Abstract
Most spinal cord injuries (SCI) occur in young adults. In the past few decades however, the average age at time of SCI and the percentage of injuries in persons over the age of 60 have increased. Studies have shown that there is an age-associated delay in the rate of remyelination following toxin-induced demyelination of the spinal cord, suggesting that there may be an age-associated difference in regenerative efficiency. Here we examine for the first time locomotor recovery, bladder recovery, and myelin pathology in young (3 months), aged (12 months), and geriatric (24 months) female rats following contusion SCI. Our assessments indicate that aged and geriatric rats have a delayed rate of locomotor recovery following contusion SCI as compared to young rats. Additionally, aged and geriatric rats have significantly slower bladder recovery as compared to young rats. Examination of myelin pathology reveals that aged and geriatric rats have significantly greater area of pathology and amount of demyelination, as well as significantly less remyelination as compared to young rats following contusion SCI. These data are the first to indicate that there is an age-associated decline in the rate and extent of both locomotor and bladder recovery following contusion SCI, and that age adversely affects the degree of general pathology, demyelination, and remyelination that accompanies contusion SCI.
Keywords: demyelination, remyelination, spinal cord injury, aging
Introduction
The average age at time of SCI has increased in the past three decades from 28.7 to 38, and the percent of SCI occurring in persons over the age of 60 has increased from 4.7% to 11.5% (NSCISC, 2006). In light of these data, it is important to determine how age at time of SCI may impact pathogenesis and functional deficit.
Several studies suggest that behavioral and pathological outcomes may be influenced by age at time of SCI. Clinical observations of humans with SCI demonstrate that older patients exhibit less recovery from injury as compared to younger patients (Scivoletto et al., 2003; Scivoletto et al., 2008). Gwak and colleagues (2004) demonstrated that aged rats exhibit slower locomotor and somatosensory recovery following hemisection SCI as compared to young rats. Similarly, Genovese and colleagues (2006) demonstrate that clip compression of the spinal cord resulted in more extensive and severe pathology, leukocyte infiltration, and nitrotyrosine levels within 24 hours of compression in 18 month old rats as compared to 3 month old rats. These studies are supported by previous findings in toxin-induced models of demyelination demonstrating that aged rats remyelinate at a slower rate than young rats following toxin-induced demyelination of the spinal cord (Shields et al., 1999). Interestingly, there is a progressive decrease in the rate at which oligodendrocyte progenitor cells (OPCs) repopulate OPC-depleted tissue with age (Chari et al., 2003).
The integrity of the myelin sheath within the central nervous system (CNS) is compromised with age. Aging studies reveal swellings within the myelin sheath, atrophy of the white matter, decrease in the conduction velocity of certain axonal populations, and a decrease in the concentration of myelin and myelin-associated proteins (Bartzokis, 2004; Ibanez et al., 2003; Melcangi et al., 1998; Peters, 1996; Peters et al., 2000; Peters et al., 2001; Peters and Sethares, 2002; Sim et al., 2000; Tanaka et al., 2005). Other age-associated changes in myelin sheaths, such as the formation of redundant myelin and increasing myelin sheath thickness, suggest that myelin formation is still occurring in the aged uninjured CNS (Peters et al., 2001; Peters and Sethares, 2003; Sturrock, 1976).
Here we document that aged and geriatric rats have significantly greater area of pathology and amount of demyelination, as well as significantly less endogenous remyelination as compared to young rats following contusion SCI. This pathology is associated with a delayed rate of locomotor and bladder recovery in aged and geriatric rats as compared to young rats. These data indicate that myelin pathogenesis and functional deficits following SCI are age-associated.
Materials and Methods
Experimental Groups
Young (n=12; 200–220 g; 6–8 weeks old), aged (n=8; 350–450 g; 12 months old), and geriatric (n=8; 430–480g; 24 months old) female Sprague-Dawley rats (Zivic Laboratories Inc, Pittsburgh, PA) were group housed with ad libitum access to food and water in a 12:12 hour light/dark vivarium.
Spinal Cord Injury
Spinal cord injuries were performed according to standard protocols (Keirstead et al., 2005). Animals were anesthetized with intraperitoneal injections of 80 mg/kg Ketamine (Phoenix Pharmaceuticals, St. Joseph, MO) and 10 mg/kg Xylazine (Phoenix Pharmaceuticals). The skin between the neck and hindlimbs, extending approximately 2 cm bilaterally from the spine, was shaved and disinfected with serial povidone and 70% ethanol scrubs. A midline incision exposed the spinal column at the level of T8–T11, and the paravertebral muscles were dissected bilaterally to visualize the transverse apophyses. A complete laminectomy was performed on the 10th thoracic vertebrae (T10). The spinal processes immediately adjacent to the laminectomy site were clamped and stabilized using a stereotactic device, and a contusion injury was induced using the Infinite Horizon Impactor (Precision Systems and Instrumentation LLC, Fairfax, VA) with a force of 200 kDynes. Following impact, the deep and superficial muscle layers were sutured in layers over the intact dura, and the skin was closed with stainless steel wound clips.
Immediately after surgery, animals were given subcutaneous saline and 2.5 mg/kg/day prophylactic enrofloxacin (Baytril; Bayer, Shawnee Mission, KS) and maintained on an isothermic pad until they were alert and mobile. Animals received manual bladder expression three times daily and were inspected for weight loss, dehydration, discomfort, and autophagia, with appropriate veterinary care as needed. All procedures were approved by the Institutional Animal Care and Use Committee at UC Irvine.
