Abstract
OBJECTIVES
The present study aimed to assess the additional value of a pocket-sized imaging device (PSID) as an adjunct to plain chest X-rays in the diagnosis of pleural effusion (PE), mainly for those requiring pleural thoracentesis.
METHODS
We performed a thoracic ultrasound examination using a PSID in 73 patients with an abnormal chest X-ray diagnostic for unilateral PE. Abundant PE was defined as an interpleural distance between the diaphragm and visceral pleura (VP) of ≥30 mm at the apex of the 50 mm bisector line of the costodiaphragmatic recess at end expiration.
RESULTS
According to PSID ultrasound evaluation, abundant PE was present in 46 patients (63%), while 27 (37%) patients showed the presence of mild PE or absence of PE. Thoracentesis was performed successfully and without procedure-induced complications in all 46 patients with abundant PE. Using the above-mentioned method, we obtained a high diagnostic accuracy (area under the curve = 0.99) and excellent sensitivity and specificity of 91.7 and 99.9%, respectively, to predict a PE >1000 ml, when VP was >6.3 cm.
CONCLUSIONS
PSID is a useful tool that may integrate and complete the physical examination, also providing additional information to chest X-ray in the clinical management of patients with suspected PE. PSID evaluation can also increase the effectiveness and safety of thoracentesis.
Keywords: Lung ultrasound, Pocket-sized imaging device, Pleural effusion
INTRODUCTION
Pleural effusion (PE) is a common clinical problem, and imaging techniques are often needed for diagnostic and monitoring purposes [1]. Plain chest X-ray has been the traditional tool used to diagnose PE. In the last few years, ultrasound examinations have been applied succesfully to detect PE, demonstrating greater sensitivity than chest X-ray and thoracic computed tomography (CT) [2].
Bedside ultrasound guidance can also improve the rate of success of pleural aspiration, either as a first-line procedure or after a failed clinical and plain chest X-ray-guided attempt [3], reducing the postprocedural incidence of iatrogenic pneumothorax and/or pulmonary haematoma [4]. Lately, as a result of significant technological advances, a wide variety of miniaturized echographic devices has become available, which can be utilized for screening patients at the bedside [5]. The extreme miniaturization of echo machines has evolved to the creation of a pocket-sized imaging device (PSID), small and light enough to fit in a hand. Such a tool provides black and white anatomical and colour-flow images in real time. The potential clinical range of applications of PSID includes its use in emergency care units and ambulances, for screening programmes in communities, and during consultations inside or outside the hospital. The use of PSID in the clinical area, including qualitative evaluation of pleural effusion, has recently been addressed in a position statement of the European Association of Echocardiography [6].
The present study aimed to assess the additional value of PSID as an adjunct to plain chest X-rays in the diagnosis of PE, mainly of those requiring pleural thoracentesis.
PATIENTS AND METHODS
Seventy-three (41 males) consecutive patients aged 68.4 ± 9.2 years, referred between December 2009 and February 2011 to the Thoracic Surgery Department with an abnormal chest X-ray consistent with unilateral PE, as assessed by an experienced single reader, were included in the study. Anterolateral and laterolateral chest radiographs where obtained in a standing position, at end expiration in all patients.
The patients were subsequently referred for evaluation by one ultrasound expert cardiologist, blinded to the results of X-ray, for assessment using a PSID. Thoracic ultrasound examinations were performed by using a single PSID (Vscan, Horten, Norway), connected to a broad-bandwith, phased array probe (1.7–3.8 MHz). Images and videos (automatic autocycle without the need for ECG) can be stored in examination folders, searchable via a gallery function and transferred to a PC or USB device through a docking station. Every patient was positioned in the upright sitting position on the bed, facing away from the investigator, with the left or right arm and hand beside the head at end expiration. PSID evaluation was used to confirm or rule out the presence of PE requiring pleural thoracentesis (T0), to guide thoracentesis, and after the end of the procedure (T1) when no more fluid could be drained (Fig. 1). PE was defined as abundant when an interpleural distance between the diaphragm and visceral pleura (VP) of ≥30 mm was present at the apex of the 50 mm bisector line of the costodiaphragmatic recess at the end of expiration (Fig. 2).
