. Early studies demonstrated that this spray cryotherapy (SCT) can be performed endoscopically and eradicated 97% cases of high-grade dysplasia in patients with Barrett's oesophagus and led to a complete response of intraluminal disease in 72% patients with T1 oesophageal cancers [1, 2].
These early data, combined with the known effects of rapid cooling on wound healing, render SCT suitable for intraluminal tissue ablation and tissue regeneration. Heat-based ablative techniques like electrocautery and argon plasma coagulation are effective, but carry limitations including the risk of perforation, stricture and airway fire [3, 4]. Cryoprobes have effectively treated endobronchial malignancies while preserving the cartilage scaffolding to prevent perforation, but are hindered by the rigid form, requirement of precise contact and risk of tears from the adherence of tissue to the probe [5, 6]. Cold-based SCT with its non-contact spray form allows for cellular death with the preservation of the tissue scaffolding, which can be distributed over a wide area.
In this pilot study, we seek to elucidate the effects of SCT on various thoracic tissues, including cellular response, depth of injury and preservation of the extracellular matrix (ECM). We will also investigate whether changing the duration of a spray cycle and the number of spray cycles for the same total dose of SCT influences these histological effects. These data will provide insight into differential patterns of injury and have practical implications to help shape the treatment guidelines for the clinical application.
MATERIALS AND METHODS
Spray cryotherapy system
The CC2-NAM system (CSA Medical, Baltimore, MD, USA) delivered liquid nitrogen at −196°C at 2–5 psi with a cooling energy of 25 W via a specialized 7 Fr catheter through a 2.8 mm working channel of the Olympus BF-1T180 flexible bronchoscope and GIF-H180 gastroscope (Olympus, Center Valley, PA, USA). The SCT application to other thoracic tissues was performed by hand through a thoracotomy.
A spray cycle began with the appearance of >50% frost coverage of the target tissue, and spray cycles were separated by at least 30 s or until the frost had disappeared completely. Except for the oesophagus, the total dose of SCT was 30 s and was divided into three different regimens to evaluate the effects of varying freeze–thaw cycles: a 5-s spray for six cycles; a 10-s spray for three cycles and a 30-s spray for one cycle. For the oesophagus, the total dose of SCT was 60 s and the three different regimens were: a 10-s spray for six cycles; a 20-s spray for three cycles and a 60-s spray for one cycle.
Animal procedure
All animal work was performed with approval from the Institutional Animal Use and Care Committee. Six Yorkshire pigs (Archer Farms, Darlington, MD, USA) weighing 50–55 kg were sedated with tiletamine/zolazepam (4.4 mg/kg), given buprenorphine (0.01 mg/kg) and glycopyrrolate (0.007 mg/kg), and intubated with a 10 Fr endotracheal tube whose inner diameter is equivalent to that of a standard rigid bronchoscope. The pigs were mechanically ventilated and maintained under general anaesthesia with isoflurane (1.5–2.5%).
Bronchoscopic SCT was performed on the right upper bronchus, right middle bronchus and left upper bronchus. The cuff of the endotracheal tube was deflated and the ventilation circuit disconnected to allow a passive escape of nitrogen gas during SCT. For SCT of the oesophagus, an orogastric tube was placed to evacuate the nitrogen gas. Endoscopic SCT was performed at the oesophagogastric junction (65 cm from the incisors), the mid-oesophagus (55 cm) and the proximal oesophagus (45 cm). Lastly, a thoracotomy was performed, and open SCT by hand was applied to the lungs, pleura, superior vena cava (SVC) and aorta.
In each pig, the airway, oesophagus and lung were resected at three time points (1, 4 and 8 h after SCT) using an Endo GIA Universal stapler (Covidien, Mansfield, MA, USA). Other tissues were harvested at the 8-h time point. Tissues for the 8-h time point were harvested immediately after euthanization of the pigs with Euthasol (pentobarbital sodium/phenytoin sodium 1 mg/4.5 kg). Specimens were fixed in formalin and stained with haematoxylin and eosin (H&E).
