Abstract
OBJECTIVES
Pulmonary metastasectomy is firmly established in the multidisciplinary management of patients with advanced sarcomas. While the number of metastases, completeness of resection, disease-free interval and grading of the primary sarcoma are well established prognostic factors in metastatic surgery, histological parameters are not widely evaluated. The objective of the present study was to evaluate the prognostic impact of intrapulmonary growth patterns of sarcoma metastases.
METHODS
We retrospectively analysed the clinicopathological characteristics of 52 sarcoma patients who underwent surgical resection of lung metastases at our centre from January 2006 to January 2009. The histological growth characteristics of all 261 metastases have been categorized and published previously. ‘Interstitial growth’ was defined as a diffuse spread of the sarcoma cells into the alveolar septae at the transition of the metastasis to the normal lung tissue and was found to be prognostic. ‘Pleural penetration’ was defined as the infiltration and destruction of all visceral pleural layers by the tumour and was found to be a risk factor for local recurrence.
RESULTS
The median post-metastasectomy overall survival was 50.3 months, and the corresponding 5-year survival rate was 44.7%. Age >55 years at metastasectomy (P = 0.02), the presence of interstitial growth (P = 0.008), size of the largest metastasis >35 mm (P = 0.023) and the presence of tumour recurrence at any site after metastasectomy (P < 0.001) were identified as risk factors for death. Pleural penetration (P = 0.007) and size of the metastasis >5 mm were found to be risk factors for local intrapulmonary recurrence.
CONCLUSIONS
Interstitial tumour growth, which is easily detected by light microscopy, can serve as a strong predictor of survival following pulmonary metastasectomy in sarcoma patients. Obvious pleural infiltration indicates the need for larger margins.
Keywords: Metastasectomy, Sarcoma metastasis, Growth patterns, Prognostic factors
INTRODUCTION
Sarcomas are a group of rare malignancies that can develop in almost any mesenchymal tissue. The histopathological growth patterns of the periphery of primary musculoskeletal sarcomas and their implications for resection have been well described [1, 2]. In contrast to the primary sarcoma, which is surrounded by the solid tissue that provides a certain barrier to uncontrolled local growth, sarcomatous lung metastases are surrounded by soft, air-containing and sponge-like tissue. Based on this obvious tissue difference, we hypothesized that lung metastases from sarcoma disseminate in a different way from their primaries, and this different way could be prognostic. The pathological criteria and description of growth patterns of a series of 52 sarcoma patients undergoing resection of lung metastases have recently been published [3]. To our knowledge, there is only one further description of sarcoma metastasis growth in the lung, which is restricted to the subtype ‘liposarcoma’ [4]. Furthermore, there are a number of prognostic factors described for pulmonary sarcoma metastases, such as disease-free interval (DFI) [5–8], completeness of resection [5, 6, 9, 10], number of metastases [5], aggressive growth pattern in liposarcoma metastases [4] and progression after chemotherapy [7]. To evaluate the prognostic significance of growth patterns of sarcoma metastases in the lung, we initiated this survival and local intrapulmonary recurrence analysis.
MATERIALS AND METHODS
We retrospectively analysed the medical records of patients who were completely resected for histologically proven pulmonary metastases derived from sarcomas at our centre. For this study, we retrospectively analysed data from 52 patients who were operated on in our department from January 2006 to January 2009. The medical records were reviewed for current biographical and survival information. In eight cases, missing data were derived from the registry offices. Until March 2012, all cases with repeated pulmonary metastasectomies were reviewed for local intrapulmonary recurrence. All metastasectomies were complete resections with no macroscopic residual tumour at the end of the procedure. All resections were conducted as antero-lateral muscle sparing thoracotomy to allow for a systematic palpation of the lung. All lung resections were performed according to the surgeon's discretion either as wedge resection or segmentectomy. The microscopic description of growth patterns was categorized as published previously [3]. Interstitial growth was defined as a diffuse growing of sarcoma cells into the alveolar septae and a tumour spreading like fingers into the surrounding tissue, visible at the surface of the lung metastases (Fig. 1). Patients with multiple metastases were counted positive when at least one lesion had obvious interstitial growth all over or more than one lesion had some areas with interstitial growth. Furthermore, the presence of a circular fibrous pseudocapsule, satellite nodules and a pleural penetration, the invasion of greater blood vessels and the presence of lymphangitic spread were microscopically evaluated in all metastases earlier [3]. To examine for prognostic differences, three sarcoma subgroups were categorized: osteogenic sarcomas, myogenic sarcomas and other soft-tissue sarcomas (Table 1). Local intrapulmonary recurrence was defined as a proven metastasis resected from the same segment and associated with scar tissue at the metastasis, visceral pleural thickening over the metastasis, metal cramps in the tumour after previous stapler resection or residual thread material on histological examination.