Figure 1:
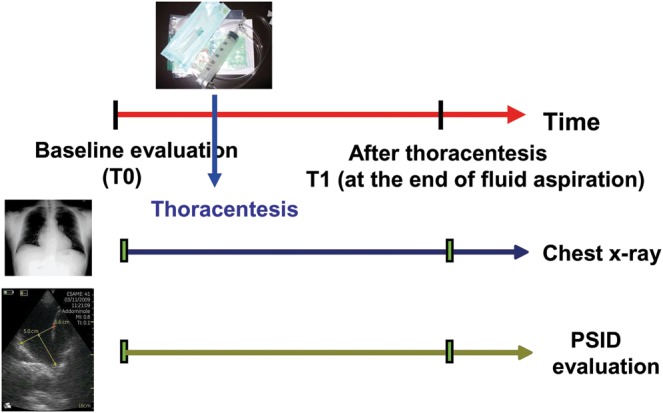
Study protocol. PSID: pocket-sized imaging device.
Figure 2:
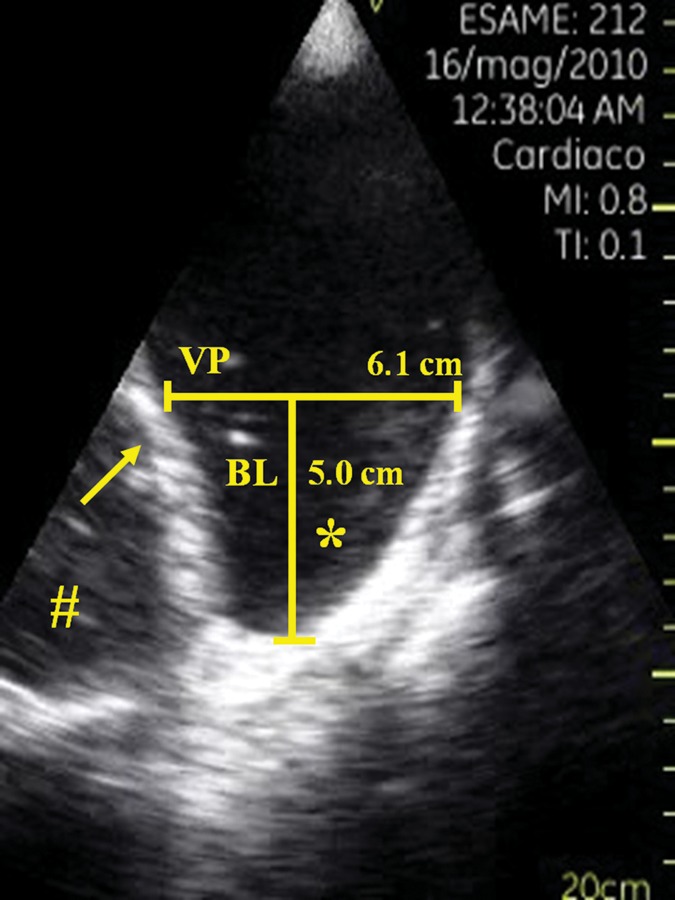
Longitudinal ultrasonographic view showing a large right pleural effusion. Abbreviations and symbols: VP indicates the distance between the diaphragm (arrow) and the visceral pleura; bisector line represents the bisector line of the costodiaphragmatic recess (*); and # indicates the liver.
The PSID was also used to select the exact thoracic location at which to perform the thoracentesis, by placing the device perpendicular to the patient's chest in the midaxillary line. Thoracentesis was performed in the presence of unilateral abundant PE, dyspnoea and peripheral oxygen saturation levels of <90% obtained without supplemental oxygen to relieve symptoms in 46 consecutive patients. Chest drainage was performed according to standard guidelines [7]. Following local anaesthesia with 1% lidocaine, we performed explorative puncture to confirm the presence of PE. If PE was present, a chest tube was inserted using a single-step trocar technique, connected to a chest drain valve placed at –20 cmH2O. The patients were then placed in a semirecumbent position. PSID evaluation was repeated when no more fluid could be aspirated (195 ± 36 min), and the ultrasound image was used also to guide the removal of the chest tube.