Locomotor Testing
Before injury, each animal was acclimated and scored using the Basso, Beattie, Bresnahan Locomotor Rating Scale (BBB) (Basso et al., 1995) and four-parameter kinematic analyses (Gonzalez et al., 2003). BBB scores were analyzed by multivariate repeated measures ANOVA to assess significance between each group over time, and by t-test for significance at each time point. The rate of locomotor recovery was determined by calculating the slope of the linear regression line between each interval of weeks post injury for each animal. The rate of locomotor recovery is reported as the average slope of the linear regression lines for animals in each group, with standard errors of the mean as the error bars. Significance was determined using the t-test for each interval of weeks post-injury.
For kinematic analyses, animals were videotaped using a Hitachi 8 mm video camcorder (VM-E555LA; Hitachi, Tokyo, Japan) from underneath Plexiglas bearing defined 1 cm grid lines. The videos were analyzed frame by frame using windows media player software and scored independently in a blinded manner. Rear paw stride length was defined as distance from the start of a step with the rear paw through to the end of that step with the same paw. Stride width was defined as the distance from the left outermost hind paw digit to the right outermost hind paw digit. Toe spread was defined as the distance from the most lateral point of the lateral digit to the most medial point of the medial digit of each hind paw. Paw rotation was defined as the angle between the axis of the rear paws with the baseline. Prior to injury, a baseline paw rotation was determined for each animal group. As a result, an adjusted paw rotation following injury was calculated by determining the angle of either inward or outward rotation from the baseline. All measurements were taken for three to five consecutive steps for each parameter, and then averaged. Kinematics were analyzed when animals regained sufficient locomotor recovery to measure plantar stepping (2 weeks post-injury for young animals and 4 weeks post-injury for aged and geriatric animals). The t-test was used to determine differences between groups.
Bladder Assessment
Animals received manual bladder expression three times daily. The need for bladder expression was based on the bladder tension during manual expression and the amount of urine expelled during the manual bladder expression. Animals needing bladder expression were given a score of zero. Animals that did not need bladder expression, as determined by a relaxed bladder with minimal urine release during manual bladder expression, were given a score of one. Scores were summed per group for each time point, and a percentage of recovered bladder function was determined by dividing the sum of the scores by the number of animals per group. Bladder recovery percentages were analyzed by multivariate repeated measures ANOVA to assess significance between each group.
Histology
Animals were sacrificed 2 months post-injury by intracardiac perfusion with 4% glutaraldehyde (Fisher Scientific, Pittsburgh, PA) in 0.1 M phosphate buffer, pH 7.4, under anesthesia. The spinal cord was removed, and the lesion-containing length was cut into 12 1-mm transverse blocks (6 blocks cranial and 6 blocks caudal to the injury site), which were then resin embedded according to standard protocols (Keirstead and Blakemore, 1997). Transverse semithin (1µm) sections were cut from the cranial face of each resin block, stained with alkaline toluidine blue (Sigma-Aldrich, St. Louis, MO), coverslipped, and examined by light microscopy on an Olympus AX-80 microscope using Olympus MicroSuite B3SV software (Olympus America, Melville, NY).
Quantification of Gross Pathology
Swelling, damaged axons, hypercellularity, and demyelination were determining features of aberrant histology within spinal cord sections. For each 1µm transverse semithin section, areas of pathology were located by viewing sections at 4× magnification, and traced using the Olympus MicroSuite B3SV software to calculate area. The accuracy of tracing was checked by viewing delineations at 200× magnification. When there was more than one area of pathology within a section, the areas of pathology were summed. The total area of pathology per section was averaged across animals within each group for corresponding 1mm transverse tissue blocks, yielding the estimated average area of pathology for each 1mm segment of spinal cord.
Quantification of Myelin Pathology
For axon quantification, images of these regions were digitally captured at 2000× magnification, and a 25µm × 25µm (625µm2) digital grid was overlaid on the images using Olympus MicroSuite B3SV software. Demyelinated and oligodendrocyte-remyelinated axons were counted on 5 × 625µm2 areas aligned on a radial oriented line, according to the line sampling technique outlined previously (Blight, 1993; Totoiu and Keirstead, 2005). The radial oriented line originated at the central canal and extended to the outermost edge of the spinal cord cross section through the middle of the area of pathology; in the absence of a central canal, the radial oriented line originated at the intersection of two digitally imposed lines, one line running from the outermost center point of the dorsal column to the outermost center point of the ventral column and the other line spanning the greatest mediolateral width of the spinal cord. The 625µm2 areas were then superimposed on this radial oriented line within the middle of the area of pathology. Oligodendrocyte-remyelinated axons were identified by their characteristically thin myelin sheaths relative to the diameter of the axons (Gilson and Blakemore, 2002; Guy et al., 1989; Hildebrand and Hahn, 1978). The number of demyelinated and oligodendrocyte-remyelinated axons was determined by counting such axons in each of 5 625µm2 regions, then averaging the numbers in the 5 regions; this number was then averaged across animal groups for each 1mm transverse tissue block.
G-Ratio Analyses
G-ratios (myelin sheath thickness/axon diameter) were determined for randomly selected normally-myelinated or oligodendrocyte-remyelinated axons within the same 625µm2 areas of pathology used for axon quantification (described above). Random sampling was accomplished by measuring the G-ratio for only those axons bisected by horizontal grid lines digitally superimposed on 2000× digitally captured images. Measurements of myelin sheath thickness and axon diameter were made using Olympus MicroSuite B3SV software.