Figure 1:
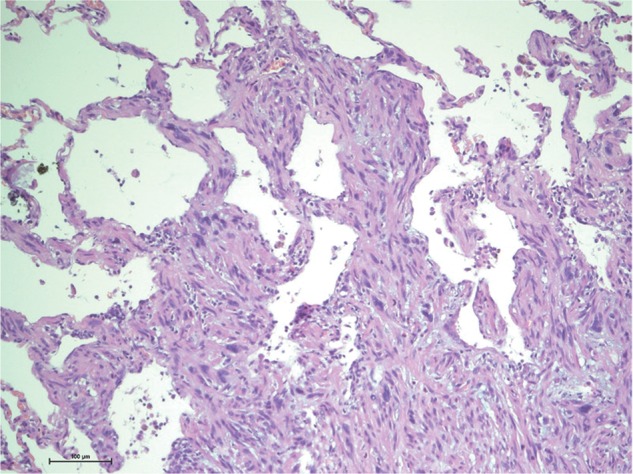
Interstitial growth at the surface of a leiomyosarcoma metastasis. Ten-fold magnification of a haematoxylin–eosin stained section of a leiomyosarcoma metastasis. The tumour grows into the alveolar septae and spreads like fingers into the surrounding lung tissue. This ‘interstitial growth’ seems to be an expression of aggressive local growth and is associated with impaired survival (P = 0.008).
Table 1:
Patient characteristics: number and size of the metastases in three different sarcoma subgroups
| Cases/category | Histology | Number of metastases (range) | Median diameter of largest metastasis [mm] (range) |
|---|---|---|---|
| 8/Osteogenic sarcomas | Chondrosarcoma, osteosarcoma, Ewing's sarcoma | 21 (1–9) | 52.5 (17–96) |
| 24/Soft-tissue sarcomas | Angiosarcoma, fibrosarcoma, clear cell sarcoma, liposarcoma, sarcoma (NOS), synovial sarcoma | 99 (1–16) | 24.5 (4–80) |
| 20/Myogenic sarcomas | Leiomyosarcoma, other myogenic sarcoma, malignant peripheral nerve sheath tumour | 141 (1–29) | 19.5 (6–115) |
Statistical analysis
Data were analysed using SPSS 15 for Windows (SPSS Inc., Chicago, IL, USA). Survival was estimated by the actuarial method derived from the Kaplan–Meier method using the date of primary sarcoma resection and the lung metastasectomy as the starting point. The prognostic influence of variables on survival was analysed using the log-rank test and differences between proportions were calculated with the χ2 test or with the Fisher's exact test when appropriate. A probability value of 0.05 or less was considered significant.
RESULTS
Patient characteristics
The patients' characteristics are listed in Table 1. Twenty-three women and 29 men had a median age of 55.8 years (19.1–80.3 years). The median DFI was 33.6 months (0–248, mean 51.6), and the site of the primary tumour was the uterus in 8, the trunk in 19 and the extremeties in 25 patients. Altogether, 261 metastases with a median of 3 per patients (mean 5.0, range 1–29) had been removed in 89 operations. The median diameter of the malignant lung nodules was 25 mm (mean 35, range 4–115). Referring to the first metastasectomy, 26 patients had a single metastasis, and at the end of the study period, 20 of 52 remained with a single metastasis only.