Chemical and histological analysis of pleural fluid obtained by thoracentesis was performed in all 46 cases according to Light's criteria [8].
All subjects gave their written informed consent for participation in the study. All work was in compliance with the Declaration of Helsinki and it was performed with the approval of local ethics committee.
RESULTS
The chest ultrasound assessment by PSID was feasible and easy to perform in all 73 patients; 146 hemithoraces were evaluated. PSID evaluations lasted 3.9 ± 0.26 min, and chest drainage was performed 8.2 ± 3.3 min following sonographic evaluation. At the time of chest ultrasound scanning (T0), abundant PE was present in 46 patients (63%), who accordingly underwent thoracentesis, while 27 (37%) patients showed the presence of mild PE or absence of PE. Thoracentesis was performed successfully and without procedure-induced pneumothorax and/or pulmonary haematoma in all 46 patients with abundant PE according to the sonographic criteria listed above. After thoracentesis, all patients had substantial complete aspiration of pleural fluid with end-expiration separation of pleural layers ≤12.5 ± 0.45 mm (Fig. 3).
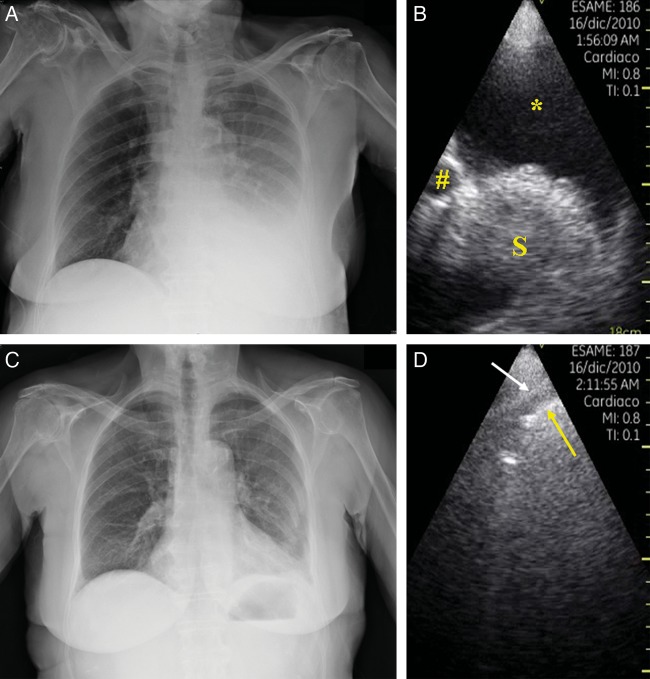
Figure 3:
Example of left-sided pleural effusion (PE) on chest X-ray (A) and corresponding pocket-sized imaging device scanning (B) that confirms the presence of PE. After thoracentesis, the chest radiograph showed the absence of residual PE (C), while ultrasound scanning (D) showed only mild residual separation between the parietal pleura (white arrow) and visceral pleura/lung surface (yellow arrow). Abbreviations and symbols: *indicates the pleural effusion; # indicates the thoracic descending aorta; S indicates the spleen.
Of the remaining 27 patients, in eight patients the PSID showed the presence of hepatic parenchyma suggestive of right diaphragm relaxation and the presence of only mild PE, in 11 a mild PE and the presence of an intrapleural parenchymatous mass and in four a lateral saccate pleural effusion (Fig. 4). The PSID ultrasound scanning confirmed the presence of a chest X-ray consensual PE, and contemporary presence of controlateral PE in four patients, which was suggestive, following echocardiographic evaluations, of a diagnosis of a cardiac origin. Moreover, use of the PSID allowed the detection of septated and non-uniform echogenic pleural effusion in six patients. In these patients, a diagnostic thoracentesis was performed, and subsequent histological and microbiological analysis of the pleural fluid confirmed the presence of haemorrhagic pleural effusion in five cases and a pleural empyema due to Staphylococcus aureus in one patient.
