The aldol reaction has long been recognized as one of the most powerful methods for new carbon-carbon bond formation.1–2 The high stereoselectivity of aldolases in C-C bond construction confers upon them tremendous applications as synthetic biocatalysts.3–5 Among the aldolases, dihydroxyacetone phosphate (DHAP)-dependent aldolases are particularly attractive as a set of four possible diastereomers of vicinal diols can be synthesized conferring upon them the potential to be used in the synthesis of rare sugars and other hydroxylated natural products.1,6–7 Unfortunately, the strict requirement for the donor substrate DHAP, a rather expensive and unstable compound, limits aldolase use in large-scale preparation.6,8–9 Therefore, the capability to generate DHAP from inexpensive sources could ultimately broaden the scope of aldolase reactions making it an attractive challenge.10–11
Rare sugars are monosaccharides, and their derivatives, that are particularly uncommon in nature.12 Importantly, rare sugars possess many potential applications in the food, pharmaceutical and nutrition industries.13 In addition, rare sugars can be used as starting materials for the synthesis of intriguing natural products with important biological activities.13–14 Unfortunately, most rare sugars are quite expensive, and their synthetic routes are both limited and costly due to the expense of costly starting materials. As a specific example, D-psicose, a rare sugar and C-3 epimer of D-fructose, has the unique property of being an ideal sucrose substitute. Compared with sucrose, it has 70% the sweetness but provides no energy due to its suppressive effect toward hepatic lipogenic enzymes.15–16 Furthermore, it has been observed that foods supplemented with D-psicose exhibit higher antioxidant activity.15 Furthermore, D-psicose can be used as a precursor in the synthesis of xylosylpsicoses, which are promising candidates for prebiotics, cosmetics and therapeutic uses.17 However, only two enzymes D-tagatose 3-epimerase from Pseudomonas cichorii18–19 and D-psicose 3-epimerase from Agrobacterium tumefaciens20 have been reported for D-psicose production. In addition, due to the fact that the interconversion between D-fructose and D-psicose is an equilibrium process, the large scale and high yield production of D-psicose remains quite challenging.
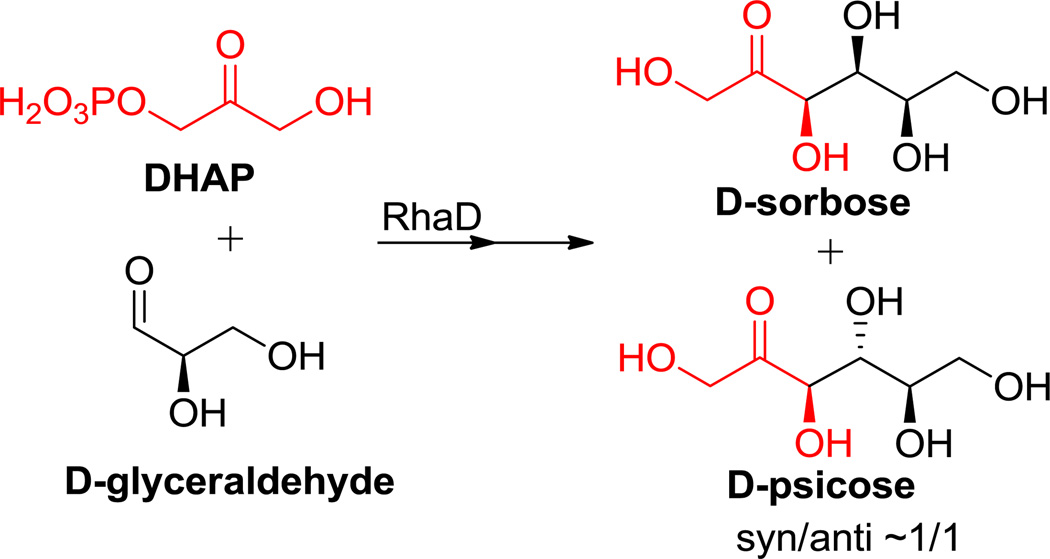
A potential solution to the above mentioned problems with rare sugar production lies in the use of aldolase reactions (i.e. DHAP aldolase reactions), which are capable of generating vicinal diol diastereomers and are thus are an ideal tool for the construction of rare sugars.21 In fact, the preparation of rare sugars, as well as their derivatives, represents the most significant applications of DHAP-dependent aldolases.8,14,22–23 In our previous work, we synthesized two rare sugars (D-sorbose and D-psicose) simultaneously with Rhamnulose-1-phosphate aldolase (RhaD) from Escherichia coli in good overall yields (Scheme 1). However, RhaD showed no stereo-preference for either product (syn/anti ~1/1) when accepting D-glyceraldehyde. Here we describe another enzyme, L-fuculose-1-phosphate aldolase, from Thermus thermophilus HB8 (FucAT.HB8) capable of stereoselectively synthesizing D-psicose from L-glycerol 3-phosphate (LGP) and D-glyceraldehyde. Alternatively, the rare sugars L-tagatose and L-fructose were synthesized efficiently with FucAT.HB8 using L-glyceraldehyde as the acceptor instead of the D-isomer.