Fifteen operations were repeated resections. Five patients had their first metastasectomy prior to the study period; hence, the evaluated metastases were specimen resected during a repeated metastasectomy. Thirty-seven patients (71.2%) had tumour recurrence at any site in the course of their disease and 15 (26.9) were identified until March 2012 with 29 local intrapulmonary recurrences. On microscopical re-examination of 261 metastases, incomplete resection margins were found in 63 (24.1%) lesions in 14 of the 52 patients (26.9%). Interstitial spread, defined as diffuse growth in the alveolar wall at the surface of the metastasis, occurred in 104 (39.8%) lesions affecting 26 (50%) patients. The presence of interstitial spread was noted in 8 of 20 (40%) patients with myogenic sarcoma, 3 of 8 (37.5%) with osteogenic sarcoma and 15 of 24 (62.5%) with soft-tissue sarcoma. A microscopically smooth surface lacking interstitial spread, satellite nodules, vascular infiltration and lymphangitic spread was found in 127 (48.7%) lesions in 13 of the 52 (25%) patients. Eighteen patients (34.6%) had two to five further pulmonary metastasectomies. Fifteen of these patients were identified, with altogether 118 metastases, of which 29 were local intrapulmonary recurrences that were treated after a median of 15 months (4–57 months). The metastases preceding local recurrence had low, median and high mitotic activity (0–9, 10–19, >19 mitoses per 10 high-power fields [HPF]) in 2, 8 and 5 patients. The mode of resection preceding the local intrapulmonary recurrence was a wedge resection in all but one, which was a lobectomy. Prior to local recurrence, incomplete resection (R1) was described for 3 and marginal R0 (0–2 mm) resection for 21 metastases. The diameter of the metastases preceding local intrapulmonary recurrence was a median of 10 mm (3–65). Pleural penetration, defined as infiltration and destruction of all visceral pleural layers by the tumour, was found in 67 of 261 (25.7%) lesions and in 10 of 29 (34.5%) metastases preceding local recurrence.
The median overall survival was updated in October 2011 and was 82.9 months (95% confidence interval [CI]: 0–165) after resection of the primary sarcoma with a 5-year survival rate of 60.7%. The overall median post-metastasectomy survival was 50.3 months (95% CI: 30–71) and the corresponding 5-year survival rate was 44.7%. Age >55 years at metastasectomy (P = 0.022), interstitial growth pattern of the metastasis (P = 0.008), size of the largest metastasis >35 mm (P = 0.023), microscopically incomplete resection (R1) (P = 0.017) and the presence of tumour recurrence at any site after metastasectomy (P < 0.001) were risk factors for death (Tables 2 and 3). No significant survival differences were found between the three sarcoma categories. The group of patients (n = 12) with a low mitotic index (1–9/10 HPF) of their metastases had 66.7% censored cases and thus, median survival could not be calculated. The mean survivals for patients with a low, medium and high mitotic index were 120, 38 and 44 months, respectively. Interstitial growth affected metastases in 26 patients, of whom only 6 were censored cases. By contrast, 17 of 26 patients without interstitial growth were censored. The 5-year survivals with and without the presence of interstitial growth were 27.8 and 64.6%, but the median survival could not be indicated for the latter subgroup (Fig. 2).