RESULTS
Spray cryotherapy of the airway
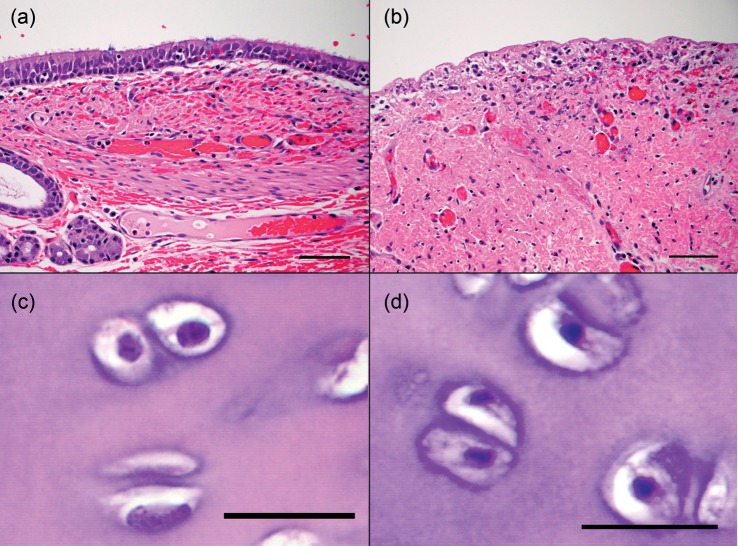
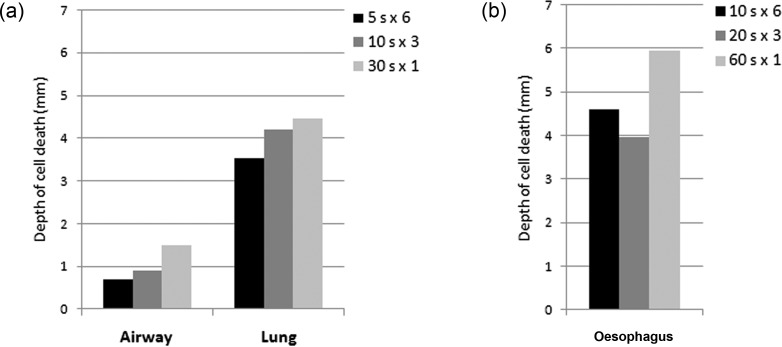
Bronchoscopic SCT was performed in 15 airways (Fig. 1). Compared with the normal tissue (Fig. 2a and c), the ablated tissue exhibited abundant mucus in the lumen, denudation of the epithelium, mild-to-moderate oedema in the submucosa and connective tissue, neutrophilic infiltrate and haemorrhage (Fig. 2b). Cellular necrosis characterized by pyknotic nuclei reached the cartilage layer (Fig. 2d) but not beyond. Cell death extended to a deeper level at the later time points (8 vs 1 h). Cell death was also deeper with a longer duration of spray (30-s spray vs 5-s spray) despite having the same total time of SCT (Fig. 3a). At 8 h, the depth of cell death was 0.7 ± 0.73 mm [0.3–1.8] with 5-s sprays, 0.85 ± 0.92 mm [0.2–1.5] with 10-s sprays and 1.45 ± 0.35 mm [1.2–1.7] with 30-s sprays. The tissue architecture and ECM in all layers remained intact despite the varying duration and number of freeze–thaw cycles.
Figure 1:
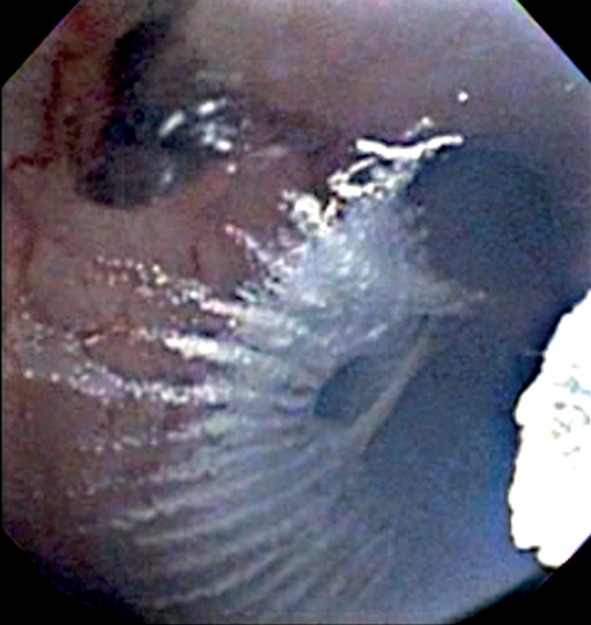
Bronchoscopic SCT. A bronchoscopic view of liquid nitrogen being sprayed onto the airway.