Scheme 1.
Enzymatic synthesis of D-sorbose and D-psicose with RhaD
FucA is a class II DHAP-dependent aldolase that catalyzes the reversible cleavage of L-fuculose 1-phosphate to DHAP and L-lactaldehyde, a central step for L-fucose metabolism in bacteria.24 Furthermore, FucA from E. coli (FucAE.coli) is a homotetramer of 215 amino acid residues containing one Zn2+ ion per subunit.25 Importantly, FucA has demonstrated its value in aldol reactions to selectively afford vicinal diols with the anti configuration, which is advantageous for D-psicose construction.26
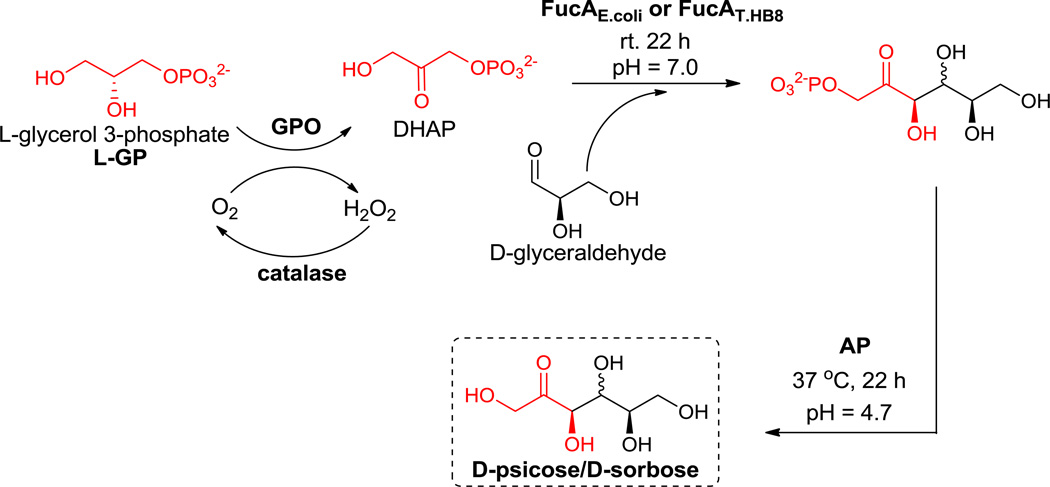
Consequently, we first employed FucAE.coli in the synthesis of D-psicose via a one-pot four enzyme reaction in which DHAP was generated from the oxidation of L-glycerol 3-phosphate (L-GP) by glycerol phosphate oxidase (GPO) (Scheme 2).27 To degrade the corresponding by-product, hydrogen peroxide, and regenerate oxygen, catalase was added to the reaction mixture. Subsequently, the DHAP generated in situ was coupled with D-glyceraldehyde by FucA to give D-psicose 1-phosphate. Lastly, the phosphate group was removed under acidic conditions by acid phosphatase (AP) to furnish D-psicose. Unfortunately, after silica gel and gel filtration purification, only a 12% yield of D-psicose could be obtained (Table 1). Compared to the high yield of D-psicose 1-phosphate when DHAP was used directly for aldol addition26, this low yield can most likely be attributed to the possibility that FucAE.coli may not be compatible with the one-pot system.
