Abstract
Background
Although the association of the dopamine transporter (DAT1) gene and ADHD has been widely-studied, far less is known about its potential interaction with environmental risk factors. Given that maltreatment is a replicated risk factor for ADHD, we explored the interaction between DAT1 and maltreatment with ADHD symptoms defined dimensionally and using latent class analysis.
Method
We tested the association of the 40 base-pair variable number of tandem repeats polymorphism in DAT1, maltreatment, and their interaction in 2,488 boys and girls from the National Longitudinal Study of Adolescent Health
Results
In boys, ADHD symptoms were optimally defined by four classes (Combined, Hyperactive/Impulsive, Inattentive, and Normal), whereas in girls, ADHD symptoms were defined by three classes (Combined, Combined-Mild, Normal). A significant DAT1 × maltreatment interaction revealed that maltreated girls homozygous for the 10-repeat allele had more symptoms of ADHD, and were also 2.5 times more likely to be classified in the Combined ADHD group than in the Normal Group
Conclusions
The underlying structure of ADHD symptoms differed between boys and girls and DAT1 interacted with maltreatment to predict ADHD symptoms and ADHD status derived from LCA. Interactive exchanges between maltreatment and DAT1 for ADHD symptoms, and their implications for intervention, are discussed
Keywords: ADHD, latent class analysis, gene-environment interaction, DAT1, maltreatment
Twin, adoption, and family studies strongly implicate genetic factors in the etiology of attention-deficit/hyperactivity disorder (ADHD). ADHD is more prevalent in first degree relatives with persistent ADHD than those with childhood-limited ADHD (Faraone et al., 2005) and the heritability of hyperactivity and inattention was estimated at 73% and 71%, respectively, in a recent meta-analysis (Nikolas & Burt, 2010). The dopamine transporter gene (DAT1; SLC6A3) is a compelling candidate gene for ADHD because stimulant medications for ADHD inhibit the dopamine (DA) transporter and increase extracellular DA (Spencer et al., 2007). The variable number of tandem repeats (VNTR) sequence in the 3’ untranslated region (UTR) affected neural activation during working memory and response inhibition tasks using functional magnetic resonance imaging (Bertolino et al., 2008; Congdon et al., 2009).
10-repeat homozygosity at DAT1 has been associated with increased mRNA expression (Mill, Ascherson, Browes, D’Souza, & Craig, 2002) as well as decreased DA transporter binding compared to non-10-repeat homozygotes (Heinz et al., 2000), although non-associations with DAT1 expression were reported from in-vitro human studies (Mill, Asherson, Craig, & D’Souza, 2005). Children with ADHD homozygous for the 10-repeat allele had increased DAT density in the basal ganglia and worse response to methylphenidate treatment compared to children without the 10/10 genotype (Cheon, Ryu, Kim, & Cho, 2004). In a double-blind study of ADHD youth, methylphenidate was less effective in reducing ADHD and impairment among youth with the 10/10 versus non-10/10 genotype (Roman et al., 2002). More importantly, striatal DA levels persisted in the extracellular space 100 times longer among mice with DAT inactivated (i.e., homozygotes) compared to mice with partial/full activation of DAT (i.e., heterozygotes and wild-type), suggesting that homozygosity significantly alters neurochemistry and behavior (Giros et al., 1996). Importantly, these studies suggest an association between DAT1 genotype and ADHD (rather than DAT1 alleles). Childhood maltreatment, in particular, strongly predicts ADHD and influences biological processes in offspring, a key consideration for selecting viable environmental risk factors in G×E research (Moffitt, 2005). Maltreated children (e.g., physical and sexual abuse, neglect) exhibited higher levels of ADHD than non-maltreated youth (Ouyang et al., 2008) and within the same household, children with ADHD were more likely to be maltreated than non-ADHD siblings (Erickson, Egeland, & Pianta, 1989). Maltreatment also affects the development of cortical and subcortical structures in the brain that influence behavioral and emotional functioning (Teicher et al., 2003). Early chronic stress in the form of maltreatment disrupts hypothalamic-pituitary-thalamic axis (HPA) functioning, which regulates stress reactivity, mental health, and DA neurotransmission (Twardosz & Lutzker, 2010; Strathearn, 2011). Prolonged maternal neglect and separation was inversely associated with DA transporter binding in rat pups (Hall et al., 1999) and poor maternal care during childhood significantly increased the release of DA in the ventral striatum among young adult humans (Pruessner, Champagne, Meaney, & Dagher, 2004). Given its negative neurobiological sequelae in humans and non-human animals, the interaction between maltreatment and genetic variation for ADHD is biologically plausible.
Beyond maltreatment, other risk factors also interact with genetic variants related to ADHD. The association between maternal alcohol use during pregnancy and offspring ADHD was moderated by the 30-bp VNTR polymorphism in intron 8 of DAT1. Youth exposed prenatally to alcohol with the 10/3-repeat haplotype had increased odds for ADHD compared to youth without the haplotype (Brookes et al., 2006). Furthermore, severe institutional deprivation prospectively predicted ADHD symptoms among youth with the 10/6-repeat combined haplotype for the 40-bp VNTR in the 3’UTR and the 30-bp VNRT in intron 8 polymorphisms in DAT1 (Stevens et al., 2009). Overall, studies involving DAT1 and ADHD suggest a possible interaction between salient environmental signals and DAT1. Few studies of DAT1 and maltreatment appear in the literature, despite their biological significance.
