Abstract
OBJECTIVES
Although lung transplantation is an accepted therapy for end-stage disease, recipient outcomes continue to be hindered by early primary graft dysfunction (PGD) as well as late rejection and bronchiolitis obliterans syndrome (BOS). We have previously shown that the pro-inflammatory cytokine response following transplantation correlates with the severity of PGD. We hypothesized that lung-transplant recipients with an increased inflammatory response immediately following surgery would also have a greater incidence of unfavorable long-term outcomes including rejection, BOS and ultimately death.
METHODS
A retrospective study of lung-transplant recipients (n = 19) for whom serial blood sampling of cytokines was performed for 24 h following transplantation between March 2002 and June 2003 at a single institution. Long-term follow-up was examined for rejection, BOS and survival.
RESULTS
Thirteen single and six bilateral lung recipients were examined. Eleven (58%) developed BOS and eight (42%) did not. Subgroup analysis revealed an association between elevated IL-6 concentrations 4 h after reperfusion of the allograft and development of BOS (P = 0.068). The correlation between IL-6 and survival time was found to be significant (corr = −0.46, P = 0.047), indicating that higher IL-6 response had shorter survival following transplantation.
CONCLUSIONS
An elevation in interleukin (IL)-6 concentration immediately following lung transplantation is associated with a trend towards development of bronchiolitis obliterans, rejection and significantly decreased survival time. Further studies are warranted to confirm the correlation between the immediate inflammatory response, PGD and BOS. Identification of patients at risk for BOS based on the cytokine response after surgery may allow for early intervention.
Keywords: Transplantation, Lung transplantation, Lung other, Inflammation
INTRODUCTION
Pulmonary transplantation has become the standard of care for many causes of end-stage lung disease [1]. However, lung-transplant recipients can suffer from early primary graft dysfunction (PGD) as well as long-term complications including allograft rejection and the development of bronchiolitis obliterans syndrome (BOS) leading to death [2]. Chronic rejection after lung transplantation is manifested by BOS, a chronic inflammatory and fibroproliferative condition in small airways [3]. Numerous studies have shown that PGD occurs in as many as 10–25% of lung-transplant recipients [4, 5]. And importantly, PGD has been associated with BOS, the greatest source of long-term mortality in lung-transplant patients. Further studies have shown that PGD is associated with an increased risk of BOS and this risk is directly related to the severity of PGD [6, 7].
Prior work at our institution has shown that the cytokine response in the first 24 h immediately following transplantation correlates with the severity of PGD following transplantation [8]. Others have also shown elevations in IL-6, 8 and 10 following transplantation [9–11]. Kaneda et al. have even shown that pre-implantation cytokine expression in donor lung grafts can be used to predict long-term survival after lung transplantation in humans [12].
The purpose of this study was to examine the long-term implications of elevated pro-inflammatory cytokine response immediately following lung transplantation. We hypothesized that lung-transplant recipients who displayed an increased inflammatory response following surgery (as shown by elevations in IL-6) would have a greater incidence of unfavourable long-term outcomes including the development of BOS, chronic allograft rejection and ultimately death.
MATERIALS AND METHODS
After Institutional Review Board approval, we performed a retrospective study of lung-transplant patients for whom serial blood sampling had been done examining the relationship between the inflammatory response and PGD at our institution [8]. This blood sampling regimen is not part of standard management of patients and had been specifically done for a limited set of patients for a limited time as part of a prior study. These patients (n = 21) had undergone single or bilateral lung transplantation between March 2002 and June 2003. One patient was excluded for perioperative mortality and another because he developed pneumonia, disqualifying him from the International Society of Heart and Lung Transplantation (ISHLT) criteria for PGD grading [13]. With the exception of serial blood sampling to measure the cytokine response, there was no deviation from our standard management of lung-transplant recipients.
Cytokines
Blood samples were obtained from a pulmonary arterial line and systemic arterial line to determine the difference in cytokine concentrations before and after perfusing the allograft. Samples were drawn baseline upon arrival in the operating room 5 min after cross-clamp release or separation from cardiopulmonary bypass (reperfusion) and at 4, 8 and 24 h after reperfusion. In the case of bilateral sequential lung transplantation, ischemia times were determined and samples obtained after the second lung was reperfused.