Scheme 2.
Synthesis of D-psicose with FucA and acid phosphatase (see Table 1 for yields and product ratio).
Table 1.
Summary of FucA catalyzed reactions using different starting materials and acceptors.
| Enzyme | Starting Saterial | Acceptor | Product(s) | Yield | Ratio | |
|---|---|---|---|---|---|---|
| FucAE.coli | L-GP | D-glyceraldehyde | D-psicose | 12% | 1/0 | |
| FucAT.HB8 | L-GP | D-glyceraldehyde | D-psicose | D-sorbose | 67% | 5.3/1 |
| FucAT.HB8 | DL-GP | D-glyceraldehyde | D-psicose | D-sorbose | 58% | 8.4/1 |
| FucAT.HB8 | DL-GP | L-glyceraldehyde | L-fructose | L-tagatose | 47% | 1.2/1 |
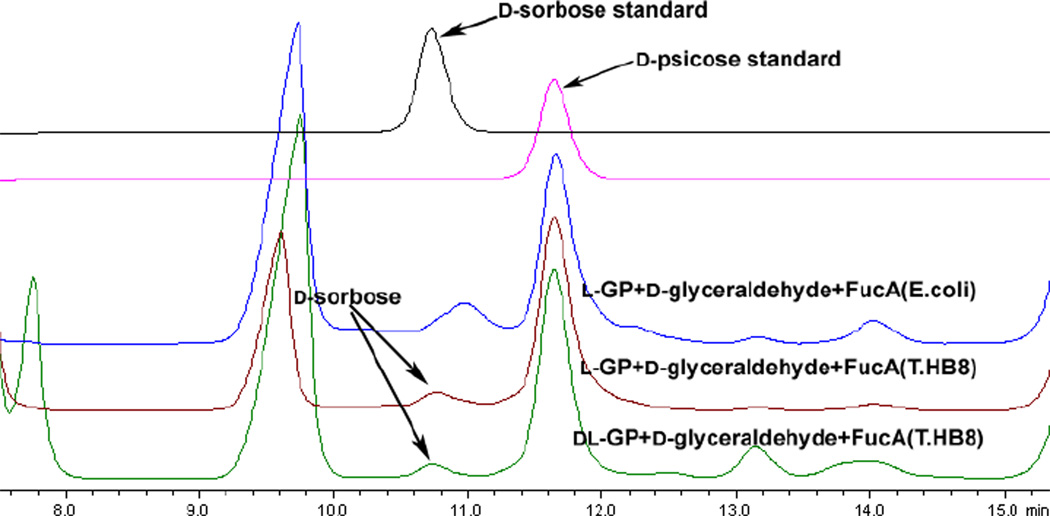
To improve the overall yield for this synthesis, we turned our attention towards enzymes from thermophilic bacteria, which show a great potential for biotechnological applications.28 Upon investigation, we noticed that the crystal structure of FucA from Thermus thermophilus HB8 (FucAT.HB8) was recently reported but the enzyme had yet to be used for synthetic purposes.29 Consequently, we expressed and purified FucAT.HB8 in E.coli (see Supporting Information) and then employed it in the one-pot reaction under the same conditions (Scheme 2). To our delight, the yield was greatly improved (Table 1, 67% vs. 12%) with the diastereomer Dsorbose being detected as a minor product when D-glyceraldehyde was used as the acceptor (Figure 1 maroon line). The product ratio of D-psicose/D-sorbose was ~5:1 as determined by ion exchange HPLC (Figure 1 maroon line).
Figure 1.
HPLC (Hydrogen form, sulfonated divinyl benzene-styrene copolymer support and eluted with 5 mM H2SO4) profile of final reaction mixture in Scheme 2 compared with authentic samples.