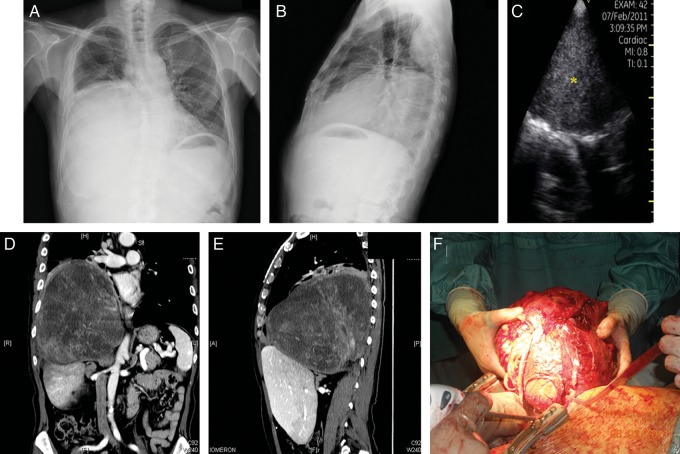
Figure 4:
Forty-five-year old man with type I neurofibromatosis. Chest radiographs revealed abnormal right chest X-ray (A and B). Chest PSID scanning (C) at the sixth intercostal space along the right scapular line showed the presence at this level of a parenchymatous mass (*), confirmed by chest computed tomography scan (D and E) and surgery (F).
We have performed a first analysis based with only semeiotics (that is simply counting intercostal spaces involved by PE) to find a parameter able to predict the presence of a PE greater than 1000 ml. With this elavulation we have observed that the presence of >3.2 intercostal spaces predicts a PE >1000 ml with a good diagnostic accuracy (area under the curve = 0.932) and a good sensitivity and specificity of 90.9 and 85.7%, respectively.
We have also performed a second analysis using the interpleural distance between the diaphragm and VP at the apex of the 50 mm bisector line of the costodiaphragmatic recess (see Fig. 2) obtained by the PSID. With this second evaluation we have obtained a high diagnostic accuracy (area under the curve = 0.99) and excellent sensitivity and specificity of 91.7 and 99.9%, respectively, to predict a PE >1000 ml, when VP was >6.3 cm (Fig. 5).
Figure 5:
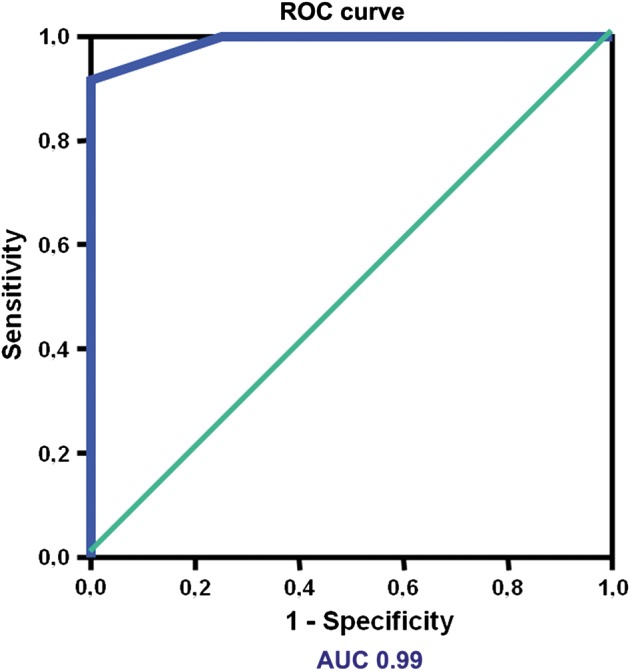
Diagnostic power of pocket-sized imaging device evaluation to predict a pleural effusion >1000 ml. Receiver operating characteristic (ROC) curve for the interpleural distance between the diaphragm and visceral pleura at the apex of the 50 mm bisector line of the costodiaphragmatic recess to predict a PE >1000 ml. AUC: area under the curve.