Figure 2:
The effects of bronchoscopic SCT of the airway. (a) Normal mucosa and submucosa. (b) After bronchoscopic SCT, the epithelium was sloughed off and the cells were necrotic. (c) Normal cartilage. (d) After ablation, cell death extended to the cartilage as depicted by pyknotic nuclei in the chondrocytes. Scale bar represents 50 µm in (a) and (b), and 20 µm in (c) and (d). H&E stain.
Figure 3:
The depth of cell death with variations in the dosimetry. (a) In spite of maintaining the same total SCT time of 30 s, a longer duration of a spray cycle led to a greater depth of cell death in the airway and lung. (b) Despite the same total SCT time of 60 s, a longer duration of a spray cycle led to a greater depth of cell death in the oesophagus.
Spray cryotherapy of the oesophagus
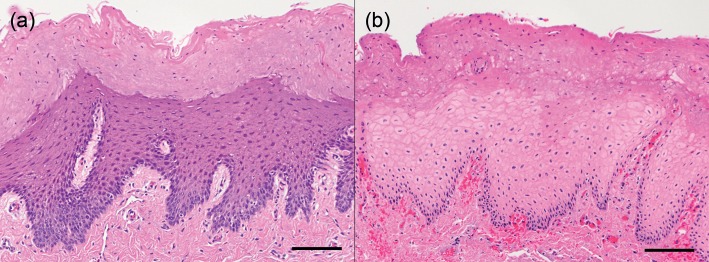
Endoscopic SCT was done in 17 oesophageal segments with two segments excluded because the harvest missed the ablated tissue. Compared with the normal tissue (Fig. 4a), the ablated oesophagus showed necrosis, marked submucosal oedema and mild neutrophilic infiltrate (Fig. 4b). Cell death extended to a deeper level at the later time points, and cell death was deeper with a longer duration of spray (Fig. 3b). At 8 h, the depth of cell death was 4.6 mm (only one specimen) with 5-s sprays, 3.97 ± 0.21 mm [3.8–4.2] with 10-s sprays and 5.95 ± 0.07 mm [5.9–6.0] with 30-s sprays. Variability in the depth of cell death was much more affected by oedema in the oesophagus than in the airway. The ECM was well-preserved in all layers.
Figure 4:
The effects of endoscopic SCT of the oesophagus. (a) Normal mucosa. (b) After endoscopic SCT, there was necrosis throughout the epithelium and lamina propria with mild haemorrhage. Scale bar represents 100 µm. H&E stain.
Spray cryotherapy of other structures
SCT of the lung was carried out in 18 areas. The ablated lung parenchyma demonstrated necrosis, marked haemorrhage, neutrophilic infiltrate and interlobular oedema. The ablated alveolar space was obliterated with oedema and blood and lined with necrotic cells with pyknotic nuclei. Cell death extended to a deeper level at the later time points, and cell death was deeper with a longer duration of spray (Fig. 3a). At 8 h, the depth of cell death was 3.53 ± 0.47 mm [3–3.9] with 5-s sprays, 4.2 ± 1.98 mm [2.8–5.6] with 10-s sprays and 4.47 ± 0.32 mm [1.2–1.7] with 30-s sprays. The ECM was intact in all except one lung specimen where it was purple, with a loss of eosinophilia which could represent a thermal artefact.
SCT in four pleural areas led to focally extensive necrosis of the pleura and the underlying fat and skeletal muscle, accompanied by mild-to-moderate haemorrhage, neutrophilic infiltrate and necrosis of the small vessels.
Four aortas were treated with SCT and had hyperaemia, a mild neutrophilic infiltrate and necrosis in the adventitial connective tissue. In only one aortic specimen was cell death present in the smooth muscle layer, extending <0.5 mm deep and still sparing the media.
SCT was performed on four SVCs, which showed hyperaemia and necrosis in the adventitial layer. In two SVC specimens, cell death reached the smooth muscle layer, being superficial in one specimen and of full-thickness in the other. The spray treatment and total dose of SCT was identical in all four treatments.