L-glycerol 3-phosphate is a reasonably stable starting material and commercially available, it is still quite expensive, and thus not ideal for large-scale synthesis. In contrast, racemic glycerol 3-phosphate (DL-GP) is much cheaper and it has been reported that GPO can exclusively oxidize the L-isomer.10,27 Therefore, it was envisioned that a racemic mixture could deliver a 50% yield of DHAP and the remaining D-glycerol 3-phosphate could be isolated during purification. Most significantly, the synthetic cost could be greatly decreased by utilizing racemic DL-glycerol 3-phosphate (DL-GP). Thus, under the same reaction conditions, staring from racemic DL-GP, a total yield of 58% (Table 1) of D-psicose/D-sorbose was obtained and the ratio was determined by HPLC (Figure 1 green line, D-psicose/D-sorbose 8.4/1). The only difference for reactions starting with DL-GP is that DL-GP was used in excess and the reaction yield was calculated using the acceptor as the limiting reagent (see supporting information for reaction stoichiometry). After silica gel and gel filtration chromatography, the mixture containing D-psicose/D-sorbose could be easily separated by a cation exchange resin column (Ca2+ form) under elevated temperature (70 °C) (see Supporting Information).
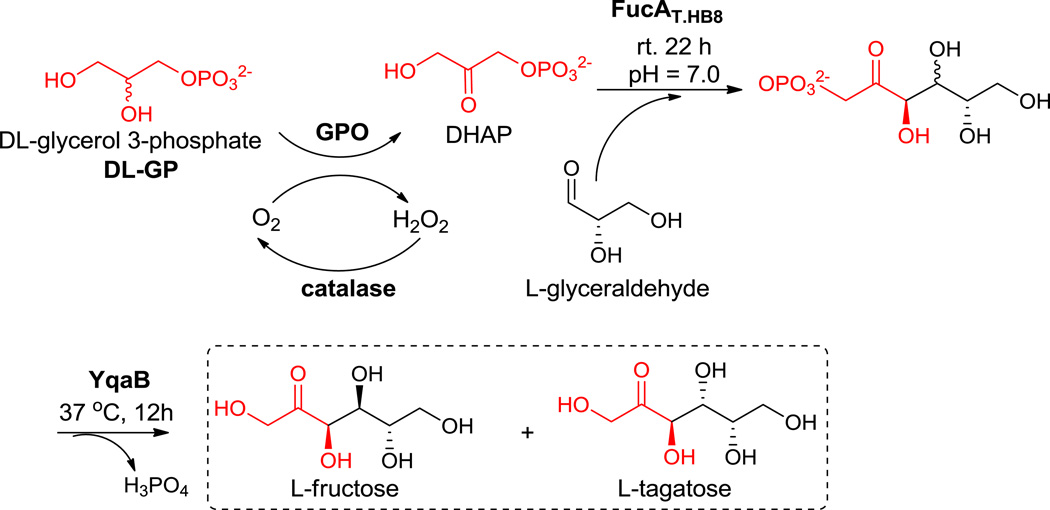
Alternatively, when L-glyceraldehyde was used as the acceptor, instead of the D-isomer, two additional rare sugars, L-fructose and L-tagatose, could accordingly be synthesized as allowed by the stereoselectivity of FucA (Scheme 3). L-fructose is a well known nonnutritive sweetener30 and an inhibitor of several glycosidases31 with its enzymatic synthesis by the aldolase RhaD being greatly exploited by Wong and co-workers.14,23 However, L-tagatose, which is a functional sweetener32 and a promising starting material for the synthesis of high value-added complex compound,33 has not yet been broadly utilized due to its high cost of production. L-Tagatose can be produced via oxidation of galactitol by Klebsiella pneumoniae 40b34 or generated via epimerization of L-sorbose by D-tagatose 3-epimerase from Psedomonas sp. ST-2435, however, both methods give low yields.
Scheme 3.
Synthesis of L-tagatose and L-fructose with FucAT.HB8 using L-glyceraldehyde.
