Abstract
The use of actigraphs, or ambulatory devices that estimate sleep-wake patterns from activity levels, has become common in pediatric research. Actigraphy provides a more objective measure than parent-report, and has gained popularity due to its ability to measure sleep-wake patterns for extended periods of time in the child’s natural environment. The purpose of this review is: (1) to provide comprehensive information on the historic and current uses of actigraphy in pediatric sleep research; (2) to review how actigraphy has been validated among pediatric populations; and (3) offer recommendations for methodological areas that should be included in all studies that utilize actigraphy, including the definition and scoring of variables commonly reported. The poor specificity to detect wake after sleep onset was consistently noted across devices and age groups, thus raising concerns about what is an “acceptable” level of specificity for actigraphy. Other notable findings from this review include the lack of standard scoring rules or variable definitions. Suggestions for the use and reporting of actigraphy in pediatric research are provided.
Keywords: accelerometer, actigraphy, actiwatch, adolescents, children, infants, pediatric, sleep, wake
Introduction
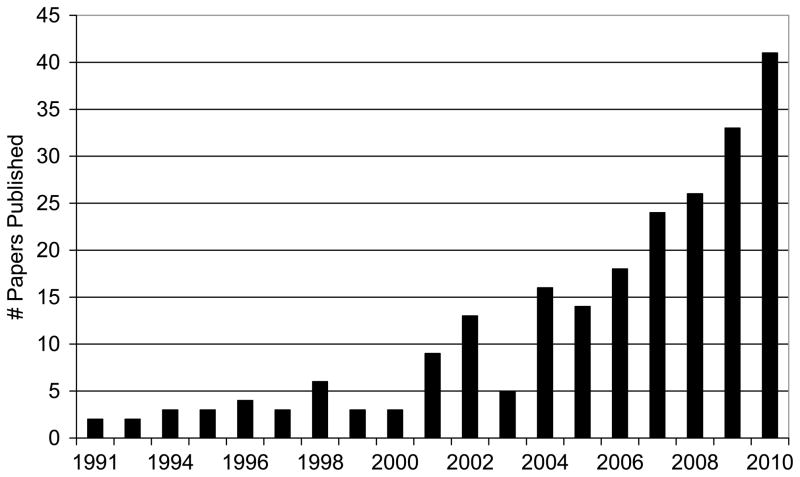
Actigraphy is an objective, non-intrusive method for estimating sleep-wake patterns using activity-based monitoring. The use of actigraphy in research has gained significant popularity over the past 20 years, to the extent that the recent growth in published research studies that used actigraphy have outpaced studies that used polysomnography (PSG).(1) Actigraphy can be a particularly valuable methodology for use among pediatric populations, where the common reliance on parental report alone may limit the range and accuracy of information about children’s sleep. Consistent with the growth of actigraphy use across sleep research domains reported by Sadeh (1), there has also been significant growth in the report of actigraphy specific to pediatric studies (Figure 1), with the number of studies published in 2010 alone (n=41) similar to the total number of studies published from 1991 to 2001 (n=38).
Figure 1.
The number of published research papers that included actigraphy in pediatric populations.
The 2007 American Academy of Sleep Medicine practice parameters state that, “Actigraphy is indicated for delineating sleep patterns, and to document treatment responses in normal infants and children (in whom traditional sleep monitoring by polysomnography can be difficult to perform and/or interpret), and in special pediatric populations.”(2) Despite this recommendation and the growth in the number of studies that utilize actigraphy, there are currently no practice standards regarding recording parameters and scoring of the recorded actigraphy signals. Thus researchers and clinicians have little guidance in the use of this technology with little consistency across the body of literature reporting actigraphy results. This lack of uniformity has resulted in our inability to compare data values across studies. Further, despite the common use of these devices, no normative values are available for pediatric populations.
Validity of Actigraphy in Pediatric Research
Another area of particular concern and utmost importance is the validity of actigraphy as a measure of sleep-wake patterns among children and adolescents of all ages. Many studies discuss the validity of actigraphy compared to PSG, but then cite studies validated on adult samples.(3–5) Actigraphy output provides valuable information about activity levels that are ideal for visual analysis, which is useful for evaluating clinical treatment efficacy or to corroborate parental report of child sleep. However, actigraphically measured sleep estimates among pediatric samples (e.g., total sleep time or wake after sleep onset) should only be used when the recording device and particular sleep value are established as valid in comparison to a ‘gold standard’ measure such as PSG or direct observation.
Correlation statistics are often used to evaluate the validity of actigraphy when compared to a gold standard like PSG. However, correlations alone are not an appropriate way to validate these devices. A perfect correlation can be found between any two instruments, even if they have widely divergent measurement scales, as long as both measures increase at the same proportional rate. Some validation studies have relied only on correlation analyses, while others have overemphasized high sensitivity and underemphasized low specificity. Sensitivity and specificity bear further explanation because they are the most appropriate statistical method for validity assessment.
Sensitivity and specificity are most commonly used in the biomedical field for determining the quality of a novel diagnostic instrument. Sensitivity describes how accurately an instrument identifies people with a disorder (“true positive” cases); the more people who are inaccurately identified as not having the disorder (“false negative” cases), the less sensitive the instrument. On the other hand, specificity describes how accurately an instrument identifies people who do not have the disorder (“true negative” cases); the more people inaccurately identified as having the disorder (“false positive” cases), the less specific the instrument.
An ideal test accurately identifies 100% of both positive cases (is highly sensitive) and negative cases (is highly specific). When it comes to actigraphy, researchers have established a convention of considering sensitivity to be the proportion of epochs scored as sleep using polysomnography that are accurately identified as sleep by actigraphy. Specificity, on the other hand, is the proportion of polysomnography-scored wake epochs accurately identified as wake by actigraphy. An actigraph, or algorithm, that incorrectly scores sleep as wake has low sensitivity, and an actigraph, or algorithm, that incorrectly scores wake as sleep has low specificity. For example, if an actigraph scores an entire sleep period as sleep, it would be 100% sensitive at the expense of poor specificity in identifying wake during the sleep period. Both sensitivity and specificity are inherently important since the incorrect scoring of sleep or wake can result in under or overestimates of reported sleep variables (e.g., total sleep time, wake after sleep onset).
Thus, examinations of sensitivity and specificity, and the relation between the two (represented by the “likelihood ratio”), are necessary for establishing the extent of actigraphy’s validity. Another assessment technique commonly used to examine instrument validity is the Bland-Altman concordance technique (6;7). This approach provides a visual representation of agreement by plotting the new method against the gold standard. The difference between the measures for each participant are fitted to lines that represent the ideal (no difference), plus either standard deviations or time discrepancies to show each participant’s deviation from the ideal.
For a more detailed description of validation and reliability issues pertaining to use of actigraphy, the reader is referred to several comprehensive reviews.(1;8;9)
Study Purpose
The purpose of this paper is to provide a comprehensive review and synthesis of the research literature that used actigraphy as a measure of sleep-wake patterns or circadian rhythms among pediatric populations (ages 0–18 years, inclusive). This review highlights areas that should be considered in the design, execution, and report of sleep-wake variables in pediatric research studies that utilize actigraphy. In addition, a thorough description of the existing research that has examined the validity of these devices is provided. It is important to note that the purpose of this paper is not to provide recommendations about which brand to use or specific ways to improve existing technologies. Further, the clinical utility of actigraphy is beyond the scope of this review. Rather we objectively report on the use of existing technologies, as well as provide evidence-based recommendations for the proper use and reporting of actigraphy in pediatric research.
Methods
A literature search was conducted using the Cumulative Index to Nursing and Allied Health (CINAHL), PsychInfo, and Pubmed. Search terms were (actigraph* or accelerometer or actimeter or actiwatch) and (newborn or infant or toddler or preschool* or child or children or adolescent* or adolescence* or youth or pediatric or premature infant*) and (sleep or rest or circadian). In addition to the electronic search, reference lists from the identified articles and other review papers were reviewed for additional studies.
Inclusion criteria for this review were: (1) a primarily pediatric study sample (ages 0–18 years); (2) an actigraphic device worn on the wrist or ankle/calf (for infants and toddlers) to measure some aspect of sleep or circadian rhythms; and (3) the study was published in English. Papers were excluded if (1) the study sample was primarily adults (with no discreet pediatric data presented); (2) the outcome variable was daytime activity, with no report of actigraphic-derived sleep variable; (3) the report was a single subject case study, (4) the paper was not published in a peer-review journal (e.g., dissertation).
As this review focused on actigraphy methodological issues (as opposed to clinical outcomes), meta-analytic techniques were not used. Instead, results are presented descriptively and are divided into the following sections: (1)Study Sample: papers reviewed and demographic information, (2) Actigraphy Use: devices, software, and placement, (3) Validation: methods and statistics, and (4) Scoring and Interpretation: algorithms and scoring rules. Where appropriate, results are further broken down by age group to highlight developmental issues.
Results
Study Sample
Using the previously described search terms, 491 abstracts for papers published through December 2010 (including electronic publications ahead of print) were identified. An additional 15 articles were identified through a review of reference lists of eligible articles or review papers. Based on a review of these 506 abstracts, 269 papers met inclusion criterion for review. An additional 41 papers were excluded during full paper review because they included primarily adults (n=10), the outcome variable was daytime activity rather than sleep or circadian rhythms (n=8), the actigraph was not placed on the wrist or ankle (e.g., belt or upper arm, n=12), wrist actigraphy was not used (e.g., piezo-electric pads, n=8), or it was a case study/report (n=3). Thus the analyses are based on a final review of 228 papers.
During the review, a number of studies were identified (e.g., same authors, same grant funding) as potentially including the same or overlapping samples and methodologies. To confirm the overlap, primary or corresponding authors were contacted via email. These articles are identified with an asterisk (*) in the References Section, highlighting that the study methodologies may not be independent. To prevent overinflating our results (e.g., the frequency with which certain devices were used), analyses were rerun using only one representative paper per sample. As seen in Table 1, no significant differences were found between the full sample and the limited sample in terms of the proportion of reported actigraph brands, placement of actigraphs, algorithms/sensitivities, or epoch lengths. Thus, the following results include all 228 papers reviewed.
Table 1.
Comparison between Full Sample of Studies (All Papers) and Subgroup of Studies with Overlapping Data Eliminated (Limited Sample)
| All Papers (n=228) | Limited Sample (n=166)a | ||
|---|---|---|---|
| % (n) | % (n) | ||
| Brand of Actigraphy | X2(4) = 1.11 | ||
| Ambulatory-Monitoring Inc. | 56.8 (130) | 56.0 (93) | |
| Mini-Mitter | 22.7 (52) | 21.7 (36) | |
| Cambridge Actiwatch | 12.6 (29) | 11.4 (19) | |
| Other Brands | 3.4 (8) | 4.8 (8) | |
| Not Reported | 4.4 (10) | 6.0 (10) | |
|
| |||
| Actigraph Placementb | X2(4) = 0.39 | ||
| Non-dominant Wrist | 44.7 (102) | 42.2 (70) | |
| Dominant Wrist | 3.9 (9) | 4.8 (8) | |
| Wrist Unspecified | 20.1 (46) | 20.5 (34) | |
| Ankle/Calf | 22.4 (51) | 22.9 (38) | |
| Not Reported | 8.3 (19) | 9.0 (15) | |
|
| |||
| AMI Algorithms | X2(3) = 0.21 | ||
| Sadeh | 65.4 (85) | 65.6 (61) | |
| Cole-Kripke | 5.4 (7) | 6.5 (6) | |
| UCSD | 1.5 (2) | 1.1 (1) | |
| Not Reported | 27.7 (36) | 26.9 (25) | |
|
| |||
| AMI Epoch Length | X2(2) = 0.28 | ||
| 30-Second | 1.5 (2) | 2.2 (2) | |
| One-Minute | 65.4 (85) | 64.4 (58) | |
| Not Reported | 33.1 (43) | 35.5 (33) | |
|
| |||
| Mini-Mitter Sensitivityc | X2(4) = 0.90 | ||
| Medium | 44.2 (23) | 36.1 (13) | |
| Automatic | 3.9 (2) | 2.8 (1) | |
| Multiple | 7.7 (4) | 11.1 (4) | |
| Study Specificy | 3.9 (2) | 2.8 (1) | |
| Not Reported | 36.5 (19) | 41.7 (15) | |
|
| |||
| Mini-Mitter Epoch Lengthd | X2(3) = 0.62 | ||
| 15-Second | 7.7 (4) | 5.6 (2) | |
| 30-Second | 11.5 (6) | 8.3 (3) | |
| One-Minute | 59.6 (31) | 58.3 (21) | |
| Not Reported | 17.3 (9) | 22.2 (8) | |
Limited sample includes only one manuscript when multiple studies were published using overlapping data
One study used both wrists
One study used the Low sensitivity and one study used the High sensitivity
Two studies used a 15-minute epoch, one study a 15-second or 30-second epoch, and one study a 10-second epoch
In total, actigraphy was reported for 16,788 subjects (mean subjects per study = 73.6, SD ± 74.9, median = 48.0, range = 3 – 387 subjects). Age was divided into developmental groups as follows: infants (0–11.9 months), toddlers (12–35.9 months), preschoolers (3–5 years), school-aged (6–12 years), and adolescents (13–18 years). Eighty-two studies (36.0%) reviewed included subjects from two age groups, 13 studies (5.7%) included subjects from three age groups, and six studies (2.6%) included subjects from four age groups. School-aged children were most commonly studied (n=136 studies, 59.6%), followed by adolescents (n=89, 39.0%), preschoolers (n=55, 24.1%), infants, (n=41, 18.0%), and toddlers (n=33, 14.5%).
Fifty-three studies (23.2%) included children with developmental disorders (attention-deficit hyperactivity disorder=23, autism spectrum disorder=19, intellectual disability=11). Forty-two studies (18.4%) included children with chronic illnesses, including cancer, asthma, chronic pain, diabetes, obesity, among many others. Insomnia was the primary sleep disorder studied (n=32, 14.0%); seven studies (3.1%) focused on youth with obstructive sleep apnea.