Adverse events
There was one intraoperative death and no other adverse events. Immediately after the first 20-s cycle of SCT on the mid-oesophageal segment, the pig's electrocardiogram showed inverted T waves that progressed rapidly to ventricular tachycardia and ventricular fibrillation. A defibrillator was not available. Emergency bilateral thoracotomies and manual cardiac massage were performed, but the pig could not be resuscitated and expired. Upon necropsy, there was no evidence of myocardial infarct on gross or histological examination, and major coronary vessels and the vagus nerve near the ablation site were normal. There was mild multifocal necrosis of Purkinje fibres in the right ventricle and rare small foci of myofibre necrosis scattered throughout the left ventricle. Mild-to-moderate endocardial, myocardial and epicardial haemorrhages were also observed. These findings were interpreted as non-specific changes that could be observed in acute cardiac death but did not suggest a particular cause.
DISCUSSION
The therapeutic use of extreme cold for ablation was first widely accepted in the field of dermatology with the advantage of minimal wound care, a low risk of infection and a short treatment time. The advent of a flexible catheter system to spray liquid nitrogen through a scope broadens the clinical application of cryotherapy to other fields. SCT has shown success in treating Barrett's oesophagus and early oesophageal cancer [1, 7] and may be safely used in patients on high levels of oxygen without the risk of airway fire.
Our study demonstrated that SCT was feasible for intraluminal and open ablation of thoracic tissues with reproducible effects. Bronchoscopic SCT ablation of the airway led to superficial necrosis extending from the mucosa to the cartilage but not beyond. Endoscopic SCT ablation of the oesophagus produced cellular necrosis and submucosal oedema. These data, combined with previous findings [1, 7], substantiate the histological effects and ablative potential of SCT in the airway and oesophagus.
This reproducible effect of SCT extended to other thoracic tissues. Similar findings as ours with SCT have been seen in the pleura, suggesting the utility of SCT for the treatment of pleural disease [8]. Our finding of the minimal effects of SCT on the outer layer of the great vessels may be attributed to the attenuation of freeze by the high blood flow within, similar to the heat-sink effect. This may offer protection to these bystander structures, lower the risk of aneurysmal formation and allow treatment of positive post-resection microscopic margins along the great vessels without the need for vascular resection.
Another consistent finding was, regardless of tissue type, the preservation of the ECM with SCT. This is in contrast to the denaturation of proteins seen with heat-based techniques [9, 10]. The preservation of the ECM may engender an environment that promotes ablation of an unwanted tissue without significant scarring [10, 11]. Our findings of a consistently intact ECM may be the histological basis for the low rate of stricture formation in the oesophagus and airway observed in both animal and human studies [1, 7, 12]. This also explains the use of SCT to treat strictures [13, 14].
We have also demonstrated that the dosimetry of SCT produces distinct and reproducible histological changes. Even with the same total dose of SCT, a longer duration of a spray led to a greater depth of necrosis. This consistent finding could be used to modulate the depth of the injury depending on the desired effects on the target tissue.
Issues that require a further study include a need for a survival model to evaluate the complete course of healing after SCT. This would be advantageous to further characterize the nature of the wound and inflammatory response as well as to assess for long-term complications like stricture and vascular aneurysm formation. Additionally, one pig had an unexplained intraoperative death. While it is unclear whether this particular event was directly related to SCT, there is a report of intraoperative deaths in patients during SCT (D.J. Finley et al., in preparation), and work to study the physiological effects of SCT is warranted before SCT is widely disseminated.
In conclusion, our study of SCT on normal thoracic tissues in a porcine model demonstrates that SCT has potential as a minimally invasive ablation technique for thoracic tissues with consistent cellular injury that is related to the specific dosimetry. It maintains the ECM, which may promote healing with reduced scar formation. We envision that SCT holds potential in a variety of different clinical applications to treat areas with limited therapeutic options.
FUNDING
This work was supported by departmental funds from the Thoracic Surgery Department at Memorial Sloan-Kettering Cancer Center.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We thank Tae Jin Song, William Krimsky, Paula Ezell, Yuman Fong and Valerie Rusch for their time, expertise and support of our research.