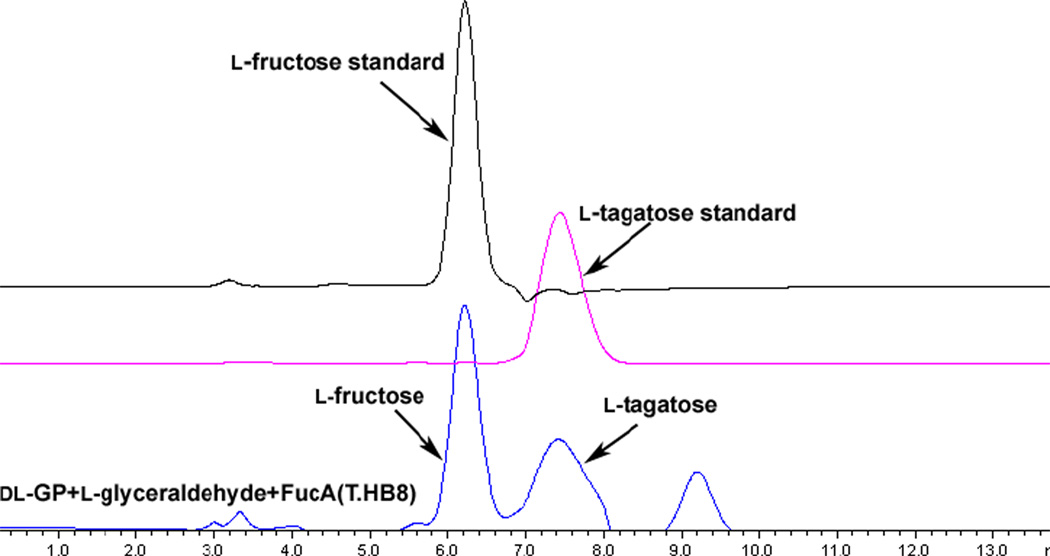
As shown in Scheme 3, we were able to successfully carry out the synthesis of L-fructose and L-tagatose in a one-pot fashion using DL-GP as starting material and L-glyceraldehyde as the acceptor. It is interesting to note that the acid phosphatase (AP) we previously used could not dephosphorylate L-tagatose 1-phosphate completely to give the desired sugar products. However, upon searching relevant literature we found that YqaB phosphatase from E.coli, belonging to the haloacid dehalogenase (HAD) superfamily, shows a remarkably broad substrate range, among which the dephosphorylation activity toward D-fructose-1-phosphate is the highest.36 We thus expressed and purified YqaB phosphatase from E.coli and the dephosphorylation reaction went quite smoothly under neutral conditions. L-Fructose and L-tagatose were afforded in moderate yield (see Table 1, total yield 47%) and the ratio was determined by HPLC (Figure 2, L-fructose/L-tagatose 1.2/1). After silica gel and gel filtration chromatography, the mixture containing L-fructose/L-tagatose could be easily separated by cation exchange resin column (Ca2+ form) under elevated temperature (70 °C) (see Supporting Information). As shown in Table 1, one thing worth noting is that FucAT.HB8 seems to lose its stereoselectivity when accepting L-glyceraldehyde–similar to our previous discovery that L-rhamnulose-1-phosphate aldoalse (RhaD) produces a single product (L-fructose) when using L-glyceraldehyde while losing its stereoselectivity when D-glyceraldehyde is the acceptor. Nonetheless, this property of aldolases could be well utilized for the synthesis of various types of rare sugars.
Figure 2.
HPLC (Calcium form, sulfonated divinyl benzene-styrene copolymer support and eluted with H2O) profile of final reaction mixture in Scheme 3 compared with authentic samples.