DISCUSSION
In the last 20 years, advances in technology and miniaturization have led to the availability of several very advanced echographic machines, including all the conventional tools such as 2D, colour Doppler and transoesophageal echocardiography [9]. In contrast, the PSID has been introduced very recently, in order to complement the physical examination. In clinical practice, the PSID has shown a relevant additional diagnostic value when combined with the sole physical examination, with a diagnostic accuracy which appears to be very good in expert ultrasound users and suboptimal in trainees [10].
The PSID is able to provide a qualitative evaluation of left and right ventricular function, pericardial effusion and extracardiac structures, including the pleura and PE. Chest X-rays are usually sufficient to diagnose PE, but it is well known that at least 175 ml of pleural fluid is necessary to permit detection of costophrenic obliteration by chest X-ray, whereas PEs with up to 500 ml of fluid can be present without costophrenic obliteration [11]. The ultrasound assessment is superior to plain radiography in diagnosing and quantifying PE and distinguishing PE from pleural thickening (that is the result of membrane thickening, which is most often due to inflammation) with high specificity, particularly when colour Doppler is employed [12]. The ultrasound assessment can also discriminate between exudative effusions, in which pleural fluid presents complex ultrasound features, septated or echogenic, from simple (anechoic) effusions, which may be exudates or transudates [7] (Fig. 6). Furthermore, ultrasound is able to detect septation within pleural fluid with greater sensitivity than CT scanning [13]; this peculiar aspect can be observed with a similar frequency in both malignant effusions and pleural infection (Fig. 7). In our study, the PSID examination was able to confirm or exclude the presence of abundant PE requiring thoracentesis, while avoiding the performance of the procedure otherwise, as well as to reducing the risk of iatrogenic complications. In the case of mild PE or in the absence of PE, the PSID has been shown to be able quickly to demonstrate the reason for hemithorax opacity at chest X-ray. In addition, all six cases of suspected haemorrhagic or exudative pleural effusion detected by ultrasound were then confirmed by thoracentesis, adding an important diagnostic element for the thoracic surgeon.
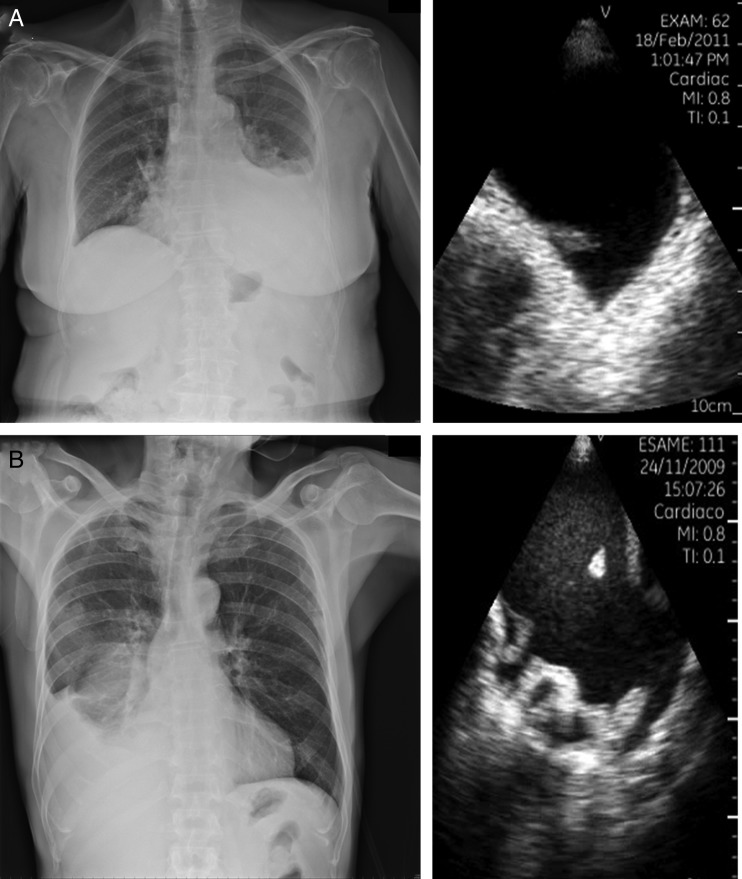
Figure 6:
Two different types of pleural effusion (PE). Above is an example of transudative PE (A); below an example of haemorrhagic pleural effusion (B).