Table 2:
Prognostic factors: clinicopathological
| Factor | Number of patients | Five-year survival (%) | Median survival (months) | Prognostic significance |
|---|---|---|---|---|
| Size of largest metastasis | ||||
| ≤35 mm | 34 (65.4%) | 58.8 | n.p. | |
| >35 mm | 18 (34.6%) | 22.2 | 23.1 | P = 0.023 |
| Number of metastases at first metastasectomy | ||||
| 1 | 26 (50.0%) | 47.5 | 53.3 | |
| 2 | 12 (23.0%) | 38.1 | 37.6 | *P = 0.800 |
| 3 | 7 (13.5%) | 57.1 | 62.7 | *P = 0.819 |
| 4 or more | 7 (13.5%) | 28.6 | 17.5 | *P = 0.113 |
| Cumulative number of operations | ||||
| 1 | 29 (55.8%) | 40.3 | 42.6 | |
| 2 | 16 (30.8%) | 45.1 | 53.3 | P = 0.474 |
| 3 or more | 7 (13.4%) | 57.1 | 70.8 | P = 0.416 |
| Location of the primary | ||||
| Uterus | 8 (15.4%) | 33.3 | 40.1 | |
| Extremities | 25 (48.1%) | 49.8 | 53.3 | P = 0.715 |
| Trunk | 19 (36.5%) | 43.4 | 42.6 | P = 0.970 |
| Sarcoma category | ||||
| Osteogenic | 8 (15.4%) | 58.3 | n.p. | |
| Myogenic | 20 (38.5%) | 44.1 | 50.3 | P = 0.222 |
| Soft tissue | 24 (46.1%) | 37.5 | 25.0 | P = 0.091 |
| Age | ||||
| ≤55 years | 24 (46.2%) | 57.3 | 70.8 | |
| >55 years | 28 (53.8%) | 34.1 | 25.0 | P = 0.022 |
| All patients | 52 (100%) | 44.7 | 50.3 | |
n.p.: Not possible.
*Probabilities are calculated vs 1 metastasis.
Probabilities are calculated vs osteogenic sarcomas.
Table 3:
Prognostic factors: histomorphological
| Histological growth patterns | Number of patients | Five-year survival (%) | Median survival (months) | P-value |
|---|---|---|---|---|
| Mitoses/10 HPF (mitotic index) | ||||
| 1–9 | 12 | 60.8 | n.p. | |
| 10–19 | 16 | 42.9 | 40 | *0.125 |
| >19 | 24 | 40.4 | 31 | *0.066 |
| Presence of a pseudocapsule | ||||
| Yes | 11 | 63.6 | n.p. | |
| No | 41 | 39.6 | 42 | 0.198 |
| Resection margins | ||||
| R0 | 36 | 59.8 | 71 | |
| R1 | 16 | 10.9 | 25 | 0.017 |
| Pleural penetration | ||||
| No | 34 | 50.9 | 63 | |
| Yes | 18 | 32.4 | 38 | 0.330 |
| Vascular infiltration | ||||
| No | 29 | 54.6 | 63 | |
| Yes | 23 | 33.1 | 35 | 0.070 |
| Interstitial growth | ||||
| No | 26 | 64.6 | n.p. | |
| Yes | 26 | 27.8 | 34 | 0.008 |
| Tumour satellites | ||||
| No | 33 | 40.5 | 71 | |
| Yes | 19 | 50.7 | 43 | 0.707 |
| Lymphangitic spread | ||||
| No | 44 | 43.5 | 50 | |
| Yes | 8 | 50 | 40 | 0.701 |
| Signs of tumour regression | ||||
| No | 33 | 52.8 | 63 | |
| Yes | 19 | 18.9 | 35 | 0.272 |
n.p.: Not possible; HPF: high-power fields.
*Probabilities are calculated vs (1–9).
Figure 2:
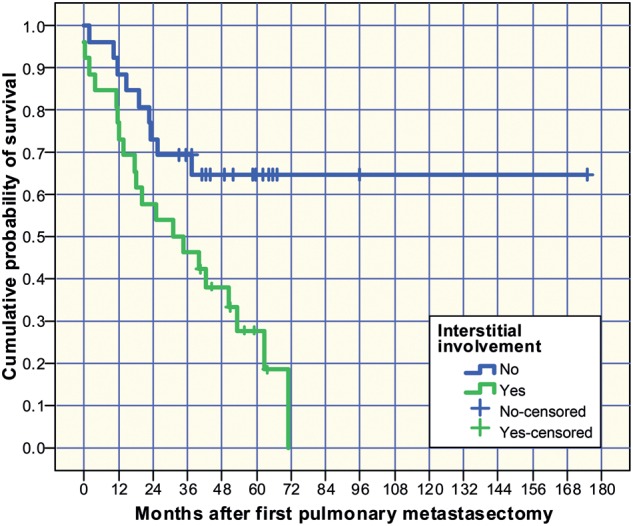
Kaplan–Meier survival estimation depending on the presence of interstitial growth described microscopically as a sign of invasive local growth.