Actigraphy Use
The majority of studies (n=130, 56.8%) used Ambulatory Monitoring Inc. actigraphs (Ardsley, New York). Mini-Mitter actigraphs (now owned by Phillips-Respironics, Bend, Oregon) were the second most commonly used (n=52, 22.7%). Cambridge Actiwatch actigraphs (Cambridge, UK) were the third most commonly used (n=29, 12.6%). Eight studies (3.4%) reported using a brand other than these three (i.e., Gaehwiler (3), American Military Inc., Healthdyne, Individual Monitoring Systems, Somnitor, Somnowatch). Ten studies (4.4%) did not specify the type of actigraph used.
Computer-based software is interfaced with devices to convert actigraphically measured activity counts into sleep and wake epochs; some software programs provide automatic scoring options for certain variables (e.g., sleep onset time). Although most investigators used the software that was provided by the actigraph’s manufacturer, 60 studies (26.3%) did not report on the type of scoring software used and 10 studies (4.4%) used a software program created specifically for that particular study or laboratory.
Actigraph placement for pediatric populations is most commonly recommended on the non-dominant wrist for older children and the ankle/calf for infants and toddlers (8). In this review, wrist placement was most common (68.5%); 102 studies (44.7%) used the non-dominant wrist, 9 studies (3.9%) used the dominant wrist, 1 study (0.4%) used both wrists, and 46 studies (20.2%) did not report which wrist was used. Fifty studies (22%) placed the actigraph on an ankle (or calf for some infants); all but one of these studies focused on young children (under five years). Actigraphy placement was not reported in 19 studies (n=8.3%).
Validation
Our review revealed 15 published validation-type studies that used pediatric samples, all but three of which were published in the last decade. Among these 15 papers, one was for the purpose of algorithm development rather than assessment (10) and two were comparisons of actigraphy placement only (11;12). Among the remaining 12 studies, two either only used correlation statistics or did not report sensitivity and specificity.(13;14) The remaining 10 validation-type studies reported sensitivity and specificity; of these, seven were direct comparisons between actigraphy and polysomnography (15–21); two compared actigraphy to direct observation (22;23), and one compared actigraphy to videosomnography.(24)
Together these 10 validation-type studies reported results from a cumulative 931 children (Table 2). Five studies (total n=449 subjects) focused specifically on infants, one study (n=11 subjects) focused on toddlers, one study (n=58 subjects) focused on preschool-age children, one study (n=16 subjects) focused on adolescents, and two studies (total n=175 subjects) included a range of ages that encompassed all of these groups. Among these, only one study included children with developmental disorders and two studies (with overlapping samples) included children with sleep disordered breathing.
Table 2.
Actigraphy Validation Studies Compared to Polysomnography (6), Direct Observation (2), or Videosomnography (1) that Report Sensitivity and Specificity
| N | Age(s) | Standard | Sensitivity | Specificity | Brand | Placement | Software | Epoch | Algorithm | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hyde et al. (2007) | 45 | 1–12 y | PSG | 96.5 | 39.4 | AW-64 | NDW | AW 3.3 | 30 s | low |
| 93.9 | 59.0 | medium | ||||||||
| 91.1 | 68.9 | high | ||||||||
| 97.7 | 39.4 | auto | ||||||||
|
| ||||||||||
| Insana et al. (2010) | 22 | 14 m | PSG | 92.4 | 58.9 | AW-64 | ankle | AW 5.5 | 15 s | medium |
|
| ||||||||||
| O’Driscoll et al. (2010) | 130 | 2–18 y | PSG: AI(>10/h) | 82.2 | 50.9 | AW-64: FI | NDW | AW 3.3 | 30 s | auto |
| PSG: OAHI(>1/h) | 12.8 | 97.6 | AW-64: FI | |||||||
| PSG: AI(>10/h) | 78.1 | 52.6 | AW-64: WB | |||||||
| PSG: OAHI(>1/h) | 14.9 | 98.8 | AW-64: WB | |||||||
|
| ||||||||||
| Sadeh et al. (1991) | 11 | 12–48 m | PSG | 87.7 | 76.9 | AMI | left leg | AMI | 1 m | Sadeh |
|
| ||||||||||
| Sadeh et al. (1994) | 16 | 10–16 y | PSG | 95.0 | 74.5 | AMI-32 | NDW | AMI | 1 m | n/a |
|
| ||||||||||
| Sadeh et al. (1995) | 10 | Newborn | Direct observation | 74.9 | 82.8 | AMI-32 | left ankle | AMI | 1 m | ** |
| 11 | 3 m | 87.3 | 92.5 | |||||||
| 10 | 6 m | 83.2 | 97.8 | |||||||
| 10 | 12 m | 99.3 | n/a | |||||||
|
| ||||||||||
| Sitnick et al. (2008) | 58 | 28–73 m | videosomnography | 97.0 | 24.0 | AW-64 | ND ankle | AW | 1 m | medium |
|
| ||||||||||
| So et al. (2005) | 8 | 2–4 wk | PSG | 96.2 | 54.6 | AW-64 | R leg | AW 3.3 | 1 m | low |
| 90.1 | 62.9 | med | ||||||||
| 80.6 | 82.6 | high | ||||||||
| 93.6 | 55.9 | auto | ||||||||
|
| ||||||||||
| 13 | 5–6 m | 88.6 | 76.3 | low | ||||||
| 78.6 | 87.6 | med | ||||||||
| 67.6 | 93.4 | high | ||||||||
| 91.2 | 69.9 | auto | ||||||||
|
| ||||||||||
| 11 | 2–4 m | 94.0 | 38.5 | low | ||||||
| 87.5 | 63.5 | med | ||||||||
| 79.8 | 61.7 | high | ||||||||
| 93.9 | 30.7 | auto | ||||||||
|
| ||||||||||
| Sung et al. (2009) | 8 | 30–33 wga | direct observation | 88.2 | 33.6 | AW-64 | R leg | AW 3.3 | 1 m | low |
| 77.5 | 46.3 | med | ||||||||
| 66.0 | 57.5 | high | ||||||||
| 83.7 | 40.4 | auto | ||||||||
|
| ||||||||||
| 20 | 34–36 wga | 93.3 | 31.5 | low | ||||||
| 86.0 | 45.9 | med | ||||||||
| 76.6 | 61.0 | high | ||||||||
| 90.8 | 39.0 | auto | ||||||||
|
| ||||||||||
| 10 | 37–40 wga | 96.8 | 31.8 | low | ||||||
| 92.9 | 46.8 | med | ||||||||
| 88.7 | 55.0 | high | ||||||||
| 96.7 | 32.3 | auto | ||||||||
|
| ||||||||||
| Tilmanne et al. (2009)* | 354 | <1 year | PSG | 81.3 | 61.2 | Healthdyne | Ankle | Not provided | 30 s | Sadeh |
| 87.1 | 53.1 | Sazonov | ||||||||
Notes. wga=weeks, gestational age, PSG=Polysomnography, AI=arousal index, OAHI=obstructive apnea/hypopnea index, AW-64=Philips, Respironics (Mini Mitter) Actiwatch 64, AMI=Ambulatory Monitoring, Inc., FI=fragmentation index, WB=wake bouts/hour, NDW=non-dominant wrist, s=seconds, m=minutes
These were the default settings. Tilmanne et al. also evaluated optimized and extended performances, as well as novel algorithms
Novel algorithm calibrated on the 3-month infant data
Although these validation-type studies utilized different devices, recording parameters, and scoring algorithms, they uniformly reported high sensitivity and low specificity (Table 2). The infant studies reported ranges of sensitivities = 83.4 – 99.3 and specificities = 17.0 – 97.8; the toddler study reported a sensitivity = 87.7 and a specificity = 76.9; the preschooler study reported a sensitivity = 97.0 and a specificity = 24.0; and the adolescents study reported a sensitivity = 95.0 and a specificity = 74.5. Studies that included multiple age groups reported a sensitivity range = 82.2 – 90.1 and a specificity range = 50.9 – 72.8. In other words, across devices, placement sites, and algorithms, actigraphy was consistently good at accurately identifying sleep periods, but less accurate in identifying wake after sleep onset among pediatric populations. In fact, 55% of the specificities reported in Table 2 were less than 60.0.
Two examples provide a meaningful and practical perspective. First, the default wake threshold values for actigraphy from 14-month old infants underestimated total sleep time by more than one hour among 55% of infants when compared to polysomnography; furthermore the range of differences between actigraphy and polysomnography for total sleep time ranged from 6 to 276 minutes.(20) Second, videosomnography on preschool-age children with and without developmental disorders underestimated sleep time and overestimated wake after sleep onset.(24) More concerning, these authors reported instances when the child was clearly awake and moving on the video recording, yet the actigraph reported that the child was asleep. Although variability in actigraphy placement (ankle versus wrist) and sensitivity thresholds may have contributed to these findings, more research and development are clearly needed to improve the validity, in particular the specificity, of actigraphy for use among all pediatric populations.
Scoring and Interpretation
Scoring algorithms or wake threshold sensitivity levels were not reported in 72 studies (31.4%). The most common epoch length studied was 1-minute (n=139, 60.4%); only 16 studies (7.0%) used 30-second epochs. Epoch length was not reported in 63 studies (27.4%). Since algorithms/sensitivities and epoch lengths are somewhat dependent on the device used, the following sections present this information for the three most commonly used actigraphy brands.
AMI devices (n=130)
The most commonly used scoring algorithm for the AMI devices was the Sadeh algorithm (n=85 studies, 65.4%). Seven studies (5.4%) used the Cole-Kripke algorithm, and two studies (1.5%) used the UCSD algorithm. No scoring algorithm was reported for 36 studies (27.7%) that used AMI devices. One-minute epochs were the most commonly reported epoch length in 85 studies (65.4%). Only two studies that used AMI devices used a 30-second epoch length (1.5%). Forty-three studies (33.1%) did not report the epoch length used. AMI devices offer three different data collection modes; 43 studies (33.0%) used the Zero Crossing Mode (ZCM), 7 studies (5.4%) used the Time Above Threshold Mode (TIM), and 1 study (0.8%) used the Proportional Integration Measure (PIM). Seventy-nine (60.8%) studies did not report the data collection mode.
Mini-Mitter devices (n=52)
The most commonly used sensitivity (wake threshold) setting for the Mini-Mitter devices was the Medium sensitivity (n=23 studies, 44.2%). Four studies (7.7%) used multiple thresholds, while other studies reported using the Low sensitivity (n=1, 1.9%), High sensitivity (n=1, 1.9%), or Automatic (n=2, 3.9%) thresholds. Two studies (3.9%) used a sensitivity threshold designed for that study, while 19 studies did not report a sensitivity/threshold setting (36.5%). One-minute epochs were the most commonly reported epoch length in 31 studies (59.6%). Six studies (11.5%) used a 30-second epoch length and 4 studies (7.7%) used a 15-second epoch length. Nine studies (17.3%) did not report the epoch length that was used.
Cambridge devices (n=29)
The most commonly used sensitivity (wake threshold) setting for the Cambridge devices was the Medium sensitivity (n=14 studies, 48.3%). Two studies used the Automatic (6.9%) threshold. One study (3.4%) used a sensitivity threshold designed for that study, while 12 studies did not report a sensitivity/threshold setting (41.4%). One-minute epochs were the most commonly reported epoch length in 17 studies (58.6%). Four studies (13.8%) used a 30-second epoch length, 3 studies (10.3%) used a 2-minute epoch length, and one study (3.4%) used a 15-second epoch length. Four studies (13.8%) did not report the epoch length that was used.
As recommended by Acebo et al. (25), five to seven nights was the most common data collection length across devices (n=106, 46.5%). Three studies (1.3%) did not report a data collection length. Less than 5 nights of actigraphy was reported in 27.6% of studies (n=63), 11 of these (4.8%) only included one night of recording (notably 9 of the 11 studies were validation studies that compared a single night of actigraphy to concurrent PSG recordings). Fifty-six studies (24%) included more than 7 nights of actigraphy data collection, 14 studies (6.1%) included more than two weeks of actigraphy. (Note: studies that included multiple non-consecutive data collection times, e.g., three nights each on three separate occasions, were coded as three nights since each measurement time was averaged across three nights, not nine nights). We attempted to determine how many weekdays and weekend days were included across studies, but over 70% of studies did not break down how many of each were included, and we avoided inference based solely on the number of days studied.
Daily sleep diaries are necessary to help score sleep onset and sleep offset times, as well as identify artifact, including times when the device is removed. Only about half of the reviewed studies reported using some form (e.g., paper, electronic) of parent-reported daily sleep diary (52.6%, n=120), while 70 studies (30.7%) used a child-reported diary. Fourteen studies (6.1%) included both a parent- and child-reported sleep diary. In addition to paper or electronic diaries, seven studies (3.1%) used a daily telephone call to or from parents, and 5 studies used a daily telephone call to or from children. Twenty-three studies (10.1%) used an event marker to indicate sleep onset and sleep offset (16 out of 23 studies also included a parent or child sleep diary).
Scoring Rules
The use of specific actigraphically measured sleep variables and their corresponding reported definitions are indicated in Table 3. The most frequently reported actigraphically measured sleep variables across studies were “sleep duration” (n=156, 68.4%), “sleep efficiency” (n=131, 57.5%), and “bedtime/sleep onset” (n=109, 47.8%). One-hundred fifty-nine studies (69.9%) provided a definition for the reported actigraphy measures.
Table 3.