The cytokines examined included IL-6, a generalized marker of inflammation; IL-8, a chemokine responsible for neutrophil recruitment and activation and IL-10, a counter-regulatory cytokine involved in suppression of the pro-inflammatory response. In our prior work, IL-6, a generalized marker of the pro-inflammatory response, showed the greatest correlation with severity of PGD and was chosen as the basis for this current study focusing on the long-term outcomes. Additional results for the other measured cytokines were published in the prior report [8]. Samples were collected in ethylenediaminetetraacetic acid, placed immediately on ice, and centrifuged at 3000 rpm (4°C) for 10 min. Supernatant was withdrawn and frozen at −70°C until samples were analyzed for cytokine concentrations using enzyme-linked immunosorbent assay kits (R&D systems, Minneapolis, MN).
Definition of primary graft dysfunction
PGD was defined and characterized based on clinical indices of oxygenation status and radiographic appearance of lung fields according to the grading system put forth by the ISHLT working group on primary lung graft dysfunction in 2005 [13]. Patients were divided into those with no lung injury (Grade 0 or 1 PGD), moderate injury (Grade 2 PGD) or severe injury (Grade 3 PGD). Moderate injury, Grade 2 PGD, was defined as (i) PaO2/FiO2 (fraction of inspired oxygen) ratio <300 within 72 h after reperfusion (PaO2 determined from systemic arterial blood gas measurements); and (ii) radiographic infiltrates consistent with pulmonary oedema. Severe injury, Grade 3 PGD, was defined as (i) PaO2/FiO2 ratio <200 within 72 h after reperfusion; and (ii) radiographic infiltrates consistent with pulmonary oedema. Any patient on extracorporeal oxygenation was automatically Grade 3. Any subject mechanically ventilated with FiO2 >0.5 on nitric oxide beyond 48 h from the time of transplant was considered to be Grade 3. If multiple blood gas values were available, the worst PaO2/FiO2 ratio following transfer to the ICU was used for the purpose of the grading scheme. For the purposes of this study, patients with a PaO2/FiO2 ratio >300 (Grade 0 or 1 PGD) were termed ‘not injured.’
Clinical follow-up and diagnosis
Postoperatively, the transplant patients were followed at our institution's pulmonary transplant clinic with forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) spirometry readings. Since the patients included in this study received their transplant in 2002 and 2003, we were able to obtain follow-up data for up to 8 years. BOS developed in some recipients who were then re-transplanted; therefore the spirometry readings were used for the most recent date prior to re-transplantation. BOS was characterized and diagnosed according to the ISHLT criteria for BOS grading; according to the most recent 2001 revision, BOS Stage 1 is defined as a decline in FEV1 from a stable post-transplant baseline by >20% [14]. Distinctions between successive classes of BOS were not included for the purposes of this study. Rejection was defined according to the recommendations of the Lung Rejection Study Group of the ISHLT as an immunologically mediated dysfunction of the transplanted organ requiring increased immunosuppression. This was diagnosed on clinical, physiological and/or histological findings in all cases. Histological data were not routinely available for all patients and thus are not presented.
Statistical analysis
Clinical variables were recorded using a Microsoft Excel database (Microsoft, Redmond, WA) and included allograft ischemia times, cardiopulmonary bypass, ratios of arterial oxygen pressure tension (PaO2) levels and FiO2; pulmonary artery and systemic haemodynamics; radiographic findings of reperfusion injury; the number of hours of required mechanical ventilation after lung transplantation; date of death (if applicable) and cause of death. Clinical notes from follow-up visits to our institution's pulmonary transplant clinical were used to identify the development of BOS, allograft rejection, spirometry readings including FEV1 and FVC, and survival.
Cytokine levels were log-transformed for computing correlation and regression models due to the substantial right skew of the distribution across subjects. Correlations between cytokine levels and other continuous variables (e.g. PaO2/FiO2) were computed using Pearson's correlations and between cytokine levels and ordinal variables (e.g. PGD) and censored variables (survival time) were computed using Spearman's correlation. The relationship between (log) cytokine levels and the binary outcomes [BOS and acute cellular rejection (ACR)] was characterized by odds ratios from fitting logistic regression models with log cytokine levels as the covariate. The relationship between (log) cytokine levels and survival was characterized by hazard ratio from fitting a Cox model with log cytokine levels as the covariate. All tests with resulting P-values <0.05 were considered to be statistically significant. All statistical analyses were conducted using SAS.