In summary, we developed a one-pot four-enzyme approach for the synthesis of the rare sugars D-psicose, D-sorbose, L-tagatose, and L-fructose with aldolase FucA. All synthesized rare sugars were characterized by 1H NMR and compared with commercially authentic samples (see supporting information). To the best of our knowledge, this is the first use of FucA from a thermophilic source (Thermus thermophilus HB8), which proved to be more efficient than its E.coli counterpart. Importantly, the one-pot four-enzyme approach does not require the use of expensive DHAP and most significantly, the inexpensive starting material DL-GP was used to greatly reduce the synthetic cost. We believe this approach could ultimately contribute to the synthesis of other rare sugars and their derivatives as well.
Supplementary Material
Acknowledgement
P.G.W. acknowledges NIH (R01 AI083754, R01 HD061935 and R01 GM085267) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iturrate L, Sanchez-Moreno I, Oroz-Guinea I, Perez-Gil J, Garcia-Junceda E. Chemistry. 2010;16:4018. doi: 10.1002/chem.200903096. [DOI] [PubMed] [Google Scholar]
- 2.Machajewski TD, Wong CH. Angew. Chem. Int. Ed. 2000;39:1352. doi: 10.1002/(sici)1521-3773(20000417)39:8<1352::aid-anie1352>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Fessner WD. Curr. Opin. Chem. Biol. 1998;2:85. doi: 10.1016/s1367-5931(98)80040-9. [DOI] [PubMed] [Google Scholar]
- 4.Castillo JA, Calveras J, Casas J, Mitjans M, Vinardell MP, Parella T, Inoue T, Sprenger GA, Joglar J, Clapes P. Org. Lett. 2006;8:6067. doi: 10.1021/ol0625482. [DOI] [PubMed] [Google Scholar]
- 5.Fessner WD, Helaine V. Curr. Opin. Biotechnol. 2001;12:574. doi: 10.1016/s0958-1669(01)00265-8. [DOI] [PubMed] [Google Scholar]
- 6.Breuer M, Hauer B. Curr. Opin. Biotechnol. 2003;14:570. doi: 10.1016/j.copbio.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Clapes P, Fessner WD, Sprenger GA, Samland AK. Curr. Opin. Chem. Biol. 2010;14:154. doi: 10.1016/j.cbpa.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama M, Hong Z, Greenberg WA, Wong CH. Bioorg. Med. Chem. 2007;15:5905. doi: 10.1016/j.bmc.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Moreno I, Garcia-Garcia JF, Bastida A, Garcia-Junceda E. Chem. Commun. (Camb) 2004:1634. doi: 10.1039/b405220j. [DOI] [PubMed] [Google Scholar]
- 10.Schumperli M, Pellaux R, Panke S. Appl. Microbiol. Biotechnol. 2007;75:33. doi: 10.1007/s00253-007-0882-3. [DOI] [PubMed] [Google Scholar]
- 11.Kramer L, Steckhan E. Tetrahedron. 1997;53:14645. [Google Scholar]
- 12.Oh DK, Kim NH, Kim HJ, Park CS, Kim SW, Ko M, Park BW, Jung MH, Yoon KH. World J. Microbiol. Biotechnol. 2007;23:559. [Google Scholar]
- 13.Poonperm W, Takata G, Ando Y, Sahachaisaree V, Lumyong P, Lumyong S, Izumori K. J. Biosci. Bioeng. 2007;103:282. doi: 10.1263/jbb.103.282. [DOI] [PubMed] [Google Scholar]
- 14.Alajarin R, Garcia-Junceda E, Wong CH. J. Org. Chem. 1995;60:4294. doi: 10.1016/0968-0896(95)00119-2. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Hayakawa S, Ogawa M, Fukada K, Izumori K. J. Agric. Food. Chem. 2008;56:4789. doi: 10.1021/jf800050d. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo T, Suzuki H, Hashiguchi M, Izumori K. J. Nutr. Sci. Vitaminol. 2002;48:77. doi: 10.3177/jnsv.48.77. [DOI] [PubMed] [Google Scholar]