Frequency (percentage) of actigraphy variables reported along with primary and secondary scoring definitions used for each variable (N = 228)
| Variable | Reported (%) | Definition (%) | Primary Scoring Definition (n, %) | Secondary Scoring Definition (n, %) |
|---|---|---|---|---|
| Bedtime/Sleep Onset | 109 (47.8) | 81 (74.3) | Time of first (predetermined) consecutive minute with decreased activity below a threshold: (n=38, 34.9) | Identified by diary: (n=13, 11.9) |
| Sleep Onset Latency | 68 (29.8) | 46 (66.2) | From bedtime to sleep onset: (n=20, 29.4) | From lights out to sleep onset: (n=17, 25.0) |
| Nocturnal Wake Frequency | 75 (32.9) | 51 (68.0) | Consecutive minutes of wake during a sleep interval: (n=30, 40.0) | Number of transitions from sleep to wake: (n=17, 22.7) |
| Nocturnal Wake Duration | 30 (13.2) | 17 (56.7) | Minutes awake between sleep onset and offset: (n=13, 44.3) | Number of awakenings: (n=3, 10.0) |
| Wake After Sleep Onset (WASO) | 44 (19.3) | 28 (63.6) | Minutes scored as wake during sleep duration period: (n=20, 45.5) | Percentage of time spent awake after sleep onset: (n=4, 9.1) |
| Midpoint | 3 (1.3) | 3 (100.0) | Half of the sleep duration: (n=3, 100.0) | - |
| Wake time/Sleep Offset | 78 (34.2) | 55 (70.5) | Time of last (predetermined) consecutive minute of sleep: (n=33, 42.3) | Identified as rise/end/sleep offset time: (n=7, 9.0) |
| Naps | 22 (9.4) | 14 (63.6) | Duration of daytime napping: (n=9, 40.9) | Percent of sleep during the daytime period: (n=3, 13.6) |
| Sleep Period | 61 (26.8) | 53 (86.9) | Time from sleep onset to awakening: (n=40, 65.6) | Time spent asleep: (n=6, 9.8) |
| Sleep Duration | 156 (68.4) | 111 (71.2) | Time spent sleeping during a determined interval: (n=69, 44.2) | Time from bedtime/sleep onset to sleep end: (n=28, 18.0) |
| Sleep Efficiency | 131 (57.5) | 86 (65.7) | Percentage of time spent asleep during a defined interval: (n=51, 38.9) | Total sleep time divided by time in bed: (n=18, 13.7) |
| Fragmentation | 14 (6.1) | 12 (85.7) | Index of activity during sleep: (n=4, 28.6) | Measure of movement or wake during sleep: (n=3, 21.4) |
| Longest Sleep | 25 (11.0) | 13 (52.0) | Longest continuous episode scored as sleep: (n=12, 48.0) | Longest duration of no activity: (n=1, 4.0) |
Note. ‘Reported (%)’ = The frequency and percent of all studies examined that reported the corresponding study variable. ‘Definition (%)’ = The frequency and percent of studies that reported the corresponding variable and also provided a scoring definition for the variable. ‘Primary Scoring Definition (%)’ = The most commonly reported scoring definition as a function of all studies that reported the corresponding variable. ‘Secondary Scoring Definition (%)’ = The second most commonly reported scoring definition as a function of all studies that reported the corresponding variable. Scoring definitions were clustered according to thematic similarity (e.g. when clustering the definitions for sleep duration ‘time scored as sleep during a sleep interval’ was similar to ‘minutes of sleep in sleep period minus minutes scored as wake’ but different than ‘time from sleep onset until waking’).
Many actigraphy variables, including “bedtime/sleep onset”, “wake time/sleep offset”, and “nocturnal wake” were calculated using specific time-based scoring rules. There is a lack of consistency in these rules. The most common scoring definition for “bedtime/sleep onset” was the number of consecutive minutes of decreased activity below a specific threshold. The three most frequently used rules for “bedtime/sleep onset” were 3 (n=18), 10 (n=9), and 15 (n=4) consecutive minutes scored as sleep, although studies also used 1, 5, and 20-minute rules. The primary scoring definition for “wake time” was the last minute of a predetermined number of minutes scored as sleep prior to wake. The three most frequently utilized time rules for “wake time” were 5 (n=18), 10 (n = 9), and 1 (n = 3) minutes scored as sleep before wake, although some studies used 3 and 15 minute rules.
The primary scoring definition for “nocturnal wake frequency” was a consecutive number of minutes scored as wake during a sleep interval. The number of minutes required to score “nocturnal wake frequency” was ≥5 minutes of wake in all studies that reported this particular scoring definition (n = 30).
Although Table 3 captures many of the commonly reported actigraphy variables, several nontraditional actigraphy measures were also found. Examples of variables that lack consistent definition across studies include: active sleep, quiet sleep, motionless sleep, activity mean, standard deviation of activity mean, variability in sleep schedule, coefficient of variation, motor activity level, actigraph pattern rating, activity per four hour interval, wake-onset instability, motionless sleep percentage, sleep quality, extremely long sleep, very short sleep index, insufficient sleep, prolonged sleep onset latency, activity level sleep transition rates, and variability in sleep schedule.
Discussion
Use of Actigraphy in Pediatric Sleep Research
The purpose of this review was to describe methodological issues with actigraphy in pediatric sleep research. Although actigraphy is by no means a “gold standard” measure of sleep, the rapid growth in its use over the past 20 years in pediatric research and the sheer volume of studies published that utilize actigraphy demonstrate the importance of these devices as an estimate of sleep-wake patterns. Despite the growth in actigraphy use, this review highlights several notable concerns for how the field of pediatric sleep is currently using and reporting on actigraphy. The cumulative results of this review support the need for the development of practice parameters for the use of actigraphy among pediatric populations, including standardization for the scoring and reporting of actigraphy variables.
The first notable finding is the lack of consistency across studies in reporting on methodological aspects of actigraphy use; including devices, software, placement, algorithms, epoch length, and scoring rules. Additionally, there is a lack of consistency in the reporting of actigraphically measured variables. With the lack of a standardized approach to the utilization and reporting of actigraphy, comparisons of results between studies are limited.
The second notable finding is the consistent report of poor specificity across all age groups and devices. While more than half of the reported specificities fell below 60%, only one in five were above 80%. Most authors tend to focus less on the low specificity and more on high specificity when concluding that actigraphy is an “acceptable” way to assess sleep-wake patterns, which is somewhat misleading. Thus with the rapid increase in the use of actigraphy in pediatric sleep research, researchers and consumers of this literature need to be aware of the limited ability of actigraphy to accurately detect wake after sleep onset among pediatric populations. This should be a priority for product improvement.
The third notable finding was that only nine (out of 228) studies examined and reported appropriate measures to assess the validity of actigraphy against a “gold-standard” sleep measure (e.g., PSG) in pediatric populations. Although these nine validation studies may not differ markedly from adult research, they encompassed different brands, scoring algorithms/sensitivity thresholds, and multiple age groups, at best providing one or two validation studies per the most frequently-used devices for each age group (with the exception of infants). A more recent validation study demonstrated that different devices and scoring algorithms do not perform equally across age groups.(26)
Areas for Further Validation
As previously described, statistics used to evaluate the validity of actigraphy devices (e.g., correlations vs. sensitivity and specificity) varied across studies, calling into further question the validity of actigraphy to estimate sleep-wake patterns among pediatric populations. Since sleep changes rapidly across development, additional research is needed to demonstrate the validity of different devices and scoring thresholds for each developmental age group. Because of known differences in pediatric sleep there are separate standards for scoring PSG for children and adults.(27–30) Similarly, developmental differences need to be considered in the validation of actigraphy.
Beyond further development of actigraphic devices and algorithms/sensitivities, additional validation studies are needed to address the appropriate placement of the devices. This review only included studies that used a wrist or ankle/calf (for infants) placement because the validity of these placements have been assessed in more than one study. Further research is needed to validate the use of actigraphs placed on the upper-arm, belt, or shirt pocket. Additional studies that compare two brands of devices side-by-side (and ideally against PSG) are also needed. In this review, only the Weiss et al. (14) study compared two brands of actigraphic devices, reporting strong correlations for TST (r = 0.88) but less strong correlations for sleep efficiency (r = 0.65), a likely result of the low sensitivity of these devices to detect wake after sleep onset.
Further research is also needed to examine the validity of algorithms and wake sensitivity thresholds. Algorithms and sensitivity thresholds are likely to be sensitive to developmental state and/or sleep disorders (e.g., sleep disordered breathing). Users should select the appropriate algorithm/sensitivity threshold based on existing validation studies, and should be cautious about simply using default settings and/or autoscoring features (e.g., AMI auto set down interval or Mini-Mitter automatic set rest interval).
Reporting of Actigraphy Data
Significant inconsistencies were noted in this review for how variables were defined, with some definitions deviating from what the variable may be traditionally understood to represent. For example, some authors described “bedtime” as the first minute of actigraphically identified sleep, whereas others used this as a definition of “sleep onset.” As another example, some authors reported “wake time” as the number of minutes the child was awake after sleep onset, whereas others used the same term to describe the parent-reported time the child awakened in the morning. Differences in variable terminology and meaning might appear subtle or fluid, or a function of semantics, but it is essential that sleep variables and their meanings be used consistently for effective communication and interpretation. Due to the variety of ways that a variable can be calculated and/or defined, standardized definitions are clearly needed. Table 4 provides a recommended list of common variable names and definitions that should be considered by researchers for inclusion in reports of studies that use actigraphy.
Table 4.
Proposed Variable Definitions for Actigraphy When Used in Pediatric Research
| Reported Variables | |
| Bedtime | Clock time attempted to fall asleep as indicated by either sleep diary or event marker |
| Wake Time | Clock time of final awakening in the morning as indicated by either sleep diary or event marker |
| Sleep Opportunity (Time in Bed) | Time between Bedtime and Wake Time (reported in minutes or hours) |
| Actigraphy Variables | |
| Sleep Onset | Clock time for first of a predetermined number of consecutive minutes of sleep following reported Bedtime |
| Sleep Offset | Clock time for last of a predetermined number of consecutive minutes of sleep prior to reported Wake Time |
| Sleep Period | Duration between Sleep Onset and Sleep Offset (reported in minutes or hours) |
| Total Sleep Time (TST) | Duration of sleep in Sleep Period (reported in minutes or hours) |
| Sleep Onset Latency | Time between Bedtime and Sleep Onset (reported in minutes) |
| Wake After Sleep Onset (WASO) | Number of minutes scored as wake during Sleep Period |
| Sleep Efficiency | (TST ÷ time in bed) × 100 (expressed as percent) [Alternatively: (TST ÷ Sleep Period) × 100 (expressed as percent)] |
| Night Waking | Predetermined number of minutes of wake (e.g., 5 minutes) preceeded and followed by predetermined number of minutes of sleep (e.g., 15 minutes) |
| Night Waking Frequency | Number of Night Wakings |
| Night Waking Duration | Sum or average of minutes scored as Night Waking |
| 24 Hour Sleep Duration | Amount of sleep in 24 hour period (reported in minutes or hours) |
| Less Commonly Reported | |
| Midpoint | Clock time halfway between Sleep Onset and Sleep Offset |
| Naps (Frequency and/or Duration) | Scoring rules similar to “Night Waking” required to define “Nap” in terms of the predetermined number of minutes asleep preceeded and followed by predetermined number of minutes of wake. Then can define Nap Frequency and Nap Duration |
Due to the lack of reported data across studies, one variable not examined in this review was the amount of data that were lost due to technical failure or participant non-adherence to wearing the device. Investigators who have used actigraphy understand that these two issues are quite common. Furthermore, data loss can be a result of a lack of diary data, which is needed to identify artifact and set sleep intervals. Acebo et al. (25) found that up to 28% of weekly recording may be unusable due to child illness, non-adherence, or device failure. Similarly, a recent study of healthy adults found that 27% of actigraphy nights were unscorable due to missing data (compared to only 1.5% of sleep diary nights).(31)
Finally, there has been little validation of many of the variables automatically calculated by manufacturer scoring programs (e.g., sleep bouts, wake bouts, motionless sleep or immobile time, circadian parameters).(9) Additional work is needed to validate these variables.
Considerations for the Use of Actigraphy in Pediatric Sleep Research
A comprehensive review of the methodological challenges and considerations for the general use of actigraphy can be found in a recent review of actigraphy use with adult populations by Berger et al. (32). The following provides a highlight of these considerations specific to pediatric sleep research.
Choosing the device
The selection of an actigraph for use in a research study should be based on a number of factors. First, researchers should consider the cost of an actigraphy system. This includes the device, which can vary in cost depending on the features (e.g., event marker, light sensor, off-wrist detection). Actigraphy interface and software are additional expenses. Some devices require new batteries to be installed every 45 days (or preferably changed for each new subject), which over the long-term can result in increased cost. Other devices have rechargeable batteries. For software, some programs may require only one license to be installed on multiple computers, while other programs may require a separate license be purchased for every computer, resulting in increased expense. Finally, it is important to consider warranties, maintenance, and technical support (for the device and software); for example, one company suggests that device maintenance occurs every 12 months at a cost of more than $100 per device.
Another area of consideration is the population of youth for the study. Actigraph devices vary by size and weight, which may impact the comfort level of participants, in particular, young children. Consideration should also be given to the durability and water resistance of the devices. Most importantly, actigraphy systems should be validated among the population of interest. If the selected device or settings have not yet been validated and reported in the literature, researchers should perform a validation study prior to use in their primary study.
Study Design
Once the actigraphy system has been chosen, researchers need to consider the use of the device in the specific study. For example, how will the actigraph be delivered to and collected from study participants? What is the length of time for data collection? Based on the existing literature, to obtain five nights of actigraphic data, a minimum of seven recording nights are usually necessary.(25) If the difference in weekday and weekend sleep is of interest, then two weeks of data should be collected. Other data collection considerations include the epoch length and the mode of data collection (e.g., ZCM, TAT, PIM, or TRI).
Variables to be assessed should be selected a priori and be well-defined. Consideration should be given to the research questions, as well as the validity of the variables of interest. For example, without accurate sleep diaries or event marker use, it is not possible to determine sleep onset latency. With this in mind, decisions need to be made about who will complete the daily sleep diary (i.e., parent, child); the frequency of diary completion (e.g., daily versus once in the morning and/or once in the evening); and the format of the diaries (e.g., paper, electronic, web-based, and/or phone call).