APPENDIX. CONFERENCE DISCUSSION
Dr C. Deschamps (Rochester, MN, USA): I think that what you've done is a great step forward. It is already being done in patients with Barrett's oesophagus, and it has a large body of publications in dermatology for skin lesions. I have two questions. One, do you think that your assessment of the cartilage, which was not clear in the manuscript, is reflective of the life of the cartilage? It's notorious that cartilage cannot be evaluated easily with simple histology, and sometimes it will take days or weeks before the cartilage dies, even if it looks very normal on histology. I would like you to comment on this, and maybe keep that in mind when you do more experiments if you have not done so.
Second, there is more work in Barrett's oesophagus than there is in pulmonary cryotherapy. You have used your pigs to the maximum. We could tell in reading your manuscript. Where do you think this should go next? Do you think it should go more to the Barrett's oesophagus world and early oesophageal cancer or should it go to endobronchial disease?
Dr Finley: To answer the first question, in terms of cartilaginous injury that is associated with this, clearly it's difficult on histology, especially in the short term, to see pyknotic nuclei, and we currently are moving forward with doing a survival model so that we can evaluate whether or not the depth of injury changes over time. We only looked at 8 h and we need to look at something around the 2-week to 3-week mark to make sure that we're not seeing continued damage that goes beyond the cartilage and also damage to the cartilage. So, yes, I agree with you. To answer that question, this is just essentially a pilot study.
Regarding the second question as to where should we go from here, there have been a couple of reports (one at our institution, 5 in total nationally) of sudden death associated with spray cryotherapy, mainly during airway procedures, but one during an oesophageal procedure. This is cardiac in nature. The advent of this entire experiment that I started with was to look at the cardiac complications, which I'm going to be publishing very shortly. Once we figure that out, I think that this specific modality is phenomenal for use within the airway. Renal cell is very difficult to treat endobronchially. You spray this, you can just rip it off the airway wall and spray it again, and 5 months later, without any treatment, there is still no regrowth of tumour. It doesn't cause strictures. With Chrish Fernando and a couple other people in the U.S., we have looked at subglottic stenosis and the ability to balloon-dilate these strictures and then spray the area where we ballooned or manually dilated, and we now have a series of 30+ patients, and one is now out at 4½ years and has not required any other intervention. These patients have sometimes had something in the vicinity of 40 lasers prior to that. So I think the specifics of the thermal injury that is occurring with liquid nitrogen versus heat are very important and I think that it will move forward for use within the airway and other tissues.
I think ablation of Barrett's oesophagus is excellent, but the only problem is that in a lot of patients you're not able to assess for T1 or T2 lesions, and we know ultrasound isn't perfect. So, unfortunately, you're not looking at treating any of the lymph nodes. So I would caution about using this for oesophageal solely, and I think moving towards the airway and within the pleura for margins would be excellent.
Dr K. Moghissi (Hull, UK): As you have not presented any clinical data, let's go into the science a bit. I don't mind about the cryotherapy in endobronchial disease. Let us, however, talk about the Barrett's oesophagus. I think you are on very dangerous ground there, because of two things. One is that you have to be absolutely certain about the depths of your necrosis, and that you can't be sure in cryotherapy, because I don't know what sort of measurement you can do to evaluate this, and it is always difficult to measure the depths of your cooling and necrosis. Secondly, things such as dysplasia in Barrett's oesophagus, what you really would want to have is a method that differentiates between dysplastic cells, particularly high dysplasia, and normal cells. You can't just go on and spray and think that it will pick up your dysplasia in preference to the normal. So my question to you is, where do you go from here? Not so much clinically, but where would you go scientifically to determine your parameters?