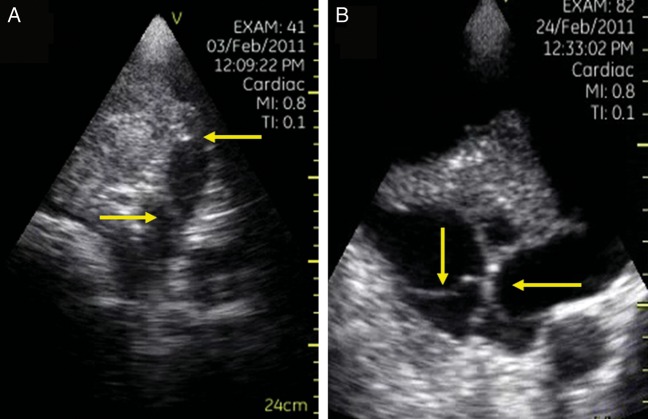
Figure 7:
Two different cases of lung ultrasound in which it is possible to observe septations within the pleural fluid (arrows). Thoracentesis demonstrated haemorrhagic pleural effusion (A) and meta-pneumonic pleural effusion (B).
The method of quantification of PE using PSID that we proposed, based on a simple monodimensional measurement, showed a high diagnostic accuracy with an area under the curve = 0.99 and with an excellent sensitivity and specificity of 91.7 and 99.9%, respectively, to predict a PE >1000 ml. This method is feasible directly at the bedside with a simple calculation, prolonging by only a few minutes the evaluation of patients with PE, using a small, light and transportable imaging device. In a previous study performed in patients with PE after cardiac surgery, the authors derived a formula to quantify pleural fluid, showing a good correlation between the measured distance between the mid-height of the diaphragm and the visceral pleura and subsequent thoracentesis [14]. There are several potential sources of bias in sonographic estimation of pleural fluid in patients with PE, the major one being the size of the thoracic cavity. In fact, especially in tall patients with large thoraces, the measure of PE obtained with ultrasound may underestimate the quantity of pleural fluid. Furthermore, in the case of lower lobe atelectasis with large effusions, the volume of pleural fluid tends to assume a geometric shape that makes it difficult to estimate with accuracy by means of ultrasound. In contrast, using our proposed method for the estimation of PE, in the case of abundant PE, the distance between the diaphragm and visceral pleura at the apex of 50 mm bisector line of the costodiaphragmatic recess increases in proportion to the increase of the pleural fluid within the pleural cavity, thus potentially overcoming the aforementioned limitations. In the case of abundant PE, a dilatation of the costodiaphragmatic recess takes place and, as a consequence, the perpendicular line to the bisector line of the costodiaphragmatic recess increases proportionally (Fig. 8). This method of ultrasound estimation of PE takes into account concomitantly the geometric shape of the thoracic cavity and the dilatation of the costodiaphragmatic recess in the case of both mild PE and abundant PE. Our study has shown that this calculation can be done directly at the bedside without any radiation risk and it can improve the efficacy and safety of the decision making regarding patients with PE before thoracentesis.
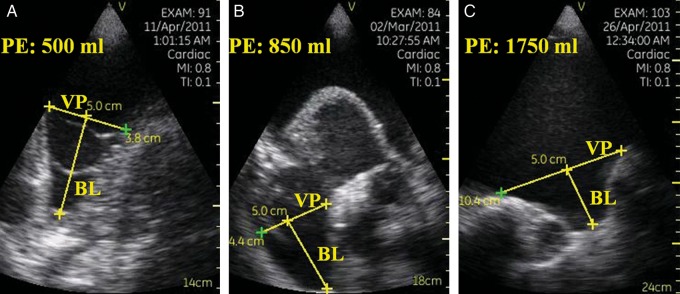
Figure 8:
Three examples of increasing degree of pleural effusion (PE), illustrating the method of ultrasound estimation of PE obtained through the pocket-sized imaging device: a mild PE (A), a moderate PE (B) and a severe PE (C). Abbreviations and symbols: VP represents the distance between the diaphragm and visceral pleura; BL represents the bisector line of the costodiaphragmatic recess.