- 17.Oshima H, Kimura I, Izumori K. J. Biosci. Bioeng. 2006;101:280. doi: 10.1263/jbb.101.280. [DOI] [PubMed] [Google Scholar]
- 18.Takeshita K, Suga A, Takada G, Izumori K. J. Biosci. Bioeng. 2000;90:453. doi: 10.1016/s1389-1723(01)80018-9. [DOI] [PubMed] [Google Scholar]
- 19.Itoh H, Sato T, Izumori K. J. Ferment. Bioeng. 1995;80:101. [Google Scholar]
- 20.Kim NH, Kim HJ, Kang DI, Jeong KW, Lee JK, Kim Y, Oh DK. Appl. Environ. Microbiol. 2008;74:3008. doi: 10.1128/AEM.00249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samland AK, Sprenger GA. Appl. Microbiol. Biotechnol. 2006;71:253. doi: 10.1007/s00253-006-0422-6. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama M, Hong Z, Liang PH, Dean SM, Whalen LJ, Greenberg WA, Wong CH. J. Am. Chem. Soc. 2007;129:14811. doi: 10.1021/ja073911i. [DOI] [PubMed] [Google Scholar]
- 23.Franke D, Machajewski T, Hsu CC, Wong CH. J. Org. Chem. 2003;68:6828. doi: 10.1021/jo030021m. [DOI] [PubMed] [Google Scholar]
- 24.Ghalambor MA, Heath EC. J. Biol. Chem. 1962;237:2427. [PubMed] [Google Scholar]
- 25.Joerger AC, Gosse C, Fessner WD, Schulz GE. Biochemistry. 2000;39:6033. doi: 10.1021/bi9927686. [DOI] [PubMed] [Google Scholar]
- 26.Fessner WD, Badia J, Eyrisch O, Schneider A, Sinerius G. Tetrahedron Lett. 1992;33:5231. [Google Scholar]
- 27.Fessner WD, Sinerius G. Angew. Chem. 1994;106:217. doi: 10.1016/0968-0896(94)85012-7. [DOI] [PubMed] [Google Scholar]
- 28.Sakuraba H, Yoneda K, Yoshihara K, Satoh K, Kawakami R, Uto Y, Tsuge H, Takahashi K, Hori H, Ohshima T. Appl. Environ. Microbiol. 2007;73:7427. doi: 10.1128/AEM.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeyakanthan J, Taka J, Kikuchi A, Kuroishi C, Yutani K, Shiro Y. Acta. Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 2005;61:1075. doi: 10.1107/S1744309105036766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin GV, Zehner LR, Saunders JP, Beadle JR. Am. J. Clin. Nutr. 1995;62:1161S. doi: 10.1093/ajcn/62.5.1161S. [DOI] [PubMed] [Google Scholar]
- 31.Muniruzzaman S, Pan YT, Zeng Y, Atkins B, Izumori K, Elbein AD. Glycobiology. 1996;6:795. doi: 10.1093/glycob/6.8.795. [DOI] [PubMed] [Google Scholar]
- 32.Rao D, Gullapalli P, Yoshihara A, Jenkinson SF, Morimoto K, Takata G, Akimitsu K, Tajima S, Fleet GW, Izumori K. J. Biosci. Bioeng. 2008;106:473. doi: 10.1263/jbb.106.473. [DOI] [PubMed] [Google Scholar]
- 33.Yoshihara A, Haraguchi S, Gullapalli P, Rao D, Morimoto K, Takata G, Jones N, Jenkinson SF, Wormald MR, Dwek RA, Fleet GWJ, Izumori K. Tetrahedron: Asymmetry. 2008;19:739. [Google Scholar]
- 34.Shimonishi T, Okumura Y, Izumori K. J. Ferment. Bioeng. 1995;79:620. [Google Scholar]
- 35.Itoh H, Izumori K. J. Ferment. Bioeng. 1996;81:351. [Google Scholar]
- 36.Kuznetsova E, Proudfoot M, Gonzalez CF, Brown G, Omelchenko MV, Borozan I, Carmel L, Wolf YI, Mori H, Savchenko AV, Arrowsmith CH, Koonin EV, Edwards AM, Yakunin AF. J. Biol. Chem. 2006;281:36149. doi: 10.1074/jbc.M605449200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.