A higher number of mitoses per 10 HPF (>19 vs <10) in the lung metastasis (P = 0.066) and vascular infiltration (P = 0.070) showed a trend towards reduced survival. In contrast, the cumulative number of metastases, primary histology, grading of the primary tumour, number of repeated lung resections, the histological growth patterns: presence of a pseudocapsule, tumour regression, vascular infiltration, tumour satellite nodules, pleural penetration and lymphangitic spread did not reach significance at univariate analysis (Tables 2 and 3). Pleural penetration (P = 0.007), partial tumour regression (P = 0.01) and size of the metastasis >5 mm (P = 0.011) were significant risk factors for local intrapulmonary recurrence (Table 4).
Table 4:
Risk factors for local intrapulmonary recurrence
| Significance P-value | |
|---|---|
| Pleural penetration | 0.007 |
| Infiltration of blood vessels | 0.536 |
| Lymphangitic spread | 0.977 |
| Interstitial spread | 0.318 |
| Signs of regression | 0.01 |
| Tumour satellites | 0.582 |
| Margins ≤2 mm | 0.105 |
| Metastasis diameter >5 mm | 0.015 |
| Metastasis diameter >10 mm | 0.052 |
| Metastasis diameter >15 mm | 0.075 |
Twenty-nine of 118 metastases resected from 14 patients preceded a proved local intrapulmonary recurrence.
DISCUSSION
Pulmonary metastasectomy is widely accepted in the multidisciplinary management of patients with oligometastases from different sarcoma subtypes. It is considered potentially beneficial and is associated with 5-year survival rates of 25–38% [5, 10–12]. The most important factor that determines the outcome in these patients is complete resection of all metastases [5, 6, 9, 10]. In addition, we could demonstrate in this series that even microscopically incomplete resection of pulmonary metastases was associated with an impaired median survival of 30.8 vs 62.7 months when resection margins were microscopically clear (P = 0.057). An increased risk of microscopically incomplete margins has been described, for interstitial growth with borderline significance (P = 0.06) [3]. The fact that microscopically incomplete resection leads to a higher rate of local recurrence has been described for primary sarcomas [1, 2, 13, 14], and for pulmonary metastases once [12]. As in the latter report, the size of the largest metastasis was a prognostic factor in our series. Patients with a largest metastasis >35 mm had a higher mortality risk (P = 0.023) than those with smaller lesions. A similar observation was described by Weiser et al. [15], with a cut-off value of 20 mm in the diameter of the largest metastasis. However, looking only at tumour size might be misleading. Multiple small metastases may add up to a higher ‘tumour load’ than one large metastasis. Thus, tumour size has to be always interpreted on the number of metastases. In our series, 18 of 52 (34.6%) patients had a largest metastasis >35 mm and 11 of the 18 had a single lesion only. A correlation of greater metastases (>35 mm) with the presence of interstitial spread was described earlier [3] and underlines the need for wide resection margins during the resection of lager metastases.
Nonanatomical resection is the standard procedure to remove pulmonary metastases. In our series, resections were performed with electrocautery, lasers or staplers, depending on the decision of the surgeon. Wedge resection (28 of 29) and marginal or microscopically incomplete resection (24 of 29) are, beyond doubt, risk factors for local recurrence in our series. Microscopically clear margins are sometimes difficult to maintain for all lesions, especially when the preservation of lung tissue is critical. The rather high rate of histologically incomplete margins in at least one lesion in 14 of 52 (26.9%) patients exposes the difficulties of wedge resections. To prevent incomplete margins, we believe that the knowledge about histological growth patterns is an important basis for resection planning. The growth patterns of the metastases in our series have been described recently [3]. We demonstrated that pleural penetration (P = 0.03), metastasis diameter >35 mm (P = 0.02) and interstitial growth (P = 0.06) were associated with an increased risk of incomplete resection, which again is a well-known risk factor for local recurrence.