Another challenge in understanding the etiology of ADHD is its heterogeneous clinical presentation. That is, variability within ADHD, attributable to differences in diagnostic and developmental subtypes (e.g., persistent ADHD) complicate studies of etiology. For example, the prevalence of ADHD in the same population varied from 3% to 16%, depending on whether DSM-IV criteria versus dimensional thresholds were used (Rowland, Lesesne, & Abramowitz, 2002). Phenotypic differences also affect estimates of heritability and genetic effects. Problematically, using a priori categories or sample-specific thresholds can be arbitrary and do not necessarily denote impairment (Blanton & Jaccard, 2006). Children who fell just short of diagnostic criteria for behavior disorders were often equally impaired as children who meet full criteria (Cho et al., 2009). Because cutoff scores introduce uncertainty into studies of etiology by collapsing across subtypes, alternative methods to defining ADHD have been exhorted (Hudziak, Achenbach, Althoff, & Pine, 2008).
Unlike diagnostic categories and dimensional approaches, empirically-anonymous approaches to classification identify groups based on severity and clinical presentation. Latent class analysis (LCA) is a person-centered approach that derives unique groups based on similar patterns of responses (McCutcheon, 1987). Latent classes consist of individuals who are homogenous and distinct from individuals in other classes, including those below the clinical threshold who are omitted from traditional diagnostic categories.Elia et al. (2009) used LCA and detected six unique classes that reflected “mild” to “moderate” DSM-IV ADHD subtypes and the inclusion of comorbid anxiety and mood disorders did not change the class structure for ADHD (Elia et al., 2009). Crucially, LCA-subtypes may reflect etiological differences. The 9-repeat DAT1 allele was associated with a combined-severe ADHD latent class, but not for any of the other seven LCA-derived classes for ADHD or when DSM-IV definitions of ADHD were used (Todd et al., 2005). In one of the first G×E studies based on LCA phenotypes, the serotonin transporter gene interacted with maltreatment to predict LCA-derived antisocial behavior phenotypes in a population-based sample (Li & Lee, 2010). These preliminary studies suggest that using LCA may minimize some of the limitations of traditional diagnostic categories (e.g., within-group heterogeneity) and improve traction on complex phenotypes in genetic studies.
Despite each being independently associated with ADHD and the plausibility of a biological interaction, to our knowledge, we know of no previous studies of G×E for DAT1 and maltreatment with ADHD. We used a large population-based study of adolescents and young adults to test the association of DAT1, maltreatment, and their interaction on self-reported ADHD using LCA and symptom counts. We predicted that the latent structure of ADHD would approximate DSM-IV subtypes, but with different levels of severity (Todd et al., 2005). We hypothesized a significant interaction between DAT1 and maltreatment, such that youth with the 10/10 DAT1 genotype (Gizer et al., 2009) would have the highest levels of ADHD symptoms in the presence of maltreatment relative to maltreated youth with other DAT1 genotypes.
Methods
Participants
The National Longitudinal Study of Adolescent Health (Add Health; Harris et al., 2008) ascertained a stratified random sample of youth from U.S. high schools. Details of the study design can be obtained at http://www.cpc.unc.edu/projects/addhealth. 20,745 adolescents were interviewed at across three waves: Wave I (grades 7–12, ages 12–20 years) was conducted during the 1994–1995 school year and consisted of 48% males; Two years later, Wave II interviews were conducted with 14,738 adolescents; Wave III included 15,197 young adults that were interviewed 7–8 years after Wave I. We analyzed the genetic subsample (obtained from full siblings and twins only) at Wave III, which consisted of 2,488 adolescents (48% male). The genetic subsample was ethnically diverse (57.5% Caucasian, 14.3% Hispanic, 18.1% African-American, 7.4% Asian, 1.7% Native American, and 0.9% “Other”).
Measures
ADHD symptoms were assessed retrospectively during an in-home interview conducted at Wave III. Respondents were asked to rate their ADHD symptoms between 5 and 12 years of age on a four-point scale (“never or rarely,” “sometimes,” “often” and “very often”). 17 ADHD items were ascertained, including: “you failed to pay close attention to details or made careless mistakes in your work,” “you had difficulty sustaining attention in tasks or fun activities” and “you had difficulty doing things quietly.” For all analyses, we dichotomized the data where all responses of “often” and “very often” were coded positively (O’Donnell, McCann, & Pluth, 2001). Cronbach’s alpha for the 17 items of ADHD was adequate (.85).
Maltreatment was also assessed retrospectively at Wave III. Respondents reported the frequency of the following events prior to age 12: (1) parents or adult-caregivers did not take care of the respondent’s basic needs (e.g., hygiene, food/clothing), (2) had been slapped, hit or kicked by parents or adult care-givers, and (3) had been touched in a sexual way, forced to touch someone else in a sexual way, or forced to have sexual relations with a parent or adult caregiver. If an event occurred at least once, it was scored as positive. 64.8% of youth reported no maltreatment history and 35.2% reported at least one episode. 11.7% of youth self-endorsed having experienced neglect, 29.5% endorsed having experienced physical abuse, and 4.8 % endorsed having experienced sexual abuse, all prior to age 12.1 These estimates approximate rates in general population-based studies of maltreatment.