Results
Locomotor Recovery
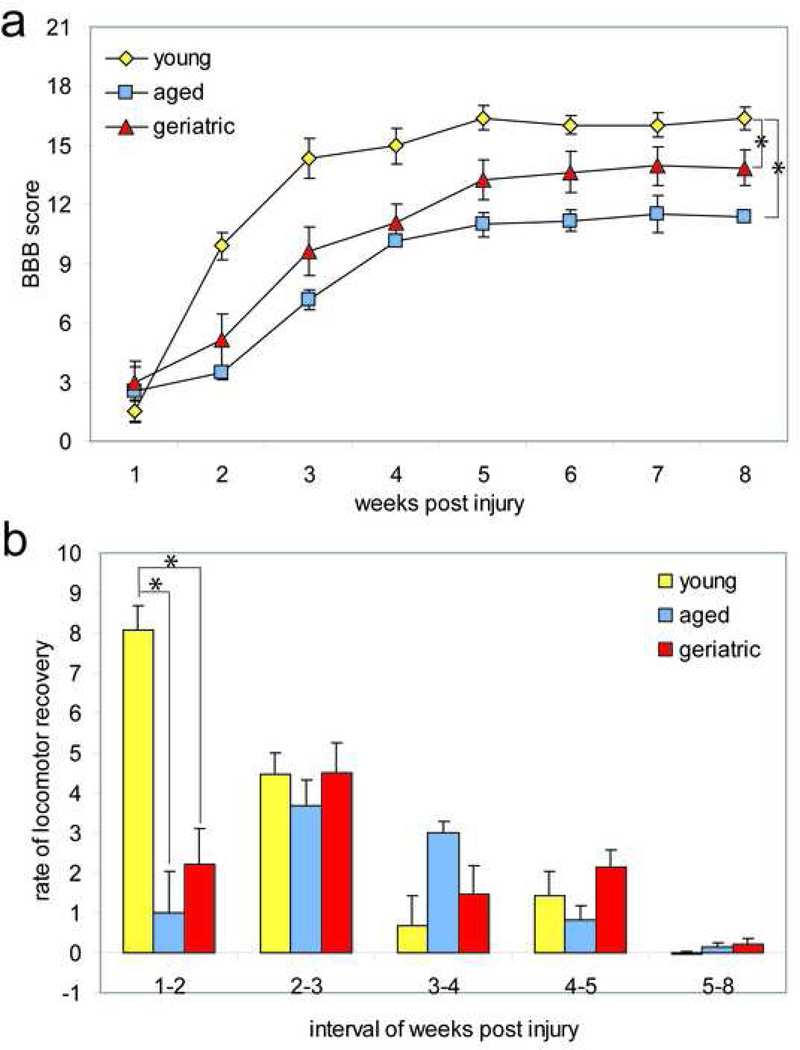
To determine whether age at time of injury affects recovery of function following contusion SCI, locomotion was assessed using the BBB locomotor scale and four-parameter kinematic analyses. There was no significant difference (p>0.05) in the BBB between the animal groups in the first week following injury, indicating that all groups had similar locomotor deficits following a 200 kDyne contusion SCI (Fig 1a). Young animals displayed a great improvement in BBB locomotor score over the first four weeks following contusion SCI, and reached a plateau at score 16 by week five (Fig 1a). Aged and geriatric animals had significantly lower BBB scores as compared to young animals by week two following contusion SCI (p<0.001) (Fig 1a). For the duration of testing, young animals had significantly greater BBB locomotor scores than aged (p<0.005) and geriatric (p<0.01) animals (Fig 1a). There was no overall significant difference in the BBB locomotor score between aged and geriatric animals (p=0.27) (Fig 1a).
Figure 1. Aging adversely affects overground locomotor recovery after SCI.
(a) Young animals demonstrated significantly greater (p<0.01) locomotor capabilities post-injury as compared to aged and geriatric animals. There was no significant difference in locomotor capabilities between aged and geriatric animals (p>0.05). (b) The average change in BBB score during one week was greatest in young animals within the first week, and gradually declined thereafter. The greatest change in BBB score for aged and geriatric animals, however, was delayed until the second week, after which the rate of recovery decreased. The change in BBB score between one week and two weeks post injury was significantly greater in young animals (p<0.01). There was no significant difference in the recovery rates between groups for all other intervals post injury (p>0.05). * p< 0.01.
Examination of the rate of recovery, as indicated by the slope of the linear regression line for each animal between each week interval post-injury, revealed that the greatest locomotor recovery occurred between 1- and 2-weeks post-injury for the young group, whereas the peak locomotor recovery was delayed by one week in the aged and geriatric group (Fig 1b). The rate of recovery between 1 and 2 weeks post-injury was significantly greater in the young group as compared to both the aged and geriatric groups (p<0.001) (Fig 1b). Similarly, the peak locomotor recovery for young animals, between weeks 1 and 2, was significantly greater than the peak locomotor recovery for aged and geriatric animals, which occurred between weeks 2 and 3 (p<0.01) (Fig 1b). There was no significant difference in the recovery rates between groups for all other intervals of weeks post injury (p>0.05) (Fig 1b). The rate of recovery patterns suggests that locomotor recovery in aged and geriatric animals is not only delayed, but also less than that of the young animals (Fig 1b).