RESULTS
Patient demographics and preoperative diagnosis
There were a total of 19 patients (12 males and 7 females) with an average age of 44.9 years at the time of transplantation. Thirteen patients underwent single lung orthotopic lung transplantation and six underwent bilateral sequential lung transplantation. Demographic information about the patients is displayed in Table 1 with the patients divided into three approximately equally-sized groups, those with a 4-h post-transplant IL-6 value <500 pg/ml, between 500 and 1500 pg/ml and >1500 pg/ml. The specific patient demographics and preoperative diagnoses, as well as perioperative results, have been discussed in further detail previously [8].
Table 1:
Demographic variables and short-term outcomes of patients separated based on IL-6 response
| Patient variables | IL-6 <500 pg/mla | IL-6 between 500 and 1500 pg/mla | IL-6 >1500 pg/mla | All |
|---|---|---|---|---|
| Number of patients | 6 | 6 | 7 | 19 |
| Age (years) at transplant: mean (SD) | 46.5 (±13.6) | 51.3 (±14.7) | 38.1 (±17.6) | 44.9 (±16) |
| Sex, male: n (%) | 4 (67%) | 5 (83%) | 3 (43%) | 12 (63%) |
| Preoperative diagnosis | ||||
| Obstructive disease | 3 (50%) | 2 (33%) | 2 (29%) | 7 (37%) |
| Restrictive disease | 1 (17%) | 2 (33%) | 1 (14%) | 4 (21%) |
| Cystic fibrosis | 3 (50%) | 1 (17%) | 0 (0%) | 4 (21%) |
| Pulmonary hypertension | 0 (0%) | 0 (0%) | 3 (43%) | 3 (16%) |
| Re-transplantation? (%) | 4 (67%) | 0 (0%) | 2 (29%) | 6 (32%) |
| Bilateral transplantation: n (%) | 1 (17%) | 1 (17%) | 4 (57%) | 6 (32%) |
| Ischaemic time (min): mean (SD)b | 253.7 (±57.5) | 221.7 (±100.8) | 301.4 (98.1) | 265.3 (±82.1) |
| Cardiopulmonary bypass: n (%) | 2 (33%) | 0 (0%) | 4 (57%) | 6 (32%) |
| Perioperative blood transfusions: n (%) | 2 (33%) | 0 (0%) | 1 (14%) | 3 (16%) |
| Postoperative pneumonia: n (%) | 3 (50%) | 2 (33%) | 2 (29%) | 7 (37%) |
| Postoperative CMV infection: n (%) | 2 (33%) | 2 (33%) | 3 (43%) | 7 (37%) |
| PaO2/FiO2 ratio: mean, (SD)c | 334 (±45.8) | 346 (±212) | 222 (±99.4) | 297 (±141) |
| Average PGD Grade (SD) | 0.67 (±0.81) | 1.33 (±1.36) | 2.28 (±0.75) | 1.47 (±1.17) |
pg: picograms; ml: millilitres; n: number; SD: standard deviation; PaO2: systemic arterial oxygen pressure; FiO2: inspired fraction of oxygen; PGD: primary graft dysfunction; CMV: cytomegalovirus.
aIL-6 levels at t = 4 h post-transplantation surgery were used.
bIn the case of bilateral transplant, the higher ischemic time of the two was used.
cWorst PaO2/FiO2 in the 72 h following transplant was used.