How to Handle Data
Prior to data collection (and download) decisions need to be made regarding how the data will be processed and analyzed. First, diary data should be used to reduce artifact, including the removal of devices (e.g. for bathing, swimming), prolonged periods of quiet activity (e.g. going to the movies, watching television in bed), periods of activity that should be scored as sleep (e.g. child sleeping in a moving vehicle), movement or night awakenings that may be due to bed sharing (i.e., parent movement that may be detected by the child’s actigraph recording during sleep), and any reason for an atypical night of sleep (e.g. child illness, sleepover).
Second, the scoring algorithm or wake threshold sensitivity level also needs to be selected. Again, the decision about how to choose the appropriate specifications for a study should be based on the study population and previously published validation studies. Rules for how to handle missing data and/or artifact should be well defined. Further, scoring rules should be clearly identified a priori (e.g., is sleep onset first of 2, 5, or 10 consecutive minutes of sleep?).
Third, decisions also need to be made about whom, or how many people, will be involved with scoring the data and how reliability will be supported. Further, if multiple personnel clean data and/or score files, inter-rater reliability should be examined. Finally, for studies involving multiple groups (e.g., children with ADHD versus typically developing children) or treatments (e.g., sleep restriction versus sleep extension) personnel who are scoring and interpreting actigraphy data should be blinded to the group/treatment. Without this blinding the ability to grade papers in systematic reviews is limited.
Reporting Actigraphy in Research Papers
While there are currently no standards for what should be reported in research papers, the following suggestions are offered for authors, reviewers, and consumers of the literature (Table 5).
Table 5.
Proposed Standard Checklist for Reporting Actigraphy in Pediatric Sleep Research Literature
| Check | |
|---|---|
| Device/System Information | |
| • The name of the device, the specific model, and the name (and location) of the manufacturer. | |
| • The placement of the device (non-dominant or dominant wrist, left or right ankle, etc.). | |
| • The measured epoch length, the mode of data collection (e.g., ZCM, TAT, PIM, or TRI), the use of the event marker, and the algorithm or wake sensitivity threshold. | |
| • Justification for the algorithm that is chosen | |
| • The type and version of software used. | |
| • Include information on sensitivity and specificity. | |
| Sleep Diary | |
| • Type of sleep diary used (e.g., paper, electronic, telephone call) | |
| • Who completed sleep diary (i.e., parent, child) | |
| • Frequency of diary completion (e.g., at bedtime only, morning and evening) | |
| Data Collection and Processing (including Missing Data) | |
| • Number of nights of data collection | |
| • Number of weekday and weekend nights (if relevant) | |
| • Methods used to identify and handle artifact | |
• How much data lost due to:
|
|
| Data Variables | |
| • Clearly define the variables, including ones automatically calculated by manufacturer scoring programs (e.g., sleep bouts, wake bouts, motionless sleep or immobile time, circadian parameters) | |
| • Clearly define the scoring rules used, using common/standardized names |
Device/System Information
A basic amount of device information should be included in all reports. This includes the name of the device, the specific model, and the name (and location) of the manufacturer. In addition, detailed information should be provided on the placement of the device (non-dominant or dominant wrist, left or right ankle, etc.). Researchers should also include the epoch length measured, the mode of data collection, and the algorithm or wake sensitivity threshold. Finally, the type and version of software used should be reported.
Data Preparation and Reporting
Multiple aspects of the data preparation are important to include in the research report. First, authors should provide detailed information about how they handled missing data or potential artifact. Second, the amount of data lost due to technical failure, artifact, or participant non-adherence to the study protocol needs to be included. Third, scoring rules should be clearly defined.
In terms of actual data reporting, a clear definition of variables (See Table 4 for suggestions) should be included; this permits cross-study comparisons of results. Finally, the number of days included (including weekdays/weekends) should be reported.
Concluding Remarks
Actigraphy has become a central part of pediatric sleep research in the past 20 years. While there are a number of benefits to actigraphy, researchers and consumers of the literature also need to be aware of the limitations and threats to validity, most notably poor specificity to detect wake after sleep onset, how artifact influences results, and the lack of consistency in terms of scoring rules and reported variables. Although we have provided some suggestions based on this review, the field of pediatric sleep would likely benefit from a task force that provides standard recommendations for the use and reporting of actigraphy in pediatric sleep research, as well as to define what is “acceptable” in terms of sensitivity and specificity.
Practice Points.
Actigraphy provides a non-intrusive, cost-effective way to objectively estimate pediatric sleep-wake patterns for an extended period of time within the child’s natural environment.
The use of actigraphy also requires concurrent sleep diaries in order to identify artifact and to corroborate or guide identification of sleep periods on actigraphy output.
The selection of devices, scoring algorithms or sensitivities, and device placement should be based on existing validation studies done within the population of interest, considering developmental stage, developmental disorders, and/or sleep disorders.
The benefits of using actigraphy should be considered alongside the limitations of these devices, in particular the poor specificity of actigraphs to detect wake after sleep onset.
Research Agenda.
Pediatric sleep researchers should work collaboratively with device-producing companies to ensure that the need for highly specific and sensitive devices is met.
Novel devices and algorithms should undergo validation assessment among youth of all ages, as well as among youth with and without sleep disorders (e.g., sleep disordered breathing).
Further development of device utilization for disordered populations (e.g., restless legs syndrome or periodic limb movement disorder) is needed.
A task force should be convened to provide standard recommendations for the use and reporting of actigraphy in pediatric sleep research, as well as to determine what is “acceptable” in terms of sensitivity and specificity of these devices.
Acknowledgments
We thank Devon Ambler for her assistance with the references. Support for this manuscript was provided by K23 MH066772.
Abbreviations
- AMI
Ambulatory Monitoring Inc
- CINAHL
Cumulative Index to Nursing and Allied Health
- PIM
Proportional Integration Mode
- PSG
Polysomnography
- TAT
Time Above Threshold Mode
- TRI
Tri-Axial Mode
- TST
Total Sleep Time
- UCSD
University of California, San Diego
- WASO
Wake After Sleep Onset
- ZCM
Zero Crossing Mode
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lisa J. Meltzer, Email: meltzerL@njhealth.org, National Jewish Health, 1400 Jackson Street, G311, Denver, CO 80206, 303-398-1837 (P), 303-270-2141 (F)
Hawley E. Montgomery-Downs, Email: Hawley.Montgomery-Downs@mail.wvu.edu, West Virginia University, PO Box 6040, Morgantown, WV 26506, 304-293-2001 x610 (P), 304-293-6606 (F)
Salvatore P. Insana, Email: insanas@upmc.edu, University of Pittsburgh Medical Center, 3811 O’Hara Street, E-1107, Pittsburgh, PA 15213, 412-246-6943 (P)
Colleen M. Walsh, Email: Colleen.walsh@uphs.upenn.edu, Drexel University, 3141 Chestnut Street, Philadelphia, PA 19104, 215-662-3189 (P)
References
* Overlapping studies
a Studies that include children with ADHD
b Studies that include children with autism spectrum disorders
c Studies that include children with intellectual disorders
d Studies that include children with chronic medical illnesses
- 1.Sadeh A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med Rev. 2011 Aug;15(4):259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Morgenthaler TI, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007 Apr 1;30(4):519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 3.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001 Sep;2(5):389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 4.Pollak CP, Tryon WW, Nagaraja H, Dzwonczyk R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep. 2001 Dec 15;24(8):957–65. doi: 10.1093/sleep/24.8.957. [DOI] [PubMed] [Google Scholar]
- 5.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992 Oct;15(5):461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 6.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–10. [PubMed] [Google Scholar]
- 7.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. The Statistician. 1983;32:307–17. [Google Scholar]
- 8.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6(2):113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 9.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006 Mar;12(1):23–30. viii. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Gnidovec B, Neubauer D, Zidar J. Actigraphic assessment of sleep-wake rhythm during the first 6 months of life. Clin Neurophysiol. 2002 Nov;113(11):1815–21. doi: 10.1016/s1388-2457(02)00287-0. [DOI] [PubMed] [Google Scholar]
- 11.Paavonen EJ, Fjallberg M, Steenari MR, Aronen ET. Actigraph placement and sleep estimation in children. Sleep. 2002 Mar 15;25(2):235–7. doi: 10.1093/sleep/25.2.235. [DOI] [PubMed] [Google Scholar]
- 12.Souders MC, Mason TBA, Valladares O, Bucan M, Levy SE, Mandell DS, et al. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32(12):1566–78. doi: 10.1093/sleep/32.12.1566. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Johnson NL, Kirchner HL, Rosen CL, Storfer-Isser A, Cartar LN, Ancoli-Israel S, et al. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: A comparison of three data modes. Sleep. 2007 Jul 1;30(7):899–905. doi: 10.1093/sleep/30.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Weiss AR, Johnson NL, Berger NA, Redline S. Validity of activity-based devices to estimate sleep. J Clin Sleep Med. 2010 Aug 15;6(4):336–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Sadeh A, Lavie P, Scher A, Tirosh E, Epstein R. Actigraphic home-monitoring of sleep-disturbed and control infants and young children: A new method for pediatric assessment of sleep-wake patterns. Pediatrics. 1991;87:494–9. [PubMed] [Google Scholar]
- 16.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep. 1994 Apr;17(3):201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 17.So K, Buckley P, Adamson TM, Horne RS. Actigraphy correctly predicts sleep behavior in infants who are younger than six months, when compared with polysomnography. Pediatr Res. 2005 Oct;58(4):761–5. doi: 10.1203/01.PDR.0000180568.97221.56. [DOI] [PubMed] [Google Scholar]
- *18.Hyde M, O’Driscoll DM, Binette S, Galang C, Tan SK, Verginis N, et al. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. J Sleep Res. 2007 Jun 15;16(2):213–6. doi: 10.1111/j.1365-2869.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 19.Tilmanne J, Urbain J, Kothare MV, Wouwer AV, Kothare SV. Algorithms for sleep-wake identification using actigraphy: a comparative study and new results. J Sleep Res. 2009 Mar;18(1):85–98. doi: 10.1111/j.1365-2869.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- 20.Insana SP, Gozal D, Montgomery-Downs HE. Invalidity of one actigraphy brand for identifying sleep and wake among infants. Sleep Med. 2010 Feb;11(2):191–6. doi: 10.1016/j.sleep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.O’Driscoll DM, Foster AM, Davey MJ, Nixon GM, Horne RS. Can actigraphy measure sleep fragmentation in children? Arch Dis Child. 2009 Oct 22; doi: 10.1136/adc.2009.166561. [DOI] [PubMed] [Google Scholar]
- 22.Sadeh A, Acebo C, Seifer R, Aytur S, Carskadon MA. Activity-based assessment of sleep wake patterns during the 1st year of life. Infant Behavior and Development. 1995 Jul;18(3):329–37. [Google Scholar]
- 23.Sung M, Adamson TM, Horne RS. Validation of actigraphy for determining sleep and wake in preterm infants. Acta Paediatr. 2009 Jan;98(1):52–7. doi: 10.1111/j.1651-2227.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- *bc24.Sitnick SL, Goodlin-Jones BL, Anders TF. The use of actigraphy to study sleep disorders in preschoolers: some concerns about detection of nighttime awakenings. Sleep. 2008 Mar 1;31(3):395–401. doi: 10.1093/sleep/31.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, et al. Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep. 1999 Feb 1;22(1):95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer LJ, Walsh CM, Traylor J, Westin AML. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2011 doi: 10.5665/sleep.1608. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 28.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004 Nov 1;27(7):1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 29.Krishna J, Sans-Capdevila O, Gozal D. Sleep studies: which technologies? Paediatr Respir Rev. 2006;7( Suppl 1):S202–S205. doi: 10.1016/j.prrv.2006.04.201. [DOI] [PubMed] [Google Scholar]