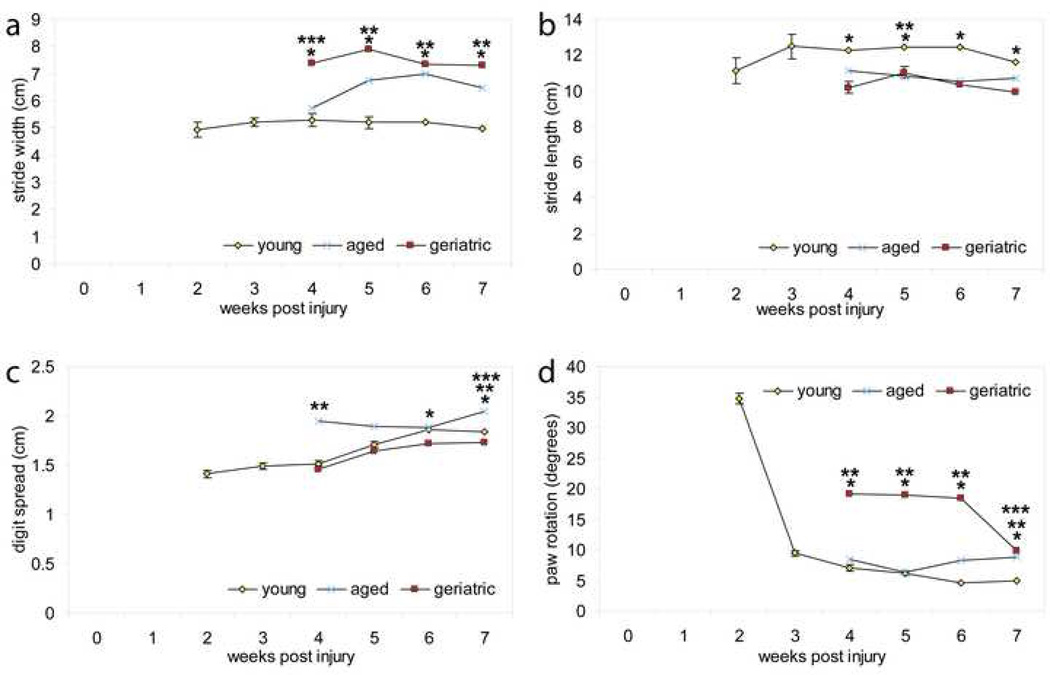
Kinematic analyses of stride width revealed that geriatric animals had a significantly greater stride width than that of young animals at 4, 5, 6, and 7 weeks post-injury (p<0.001) (Fig 2a); furthermore, aged animals had a significantly greater stride width than that of young animals at 5, 6, and & 7 weeks post-injury (p<0.001) (Fig 2a). Only at 4 weeks post-injury did geriatric animals have a significantly greater stride width than that of aged animals (p<0.05) (Fig. 2a). Examination of the rate in stride width recovery reveals that although there was no significant difference between aged and geriatric animals besides 4 weeks post-injury, both of these groups remained significantly different from young animals (excluding aged and young 4 weeks post-injury) indicating that they do not recover as well.
Figure 2. Aging adversely affects kinematic locomotor recovery following SCI.
(a) Kinematic analysis of stride width indicated that aged and geriatric animals had a significantly higher stride width than young animals at multiple time points following contusion SCI (p<0.001). (b) Kinematic analysis of stride length indicated that aged and geriatric animals had significantly shorter stride lengths at multiple time points following contusion SCI as compared to young (p<0.05). (c) Kinematic analysis of digit spread indicated that aged animals had significantly greater digit spread at 4 and 7 weeks following contusion SCI (p<0.05). (d) Kinematic analysis of paw rotation indicated that aged and geriatric animals had significantly greater paw rotation at 4, 5, 6, and 7 weeks post contusion SCI (p<0.05). (* indicates significance in difference between geriatric and young animal groups; ** indicates significance in difference between aged and young animal groups; *** indicates significance in difference between aged and geriatric animal groups).
Kinematic analyses of stride length following contusion SCI revealed that geriatric animals had a significantly shorter stride length than young animals at 4, 5, 6, and 7 weeks post-injury (p<0.001) (Fig 2a). Young animals had significantly longer stride lengths than aged animals at 5 weeks post injury (p< 0.05) (Fig 2b). Examination of the rate of stride length recovery revealed that young and aged animals plateau in recovery at 3 and 4 weeks post-injury, respectively, and remain constant throughout the duration of the study (Fig 2b). Geriatric animals had smaller stride widths than aged animals, except at 5 weeks post-injury, indicating that they did not recover as well as aged animals, which did not recover as well as young animals. Lastly, young animals retained the same stride length up to 7 weeks post-injury, which was noticeably greater than both aged and geriatric animals, suggesting optimal recovery for this age group.
Kinematic analyses of toe spread revealed that young animals had a significantly greater toe spread than that of geriatric animals 6, and 7 weeks post-injury (p<0.05) (Fig 2c). Interestingly, aged animals had a significantly greater toe spread than young animals at 4 and 7 weeks post-injury (p<0.001) (Fig 2c), and a significantly greater toe spread than geriatric animals at 7 weeks post-injury (p<0.001) (Fig. 2c). Examination of the rate of toe spread recovery suggested that geriatric animals generally did not recover as efficiently as young animals. Furthermore, young animals had the most consistent improvement in toe spread recovery following injury, while geriatric reached a plateau 6 to 7 weeks post injury, and aged animals decreased toe spread until week 7.
Kinematic analyses of paw rotation revealed that young animals recovered from either paw inner or outward rotation due to injury between 2 and 3 weeks post-injury and then maintained a plateau for the remaining weeks (Fig 2d). Both geriatric and aged animals had significantly higher paw rotations than young animals at 4, 5, 6, and 7 weeks post-injury (p<0.05) (Fig. 2d). Examination of the rate of paw rotation recovery suggests geriatric animals take longer to recover than aged and young animals, with young animals recovering most efficiently (Fig 2d).