Genotyping
Saliva samples were collected from full siblings or twins and genotyped. Genomic DNA was isolated from buccal cells using standard methods. Dopamine transporter (DAT, SLC6A3) contains a 40-bp VNTR polymorphism in the 3’ UTR. The 9-repeat (440 bp) and 10-repeat (480 bp) polymorphisms are the two most common alleles in the population. The primer sequences were: forward, 5’TGTGGTGTAGGGAACGGCCTGAG-3’ (fluorescently labeled), and reverse: 5’CTTCCTGGAGGTCACGGCTCAAGG-3’. The DAT1 analyses compared individuals homozygous for the 10-repeat allele (i.e., 10/10 repeat) versus individuals with at least a one copy of the 9-repeat allele (i.e., 9/9 + 9/10 repeat groups).
Statistical Analyses
To test the independent and interactive effects of maltreatment and DAT1 with ADHD, we controlled for age and race-ethnicity and fit a zero-inflated negative binomial regression given that 31% (n = 4,549) of youth did not endorse any ADHD symptoms. We conducted LCA on the 17 ADHD items using MPlus 4.0 (Muthen & Muthen, 2006) by fitting a two-class solution followed by successive models with an additional class until the best fitting model was indicated. We examined model fit using the Bayesian Information Criterion (BIC), adjusted BIC, as well as the Lo-Mendell-Rubin likelihood ratio test (LMR-LRT), which computes a p-value comparing the fit of a given model to a model with one less class. We then used multinomial logistic regression to examine the association of maltreatment, DAT1, and their interaction on membership in the LCA-derived subtypes based on self-reported ADHD.
Results
Population Stratification and Gene-Environment Correlation (rGE)
Population stratification may confound the interpretation of potential G×E effects. DAT1 genotypes were related to race-ethnicity (X2 = 46.58, df = 3, p < .01), but not sex (X2 =.65, df = 1, p = .42). Thus, we controlled for race-ethnicity in all models. Exposure to maltreatment was unrelated to DAT1 (X2 = 0.77, df = 1, p = .38), thereby minimizing the possibility that rGE confounded our tests/interpretation of G×E.
Predicting Counts of ADHD Symptoms
We analyzed the association of DAT1, maltreatment, and their interaction with the total number of ADHD symptoms using zero-inflated negative binomial regression. The interaction of DAT1 and maltreatment was not significant for the overall sample (β = .03, SE = .09, p = .74), although there was a significant three-way interaction between sex, DAT1 and maltreatment (β = .57, SE = .18, p < .001). Among boys, significant main effects emerged for DAT1 genotype and maltreatment (β = .28, SE = .11, p < .05 and β = .38, SE = .15, p < .05, respectively) but their interaction was marginally significant (β = −.22, SE = .12, p = .07). In girls, there were no significant effects for DAT1 or maltreatment (β = −.23, SE = .13, p = .09 and β = −.17, SE = .14, p = .22, respectively), but their interaction was significant (β = .33, SE = .13, p < .01) such that maltreatment positively predicted ADHD symptoms for girls with the 10/10 genotype (β = .23, SE = .08, p < .001), but not the 9/10 and 9/9 genotypes (β = −.13, SE = .10, p = .21).
Latent Class Models
LCA fit indices are summarized in Table 1. Across the entire sample, the five-class solution was the best fit (BIC = 174717.64, p < .001). However, based on the significant three-way interaction between sex, maltreatment and DAT1 in our dimensional model, we also examined sex differences in the LCA. The probability of class membership differed significantly by sex (X2 = 3.50, df = 4, p < .001), suggesting that the underlying structure of ADHD differed between boys and girls. For boys, a four-class solution was optimal (BIC = 92990.04, p = .03). When a five-class solution was modeled, the BIC dropped to 92698.55, but the LMR-LRT was no longer significant (p = .64), indicating that the four-class solution was optimal. For girls, a three-class solution was the best fit to the data (BIC = 80908.48, p < .001), given that LMR-LRT for the four-class solution was not significant (BIC = 80395.19, p = .13).
Table 1.
Fit indices for LCA models with 2–6 classes
| Number of Classes |
BIC | Sample Size Adjusted BIC |
p | |
|---|---|---|---|---|
| Combined (n = 13,963) | 2 | 181392.43 | 181274.84 | <.01 |
| 3 | 176495.42 | 176317.46 | <.01 | |
| 4 | 175516.74 | 175278.40 | <.01 | |
| 5 | 174717.64 | 174418.92 | <.01 | |
| 6 | 174343.40 | 173984.30 | .08 | |
| Boys (n = 6,611) | 2 | 95825.82 | 95708.25 | <.01 |
| 3 | 93425.09 | 93247.14 | <.01 | |
| 4 | 92990.04 | 92751.71 | .03 | |
| 5 | 92698.55 | 92399.84 | .64 | |
| 6 | 92526.43 | 92167.34 | .11 | |
| Girls (n= 7,352) | 2 | 83222.25 | 83104.68 | <.01 |
| 3 | 80908.48 | 80730.53 | <.01 | |
| 4 | 80395.19 | 80156.86 | .13 | |
| 5 | 80021.26 | 79722.55 | .02 | |
| 6 | 79908.23 | 79549.14 | .33 |
Note. Bold indicates best fitting model; BIC = Bayesian Information Criterion; p = Lo-Mendell-Rubin likelihood ratio test for k versus k - 1 classes.