For the present report, the survival information of our case series was updated, and the prognostic factors were calculated particularly focussing on histological growth patterns. To our knowledge, there is only one publication describing the prognostic influence of certain patterns of metastatic growth in the lung: Nicolas et al. [4] published a series of 24 cases with liposarcoma metastases in the lung in which they observed a correlation between aggressive histological patterns in the lung and an inferior disease outcome. At the border between lung tissue and metastasis, they observed invading tumours, dispersed single cells or tight clusters.
A similar observation was made in our series: interstitial growth as an expression of the invading tumour was significantly associated with reduced survival (P = 0.008; Table 3). Before initiating this study, we hypothesized that aggressive patterns of metastatic growth should lead to a higher incidence of incomplete resection, higher rate of local recurrence and impaired survival. Surprisingly, only interstitial growth proved to be a strong predictor of survival. The survival curves of all other patterns of aggressive growth (pleural penetration, vascular infiltration, satellite nodules and lymphangitic spread) showed impaired survival, but they did not reach significance. A greater sample size might discover other growth patterns being risk factors or prognosticators as well. Furthermore, the exact allocation of metastases preceding local intrapulmonary recurrence in this series discovered ‘Pleural Penetration and Partial tumour regression’ as the only growth pattern associated with a significantly increased risk of recurrence. One explanation might be the broad discoidal spread within the pleura that can exceed the palpable diameter of the metastasis. ‘Regression’ was found in several metastases after chemotherapy. Why its presence leads to a higher rate of local recurrence is unclear. At least, it could be noticed that regressive areas were always localized in the middle of the lesions. The surface might consist of chemoresistant cells, which might be more aggressive.
High-grade primary sarcomas are well known to be associated with an increased risk of local recurrence [2], increased incidence of pulmonary metastases [9] and reduced survival [16, 17]. Once pulmonary metastases are present, a higher mitotic index (>19/10 HPF) tends to be related to an impaired prognosis, as demonstrated in our study (mean survival 45 vs 120 months, P = 0.066).
Lymph node involvement is another prognostic factor first described for sarcoma lung metastases by Pfannschmidt et al. [18]. Performing a radical lymphadenectomy in 69 cases with pulmonary metastases from sarcomas, they found 23.2% lymph node involvement. By contrast, we performed lymph node sampling only, which failed to reveal involved nodes in this series. However, 15.4% patients had at least one metastasis with lymphangitic spread.
Study limitations
The limitations of this study are its retrospective design of a single centre, the relatively small sample size and 26 of the 52 (44%) censored cases. However, the median follow-up of all censored cases was 40.2 (33.1–174.9) months, which makes it clear that even the death of several patients in the near future would not significantly change the survival results.
CONCLUSION
Besides, the well known prognostic factors such as completeness of resection, number of metastases and higher age after resection of pulmonary metastases from sarcomas, a simple description of interstitial growth at the surface of the metastasis can predict an increased risk of death and a description of microscopically visible pleural penetration, which means for the surgeon, superficial pleura-attached metastases are associated with an increased risk of intrapulmonary recurrence and need larger lateral resection margins.
Conflict of interest: none declared.