- 30.Jenni OG, Carskadon MA. SRS Basics of Sleep Guide. Westchester, IL: Sleep Researcher Society; 2005. Normal human sleep at different ages: Infants to adolescents; pp. 11–9. [Google Scholar]
- 31.Ustinov Y, Lichstein KL. Actigraphy reliability. Sleep. 2011;34:A330. doi: 10.1080/15402002.2012.688779. [DOI] [PubMed] [Google Scholar]
- 32.Berger AM, Wielgus KK, Young-McCaughan S, Fischer P, Farr L, Lee KA. Methodological challenges when using actigraphy in research. J Pain Symptom Manage. 2008:1–9. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *33.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005 Dec 1;28(12):1568–77. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- *b34.Allik H, Larsson JO, Smedje H. Insomnia in school-age children with Asperger syndrome or high-functioning autism. BMC Psychiat. 2006;6:18. doi: 10.1186/1471-244X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *b35.Allik H, Larsson JO, Smedje H. Sleep patterns of school-age children with Asperger syndrome or high-functioning autism. J Autism Dev Disord. 2006 Apr;36(5):585–95. doi: 10.1007/s10803-006-0099-9. [DOI] [PubMed] [Google Scholar]
- *b36.Allik H, Larsson JO, Smedje H. Sleep patterns in school-age children with Asperger Syndrome or high-functioning autism: A follow-up study. J Autism Dev Disord. 2008 Feb 22; doi: 10.1007/s10803-008-0543-0. [DOI] [PubMed] [Google Scholar]
- d37.Amin R, Bean J, Burklow K, Jeffries J. The relationship between sleep disturbance and pulmonary function in stable pediatric cystic fibrosis patients. Chest. 2005 Sep;128(3):1357–63. doi: 10.1378/chest.128.3.1357. [DOI] [PubMed] [Google Scholar]
- *38.Anderson B, Storfer-Isser A, Taylor HG, Rosen CL, Redline S. Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics. 2009 Apr;123(4):e701–e707. doi: 10.1542/peds.2008-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d39.Angulo-Kinzler RM, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum Dev. 2002 Dec;70(1–2):85–101. doi: 10.1016/s0378-3782(02)00092-0. [DOI] [PubMed] [Google Scholar]
- 40.Armitage R, Flynn H, Hoffmann R, Vazquez D, Lopez J, Marcus S. Early developmental changes in sleep in infants: The impact of maternal depression. Sleep. 2009;32(5):693–6. doi: 10.1093/sleep/32.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asaka Y, Takada S. Activity-based assessment of the sleep behaviors of VLBW preterm infants and full-term infants at around 12 months of age. Brain Dev. 2010 Feb;32(2):150–5. doi: 10.1016/j.braindev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- d42.Beebe DW, Lewin D, Zeller M, McCabe M, MacLeod K, Daniels SR, et al. Sleep in overweight adolescents: Shorter sleep, poorer sleep quality, sleepiness, and sleep-disordered breathing. J Pediatr Psychol. 2007 Jan;32(1):69–79. doi: 10.1093/jpepsy/jsj104. [DOI] [PubMed] [Google Scholar]
- 43.Beebe DW, Fallone G, Godiwala N, Flanigan M, Martin D, Schaffner L, et al. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. J Child Psychol Psychiatry. 2008 Sep;49(9):915–23. doi: 10.1111/j.1469-7610.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- 44.Beijamini F, Silva AG, Peixoto CA, Louzada FM. Influence of gender on psychomotor vigilance task performance by adolescents. Braz J Med Biol Res. 2008 Aug;41(8):734–8. doi: 10.1590/s0100-879x2008000800016. [DOI] [PubMed] [Google Scholar]
- 45.Blumer JL, Reed MD, Steinberg F, O’Riordan MA, Rosen CL, Springer MA, et al. Potential pharmacokinetic basis for zolpidem dosing in children with sleep difficulties. Clin Pharmacol Ther. 2008 Apr;83(4):551–8. doi: 10.1038/sj.clpt.6100380. [DOI] [PubMed] [Google Scholar]
- 46.Bruni O, Russo PM, Violani C, Guidetti V. Sleep and migraine: an actigraphic study. Cephalalgia. 2004 Feb;24(2):134–9. doi: 10.1111/j.1468-2982.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- *47.Buckhalt JA, El-Sheikh M, Keller P. Children’s sleep and cognitive functioning: race and socioeconomic status as moderators of effects. Child Dev. 2007 Jan;78(1):213–31. doi: 10.1111/j.1467-8624.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- *48.Buckhalt JA, El-Sheikh M, Keller PS, Kelly RJ. Concurrent and longitudinal relations between children’s sleep and cognitive functioning: the moderating role of parent education. Child Dev. 2009 May;80(3):875–92. doi: 10.1111/j.1467-8624.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 49.Burnham MM. The ontogeny of diurnal rhythmicity in bed-sharing and solitary-sleeping infants: a preliminary report. Inf Child Dev. 2007 Aug;16(4):341–57. [Google Scholar]
- d50.Bursztein C, Steinberg T, Sadeh A. Sleep, sleepiness, and behavior problems in children with headache. J Child Neurol. 2006 Dec;21(12):1012–9. doi: 10.1177/7010.2006.00239. [DOI] [PubMed] [Google Scholar]
- 51.Caldwell-Andrews AA, Kain ZN. Psychological predictors of postoperative sleep in children undergoing outpatient surgery. Paediatr Anaesth. 2006 Feb;16(2):144–51. doi: 10.1111/j.1460-9592.2005.01706.x. [DOI] [PubMed] [Google Scholar]
- 52.Campbell IG, Higgins LM, Trinidad JM, Richardson P, Feinberg I. The increase in longitudinally measured sleepiness across adolescence is related to the maturational decline in low-frequency EEG power. Sleep. 2007 Dec 1;30(12):1677–87. doi: 10.1093/sleep/30.12.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Bio Rhythm. 1997 Jun;12(3):278–89. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- 54.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998 Dec 15;21(8):871–81. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- d55.Cavallo A, Daniels SR, Dolan LM, Bean JA, Khoury JC. Blood pressure-lowering effect of melatonin in type 1 diabetes. J Pineal Res. 2004 May;36(4):262–6. doi: 10.1111/j.1600-079X.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- a56.Corkum P, Tannock R, Moldofsky H, Hogg-Johnson S, Humphries T. Actigraphy and parental ratings of sleep in children with Attention-Deficit/Hyperactivity Disorder (ADHD) Sleep. 2001 May 1;24(3):303–12. doi: 10.1093/sleep/24.3.303. [DOI] [PubMed] [Google Scholar]
- *a57.Corkum P, Panton R, Ironside S, Macpherson M, Williams T. Acute impact of immediate release methylphenidate administered three times a day on sleep in children with attention-deficit/hyperactivity disorder. J Pediatr Psychol. 2007 Dec 3;33(4) doi: 10.1093/jpepsy/jsm106. [DOI] [PubMed] [Google Scholar]
- a58.Crabtree VM, Ivanenko A, Gozal D. Clinical and parental assessment of sleep in children with attention-deficit/hyperactivity disorder referred to a pediatric sleep medicine center. Clin Pediatr (Phila) 2003 Nov;42(9):807–13. doi: 10.1177/000992280304200906. [DOI] [PubMed] [Google Scholar]
- 59.Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010 Aug;27(7):1469–92. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cubero J, Narciso D, Terron P, Rial R, Esteban S, Rivero M, et al. Chrononutrition applied to formula milks to consolidate infants’ sleep/wake cycle. Neuro Endocrinol Lett. 2007 Aug;28(4):360–6. [PubMed] [Google Scholar]
- 61.Cubero J, Chanclon B, Sanchez S, Rivero M, Rodriguez AB, Barriga C. Improving the quality of infant sleep through the inclusion at supper of cereals enriched with tryptophan, adenosine-5′-phosphate, and uridine-5′-phosphate. Nutr Neurosci. 2009 Dec;12(6):272–80. doi: 10.1179/147683009X423490. [DOI] [PubMed] [Google Scholar]
- a62.Dagan Y, Zeevi-Luria S, Sever Y, Hallis D, Yovel I, Sadeh A, et al. Sleep quality in children with attention deficit hyperactivity disorder: an actigraphic study. Psychiatry Clin Neurosci. 1997 Dec;51(6):383–6. doi: 10.1111/j.1440-1819.1997.tb02604.x. [DOI] [PubMed] [Google Scholar]
- d63.de Castro-Silva C, de BV, Cunha GM, Nunes DM, Medeiros CA, de Bruin PF. Melatonin improves sleep and reduces nitrite in the exhaled breath condensate in cystic fibrosis--a randomized, double-blind placebo-controlled study. J Pineal Res. 2010 Jan;48(1):65–71. doi: 10.1111/j.1600-079X.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- c64.de LH, de Blois MC, Claustrat B, Romana S, Albrecht U, Von Kleist-Retzow JC, et al. Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J Pediatr. 2001 Jul;139(1):111–6. doi: 10.1067/mpd.2001.115018. [DOI] [PubMed] [Google Scholar]
- 65.El-Sheikh M, Buckhalt JA. Vagal regulation and emotional intensity predict children’s sleep problems. Dev Psychobiol. 2005 May;46(4):307–17. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- *66.El-Sheikh M, Buckhalt JA, Mize J, Acebo C. Marital conflict and disruption of children’s sleep. Child Dev. 2006 Jan;77(1):31–43. doi: 10.1111/j.1467-8624.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- *67.El-Sheikh M, Erath SA, Keller PS. Children’s sleep and adjustment: the moderating role of vagal regulation. J Sleep Res. 2007 Dec;16(4):396–405. doi: 10.1111/j.1365-2869.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- *68.El-Sheikh M, Buckhalt JA, Keller PS, Cummings EM, Acebo C. Child emotional insecurity and academic achievement: the role of sleep disruptions. J Fam Psychol. 2007 Mar;21(1):29–38. doi: 10.1037/0893-3200.21.1.29. [DOI] [PubMed] [Google Scholar]
- *69.El-Sheikh M, Buckhalt JA, Mark CE, Keller P. Sleep disruptions and emotional insecurity are pathways of risk for children. J Child Psychol Psychiatry. 2007 Jan;48(1):88–96. doi: 10.1111/j.1469-7610.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- *70.El-Sheikh M, Buckhalt JA, Granger DA, Erath SA, Acebo C. The association between children’s sleep disruption and salivary interleukin-6. J Sleep Res. 2007 Jun;16(2):188–97. doi: 10.1111/j.1365-2869.2007.00593.x. [DOI] [PubMed] [Google Scholar]
- *71.El-Sheikh M, Buckhalt JA, Keller PS, Granger DA. Children’s objective and subjective sleep disruptions: links with afternoon cortisol levels. Health Psychol. 2008 Jan;27(1):26–33. doi: 10.1037/0278-6133.27.1.26. [DOI] [PubMed] [Google Scholar]
- *72.El-Sheikh M, Hinnant JB, Kelly RJ, Erath S. Maternal psychological control and child internalizing symptoms: vulnerability and protective factors across bioregulatory and ecological domains. J Child Psychol Psychiatry. 2010 Feb;51(2):188–98. doi: 10.1111/j.1469-7610.2009.02140.x. [DOI] [PubMed] [Google Scholar]
- *73.El-Sheikh M, Kelly RJ, Buckhalt JA, Benjamin HJ. Children’s sleep and adjustment over time: the role of socioeconomic context. Child Dev. 2010 May;81(3):870–83. doi: 10.1111/j.1467-8624.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- *74.El-Sheikh M, Arsiwalla DD. Children’s sleep, skin conductance level and mental health. J Sleep Res. 2011 Jun;20(2):326–37. doi: 10.1111/j.1365-2869.2010.00880.x. [DOI] [PubMed] [Google Scholar]
- 75.Epstein R, Herer P, Tzischinsky O, Lavie P. Changing from communal to familial sleep arrangement in the Kibbutz: effects on sleep quality. Sleep. 1997 May;20(5):334–9. [PubMed] [Google Scholar]
- 76.Fallone G, Seifer R, Acebo C, Carskadon MA. How well do school-aged children comply with imposed sleep schedules at home? Sleep. 2002 Nov 1;25(7):739–45. doi: 10.1093/sleep/25.7.739. [DOI] [PubMed] [Google Scholar]
- 77.Ferber SG, Laudon M, Kuint J, Weller A, Zisapel N. Massage therapy by mothers enhances the adjustment of circadian rhythms to the nocturnal period in full-term infants. J Dev Behav Pediatr. 2002 Dec;23(6):410–5. doi: 10.1097/00004703-200212000-00003. [DOI] [PubMed] [Google Scholar]
- d78.Floro JN, Dunton GE, Delfino RJ. Assessing physical activity in children with asthma: convergent validity between accelerometer and electronic diary data. Res Q Exerc Sport. 2009 Jun;80(2):153–63. doi: 10.1080/02701367.2009.10599549. [DOI] [PubMed] [Google Scholar]
- d79.Franck LS, Johnson LM, Lee K, Hepner C, Lambert L, Passeri M, et al. Sleep disturbances in children with human immunodeficiency virus infection. Pediatrics. 1999 Nov;104(5):e62. doi: 10.1542/peds.104.5.e62. [DOI] [PubMed] [Google Scholar]
- *80.Gaina A, Sekine M, Chen X, Hamanishi S, Kagamimori S. Validity of child sleep diary questionnaire among junior high school children. J Epidemiol. 2004 Jan;14(1):1–4. doi: 10.2188/jea.14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *81.Gaina A, Sekine M, Chen X, Hamanishi S, Kagamimori S. Sleep parameters recorded by Actiwatch in elementary school children and junior high school adolescents: schooldays vs weekends. Sleep and Hypnosis. 2004;6(2):66–77. [Google Scholar]
- *82.Gaina A, Sekine M, Hamanishi S, Chen X, Kagamimori S. Gender and temporal differences in sleep-wake patterns in Japanese school children. Sleep. 2005 Mar 1;28(3):337–42. [PubMed] [Google Scholar]