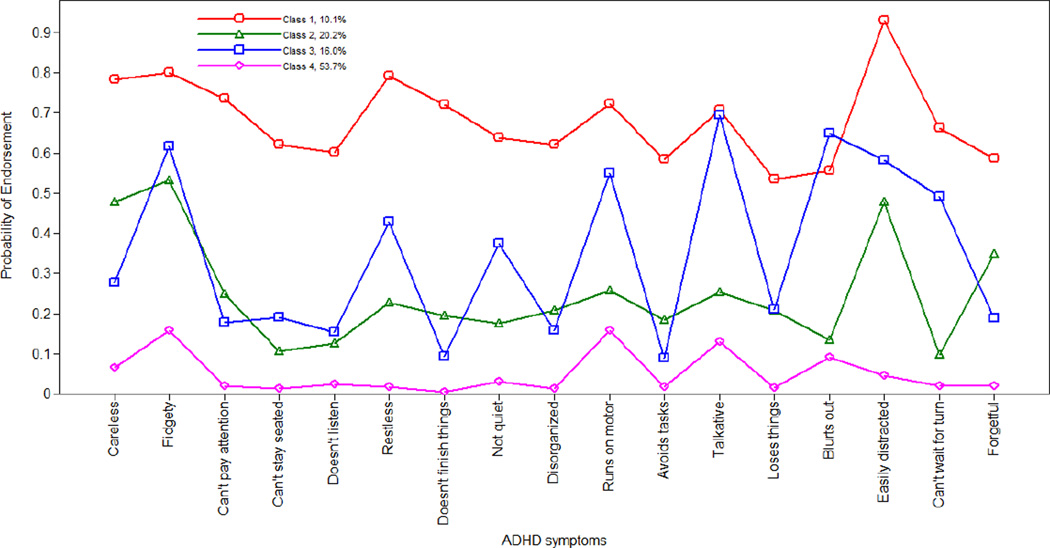
Figure 1 shows the prevalence of group membership for boys. Class 1 (10% of boys) was characterized by high probabilities for endorsing all ADHD symptoms (Combined Type), including symptoms of inattention [“easily distracted” (.93), “careless” (.78), and “can’t pay attention” (.74)] and hyperactivity/impulsivity [“fidgety” (.80), “restless” (.79) and “runs on motor” (.72)]. Next, 20.2% of boys fell into Class 2 (Inattentive), characterized by distinctively high probabilities for endorsing inattention [“easily distracted” (0.48) and “forgetful” (0.35)] but low probabilities for hyperactivity/impulsivity [“can’t stay seated” (.11), “not quiet” (.18), and “blurts out” (.13)], with the exception of “fidgety” (.53). Class 3 (Hyperactive/Impulsive) consisted of 15.2% of boys, and was characterized by relatively high probabilities for endorsing hyperactivity symptoms [“talkative” (.70), “blurts out” (.65), “fidgety” (.62) and “runs on motor” (.55)], but not inattention [“can’t pay attention” (.18) and “disorganized” (.16)]. Finally, Class 4 constituted 53.7% of boys, as this group was characterized by having minor ADHD symptoms (Normal Group) (i.e., probabilities of endorsing ADHD symptoms were all below 20%).
Figure 1.
Latent class membership for boys
Note. Class 1 = Combined Group; Class 2 = Inattentive Group; Class 3 = Hyperactive Group; Class 4 = Normal group (reference class).
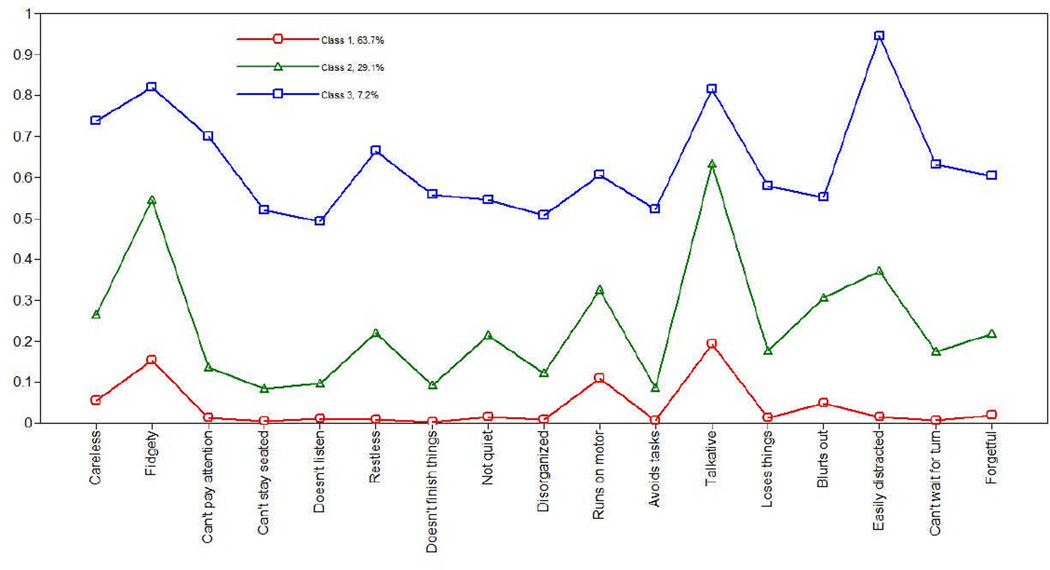
Figure 2 shows the prevalence of class membership for girls. Nearly 64% of girls fell into Class 1, characterized by few ADHD symptoms (Normal Group). These individuals had low probabilities of endorsing all symptoms (i.e., < .20 for all items). Class 2 (Mild Combined) consisted of 29% of girls with higher probabilities for “fidgety” (.55), “runs on motor” (.32), “talkative” (.63), “blurts out” (.31), and “easily distracted” (.37), compared to the Normal Group. However, this group was also low on “avoids tasks” (.09), “does not finish things” (.09), and “can’t stay seated” (.08). 7% of girls fell into Class 3 (Combined), which had high probabilities of endorsing most ADHD symptoms (i.e., > .50 for most items), although some symptoms were quite high [e.g., “fidgety” (.82), “talkative” (.82), and “easily distracted” (.95)].