Bladder Recovery
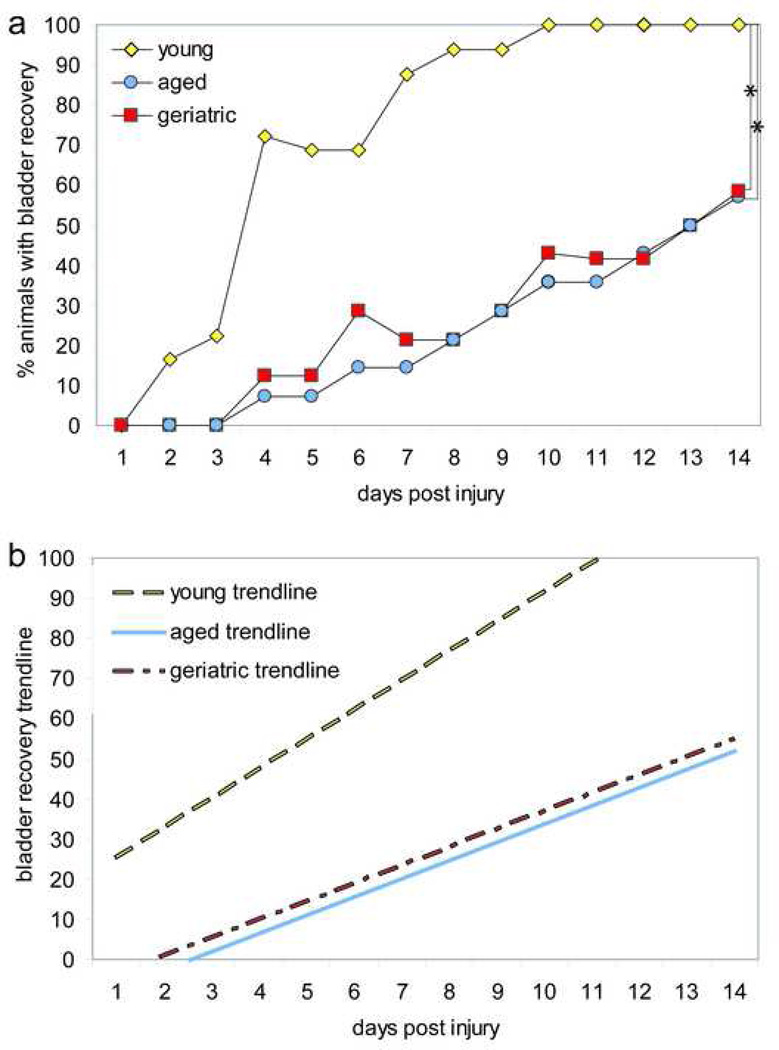
All animals in young, aged, and geriatric groups required bladder expression one day following contusion SCI (Fig 3a). Both aged and geriatric groups maintained 0% bladder recovery for three days following SCI, whereas 16.66% and 22.22% of young animals recovered bladder function two days and three days following SCI, respectively (Fig 3a). Young animals reached 100% bladder recovery at ten days following SCI (Fig 3a). Only 57.14% of aged animals and 58.33% of geriatric animals recovered bladder function by fourteen days following SCI, indicating that aged and geriatric animals have a delayed and slower rate of bladder recovery as compared to young animals following SCI (Fig 3a). At the end of the study, the recovery of bladder function was significantly greater in young animals as compared to both aged and geriatric animals (p<0.001) (Fig 3a). There was no significant difference in bladder recovery between aged and geriatric animals (p>0.05) (Fig 3a).
Figure 3. Aging adversely affects bladder recovery following SCI.
(a) The percent of animals with bladder recovery was significantly different between young and both aged and geriatric animals (p<0.001). Young animals had 100% bladder recovery at fourteen days following SCI, whereas aged and geriatric animals reached only 57.14% and 58.33% bladder recovery, respectively, by fourteen days following SCI. (b) Linear regression analysis of bladder recovery following SCI demonstrated a greater recovery rate for young animals as compared to aged and geriatric animals. The linear equations reveal that young animals had a slope of 7.3596, whereas aged and geriatric animals had a slope of 4.5526 and 4.4819, respectively. * p<0.001.
Examination of the linear trendline for young, aged, and geriatric bladder recovery reveals a significant difference in the rate of bladder recovery (p<0.001) (Fig 3b). The linear equation for the bladder recovery of the young animal group reveals a slope of 7.3596, whereas the aged and geriatric animal groups had slopes of 4.4819 and 4.5526, respectively (Fig 3b).