Dr Finley: I completely agree with the Barrett's, but, unfortunately, right now we're seeing a significant number of patients being treated with high-grade dysplasia with various thermal modalities. Actually, in our data here, we had 18 different specimens for each area, and in all of those areas we were off by about 0.03, 0.04 mm in terms of the depth of injury for each of these, and these were done over a 6-month period. So I do think that we can actually pretty much quantify the depth of injury depending on the location and the type of tissue and how much you spray. More importantly, we have had follow-up with over 130 patients that we have done with airway spray cryotherapy, and in those patients we have shown re-mucosalization of the airway in the area that we sprayed, with minimal stricture formation or scarring. So I think that the difference is even if you have full-thickness necrosis, which has been shown in the oesophageal data where they sprayed patients and then they underwent an oesophagectomy, even in those patients you don't cause stricture formation because the extracellular matrix is left intact, and you don't cause perforation, which would be my biggest concern in the oesophagus. So I think from a scientific standpoint, having a survival model will allow us to evaluate what happens 2, 3, 4 weeks after we do this, and from a histologic standpoint, where are we going and what we see. The second thing is, are we going to cause a problem? Is there going to be some untoward event that we're not recognizing now that we will see when we do these models? If it is just the extracellular matrix being left intact that allows for the structures to reform with minimal scar, then I think that we can go forward with expanding this into different modalities around the body, how to treat microscopic disease in various places, and some people are talking about when you're getting ready to re-excise, you can just spray the base of where you were if you had a positive margin, allowing you to kill to a certain depth based upon a nomogram that can be formulated beforehand in the animal models.
REFERENCES
- 1.Greenwald BD, Dumot JA, Abrams JA, Lightdale CJ, David DS, Nishioka NS, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686–93. doi: 10.1016/j.gie.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Greenwald BD, Peery AF, Dumot JA, Nishioka NS, Wolfsen HC, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680–5. doi: 10.1016/j.gie.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolliger CT, Sutedja TG, Strausz J, Freitag L. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258–71. doi: 10.1183/09031936.06.00013906. [DOI] [PubMed] [Google Scholar]
- 4.Dumot JA, Greenwald BD. Argon plasma coagulation, bipolar cautery, and cryotherapy: ABC's of ablative techniques. Endoscopy. 2008;40:1026–32. doi: 10.1055/s-0028-1103414. [DOI] [PubMed] [Google Scholar]
- 5.Schumann C, Hetzel M, Babiak AJ, Hetzel J, Merk T, Wibmer T, et al. Endobronchial tumor debulking with a flexible cryoprobe for immediate treatment of malignant stenosis. J Thorac Cardiovasc Surg. 2010;139:997–1000. doi: 10.1016/j.jtcvs.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Schumann C, Hetzel J, Babiak AJ, Merk T, Wibmer T, Moller P, et al. Cryoprobe biopsy increases the diagnostic yield in endobronchial tumor lesions. J Thorac Cardiovasc Surg. 2010;140:417–21. doi: 10.1016/j.jtcvs.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Dumot JA, Vargo JJ, 2nd, Falk GW, Frey L, Lopez R, Rice TW. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635–44. doi: 10.1016/j.gie.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Downie G, Krimsky W. Response to spray cryotherapy in a patient with adenocarcinoma in the parietal pleura. Respiration. 2010;80:73–7. doi: 10.1159/000271866. [DOI] [PubMed] [Google Scholar]
- 9.Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med. 1999;20:123–38. doi: 10.1016/s0272-5231(05)70131-3. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd JP, Dawber RP. Wound healing and scarring after cryosurgery. Cryobiology. 1984;21:157–69. doi: 10.1016/0011-2240(84)90207-4. [DOI] [PubMed] [Google Scholar]
- 11.Fernando HC, Sherwood JT, Krimsky W. Endoscopic therapies and stents for benign airway disorders: where are we, and where are we heading? Ann Thorac Surg. 2010;89:S2183–7. doi: 10.1016/j.athoracsur.2010.02.106. [DOI] [PubMed] [Google Scholar]
- 12.Johnston CM, Schoenfeld LP, Mysore JV, Dubois A. Endoscopic spray cryotherapy: a new technique for mucosal ablation in the esophagus. Gastrointest Endosc. 1999;50:86–92. doi: 10.1016/s0016-5107(99)70352-4. [DOI] [PubMed] [Google Scholar]
- 13.Fernando HC, Dekeratry D, Downie G, Finley D, Sullivan V, Sarkar S, et al. Feasibility of spray cryotherapy and balloon dilation for non-malignant strictures of the airway. Eur J Cardiothorac Surg. 2011;40:1177–80. doi: 10.1016/j.ejcts.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 14.Krimsky WS, Rodrigues MP, Malayaman N, Sarkar S. Spray cryotherapy for the treatment of glottic and subglottic stenosis. Laryngoscope. 2010;120:473–7. doi: 10.1002/lary.20794. [DOI] [PubMed] [Google Scholar]