Long-term clinical outcomes and bronchiolitis obliterans syndrome
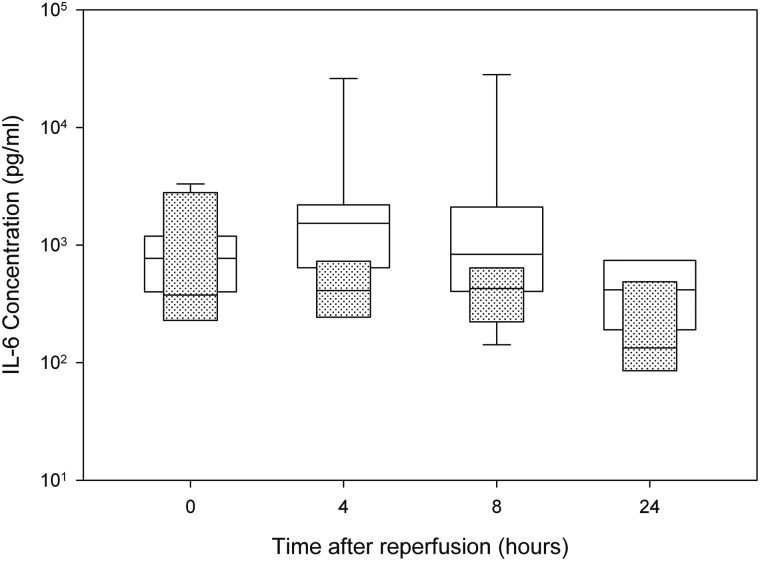
Four-hour postoperative IL-6 levels for lung-transplant recipients were higher in patients who later went on to develop BOS (Fig. 1). The risk of developing BOS before 7 years post-transplant (time from transplant until publication of this study) had a positive relationship with the log of immediate postoperative IL-6 (P = 0.068) with the predicted probability (and 95% confidence intervals) of BOS having the values 0.20 (0.04–0.58), 0.32 (0.12–0.62) and 0.56 (0.28–0.81) for IL-6 levels of 500, 1000 and 1500 pg/ml. Odds ratios for a change in IL-6 from 500 to 1000, 1000 to 1500 and 500 to 1500 were 2.8 (0.93–8.3), 1.8 (0.96–3.5) and 5.1 (0.88–29), respectively; odds ratios for a 50, 200 and 300% change were 0.36 (0.12–1.08), 2.8 (0.93–8.3) and 5.1 (0.88–29), respectively. Logistic regression models were also fit using IL-6 concentrations at the other time points post surgery (all P-values > 0.05).
Figure 1:
Immediate postoperative IL-6 levels (median) in patients who developed BOS (n = 11), and those who did not (n = 8). (White = BOS; Dotted = no BOS; boxes represent medians with 25th and 75th percentile ranges as well as 10th and 90th where available).
Acute rejection
ACR in patients' allografts had a positive relationship with 4-h postoperative IL-6 values (as measured by a logistic regression of ACR on log IL-6 values) with the predicted probability (and 95% confidence intervals) of ACR having the values 0.69 (0.34–0.91), 0.96 (0.46–1.0) and 0.99 (0.44–1.0) for IL-6 levels of 500, 1000 and 1500 pg/ml, respectively (P = 0.08). Odds ratios for rejection for a change in IL-6 from 500 to 1000, 1000 to 1500 and 500 to 1500 were 11.7 (0.72–189), 4.2 (0.83–21) and 49 (0.60–4052), respectively; odds ratios for rejection for a 50, 200 and 300% change in IL-6 levels were 0.09 (0.005–1.4), 11.7 (0.72–189) and 49 (0.60–4052), respectively.
Survival
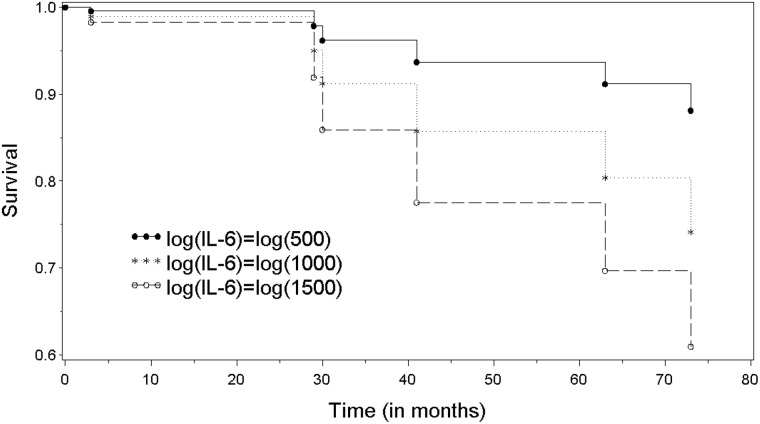
Of the patients involved in the study, 68% (n = 13) were still alive in June 2010. The correlation between IL-6 levels at 4 h postoperatively and survival time (in months) was found to be significant (corr = −0.46, P = 0.047), indicating that higher IL-6 response had shorter survival following transplant. This relationship was also assessed using a Cox model and the relationship was significant (P = 0.013). The hazard ratios for a 50%, 200% or 300% change in IL-6 were 0.42 (0.21–0.83), 2.4 (1.2–4.7) and 3.9 (1.3–11.5), respectively. Kaplan–Meier survival curves for IL-6 concentrations of <500, 500–1500 and >1500 pg/ml are given in Fig. 2.