Area of Pathology
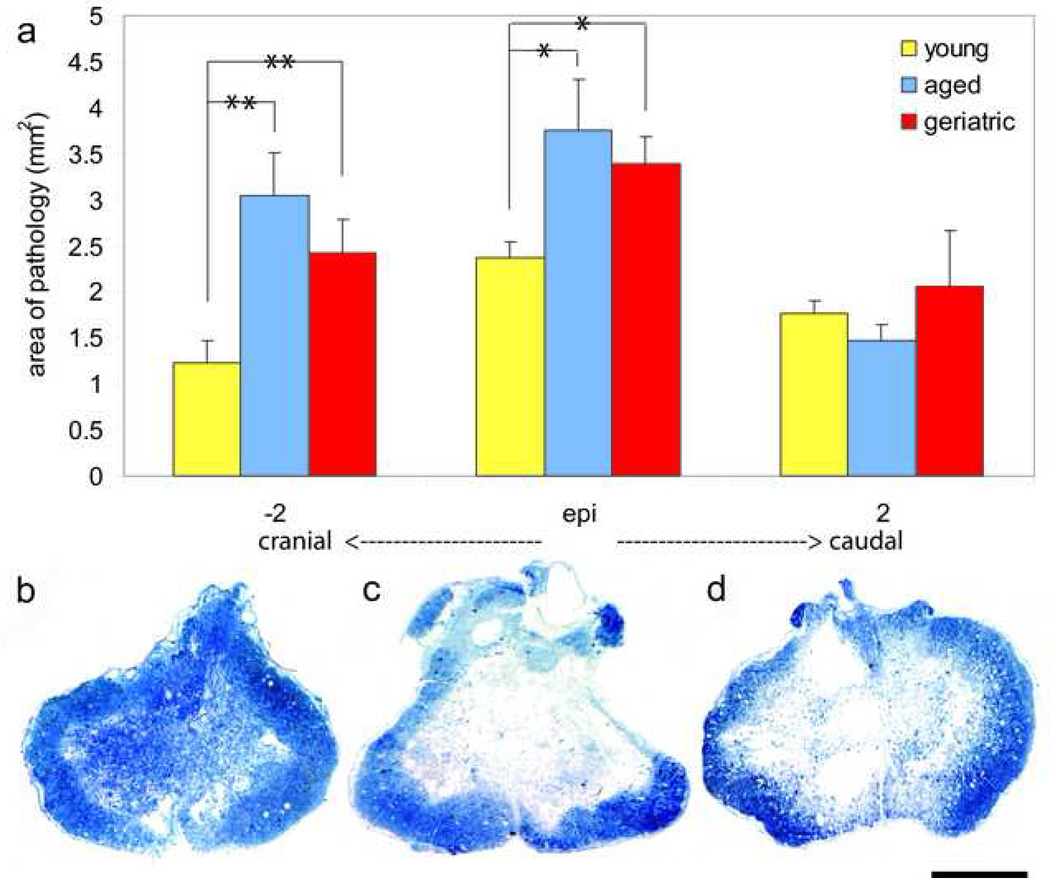
Contusion injury resulted in cavitation and loss of gross tissue structure within the central region of the spinal cord at the injury epicenter in all animal groups. Tissue swelling, demyelination, axonal death, and inflammation were abundant at the injury epicenter and extended several millimeters either side of the injury epicenter in all animal groups. Aged and geriatric animals had an average area of pathology of 3.75 mm2 and 3.39 mm2 at the injury epicenter, respectively, both of which are significantly larger than that of young animals, which had an average area of 2.37 mm2 (p<0.001) (Fig 4a). Similarly, aged and geriatric animals had an average area of pathology of 3.04 mm2 and 2.42 mm2 cranial to the injury epicenter, respectively, both of which are significantly larger than that of young animals, which had an average area of 1.22 mm2 (p<0.05) (Fig 4a). There were no significant differences in area of pathology between groups caudal to the injury epicenter (Fig 4a). These data indicate that there is an age-associated increase in the area of pathology following contusion SCI, which is specifically seen at the injury epicenter and cranial to the epicenter. Figures 4b, c, and d qualitatively illustrate the differences in area of pathology between young, aged, and geriatric animals at the injury epicenter.
Figure 4. Aging increases area of pathology following SCI.
(a) Aged and geriatric animals had significantly greater area of pathology as compared to young animals both at the injury epicenter (p<0.001) and 2 mm cranial to the injury epicenter (p<0.05). There were no significant differences in area of pathology between aged and geriatric animals at either location examined (p>0.05), nor were there differences in area of pathology between all three groups 2 mm caudal to the injury epicenter. (b), (c), (d) Low magnification imaging of transverse spinal cord sections at the injury epicenter of young (b), aged (c), and geriatric (d) animals reveals qualitative differences in the area of pathology. * p<0.001; **p< 0.05. Scale bar= 1mm.