- 83.Gallagher MR, Cron SG, Meninger JC. Nocturnal sleep duration and its relation to obesity in Latino women and preschool children. Hispanic Health Care International. 2010;8(4):209–16. [Google Scholar]
- 84.Gedaly-Duff V, Lee KA, Nail LM, Nicholson S, Johnson KP. Pain, sleep disturbance, and fatigue in children with Leukemia and their parents: A pilot study. Oncol Nurs Forum. 2006;33(3):641–6. doi: 10.1188/06.ONF.641-646. [DOI] [PubMed] [Google Scholar]
- *85.Geiger A, Achermann P, Jenni OG. Association between sleep duration and intelligence scores in healthy children. Dev Psychol. 2010 Jul;46(4):949–54. doi: 10.1037/a0019679. [DOI] [PubMed] [Google Scholar]
- d86.German A, Suraiya S, Tenenbaum-Rakover Y, Koren I, Pillar G, Hochberg Z. Control of childhood congenital adrenal hyperplasia and sleep activity and quality with morning or evening glucocorticoid therapy. J Clin Endocrinol Metab. 2008 Dec;93(12):4707–10. doi: 10.1210/jc.2008-0519. [DOI] [PubMed] [Google Scholar]
- 87.Gershoni-Baruch R, Epstein R, Tzischinsky O, Lavie P, Brandes JM. Actigraphic home-monitoring of the sleep patterns of in vitro fertilization children and their matched controls. Dev Med Child Neurol. 1994 Jul;36(7):639–45. doi: 10.1111/j.1469-8749.1994.tb11902.x. [DOI] [PubMed] [Google Scholar]
- 88.Gertner S, Greenbaum CW, Sadeh A, Dolfin Z, Sirota L, Ben-Nun Y. Sleep-wake patterns in preterm infants and 6 month’s home environment: implications for early cognitive development. Early Hum Dev. 2002 Jul;68(2):93–102. doi: 10.1016/s0378-3782(02)00018-x. [DOI] [PubMed] [Google Scholar]
- a89.Giblin JM, Strobel AL. Effect of lisdexamfetamine dimesylate on sleep in children with ADHD. J Atten Disord. 2010 Jun 23; doi: 10.1177/1087054710371195. [DOI] [PubMed] [Google Scholar]
- b90.Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, Malow BA. Defining the sleep phenotype in children with autism. Dev Neuropsychol. 2009 Sep;34(5):560–73. doi: 10.1080/87565640903133509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *bc91.Goodlin-Jones B, Tang K, Liu J, Anders TF. Sleep problems, sleepiness and daytime behavior in preschool-age children. J Child Psychol Psyc. 2009 Jul 1; doi: 10.1111/j.1469-7610.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- *bc92.Goodlin-Jones B, Schwichtenberg AJ, Iosif AM, Tang K, Liu J, Anders TF. Six-month persistence of sleep problems in young children with autism, developmental delay, and typical development. J Am Acad Child Adolesc Psychiatry. 2009 Aug;48(8):847–54. doi: 10.1097/CHI.0b013e3181a8135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *bc93.Goodlin-Jones BL, Sitnick SL, Tang K, Liu J, Anders TF. The Children’s Sleep Habits Questionnaire in toddlers and preschool children. J Dev Behav Pediatr. 2008 Apr;29(2):82–8. doi: 10.1097/dbp.0b013e318163c39a. [DOI] [PubMed] [Google Scholar]
- *bc94.Goodlin-Jones BL, Tang K, Liu J, Anders TF. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. J Am Acad Child Psy. 2008 Aug;47(8):930–8. doi: 10.1097/CHI.ObO13e3181799f7c. [DOI] [PubMed] [Google Scholar]
- *bc95.Goodlin-Jones BL, Waters S, Anders TF. Objective sleep measurement in typically and atypically developing preschool children with ADHD-like profiles. Child Psychiat Hum D. 2009 Jun;40(2):257–68. doi: 10.1007/s10578-009-0124-2. [DOI] [PubMed] [Google Scholar]
- *a96.Gruber R, Sadeh A, Raviv A. Instability of sleep patterns in children with Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Psy. 2000 Apr;39(4):495–501. doi: 10.1097/00004583-200004000-00019. [DOI] [PubMed] [Google Scholar]
- *a97.Gruber R, Sadeh A. Sleep and neurobehavioral functioning in boys with Attention-deficit/Hyperactivity Disorder and no reported breathing problems. Sleep. 2004;27(2):267–73. doi: 10.1093/sleep/27.2.267. [DOI] [PubMed] [Google Scholar]
- *a98.Gruber R, Grizenko N, Schwartz G, Ben AL, Gauthier J, de GR, et al. Sleep and COMT polymorphism in ADHD children: preliminary actigraphic data. J Am Acad Child Adolesc Psychiatry. 2006 Aug;45(8):982–9. doi: 10.1097/01.chi.0000220848.48650.10. [DOI] [PubMed] [Google Scholar]
- *a99.Gruber R, Grizenko N, Schwartz G, Bellingham J, Guzman R, Joober R. Performance on the continuous performance test in children with ADHD is associated with sleep efficiency. Sleep. 2007 Aug 1;30(8):1003–9. doi: 10.1093/sleep/30.8.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gruber R, Laviolette R, Deluca P, Monson E, Cornish K, Carrier J. Short sleep duration is associated with poor performance on IQ measures in healthy school-age children. Sleep Med. 2010 Mar;11(3):289–94. doi: 10.1016/j.sleep.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 101.Guilleminault C, Leger D, Pelayo R, Gould S, Hayes B, Miles L. Development of circadian rhythmicity of temperature in full-term normal infants. Neurophysiol Clin. 1996;26(1):21–9. doi: 10.1016/0987-7053(96)81531-0. [DOI] [PubMed] [Google Scholar]
- 102.Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol. 2002 Nov;14(6):762–8. doi: 10.1002/ajhb.10093. [DOI] [PubMed] [Google Scholar]
- d103.Haim A, Pillar G, Pecht A, Lerner A, Tov N, Jaffe M, et al. Sleep patterns in children and adolescents with functional recurrent abdominal pain: objective versus subjective assessment. Acta Paediatr. 2004 May;93(5):677–80. [PubMed] [Google Scholar]
- 104.Hall WA, Saunders RA, Clauson M, Carty EM, Janssen PA. Effects of an intervention aimed at reducing night waking and signaling in 6- to 12-month-old infants. Behav Sleep Med. 2006;4(4):242–61. doi: 10.1207/s15402010bsm0404_4. [DOI] [PubMed] [Google Scholar]
- d105.Hasler BP, Bootzin RR, Cousins JC, Fridel K, Wenk GL. Circadian phase in sleep-disturbed adolescents with a history of substance abuse: a pilot study. Behav Sleep Med. 2008;6(1):55–73. doi: 10.1080/15402000701796049. [DOI] [PubMed] [Google Scholar]
- 106.Hatzinger M, Brand S, Perren S, Stadelmann S, von WA, von KK, et al. Sleep actigraphy pattern and behavioral/emotional difficulties in kindergarten children: association with hypothalamic-pituitary-adrenocortical (HPA) activity. J Psychiatr Res. 2010 Mar;44(4):253–61. doi: 10.1016/j.jpsychires.2009.08.012. [DOI] [PubMed] [Google Scholar]
- d107.Heikkila E, Hatonen TH, Telakivi T, Laakso ML, Heiskala H, Salmi T, et al. Circadian rhythm studies in neuronal ceroid-lipofuscinosis (NCL) Am J Med Genet. 1995 Jun 5;57(2):229–34. doi: 10.1002/ajmg.1320570223. [DOI] [PubMed] [Google Scholar]
- b108.Hering E, Epstein R, Elroy S, Iancu DR, Zelnik N. Sleep patterns in autistic children. J Autism Dev Disord. 1999 Apr;29(2):143–7. doi: 10.1023/a:1023092627223. [DOI] [PubMed] [Google Scholar]
- *d109.Hinds PS, Hockenberry M, Rai SN, Zhang L, Razzouk BI, Cremer L, et al. Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. J Pain Symptom Manage. 2007 Jun;33(6):686–97. doi: 10.1016/j.jpainsymman.2006.09.025. [DOI] [PubMed] [Google Scholar]
- *d110.Hinds PS, Hockenberry M, Rai SN, Zhang L, Razzouk BI, McCarthy K, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncol Nurs Forum. 2007 Mar;34(2):393–402. doi: 10.1188/07.ONF.393-402. [DOI] [PubMed] [Google Scholar]
- *d111.Hinds PS, Hockenberry MJ, Gattuso JS, Srivastava DK, Tong X, Jones H, et al. Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer. 2007 Nov 15;110(10):2321–30. doi: 10.1002/cncr.23039. [DOI] [PubMed] [Google Scholar]
- d112.Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K, Sambuco G, Krull K. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol Nurs Forum. 2010 Jan;37(1):E16–E27. doi: 10.1188/10.ONF.E16-E27. [DOI] [PubMed] [Google Scholar]
- 113.Holley S, Hill CM, Stevenson J. A comparison of actigraphy and parental report of sleep habits in typically developing children aged 6 to 11 years. Behav Sleep Med. 2010;8(1):16–27. doi: 10.1080/15402000903425462. [DOI] [PubMed] [Google Scholar]
- 114.Hourmand-Ollivier I, Piquet MA, Toudic JP, Denise P, Dao T. Actigraphy: A new diagnostic tool for hepatic encephalopathy. World J Gastroenterol. 2006 Apr 14;12(14):2243–4. doi: 10.3748/wjg.v12.i14.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d115.Huynh C, Guile JM, Breton JJ, Desrosiers L, Cohen D, Godbout R. Is it possible to study sleep-wake patterns in adolescent borderline personality disorder? An actigraphic feasibility study. Int J Adolesc Med Health. 2010 Oct;22(4):547–60. doi: 10.1515/ijamh.2010.22.4.547. [DOI] [PubMed] [Google Scholar]
- a116.Hvolby A, Jorgensen J, Bilenberg N. Actigraphic and parental reports of sleep difficulties in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2008 Apr;162(4):323–9. doi: 10.1001/archpedi.162.4.323. [DOI] [PubMed] [Google Scholar]
- a117.Hvolby A, Bilenberg N. Use of ball blanket in attention-deficit/hyperactivity disorder sleeping problems. Nord J Psychiatry. 2011;65(2):89–94. doi: 10.3109/08039488.2010.501868. [DOI] [PubMed] [Google Scholar]
- *a118.Ironside S, Davidson F, Corkum P. Circadian motor activity affected by stimulant medication in children with attention-deficit/hyperactivity disorder. J Sleep Res. 2010 Dec;19(4):546–51. doi: 10.1111/j.1365-2869.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- *119.Iwasaki M, Iwata S, Iemura A, Yamashita N, Tomino Y, Anme T, et al. Utility of subjective sleep assessment tools for healthy preschool children: a comparative study between sleep logs, questionnaires, and actigraphy. J Epidemiol. 2010 Mar 5;20(2):143–9. doi: 10.2188/jea.JE20090054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *120.Iwata S, Iwata O, Iemura A, Iwasaki M, Matsuishi T. Determinants of sleep patterns in healthy Japanese 5-year-old children. Int J Dev Neurosci. 2011 Feb;29(1):57–62. doi: 10.1016/j.ijdevneu.2010.09.004. [DOI] [PubMed] [Google Scholar]
- *121.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep Quality and Elevated Blood Pressure in Adolescents. Circulation. 2008 Aug 18;:118. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *122.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. J Pediatr. 2011 Apr;158(4):617–23. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jenni OG, Deboer T, Achermann P. Development of the 24-h rest-activity pattern in human infants. Infant Behav Dev. 2006 Apr;29(2):143–52. doi: 10.1016/j.infbeh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 124.Jones SH, Tai S, Evershed K, Knowles R, Bentall R. Early detection of bipolar disorder: a pilot familial high-risk study of parents with bipolar disorder and their adolescent children. Bipolar Disord. 2006 Aug;8(4):362–72. doi: 10.1111/j.1399-5618.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- a125.Jonsdottir S, Bouma A, Sergeant JA, Scherder EJ. Effects of transcutaneous electrical nerve stimulation (TENS) on cognition, behavior, and the rest-activity rhythm in children with attention deficit hyperactivity disorder, combined type. Neurorehabil Neural Repair. 2004 Dec;18(4):212–21. doi: 10.1177/1545968304270759. [DOI] [PubMed] [Google Scholar]
- 126.Kain ZN, Mayes LC, Caldwell-Andrews AA, Alexander GM, Krivutza D, Teague BA, et al. Sleeping characteristics of children undergoing outpatient elective surgery. Anesthesiology. 2002 Nov;97(5):1093–101. doi: 10.1097/00000542-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 127.Kain ZN, Caldwell-Andrews AA, Weinberg ME, Mayes LC, Wang SM, Gaal D, et al. Sevoflurane versus halothane: postoperative maladaptive behavioral changes: a randomized, controlled trial. Anesthesiology. 2005 Apr;102(4):720–6. doi: 10.1097/00000542-200504000-00005. [DOI] [PubMed] [Google Scholar]
- d128.Kaufman Y, Tzischinsky O, Epstein R, Etzioni A, Lavie P, Pillar G. Long-term sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 2001 Feb;24(2):129–34. doi: 10.1016/s0887-8994(00)00254-x. [DOI] [PubMed] [Google Scholar]
- *129.Keller PS, El-Sheikh M, Buckhalt JA. Children’s attachment to parents and their academic functioning: sleep disruptions as moderators of effects. J Dev Behav Pediatr. 2008 Dec;29(6):441–9. doi: 10.1097/DBP.0b013e318182a9b4. [DOI] [PubMed] [Google Scholar]
- d130.Kieckhefer GM, Ward TM, Tsai SY, Lentz MJ. Nighttime sleep and daytime nap patterns in school age children with and without asthma. J Dev Behav Pediatr. 2008 Oct;29(5):338–44. doi: 10.1097/DBP.0b013e318182a99e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kohyama J. Early rising children are more active than late risers. Neuropsychiatr Dis Treat. 2007 Dec;3(6):959–63. doi: 10.2147/ndt.s2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Landau R, Sadeh A, Vassoly P, Berger A, Atzaba-Poria N, Auerbach JG. Sleep patterns of 7-week-ols infants at familial risk for attention deficit hyperactivity disorder. Inf Mental Hlth J. 2010;31(6):630–46. doi: 10.1002/imhj.20275. [DOI] [PubMed] [Google Scholar]
- *133.Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, Johnson NL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005 Apr 19;111(15):1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 134.Lavie P, Amit Y, Epstein R, Tzischinsky O. Children’s sleep under the threat of attack by ballistic missiles. J Sleep Res. 1993 Mar;2(1):34–7. doi: 10.1111/j.1365-2869.1993.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 135.Leitner Y, Bloch AM, Sadeh A, Neuderfer O, Tikotzky L, Fattal-Valevski A, et al. Sleep-wake patterns in children with intrauterine growth retardation. J Child Neurol. 2002 Dec;17(12):872–6. doi: 10.1177/08830738020170121901. [DOI] [PubMed] [Google Scholar]