Figure 2:
Kaplan–Meier survival curves for patients with 4-h post-transplant IL-6 levels of >500 pg/ml (solid, n = 19), >1000 pg/ml (dotted, n = 13) and >1500 pg/ml (dashed, n = 7). The correlation between IL-6 and survival time (in months) indicates that higher IL-6 response had shorter survival following transplant (corr = −0.46, log rank P = 0.047).
DISCUSSION
Chronic rejection of the allograft following lung transplantation often manifests in the form of BOS. Because BOS is the primary cause of late death, there is tremendous interest in a useful biomarker to predict the onset of PGD and later BOS. Prior work at our institution has shown that the immediate cytokine response following transplantation correlates with the severity of PGD [8]. Monitoring the inflammatory response following transplantation might help in better identification and management of patients at risk of developing BOS and chronic rejection. In this study, we found a correlation between elevated IL-6 levels at 4 h post-transplant and length of postoperative survival time, suggesting that IL-6 could serve as a marker for late outcomes.
Acute rejection, PGD, and infection are marked by a vigorous immune and inflammatory response: the lung allograft undergoes a natural process of healing, with consequent tissue remodelling and fibrosis. The resulting heightened inflammatory response leads to increased immunogenicity and subsequent development of fibrosis and BOS [15, 16]. Bharat et al. explored the so-called ‘injury response hypothesis’ and showed that inflammation associated with PGD promotes the development of alloimmunity after lung transplantation, leading to the immunopathogenesis of BOS [17]. Our work expands this theory to link the immediate pro-inflammatory cytokine response to long-term survival.
Increased levels of IL-6 are often found in injured airways and lung tissue after an inflammatory stimulus; however, the functional significance of that finding is unknown at present [11, 18]. We have previously shown that changes in cytokine and leukocyte expression are frequently evident prior to surgery, suggesting a possible patient predisposition to heightened inflammatory response and adverse outcomes [19]. High levels of IL-6 in bronchoalveolar lavage fluid have also been reported in refractory cases of acute rejection [20]. Multiple studies have associated genetic polymorphisms of the IL-6 gene with an earlier onset of bronchiolitis obliterans [9, 11, 15]. A large pro-inflammatory response has also been associated with co-morbidity following complex cardiac surgery as we have previously shown elevated pro-inflammatory cytokine levels correlate with prolonged mechanical ventilation, renal injury and increased ICU stay [21]. These studies support an association between the immediate inflammatory response in lung-transplant recipients and long-term outcomes including the development of BOS and survival.
Identifying patients at risk for worse outcomes offers an opportunity for intervention. Specifically, attenuating the inflammatory response associated with PGD immediately following surgery may improve late outcomes as Kreisel et al. have previously shown that severe PGD is associated with lower survival rates at 1, 5 and 10 years [22].
LIMITATIONS
While this study unfortunately has a number of shortcomings, most notably the small sample size (n = 19) and confounding variables such as indication for transplant and re-transplantation, the results are striking in that they show a link between early inflammation and late events following lung transplantation. In addition to showing a potential trend between IL-6 and the development of BOS and acute rejection, we found a significant association between IL-6 elevation and survival. This study is consistent with the work of others showing that heightened alloimmunity leads to worse prognosis over time [17, 18]. Further studies with larger numbers of patients and more complete multiplex cytokine panels that can measure over 40 cytokines with one serum sample are warranted.
CONCLUSION
In summary, we found an association between the immediate postoperative inflammatory response and late outcomes of lung transplantation. Elevations in IL-6 concentration 4 h following lung transplantation are associated with trends towards higher BOS and rejection, and a greater probability of death. Earlier identification of patients at risk for BOS and rejection of the allograft based on the pro-inflammatory cytokine response following surgery may allow for early intervention. Future efforts should focus on attenuating the pro-inflammatory response following lung reperfusion to minimize early and long-term effects.
FUNDING
This work was supported by a National Institutes of Health (NIH) T35 Training Grant.
Conflict of interest: none declared.
ACKNOWLEDGEMENT
We would like to thank Christina Eagan, RN, BSN, CPTC for her help in gathering essential data for the preparation of the manuscript.