Myelin Pathology
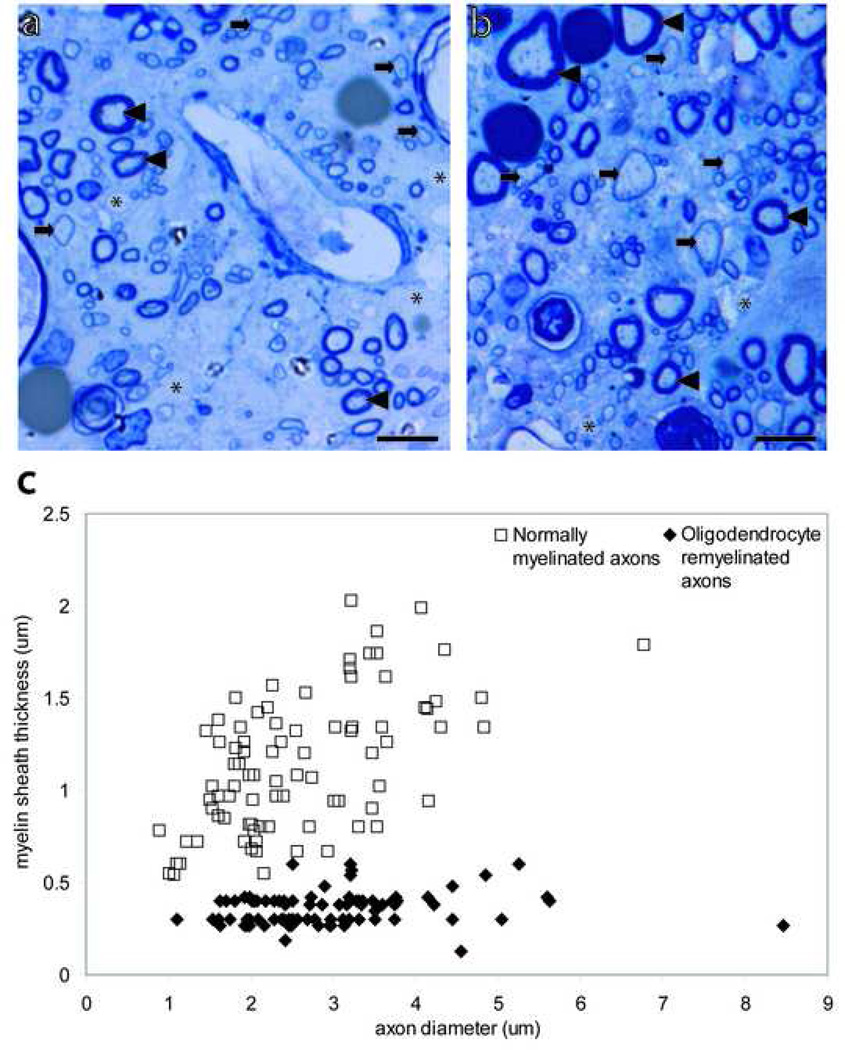
High magnification imaging within areas of pathology revealed axonal and myelin pathology in all animal groups. Normally-myelinated (arrowheads), demyelinated (asterisk), and remyelinated axons (arrows) were visible in the high magnification images and were distinguishable based on the absence or thickness of the myelin sheath (Fig 5a, b). Oligodendrocyte-remyelinated axons can be distinguished from normally-myelinated axons due to the characteristically thin myelin sheaths. The mean G ratio of normally-myelinated axons, 47 ± 0.2, was significantly greater (p<0.001) than the mean G ratio of oligodendrocyte-remyelinated axons, 13 ± 0.05 (Fig 5c). These data indicate that oligodendrocyte-remyelinated axons can accurately be distinguished from normally-myelinated axons (Fig 5c).
Figure 5. Oligodendrocyte remyelinated axons can be distinguished from normally-myelinated axons.
(a), (b) Toluidine blue-stained transverse sections of contused spinal cord at the magnification used for quantification and identification of normally-myelinated axons (arrowhead), oligodendrocyte remyelinated axons (arrows), and demyelinated axons (asterisk). (c) Myelin sheath thickness against axon diameter of normally-myelinated and oligodendrocyte remyelinated axons. The G ratio was 47 ± .2 (85)† for normally-myelinated axons and 13 ± .05 (85)† for oligodendrocyte remyelinated axons. † Data are expressed as mean ± SEM; number in parentheses shows the number of axons scored. Scale bars= 5µm.
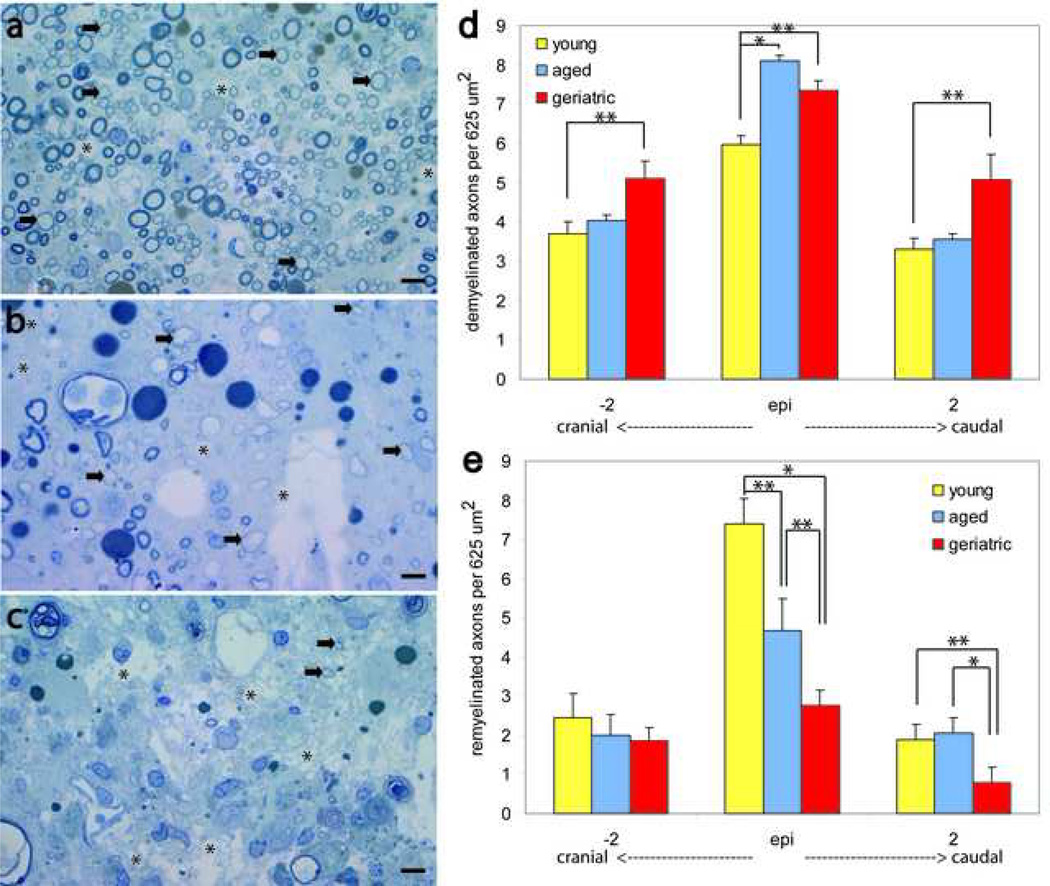
High magnification imaging was used to quantify demyelinated and oligodendrocyte-remyelinated axons within 625µm2 sampling regions using systematic random sampling. Figures 6a, b, and c illustrate qualitative differences in myelin pathology of young, aged, and geriatric animals following SCI. Aged and geriatric animals had a significantly greater amount of demyelination at the injury epicenter as compared to young animals (Fig. 6d; p<0.05). Aged animals did not have significant differences in the amount of demyelination 2 mm cranial and caudal when compared to young and geriatric animals (Fig. 6d; p>0.05). Geriatric animals, however, did have significantly greater demyelination as compared to young animals both 2 mm cranial and caudal to the injury epicenter (p<0.05), indicating that demyelination following SCI is progressively greater and more extensive with age (Fig 6d). Both aged and geriatric animals had significantly less remyelination at the injury epicenter as compared to young animals (Fig. 6e; p<0.05). Furthermore, geriatric animals had significantly less remyelination at the injury epicenter as compared to aged animals (p<0.05), indicating that remyelination progressively decreases following SCI with age (Fig 6e). There were no significant differences between groups in the amount of remyelination 2 mm cranial to the injury epicenter (Fig 6e; p>0.05). Geriatric animals had significantly less remyelination as compared to both young and aged animals 2 mm caudal to the injury epicenter (p<0.05), indicating that remyelination following SCI is progressively greater and more extensive with age, particularly caudal to the injury epicenter (Fig 6e).
Figure 6. Aging increases demyelination and decreases remyelination following contusion SCI.