Figure 2.
Latent class membership for girls
Note. Class 1 = Normal group; Class 2 = Combined-Mild Group; Class 3 = Combined Group.
Predicting Latent Class Membership
We then separately modeled the probability of class membership using multinomial logistic regression for boy and girls (Tables 2 and 3). The odd ratios represent the likelihood of membership in each class relative to the Normal Group. First, we compared the probability of membership in the Combined Group versus Normal Group in boys. We detected a significant main effect for maltreatment, but not for DAT1 (B = 1.69, SE = .77, p = .03 and B = .43, SE = .30, p = .16, respectively). Although maltreated boys were 5.44 times more likely to be in the Combined Group than in the Normal Group, the interaction term was not significant (B = −.69, SE = .46, p = .13). We then compared the Inattentive Group versus the Normal Group for boys. No significant effects were detected for DAT1, maltreatment (B = .13, SE = .23, p = .58 and B = .31, SE = .66, p = .64, respectively), or their interaction (B < .01, SE = .39 p = .99). Finally, we compared the Hyperactive/Impulsive Group versus the Normal Group for boys. No significant effects for DAT1, maltreatment (B = .08, SE = .25, p = .73 and B = .71, SE = .68, p = .30, respectively), or their interaction (B = −.26, SE = .41 p = .53) were observed for this comparison.
Table 2.
Multinomial logistic regression analyses: Predicting class membership for boys
| 95% Confidence Interval |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Variable | B | SE | OR | Lower bound | Upper bound | Sig. |
| Combined Group | Age | −0.07 | 0.07 | 0.94 | 0.81 | 1.06 | .32 |
| (Class 1) | Race-ethnicity | −0.51 | 0.16 | 0.60 | 0.29 | 0.92 | .00 |
| DAT1 | 0.43 | 0.30 | 1.53 | 0.94 | 2.12 | .16 | |
| Maltreatment | 1.69 | 0.77 | 5.44 | 3.93 | 6.94 | .03 | |
| DAT1 × Maltreatment | −0.69 | 0.46 | 0.50 | −0.40 | 1.40 | .13 | |
| Inattentive Group | Age | −0.03 | 0.05 | 0.97 | 0.86 | 1.07 | .56 |
| (Class 2) | Race-ethnicity | −0.04 | 0.09 | 0.96 | 0.77 | 1.14 | .66 |
| DAT1 | 0.13 | 0.23 | 1.14 | 0.68 | 1.60 | .58 | |
| Maltreatment | 0.31 | 0.66 | 1.36 | 0.07 | 2.65 | .64 | |
| DAT1 × Maltreatment | 0.00 | 0.39 | 1.00 | 0.25 | 1.76 | .99 | |
| Hyperactive Group | Age | −0.11 | 0.06 | 0.90 | 0.79 | 1.01 | .07 |
| (Class 3) | Race-ethnicity | −0.35 | 0.13 | 0.71 | 0.46 | 0.95 | .01 |
| DAT1 | 0.08 | 0.25 | 1.09 | 0.61 | 1.57 | .73 | |
| Maltreatment | 0.71 | 0.68 | 2.03 | 0.70 | 3.36 | .30 | |
| DAT1 × Maltreatment | −0.26 | 0.41 | 0.77 | −0.03 | 1.58 | .53 | |
Note. All analyses are compared against the Normal group (Class 4); DAT1 = dopamine transporter genotype (9/9 + 9/10 versus 10/10); B = parameter estimate; SE = standard error; OR = odds ratio.
Table 3.
Multinomial logistic regression: Predicting class membership for girls
| 95% Confidence Interval |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Variable | B | SE | OR | Lower bound | Upper bound | Sig. |
| Combined Mild | Age | −0.05 | 0.04 | 0.95 | 0.87 | 1.04 | .25 |
| (Class 2) | Race-ethnicity | −0.10 | 0.08 | 0.90 | 0.74 | 1.06 | .21 |
| DAT1 | −0.20 | 0.18 | 0.82 | 0.46 | 1.17 | .26 | |
| Maltreatment | 0.43 | 0.51 | 1.53 | 0.53 | 2.54 | .40 | |
| DAT1 × Maltreatment | 0.05 | 0.30 | 1.05 | 0.46 | 1.64 | .87 | |
| Combined | Age | −0.02 | 0.08 | 0.98 | 0.82 | 1.15 | .85 |
| (Class 3) | Race-ethnicity | −0.23 | 0.17 | 0.79 | 0.45 | 1.14 | .19 |
| DAT1 | −0.25 | 0.35 | 0.78 | 0.08 | 1.47 | .48 | |
| Maltreatment | −2.97 | 1.57 | 0.05 | −3.02 | 3.13 | .06 | |
| DAT1 × Maltreatment | 1.94 | 0.84 | 6.97 | 5.33 | 8.61 | .02 | |
Note. All analyses are compared against the Normal group (Class 1); DAT1 = dopamine transporter genotype (9/9 + 9/10 versus 10/10); B = parameter estimate; SE = standard error; OR = odds ratio.