REFERENCES
- 1.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-second official adult lung and heart-lung transplant report–2005. J Heart Lung Transplant. 2005;24:956–67. doi: 10.1016/j.healun.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Zander DS, Baz MA, Visner GA, Staples ED, Donnelly WH, Faro A, et al. Analysis of early deaths after isolated lung transplantation. Chest. 2001;120:225–32. doi: 10.1378/chest.120.1.225. [DOI] [PubMed] [Google Scholar]
- 3.Sato M, Keshavjee S. Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling. Semin Thorac Cardiovasc Surg. 2008;20:173–82. doi: 10.1053/j.semtcvs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Lee JC, Christie JD. Primary graft dysfunction. Proc Am Thorac Soc. 2009;6:39–46. doi: 10.1513/pats.200808-082GO. [DOI] [PubMed] [Google Scholar]
- 5.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Resp Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 6.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Resp Crit Care Med. 2007;175:507–13. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 7.Huang HJ, Yusen RD, Meyers BF, Walter MJ, Mohanakumar T, Patterson GA, et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8:2454–62. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathur A, Baz M, Staples ED, Bonnell M, Speckman JM, Hess PJ, et al. Cytokine profile after lung transplantation: correlation with allograft injury. Ann Thorac Surg. 2006;81:1844–9. doi: 10.1016/j.athoracsur.2005.11.053. discussion 9–50. [DOI] [PubMed] [Google Scholar]
- 9.Mal H, Dehoux M, Sleiman C, Boczkowski J, Leseche G, Pariente R, et al. Early release of proinflammatory cytokines after lung transplantation. Chest. 1998;113:645–51. doi: 10.1378/chest.113.3.645. [DOI] [PubMed] [Google Scholar]
- 10.De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M, et al. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Resp Crit Care Med. 2002;165:211–5. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 11.Pham SM, Yoshida Y, Aeba R, Hattler BG, Iwaki Y, Zeevi A, et al. Interleukin-6, a marker of preservation injury in clinical lung transplantation. J Heart Lung Transplant. 1992;11:1017–24. [PubMed] [Google Scholar]
- 12.Kaneda H, Waddell TK, de Perrot M, Bai XH, Gutierrez C, Arenovich T, et al. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant. 2006;6:544–51. doi: 10.1111/j.1600-6143.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 13.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT working group on primary lung graft dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 15.Lu KC, Jaramillo A, Lecha RL, Schuessler RB, Aloush A, Trulock EP, et al. Interleukin-6 and interferon-gamma gene polymorphisms in the development of bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2002;74:1297–302. doi: 10.1097/00007890-200211150-00017. [DOI] [PubMed] [Google Scholar]
- 16.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–7. doi: 10.1016/s0003-4975(01)03606-2. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 17.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83:150–8. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 18.Farivar AS, Merry HE, Fica-Delgado MJ, McCourtie AS, Mackinnon-Patterson BC, Mulligan MS. Interleukin-6 regulation of direct lung ischemia reperfusion injury. Ann Thorac Surg. 2006;82:472–8. doi: 10.1016/j.athoracsur.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 19.Feezor RJ, Baker HV, Xiao W, Lee WA, Huber TS, Mindrinos M, et al. Genomic and proteomic determinants of outcome in patients undergoing thoracoabdominal aortic aneurysm repair. J Immunol. 2004;172:7103–9. doi: 10.4049/jimmunol.172.11.7103. [DOI] [PubMed] [Google Scholar]
- 20.Scholma J, Slebos DJ, Boezen HM, van den Berg JW, van der Bij W, de Boer WJ, et al. Eosinophilic granulocytes and interleukin-6 level in bronchoalveolar lavage fluid are associated with the development of obliterative bronchiolitis after lung transplantation. Am J Resp Crit Care Med. 2000;162:2221–5. doi: 10.1164/ajrccm.162.6.9911104. [DOI] [PubMed] [Google Scholar]
- 21.Kim T, Arnaoutakis GJ, Bihorac A, Martin TD, Hess PJ, Jr, Klodell CT, et al. Early blood biomarkers predict organ injury and resource utilization following complex cardiac surgery. J Surg Res. 2011;168:168–72. doi: 10.1016/j.jss.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreisel D, Krupnick AS, Puri V, Guthrie TJ, Trulock EP, Meyers BF, et al. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg. 2011;141:215–22. doi: 10.1016/j.jtcvs.2010.09.009. [DOI] [PubMed] [Google Scholar]