- d136.Lewandowski AS, Palermo TM, De la MS, Fu R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 2010 Oct;151(1):220–5. doi: 10.1016/j.pain.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.MacLaren JE, Kain ZN. Prevalence and predictors of significant sleep disturbances in children undergoing ambulatory tonsillectomy and adenoidectomy. J Pediatr Psychol. 2008 Apr;33(3):248–57. doi: 10.1093/jpepsy/jsm073. [DOI] [PubMed] [Google Scholar]
- b138.McArthur AJ, Budden SS. Sleep dysfunction in Rett syndrome: a trial of exogenous melatonin treatment. Dev Med Child Neurol. 1998 Mar;40(3):186–92. doi: 10.1111/j.1469-8749.1998.tb15445.x. [DOI] [PubMed] [Google Scholar]
- 139.Mello L, Louzada F, Menna-Barreto L. Effects of school schedule transition on sleep-wake cycle of Brazilian adolescents. Sleep and Hypnosis. 2001;3(3):106–11. [Google Scholar]
- 140.Mennella JA, Gerrish CJ. Effects of exposure to alcohol in mother’s milk on infant sleep. Pediatrics. 1998 May;101(5):E2. doi: 10.1542/peds.101.5.e2. [DOI] [PubMed] [Google Scholar]
- 141.Mennella JA, Garcia-Gomez PL. Sleep disturbances after acute exposure to alcohol in mothers’ milk. Alcohol. 2001 Nov;25(3):153–8. doi: 10.1016/s0741-8329(01)00175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mennella JA, Yourshaw LM, Morgan LK. Breastfeeding and smoking: short-term effects on infant feeding and sleep. Pediatrics. 2007 Sep;120(3):497–502. doi: 10.1542/peds.2007-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d143.Milroy G, Dorris L, McMillan TM. Sleep disturbances following mild traumatic brain injury in childhood. J Pediatr Psychol. 2008 Apr;33(3):242–7. doi: 10.1093/jpepsy/jsm099. [DOI] [PubMed] [Google Scholar]
- *144.Montgomery-Downs HE, Gozal D. Toddler behavior following polysomnography: effects of unintended sleep disturbance. Sleep. 2006 Oct 1;29(10):1282–7. doi: 10.1093/sleep/29.10.1282. [DOI] [PubMed] [Google Scholar]
- b145.Moon EC, Corkum P, Smith IM. Case study: A case-series evaluation of a behavioral sleep intervention for three children with autism and primary insomnia. J Pediatr Psychol. 2011 Jan;36(1):47–54. doi: 10.1093/jpepsy/jsq057. [DOI] [PubMed] [Google Scholar]
- *146.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, ncoli-Israel S, et al. Relationships among sleepiness, sleep time, and psychological functioning in adolescents. J Pediatr Psychol. 2009 Jun 3; doi: 10.1093/jpepsy/jsp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nishihara K, Horiuchi S, Eto H, Uchida S. The development of infants’ circadian rest-activity rhythm and mothers’ rhythm. Physiol Behav. 2002 Sep;77(1):91–8. doi: 10.1016/s0031-9384(02)00846-6. [DOI] [PubMed] [Google Scholar]
- d148.Ohinata J, Suzuki N, Araki A, Takahashi S, Fujieda K, Tanaka H. Actigraphic assessment of sleep disorders in children with chronic fatigue syndrome. Brain Dev. 2008 May;30(5):329–33. doi: 10.1016/j.braindev.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 149.Ohrstrom E, Hadzibajramovic E, Holmes M, Svensson H. Effects of road traffic noise on sleep: Studies on children and adults. Journal of Environmental Psychology. 2006;26(2):116–26. [Google Scholar]
- 150.Okawa M, Uchiyama M, Ozaki S, Shibui K, Kamei Y, Hayakawa T, et al. Melatonin treatment for circadian rhythm sleep disorders. Psychiatry Clin Neurosci. 1998 Apr;52(2):259–60. doi: 10.1111/j.1440-1819.1998.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 151.Ophir-Cohen M, Epstein R, Tzischinsky O, Tirosh E, Lavie P. Sleep patterns of children sleeping in residential care, in kibbutz dormitories and at home--a comparative study. Sleep. 1993 Aug;16(5):428–32. [PubMed] [Google Scholar]
- *a152.Owens J, Sangal RB, Sutton VK, Bakken R, Allen AJ, Kelsey D. Subjective and objective measures of sleep in children with attention-deficit/hyperactivity disorder. Sleep Med. 2009 Apr;10(4):446–56. doi: 10.1016/j.sleep.2008.03.013. [DOI] [PubMed] [Google Scholar]
- b153.Oyane NM, Bjorvatn B. Sleep disturbances in adolescents and young adults with autism and Asperger syndrome. Autism. 2005 Feb;9(1):83–94. doi: 10.1177/1362361305049031. [DOI] [PubMed] [Google Scholar]
- b154.Paavonen EJ, Nieminen-von WT, Vanhala R, Aronen ET, von WL. Effectiveness of melatonin in the treatment of sleep disturbances in children with Asperger disorder. J Child Adolesc Psychopharmacol. 2003;13(1):83–95. doi: 10.1089/104454603321666225. [DOI] [PubMed] [Google Scholar]
- *155.Paavonen EJ, Raikkonen K, Lahti J, Komsi N, Heinonen K, Pesonen A, et al. Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics. 2009 May;123(5):e857–e864. doi: 10.1542/peds.2008-2164. [DOI] [PubMed] [Google Scholar]
- 156.Paavonen EJ, Raikkonen K, Pesonen AK, Lahti J, Komsi N, Heinonen K, et al. Sleep quality and cognitive performance in 8-year-old children. Sleep Med. 2010 Apr;11(4):386–92. doi: 10.1016/j.sleep.2009.09.009. [DOI] [PubMed] [Google Scholar]
- *d157.Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clin J Pain. 2007 Nov;23(9):812–20. doi: 10.1097/AJP.0b013e318156ca63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *d158.Palermo TM, Fonareva I, Janosy NR. Sleep quality and efficiency in adolescents with chronic pain: relationship with activity limitations and health-related quality of life. Behav Sleep Med. 2008;6(4):234–50. doi: 10.1080/15402000802371353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Peixoto CA, da Silva AG, Carskadon MA, Louzada FM. Adolescents living in homes without electric lighting have earlier sleep times. Behav Sleep Med. 2009;7(2):73–80. doi: 10.1080/15402000902762311. [DOI] [PubMed] [Google Scholar]
- d160.Perfect MM, Elkins GR, Lyle-Lahroud T, Posey JR. Stress quality of sleep among individuals diagnosed with diabetes. Stress and Health. 2010 Feb;26(1):61–74. [Google Scholar]
- *161.Pesonen AK, Raikkonen K, Matthews K, Heinonen K, Paavonen JE, Lahti J, et al. Prenatal origins of poor sleep in children. Sleep. 2009 Aug 1;32(8):1086–92. doi: 10.1093/sleep/32.8.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *162.Pesonen AK, Raikkonen K, Paavonen EJ, Heinonen K, Komsi N, Lahti J, et al. Sleep Duration and Regularity are Associated with Behavioral Problems in 8-year-old Children. Int J Behav Med. 2009 Oct 21; doi: 10.1007/s12529-009-9065-1. [DOI] [PubMed] [Google Scholar]
- c163.Pillar G, Shahar E, Peled N, Ravid S, Lavie P, Etzioni A. Melatonin improves sleep-wake patterns in psychomotor retarded children. Pediatr Neurol. 2000 Sep;23(3):225–8. doi: 10.1016/s0887-8994(00)00161-2. [DOI] [PubMed] [Google Scholar]
- d164.Radan I, Rajer E, Ursic BN, Neubauer D, Krzisnik C, Battelino T. Motor activity during asymptomatic nocturnal hypoglycemia in adolescents with type 1 diabetes mellitus. Acta Diabetol. 2004 Jun;41(2):33–7. doi: 10.1007/s00592-004-0141-3. [DOI] [PubMed] [Google Scholar]
- *165.Raikkonen K, Matthews KA, Pesonen AK, Pyhala R, Paavonen EJ, Feldt K, et al. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab. 2010 May;95(5):2254–61. doi: 10.1210/jc.2009-0943. [DOI] [PubMed] [Google Scholar]
- *166.Ravid S, Afek I, Suraiya S, Shahar E, Pillar G. Sleep disturbances are associated with reduced school achievements in first-grade pupils. Dev Neuropsychol. 2009 Sep;34(5):574–87. doi: 10.1080/87565640903133533. [DOI] [PubMed] [Google Scholar]
- *167.Ravid S, Afek I, Suraiya S, Shahar E, Pillar G. Kindergarten children’s failure to qualify for first grade could result from sleep disturbances. J Child Neurol. 2009 Feb 2;000(00):1–7. doi: 10.1177/0883073808330766. [DOI] [PubMed] [Google Scholar]
- b168.Reed HE, McGrew SG, Artibee K, Surdkya K, Goldman SE, Frank K, et al. Parent-based sleep education workshops in autism. J Child Neurol. 2009 Aug;24(8):936–45. doi: 10.1177/0883073808331348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reinberg A, Bicakova-Rocher A, Mechkouri M, Ashkenazi I. Right- and left-brain hemisphere. Rhythm in reaction time to light signals is task-load-dependent: age, gender, and handgrip strength rhythm comparisons. Chronobiol Int. 2002 Nov;19(6):1087–106. doi: 10.1081/cbi-120015959. [DOI] [PubMed] [Google Scholar]
- d170.Rivkees SA, Fink C, Nelson M, Borchert M. Prevalence and risk factors for disrupted circadian rhythmicity in children with optic nerve hypoplasia. Br J Ophthalmol. 2010 Oct;94(10):1358–62. doi: 10.1136/bjo.2009.175851. [DOI] [PubMed] [Google Scholar]
- *d171.Roemmich JN, Barkley JE, D’Andrea L, Nikova M, Rogol AD, Carskadon MA, et al. Increases in overweight after adenotonsillectomy in overweight children with obstructive sleep-disordered breathing are associated with decreases in motor activity and hyperactivity. Pediatrics. 2006 Feb;117(2):e200–e208. doi: 10.1542/peds.2005-1007. [DOI] [PubMed] [Google Scholar]
- d172.Rosen G, Brand SR. Sleep in children with cancer: Case review of 70 children evaluated in a comprehensive pediatric sleep center. Support Care Cancer. 2010 Jun 2; doi: 10.1007/s00520-010-0921-y. [DOI] [PubMed] [Google Scholar]
- d173.Ross KR, Hart MA, Storfer-Isser A, Kibler AM, Johnson NL, Rosen CL, et al. Obesity and obesity related co-morbidities in a referral population of children with asthma. Pediatr Pulmonol. 2009 Sep;44(9):877–84. doi: 10.1002/ppul.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sadeh A. Assessment of intervention for infant night waking: Parental reports and activity-based home monitoring. J Consult Clin Psych. 1994 Feb;62(1):63–8. doi: 10.1037//0022-006x.62.1.63. [DOI] [PubMed] [Google Scholar]
- 175.Sadeh A, McGuire JP, Sachs H, Seifer R, Tremblay A, Civita R, et al. Sleep and psychological characteristics of children on a psychiatric inpatient unit. J Am Acad Child Psy. 1995 Jun;34(6):813–9. doi: 10.1097/00004583-199506000-00023. [DOI] [PubMed] [Google Scholar]
- 176.Sadeh A. Evaluating night wakings in sleep-disturbed infants: a methodological study of parental reports and actigraphy. Sleep. 1996 Dec;19(10):757–62. doi: 10.1093/sleep/19.10.757. [DOI] [PubMed] [Google Scholar]
- d177.Sadeh A, Horowitz I, Wolach-Benodis L, Wolach B. Sleep and pulmonary function in children with well-controlled, stable asthma. Sleep. 1998;21(4):379–84. doi: 10.1093/sleep/21.4.379. [DOI] [PubMed] [Google Scholar]
- *178.Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000 May;36(3):291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- *179.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002 Mar 1;73(2):405–17. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- 180.Sadeh A. A brief screening questionnaire for infant sleep problems: Validation and findings for an Internet sample. Pediatrics. 2004 Jun;113(6):e570–e577. doi: 10.1542/peds.113.6.e570. [DOI] [PubMed] [Google Scholar]
- 181.Sadeh A, Flint-Ofir E, Tirosh T, Tikotzky L. Infant sleep and parental sleep-related cognitions. J Fam Psychol. 2007 Mar;21(1):74–87. doi: 10.1037/0893-3200.21.1.74. [DOI] [PubMed] [Google Scholar]
- 182.Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009 Dec 1;32(12):1602–9. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *d183.Sanford SD, Okuma JO, Pan J, Srivastava DK, West N, Farr L, et al. Gender differences in sleep, fatigue, and daytime activity in a pediatric oncology sample receiving dexamethasone. J Pediatr Psychol. 2008 Apr;33(3):298–306. doi: 10.1093/jpepsy/jsm110. [DOI] [PubMed] [Google Scholar]
- *a184.Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, Kelsey D. Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep. 2006 Dec 1;29(12):1573–85. doi: 10.1093/sleep/29.12.1573. [DOI] [PubMed] [Google Scholar]
- *185.Scher A, Tirosh E, Lavie P. The relationship between sleep and temperament revisited: evidence for 12-month-olds: a research note. J Child Psychol Psychiatry. 1998 Jul;39(5):785–8. [PubMed] [Google Scholar]
- *186.Scher A. Attachment and sleep: a study of night waking in 12-month-old infants. Dev Psychobiol. 2001 May;38(4):274–85. doi: 10.1002/dev.1020. [DOI] [PubMed] [Google Scholar]
- *187.Scher A, Epstein R, Tirosh E. Stability and changes in sleep regulation: A longitudinal study from 3 months to 3 years. International Journal of Behavioral Development. 2004 May;28(3):268–74. [Google Scholar]
- *188.Scher A. Infant sleep at 10 months of age as a window to cognitive development. Early Hum Dev. 2005 Mar;81(3):289–92. doi: 10.1016/j.earlhumdev.2004.07.005. [DOI] [PubMed] [Google Scholar]
- *189.Scher A. Crawling in and out of sleep. Inf Child Dev. 2005;14(5):491–500. [Google Scholar]
- 190.Scher A, Hall WA, Zaidman-Zait A, Weinberg J. Sleep quality, cortisol levels, and behavioral regulation in toddlers. Dev Psychobiol. 2010 Jan;52(1):44–53. doi: 10.1002/dev.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d191.Schmitt B, Martin F, Critelli H, Molinari L, Jenni OG. Effects of valproic acid on sleep in children with epilepsy. Epilepsia. 2009 Aug;50(8):1860–7. doi: 10.1111/j.1528-1167.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- a192.Schwartz G, Amor LB, Grizenko N, Lageix P, Baron C, Boivin DB, et al. Actigraphic monitoring during sleep of children with ADHD on methylphenidate and placebo. J Am Acad Child Adolesc Psychiatry. 2004 Oct;43(10):1276–82. doi: 10.1097/01.chi.0000135802.94090.93. [DOI] [PubMed] [Google Scholar]