For girls, we compared the Combined-Mild Group versus the Normal Group. There were no main effects for DAT1, maltreatment (B = −.20, SE = .18, p = .26 and B = .43, SE = .51, p = .40, respectively), or their interaction (B = .05, SE = .30, p = .87). Similarly, for the Combined Type Group versus the Normal Group, no main effects were detected for DAT1 or maltreatment (B = −.25, SE = .35, p = .48 and B = −2.97, SE = 1.57, p = .06, respectively), but their interaction was significant (B = 1.94, SE = .84, p = .02). Post-hoc analyses indicated that maltreated girls with the 10/10 genotype were 2.55 more likely to be in the Combined Group than in the Normal Group (B = .93, SE = .34, p < .01). Conversely, there was no association of maltreatment and latent class membership among girls with the 9/9 or 9/10 genotype (B = −1.14, SE = .77, p = .14).
Dopamine D4 Receptor (DRD4) and Monoamine Oxidase –A (MAOA)
Given their potential role in the etiology of ADHD, as well as their availability in Add Health, we also examined the association of DRD4 (7-repeat allele carriers vs. no 7-repeat) and MAOA (0, 1 or 2 copies of the high activity allele), maltreatment and their respective interactions. There were no significant maltreatment×DRD4 and maltreatment×MAOA effects for ADHD defined by symptom counts or latent class analysis (results available upon request). We believe these findings for DRD4 and MAOA reinforce the specificity of DAT1 for ADHD.2
Discussion
Despite their independent associations with ADHD, there is relatively little knowledge about the potential interactive effects of DAT1 and maltreatment. To address the phenotypic complexity of ADHD, we conducted LCA using a large population-based sample of adolescents and young adults and found four classes of ADHD in boys (Combined, Inattentive, Hyperactive/Impulsive, and Normal) and three classes in girls (Combined, Combined-Mild, and Normal). Based on ADHD symptoms, maltreated girls homozygous for the 10-repeat allele had more ADHD symptoms than girls with the 9/9 or 9/10 genotypes. This pattern was replicated using LCA where maltreated girls with the 10/10 genotype had higher odds of being in the Combined ADHD group than maltreated girls with the 9/9 or 9/10 genotype, suggesting a potential G×E. No significant G×E was observed in boys using ADHD symptoms or LCA.
The latent structure of ADHD in boys approximated DSM-IV subtypes. However, they differed in that LCA classes included individuals who were unlikely to meet symptom criteria for ADHD. Interestingly, the latent structure for girls deviated from DSM-IV subtypes given that classes were organized around differences in severity rather than the nominal differences. This finding in girls diverges somewhat from a previous LCA study of adolescent female twins where different classes of severity corresponded well to DSM-IV subtypes (Hudziak et al., 1998). Indeed, sex differences in the underlying structure for ADHD symptoms were not entirely surprising given sex differences in the prevalence of ADHD as in neurocognitive and academic correlates (Gershon, 2002). It is also possible that including internalizing symptoms into the LCA may have changed the nature of the classes identified in girls (Neuman et al., 2001).
Among girls with the 10/10 DAT1 genotype, maltreatment positively predicted ADHD symptoms, but not in girls with the 9/9 and 9/10 genotype. LCA improved the specificity of our findings given that maltreated girls with the 10/10 genotype were nearly three times more likely to be in the Combined Group versus the Normal Group. However, this effect was only detected in the most severe class of ADHD for girls. One explanation may be that girls with the 10/10 genotype are the most sensitive to environmental stress (Caspi et al., 2010), which resonates with sex differences in sensitivity to stressful events in human and non-human studies (Oldehinkel & Bouma, 2010). For example, acute stress enhanced learning and memory in male rats, but impaired these functions in female rats (Shors, 2004). Similarly, adolescent girls experienced significantly more interpersonal stress (e.g., peers, parents) than boys, as well as higher levels of depression and anxiety as function of these stressors. Thus, girls may be more vulnerable to interpersonal stressors than boys, including potentially maltreatment (Rudolph & Hammen, 1999). Furthermore, the 10/10 genotype may confer additional sensitivity to positive and negative environmental factors through disrupted DA neurotransmission (Strathearn, 2011). Adolescents homozygous for the 10-repeat allele had the most inattention under high psychosocial adversity, but concurrently had the lowest inattention under low psychosocial adversity (Laucht et al., 2007). The rate of DA binding and subcortical reactivity may be contingent upon the salience of the event (i.e., highly positive or negative stimuli) such that DA neurons exhibit the highest firing rate prior to a salient reward, but the firing rate falls below baseline in anticipation of a harsh punishment (Mirenowicz & Schultz, 1996).
There were several limitations to our study. ADHD was assessed via retrospective self-report, which may have been influenced by mood, psychopathology or inaccurate recall. However, adults with ADHD are better at self-reporting their own ADHD symptoms than their partners or their parents (Kooij et al., 2008), suggesting that patients may remember their externalizing behavior better than other forms of psychopathology. Although items related to other dimensions of psychopathology were ascertained, these data were only available for concurrent psychopathology (i.e., when participants were ages 12–20) rather than for retrospective psychopathology as it was for ADHD (ages 5–12). Moreover, because ADHD symptoms were not assessed using a formal diagnostic interview, the groups that were ascertained in the LCA should not be interpreted as clinical groups. Finally, latent transition analysis would have provided improved traction on the developmental trajectory of ADHD symptoms. However, the present study was cross-sectional because ADHD data were retrospective and only available at Wave III.