REFERENCES
- 1.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–20. [PubMed] [Google Scholar]
- 2.Enneking WF, Spanier SS, Malawer MM. The effect of the anatomic setting on the results of surgical procedures for soft parts sarcoma of the thigh. Cancer. 1981;47:1005–22. doi: 10.1002/1097-0142(19810301)47:5<1005::aid-cncr2820470532>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Welter S, Grabellus F, Bauer S, Schmid KW, Stamatis G, Tötsch M. Growth patterns of lung metastases from sarcomas. Virchows Arch. 2011;459:213–9. doi: 10.1007/s00428-011-1116-8. [DOI] [PubMed] [Google Scholar]
- 4.Nicolas M, Moran CA, Suster S. Pulmonary metastasis from liposarcoma. A clinicopathologic and immunohistochemical study of 24 cases. Am J Clin Pathol. 2005;123:265–75. doi: 10.1309/blym-30cd-jjc6-g1nq. [DOI] [PubMed] [Google Scholar]
- 5.Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 6.Smith R, Pak Y, Kraybill W, Kane JM. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol. 2009;35:356–61. doi: 10.1016/j.ejso.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Stephens EH, Blackmon SH, Correa AM, Roth JA, Rice DC, Hofstetter W, et al. Progression after chemotherapy is a novel predictor of poor outcomes after pulmonary metastasectomy in sarcoma patients. J Am Coll Surg. 2011;212:821–6. doi: 10.1016/j.jamcollsurg.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 8.García Franco CE, Torre W, Tamura A, Guillén-Grima F, San-Julian M, Martin-Algarra S, et al. Long-term results after resection for bone sarcoma pulmonary metastases. Eur J Cardiothorac Surg. 2010;37:1205–8. doi: 10.1016/j.ejcts.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Billingsley KG, Burt ME, Jara E, Ginsberg RJ, Woodruff JM, Leung DH, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg. 1999;229:602–10. doi: 10.1097/00000658-199905000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfannschmidt J, Klode J, Muley T, Dienemann H, Hoffmann H. Pulmonary metastasectomy in patients with soft tissue sarcomas: experiences in 50 patients. Thorac Cardiovasc Surg. 2006;54:489–92. doi: 10.1055/s-2006-924248. [DOI] [PubMed] [Google Scholar]
- 11.Rehders A, Hosch SB, Scheunemann P, Stoecklein NH, Knoefel WT, Peiper M. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Arch Surg. 2007;142:70–5. doi: 10.1001/archsurg.142.1.70. [DOI] [PubMed] [Google Scholar]
- 12.van Geel AN, Pastorino U, Jauch KW, Judson IR, van Coevorden F, Buesa JM, et al. Surgical treatment of lung metastases: the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group Study of 255 patients. Cancer. 1996;77:675–82. doi: 10.1002/(sici)1097-0142(19960215)77:4<675::aid-cncr13>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Sampo M, Tarkkanen M, Huuhtanen R, Tukiainen E, Bohling T, Blomqvist C. Impact of the smallest surgical margin on local control in soft tissue sarcoma. Br J Surg. 2008;95:237–43. doi: 10.1002/bjs.5906. [DOI] [PubMed] [Google Scholar]
- 14.Gerrand CH, Wunder JS, Kandel RA, O'Sullivan B, Catton CN, Bell RS, et al. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br. 2001;83:1149–55. doi: 10.1302/0301-620x.83b8.12028. [DOI] [PubMed] [Google Scholar]
- 15.Weiser MR, Downey RJ, Leung DH, Brennan MF. Repeat resection of pulmonary metastases in patients with soft-tissue sarcoma. J Am Coll Surg. 2000;191:184–90. doi: 10.1016/s1072-7515(00)00306-9. [DOI] [PubMed] [Google Scholar]
- 16.Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol. 2006;130:1448–53. doi: 10.5858/2006-130-1448-GOSTSR. [DOI] [PubMed] [Google Scholar]
- 17.Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchere D, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91:1914–26. doi: 10.1002/1097-0142(20010515)91:10<1914::aid-cncr1214>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Pfannschmidt J, Klode J, Muley T, Dienemann H, Hoffmann H. Nodal involvement at the time of pulmonary metastasectomy: experiences in 245 patients. Ann Thorac Surg. 2006;81:448–54. doi: 10.1016/j.athoracsur.2005.08.049. [DOI] [PubMed] [Google Scholar]