- *193.Smits MG, Nagtegaal EE, van der Heijden J, Coenen AM, Kerkhof GA. Melatonin for chronic sleep onset insomnia in children: A randomized placebo-controlled trial. J Child Neurol. 2001 Feb;16(2):86–92. doi: 10.1177/088307380101600204. [DOI] [PubMed] [Google Scholar]
- 194.So K, Adamson TM, Horne RS. The use of actigraphy for assessment of the development of sleep/wake patterns in infants during the first 12 months of life. J Sleep Res. 2007 Jun;16(2):181–7. doi: 10.1111/j.1365-2869.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 195.Spruyt K, Aitken RJ, So K, Charlton M, Adamson TM, Horne RS. Relationship between sleep/wake patterns, temperament and overall development in term infants over the first year of life. Early Hum Dev. 2008 May;84(5):289–96. doi: 10.1016/j.earlhumdev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 196.Steenari MR, Vuontela V, Paavonen EJ, Carlson S, Fjallberg M, Aronen E. Working memory and sleep in 6- to 13-year-old school children. J Am Acad Child Adolesc Psychiatry. 2003 Jan;42(1):85–92. doi: 10.1097/00004583-200301000-00014. [DOI] [PubMed] [Google Scholar]
- a197.Stein MA, Blondis TA, Schnitzler ER, O’Brien T, Fishkin J, Blackwell B, et al. Methylphenidate dosing: twice daily versus three times daily. Pediatrics. 1996 Oct;98(4 Pt 1):748–56. [PubMed] [Google Scholar]
- 198.Stremler R, Hodnett E, Lee K, MacMillan S, Mill C, Ongcangco L, et al. A behavioral-educational intervention to promote maternal and infant sleep: a pilot randomized, controlled trial. Sleep. 2006 Dec 1;29(12):1609–15. doi: 10.1093/sleep/29.12.1609. [DOI] [PubMed] [Google Scholar]
- *199.Suratt PM, Barth JT, Diamond R, D’Andrea L, Nikova M, Perriello VA, Jr, et al. Reduced time in bed and obstructive sleep-disordered breathing in children are associated with cognitive impairment. Pediatrics. 2007 Feb;119(2):320–9. doi: 10.1542/peds.2006-1969. [DOI] [PubMed] [Google Scholar]
- *200.Teixeira LR, Fischer FM, de Andrade MM, Louzada FM, Nagai R. Sleep patterns of day-working, evening high-schooled adolescents of Sao Paulo, Brazil. Chronobiol Int. 2004 Mar;21(2):239–52. doi: 10.1081/cbi-120037808. [DOI] [PubMed] [Google Scholar]
- *201.Teixeira LR, Fischer FM, Nagai R, Turte SL. Teen at work: the burden of a double shift on daily activities. Chronobiol Int. 2004;21(6):845–58. doi: 10.1081/cbi-200036878. [DOI] [PubMed] [Google Scholar]
- *202.Teixeira LR, Lowden A, Turte SL, Nagai R, Moreno CR, Latorre MR, et al. Sleep and sleepiness among working and non-working high school evening students. Chronobiol Int. 2007;24(1):99–113. doi: 10.1080/07420520601139763. [DOI] [PubMed] [Google Scholar]
- *203.Teixeira LR, Lowden A, Moreno CR, Turte SL, Nagai R, Latorre MR, et al. Work and excessive sleepiness in Brazillian evening high school students. International Journal of Occupational and Behavioral Health. 2010;16(2):172–7. [PubMed] [Google Scholar]
- c204.Thackeray EJ, Richdale AL. The behavioral treatment of sleep difficulties in children with an intellectual disability. Behavioral Interventions. 2002 Oct;17(4):211–31. [Google Scholar]
- *205.Thomas KA, Burr RL. Circadian research in mothers and infants: how many days of actigraphy data are needed to fit cosinor parameters? J Nurs Meas. 2008;16(3):201–6. doi: 10.1891/1061-3749.16.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tikotzky L, Sadeh A. Sleep patterns and sleep disruptions in kindergarten children. J Clin Child Psychol. 2001 Nov;30(4):581–91. doi: 10.1207/S15374424JCCP3004_13. [DOI] [PubMed] [Google Scholar]
- *207.Tikotzky L, Sadeh A. Maternal sleep-related cognitions and infant sleep: a longitudinal study from pregnancy through the 1st year. Child Dev. 2009 May;80(3):860–74. doi: 10.1111/j.1467-8624.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- *208.Tikotzky L, de MG, Har-Toov J, Dollberg S, Bar-Haim Y, Sadeh A. Sleep and physical growth in infants during the first 6 months. J Sleep Res. 2009 Oct 14; doi: 10.1111/j.1365-2869.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- *209.Tikotzky L, Sadeh A, Glickman-Gavrieli T. Infant sleep and paternal involvement in infant caregiving during the first 6 months of life. J Pediatr Psychol. 2010 May 5; doi: 10.1093/jpepsy/jsq036. [DOI] [PubMed] [Google Scholar]
- *210.Tininenko JR, Fisher PA, Brummett BH, Pears KC. Sleep disruption in young foster children. Child Psychiat Hum D. 2010;41:409–24. doi: 10.1007/s10578-010-0177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *211.Tininenko JR, Fisher PA, Bruce J, Pears KC. Associations between sleep and inattentive/hyperactive problem behaviors among foster community children. J Dev Behav Pediatr. 2011;31:668–74. doi: 10.1097/DBP.0b013e3181f1773b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *212.Tremaine RB, Dorrian J, Blunden S. Measuring sleep habits using the Sleep Timing Questionnaire: A validation study for school-age children. Sleep and Biological Rhythms. 2010;8:194–202. [Google Scholar]
- *213.Tremaine RB, Dorrian J, Blunden S. Subjective and objective sleep in children and asolescents: Measurement, age, adn gender differences. Sleep and Biological Rhythms. 2010;8:229–38. [Google Scholar]
- d214.Tsai SY, Labyak SE, Richardson LP, Lentz MJ, Brandt PA, Ward TM, et al. Actigraphic sleep and daytime naps in adolescent girls with chronic musculoskeletal pain. J Pediatr Psychol. 2008 Apr;33(3):307–11. doi: 10.1093/jpepsy/jsm117. [DOI] [PubMed] [Google Scholar]
- *215.Tsai SY, Burr RL, Thomas KA. Effect of external motion on correspondence between infant actigraphy and maternal diary. Infant Behav Dev. 2009 Jun;32(3):340–3. doi: 10.1016/j.infbeh.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d216.Tzischinsky O, Skene D, Epstein R, Lavie P. Circadian rhythms in 6-sulphatoxymelatonin and nocturnal sleep in blind children. Chronobiol Int. 1991;8(3):168–75. doi: 10.3109/07420529109063923. [DOI] [PubMed] [Google Scholar]
- d217.Tzischinsky O, Latzer Y. Sleep-wake cycles in obese children with and without binge-eating episodes. J Paediatr Child Health. 2006 Nov;42(11):688–93. doi: 10.1111/j.1440-1754.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- d218.Uberos J, Augustin-Morales MC, Molina CA, Florido J, Narbona E, Munoz-Hoyos A. Normalization of the sleep-wake pattern and melatonin and 6-sulphatoxy-melatonin levels after a therapeutic trial with melatonin in children with severe epilepsy. J Pineal Res. 2011 Mar;50(2):192–6. doi: 10.1111/j.1600-079X.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- 219.Urner M, Tornic J, Bloch KE. Sleep patterns in high school and university students: a longitudinal study. Chronobiol Int. 2009 Aug;26(6):1222–34. doi: 10.3109/07420520903244600. [DOI] [PubMed] [Google Scholar]
- *220.Vallance KLW, Mandrell BN, Panetta JC, Gattuso JS, Hockenberry MJ, Zupanec S, et al. Mechanisms of dexamethasone-induced disturbed sleep and fatigue in pediatric patients receiving treatment for ALL. Eur J Cancer. 2010;46:1848–55. doi: 10.1016/j.ejca.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *a221.Van der Heijden KB, Smits MG, Van Someren EJ, Boudewijn GW. Prediction of melatonin efficacy by pretreatment dim light melatonin onset in children with idiopathic chronic sleep onset insomnia. J Sleep Res. 2005 Jun;14(2):187–94. doi: 10.1111/j.1365-2869.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- *a222.Van der Heijden KB, Smits MG, Van Someren EJ, Gunning WB. Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int. 2005;22(3):559–70. doi: 10.1081/CBI-200062410. [DOI] [PubMed] [Google Scholar]
- *a223.Van der Heijden KB, Blok MJ, Spee K, Archer SN, Smits MG, Curfs LM, et al. No evidence to support an association of PER3 clock gene polymorphism with ADHD-related idiopathic chronic sleep onset insomnia. Biological Rhythm Research. 2005 Dec;36(5):381–8. [Google Scholar]
- *a224.Van der Heijden KB, Smits MG, Gunning WB. Sleep hygiene and actigraphically evaluated sleep characteristics in children with ADHD and chronic sleep onset insomnia. J Sleep Res. 2006 Mar;15(1):55–62. doi: 10.1111/j.1365-2869.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- *a225.Van der Heijden KB, Smits MG, Van Someren EJ, Ridderinkhof KR, Gunning WB. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Psy. 2007 Feb;46(2):233–41. doi: 10.1097/01.chi.0000246055.76167.0d. [DOI] [PubMed] [Google Scholar]
- 226.Van GI, Van der Heijden KB, Egberts AC, Korzilius HP, Smits MG. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl) 2010 Oct;212(3):379–91. doi: 10.1007/s00213-010-1962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wall SP, Mattacola CG, Swanik CB, Levenstein S. Sleep efficiency and overreaching in swimmers. Journal of Sport Rehabilitation. 2003;12(1):1–12. [Google Scholar]
- *228.Ward TM, Gay C, Anders TF, Alkon A, Lee KA. Sleep and napping patterns in 3-to-5-year old children attending full-day childcare centers. J Pediatr Psychol. 2008 Jul;33(6):666–72. doi: 10.1093/jpepsy/jsm102. [DOI] [PubMed] [Google Scholar]
- *229.Ward TM, Gay C, Alkon A, Anders TF, Lee KA. Nocturnal sleep and daytime nap behaviors in relation to salivary cortisol levels and temperament in preschool-age children attending child care. Biol Res Nurs. 2008 Jan;9(3):244–53. doi: 10.1177/1099800407310158. [DOI] [PubMed] [Google Scholar]
- c230.Wasdell MB, Jan JE, Bomben MM, Freeman RD, Rietveld WJ, Tai J, et al. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J Pineal Res. 2008 Jan;44(1):57–64. doi: 10.1111/j.1600-079X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- d231.Webb EA, O’Reilly MA, Orgill J, Dale N, Salt A, Gringras P, et al. Rest-activity disturbances in children with septo-optic dysplasia characterized by actigraphy and 24-hour plasma melatonin profiles. J Clin Endocrinol Metab. 2010 Oct;95(10):E198–E203. doi: 10.1210/jc.2010-0027. [DOI] [PubMed] [Google Scholar]
- d232.Wee R, Van Gelder RN. Sleep disturbances in young subjects with visual dysfunction. Ophthalmology. 2004 Feb;111(2):297–302. doi: 10.1016/j.ophtha.2003.05.014. [DOI] [PubMed] [Google Scholar]
- *233.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010 Sep 1;33(9):1201–9. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *234.Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children’s sleep patterns. Arch Pediatr Adolesc Med. 2008 Apr;162(4):350–8. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- *235.Werner H, LeBourgeois MK, Geiger A, Jenni OG. Assessment of chronotype in four- to eleven-year-old children: reliability and validity of the Children’s Chronotype Questionnaire (CCTQ) Chronobiol Int. 2009 Jul;26(5):992–1014. doi: 10.1080/07420520903044505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d236.Wiggins SA. Family exemplars during implementation of a home pain management intervention. Issues Compr Pediatr Nurs. 2009 Dec;32(4):160–79. doi: 10.3109/01460860903281341. [DOI] [PubMed] [Google Scholar]
- b237.Wiggs L, Stores G. Sleep patterns and sleep disorders in children with autistic spectrum disorders: Insights using parent report and actigraphy. Dev Med Child Neurol. 2004 Jun;46(6):372–80. doi: 10.1017/s0012162204000611. [DOI] [PubMed] [Google Scholar]
- *a238.Wiggs L, Montgomery P, Stores G. Actigraphic and parent reports of sleep patterns and sleep disorders in children with subtypes of attention-deficit hyperactivity disorder. Sleep. 2005 Nov 1;28(11):1437–45. doi: 10.1093/sleep/28.11.1437. [DOI] [PubMed] [Google Scholar]
- bc239.Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med. 2009 Apr 15;5(2):145–50. [PMC free article] [PubMed] [Google Scholar]
- 240.Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003 Mar 15;26(2):213–6. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- 241.Wulff K, Dedek A, Siegmund R. Circadian and ultradian time patterns in human behavior: Part 2: Social synchronisation during the development of the infant’s diurnal activity-rest pattern. Biological Rhythm Research. 2001 Dec;32(5):529–46. [Google Scholar]
- 242.Yaron M, Lindgren K, Halbower AC, Weissberg M, Reite M, Niermeyer S. Sleep disturbance after rapid ascent to moderate altitude among infants and preverbal young children. High Alt Med Biol. 2004;5(3):314–20. doi: 10.1089/ham.2004.5.314. [DOI] [PubMed] [Google Scholar]
- d243.Yetman RJ, Portman RJ, Thomas VV, Chan W, Smolensky M, Franco K. Non-invasive ambulatory blood pressure monitoring: effect on nocturnal sleep of children and adults. Blood Press Monit. 1996 Apr;1(2):111–3. [PubMed] [Google Scholar]
- d244.Yuksel H, Sogut A, Yilmaz H, Yilmaz O, Dinc G. Sleep actigraphy evidence of improved sleep after treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2009 Oct;103(4):290–4. doi: 10.1016/S1081-1206(10)60527-3. [DOI] [PubMed] [Google Scholar]