Conclusions
A PSID is a useful tool that may integrate and complete the physical examination and may also provide additional information to chest X-ray for the clinical management of patients with suspected PE. The PSID-derived estimates regarding the nature and quantity of PE may be applied in the decision making of thoracentesis, and the use of PSID increases the effectiveness and safety of the procedure.
Conflict of interest: none declared.
References
- 1.Sahn SA, Heffner JE. Pleural fluid analysis. In: Light RW, Lee YCG, editors. Textbook of Pleural Diseases. 2nd edn. London: Arnold Press; 2008. pp. 209–26. [Google Scholar]
- 2.Hooper C, Lee YCG, Maskell N. BTS Pleural Guidelines Group. Investigation of a unilateral effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65:ii4–17. doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 3.O'Moore PV, Mueller PR, Simeone JF, Saini S, Butch RJ, Hahn PF, et al. Sonographic guidance in diagnostic and therapeutic interventions in the pleural space. AJR Am J Roentgenol. 1987;149:1–5. doi: 10.2214/ajr.149.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33:442–6. doi: 10.1002/jcu.20163. [DOI] [PubMed] [Google Scholar]
- 5.Mondillo S, Giannotti G, Innelli P, Ballo PC, Galderisi M. Hand-held echocardiography: its use and usefulness. Int J Cardiol. 2006;111:1–5. doi: 10.1016/j.ijcard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Sicari R, Galderisi M, Voigt JU, Habib G, Zamorano JL, Lancellotti P, et al. The use of pocket-size imaging devices: a position statement of the European Association of Echocardiography. Eur J Echocardiogr. 2011;12:85–7. doi: 10.1093/ejechocard/jeq184. [DOI] [PubMed] [Google Scholar]
- 7.Havelock T, Teoh R, Laws D, Gleeson F. on behalf of the BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British thoracic society pleural disease guideline 2010. Thorax. 2010;65:61–76. doi: 10.1136/thx.2010.137026. [DOI] [PubMed] [Google Scholar]
- 8.Light RW, MacGreggor I, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–13. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 9.Marwick TH. The future of echocardiography. Eur J Echocardiogr. 2009;10:594–601. doi: 10.1093/ejechocard/jep056. [DOI] [PubMed] [Google Scholar]
- 10.Galderisi M, Santoro A, Versiero M, Lomoriello VS, Esposito R, Raia R, et al. Improved cardiovascular diagnostic accuracy by pocket size imaging device in non-cardiologic outpatients: the NaUSiCa (Naples Ultrasound Stethoscope in Cardiology) study. Cardiovasc Ultrasound. 2010;8:51. doi: 10.1186/1476-7120-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins JD, Burwell D, Furmanski S, Lorber P, Steckel RJ. Minimal detectable pleural effusions. A roentgen pathology model. Radiology. 1972;105:51–3. doi: 10.1148/105.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Roch A, Bojan M, Michelet P, Romain F, Bregeon F, Papazian L, et al. Usefulness of ultrasonography in predicting pleural effusions >500 ml in patients receiving mechanical ventilation. Chest. 2005;127:224–32. doi: 10.1378/chest.127.1.224. [DOI] [PubMed] [Google Scholar]
- 13.Kearney SE, Davies CWH, Davies RJO, Gleeson FV. Computed tomography and ultrasound in parapneumonic effusions and empyema. Clin Radiol. 2000;55:542–7. doi: 10.1053/crad.1999.0480. [DOI] [PubMed] [Google Scholar]
- 14.Usta E, Mustafi M, Ziemer G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact CardioVasc Thorac Surg. 2010;10:204–7. doi: 10.1510/icvts.2009.222273. [DOI] [PubMed] [Google Scholar]