This study characterized the latent structure of ADHD in a population-based sample with evidence that a biologically-plausible interaction between DAT1 and maltreatment predicted severe ADHD, but only among girls. These findings have potential clinical implications insofar as future G×E research may facilitate efforts to identify subgroups of resilient youth and to prevent the development of psychopathology among individuals who experience severe and/or chronic psychosocial adversity (Kim-Cohen & Gold, 2009). Within the context of differential susceptibility (Belsky & Pleuss, 2009), some genetic variants that predict psychopathology in the context of environmental adversity may also increase responsiveness to environmental enrichment. Thus, genetic factors may help to identify youth who are most likely to benefit from enhancements to their environment. Finally, diagnoses of DSM-IV ADHD should also be done cautiously as traditional diagnostic approaches may include phenotypically heterogeneous subgroups that inadvertently reduce statistical power, a longstanding concern in genetic association studies. We anticipate that integrated models of risk, incorporating genetic and environmental constructs with empirically-derived groups of youth, will facilitate the development and implementation of targeted interventions to reduce the considerable burden associated with significant ADHD.
Key Points.
Genetic studies are often complicated by phenotypic heterogeneity, which significantly reduces power to detect meaningful effect sizes. Latent class analysis may improve power in genetic studies by creating more homogenous ADHD subgroups.
Our findings indicate that maltreatment interacts with DAT1 genotype to predict ADHD symptoms in youth. Girls with 10/10 DAT1 genotype are especially sensitive to the effects of maltreatment, and are 2.55 times more likely to belong to the most severe class of ADHD than in the typically-developing class when they are maltreated.
Future studies should incorporate genetic and environmental constructs with empirically-derived groups of youth, which will facilitate the development and implementation of targeted interventions to reduce the considerable burden associated with ADHD.
Acknowledgments
This work was supported by the Consortium of Neuropsychiatric Phenomics (NIH Roadmap for Medical Research grant UL1-DE019580, RL1DA024853).
Abbreviations
- ADHD
attention-deficit/hyperactivity disorder
- DA
dopamine
- DAT1
dopamine transporter
- G×E
gene-environment interaction
- LCA
latent class analysis
- LMR-LRT
Lo-Mendell-Rubin likelihood ratio test
- MAO-A
monoamine oxidase-A
- rGE
gene-environment correlation
- VNTR
variable number of tandem repeats
Footnotes
The authors have declared that they have no competing or potential conflicts of interest.
There were no sex differences in physical abuse [χ2 (1) = 2.06, p = .15] or sexual abuse [χ2 (1) = 2.73, p = .10], although neglect was more prevalent in boys than in girls [χ2 (1) = 24.65, p < .001]. There was no main effect of neglect on ADHD symptoms, and the sex difference in neglect did not change the G×E results in any of the models (results available upon request).
We thank the editor and anonymous review for suggesting the inclusion of these specific analyses.
References
- Belsky J, Pleuss M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Di Giorgio A, Blasi G, Sambataro F, Caforio G, Sinibaldi L, et al. Epistasis between dopamine regulating genes identifies a nonlinear response of the human hippocampus during memory tasks. Biological Psychiatry. 2008;64:226–234. doi: 10.1016/j.biopsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Blanton H, Jaccard J. Arbitrary metrics in psychology. American Psychologist. 2006;61:27–41. doi: 10.1037/0003-066X.61.1.27. [DOI] [PubMed] [Google Scholar]
- Brookes KJ, Mill J, Guindalini C, Curran S, Xu X, Knight J, et al. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Archives of General Psychiatry. 2006;63:74–81. doi: 10.1001/archpsyc.63.1.74. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Kim JW, Cho DY. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. European Neuropsychopharmacology. 2005;15:95–101. doi: 10.1016/j.euroneuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Cho SC, Kim BN, Kim JW, Rohde L, Hwang JW, Chungh DS, et al. Full syndrome and subthreshold attention-deficit/hyperactivity disorder in a Korean community sample: comorbidity and temperament findings. European Child and Adolescent Psychiatry. 2009;18:447–457. doi: 10.1007/s00787-009-0755-7. [DOI] [PubMed] [Google Scholar]
- Congdon E, Constable RT, Lesch KP, Canli T. Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biological Psychology. 2009;81:144–152. doi: 10.1016/j.biopsycho.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, Arcos-Burgos M, Bolton KL, Ambrosini PJ, Berrettini W, Muenke M. ADHD latent class clusters: DSM-IV subtypes and comorbidity. Psychiatry Research. 2009;170:192–198. doi: 10.1016/j.psychres.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MF, Egeland B, Pianta R. The effects of maltreatment on the development of young children. In: Cicchetti D, Carlson V, editors. Child maltreatment: Theory and research on the causes and consequences of child abuse and neglect. New York, NY, US: Cambridge University Press; 1989. pp. 647–684. [Google Scholar]
- Faraone SV, Biederman J, Friedman D. Validity of DSM-IV subtypes of attention-deficit/hyperactivity disorder: a family study perspective. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:300–307. doi: 10.1097/00004583-200003000-00011. [DOI] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders. 2002;5:143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gizer I, Ficks C, Waldman I. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Hall F, Wilkinson L, Humby T, Inglis W, Kendall D, Marsden C, et al. Isolation rearing in rats: Pre-and postsynaptic changes in striatal dopaminergic systems. Pharmacology Biochemistry and Behavior. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Harris KM. The National Longitudinal Study of Adolescent Health (Add Health), Waves I & II, 1994–1996; Wave III, 2001–2002 [machine-readable data file and documentation] Chapel Hill, NC: Carolina Population Center, University of North Carolina at Chapel Hill; 2008. [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. International Journal of Methods in Psychiatric Research. 2008;16:16–23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak JJ, Heath AC, Madden PF, Reich W, Bucholz KK, Slutske W, et al. Latent class and factor analysis of DSM-IV ADHD: A twin study of female adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:848–857. doi: 10.1097/00004583-199808000-00015. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Gold AL. Measured gene-environment interactions and mechanisms promoting resilient development. Current Directions in Psychological Science. 2009;18:138–142. [Google Scholar]
- Kooij JS, Boonstra AM, Vermeulen SH, Heister AG, Burger H, Buitelaar JK, et al. Response to methylphenidate in adults with ADHD is associated with a polymorphism in SLC6A3 (DAT1) American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147:201–208. doi: 10.1002/ajmg.b.30586. [DOI] [PubMed] [Google Scholar]
- Laucht M, Skowronek MH, Becker K, Schmidt MH, Esser G, Schulze TG, et al. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symptoms among 15-year-olds from a high-risk community sample. Archives of General Psychiatry. 2007;64:585–590. doi: 10.1001/archpsyc.64.5.585. [DOI] [PubMed] [Google Scholar]
- Li JJ, Lee SS. Latent class analysis of antisocial behavior: interaction of serotonin transporter genotype and maltreatment. Journal of Abnormal Child Psychology. 2010;38:789–801. doi: 10.1007/s10802-010-9409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3 UTR VNTR: Evidence from brain and lymphocytes using quantitative RT PCR. American Journal of Medical Genetics. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Craig I, D'Souza U. Transient expression analysis of allelic variants of a VNTR in the dopamine transporter gene (DAT1) BMC Genetics. 2005;6:3. doi: 10.1186/1471-2156-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon AL. Latent Class Analysis. Sage Publications, Inc; 1987. [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Muthén L, Muthén B. Mplus Version 4.1. Los Angeles, CA: Muthén & Muthén; 2006. [Google Scholar]
- Neuman RJ, Heath A, Reich W, Bucholz KK, Madden PAF, Sun L, et al. Latent class analysis of ADHD and comorbid symptoms in a population sample of adolescent female twins. Journal of Child Psychology and Psychiatry. 2001;42:933–942. doi: 10.1111/1469-7610.00789. [DOI] [PubMed] [Google Scholar]
- Nikolas MA, Burt SA. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. Journal of Abnormal Psychology. 2010;119:1–17. doi: 10.1037/a0018010. [DOI] [PubMed] [Google Scholar]
- O’Donnell JP, McCann KK, Pluth S. Assessing adult ADHD using a self-report symptom checklist. Psychological Reports. 2001;88:871–881. doi: 10.2466/pr0.2001.88.3.871. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Bouma E. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: A review of gender differences. Neuroscience and Biobehavioral Reviews. 2010;35:1757–1770. doi: 10.1016/j.neubiorev.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Ouyang L, Fang X, Mercy J, Perou R, Grosse SD. Attentiondeficit/ hyperactivity disorder symptoms and child maltreatment: A population-based study. The Journal of Pediatrics. 2008;153:851–856. doi: 10.1016/j.jpeds.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C] raclopride. The Journal of Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman T, Szobot C, Martins S, Biederman J, Rohde LA, Hutz MH. Dopamine transporter gene and response to methylphenidate in attentiondeficit/ hyperactivity disorder. Pharmacogenetics and Genomics. 2002;12:497–499. doi: 10.1097/00008571-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Rowland AS, Lesesne CA, Abramowitz AJ. The epidemiology of attention deficit/hyperactivity disorder (ADHD): a public health view. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:162–170. doi: 10.1002/mrdd.10036. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Learning during stressful times. Learning and Memory. 2004;11:137–144. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Adler LA, McGough JJ, Muniz R, Jiang H, Pestreich L. Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1380–1387. doi: 10.1016/j.biopsych.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Stevens SE, Kumsta R, Kreppner JM, Brookes KJ, Rutter M, Sonuga Barke EJS. Dopamine transporter gene polymorphism moderates the effects of severe deprivation on ADHD symptoms: Developmental continuities in gene–environment interplay. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150:753–761. doi: 10.1002/ajmg.b.31010. [DOI] [PubMed] [Google Scholar]
- Strathearn L. Maternal neglect: Oxytocin, dopamine and the neurobiology of attachment. Journal of Neuroendocrinology. 2011;23:1054–1065. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews. 27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Smalley SL, Nelson SF, Willcutt EG, Pennington BF, et al. Collaborative analysis of DRD4 and DAT genotypes in population defined ADHD subtypes. Journal of Child Psychology and Psychiatry. 2005;46:1067–1073. doi: 10.1111/j.1469-7610.2005.01517.x. [DOI] [PubMed] [Google Scholar]
- Twardosz S, Lutzker JR. Child maltreatment and the developing brain: A review of neuroscience perspectives. Aggression and Violent Behavior. 2010;15:59–68. [Google Scholar]