(a), (b), (c) High magnification imaging of toluidine-blue stained transverse spinal cord sections at the injury epicenter of young (a), aged (b), and geriatric (c) animals reveals qualitative differences in myelin pathology. (d) Aged and geriatric animals had significantly more demyelinated axons as compared to young animals at the injury epicenter (p<0.05). Geriatric animals had significantly more demyelinated axons 2 mm cranial and caudal to the injury epicenter as compared to young animals (p<0.05). (e) Aged and geriatric animals had significantly fewer remyelinated axons as compared to young animals at the injury epicenter (p<0.05), and geriatric animals had significantly fewer remyelinated axons as compared to aged animals at the injury epicenter (p<0.05). Geriatric animals had significantly fewer remyelinated axons as compared to both young and aged animals 2 mm caudal to the injury epicenter (p<0.05). There were no significant differences cranial to the injury epicenter. * p<0.01; **p< 0.05. Scale bar= 5µm.
Discussion
This study indicates that myelin pathogenesis and functional deficits following SCI are age-associated. Our data support and extend an increasing number of studies which demonstrate age-associated decline in neural maintenance, protection, repair, and recovery in the CNS. The aged CNS differs from the young CNS in many regards, including the amount of oxidative stress, mitochondrial damage, glial activation, and level of circulating growth factors, hormones, and neurosteroids that provide neuroprotection (Genazzani et al., 1998; Hayashi et al., 1997; Hoffman et al., 1992; Keller et al., 2000; Kyrkanides et al., 2001; Morales et al., 1998; Navarro and Boveris, 2007a; Navarro and Boveris, 2007b). These changes predispose the aged CNS to loss of integrity and function, which may increase susceptibility to injury (Azcoitia et al., 2003; Ciriza et al., 2004a; Ciriza et al., 2004b; Hayashi et al., 1997; Hoffman et al., 1992; Kyrkanides et al., 2001; Moorthy et al., 2004; Morales et al., 1998; Veiga et al., 2003). The age-associated decline of CNS integrity is evidenced by compromised myelin integrity with age (Kovari et al., 2004; Kullberg et al., 1998; Peters and Sethares, 2003; Raz et al., 2005; Raz and Rodrigue, 2006).
Our data indicate for the first time that there is an age-associated increase in demyelination, accompanied by an age-associated progressive decrease in remyelination following contusion SCI. These findings are supported by studies of toxin-induced demyelination, which indicate that aged rats remyelinate at a slower rate than young rats (Shields et al., 1999). This age-associated difference has been attributed to delayed growth factor gene expression, a decline in OPC colonization of OPC-depleted spinal cord tissue, and differences in early inflammatory responses (Chari et al., 2003; Hinks and Franklin, 2000; Zhao et al., 2006). Our data also indicate that aged and geriatric rats recover bladder and locomotor function at a slower rate than young rats following contusion SCI. Although our findings are the first to demonstrate an age-associated effect on bladder recover following SCI, our data concerning an age-associated effect on locomotor function are supported by several studies using various models of CNS injury and outcomes. Aged rats exhibit slower locomotor and somatosensory recovery following transection SCI as compared to young rats (Gwak et al., 2004). Similarly, clip compression of the spinal cord results in more extensive and severe pathology, leukocyte infiltration, and nitrotyrosine levels, accompanied by decreased behavioral recovery in 18 month old rats as compared to 3 month old rats (Genovese et al., 2006). Age has also been shown to adversely influence recovery following brain injury and experimental stroke (Davis et al., 1995; Erdincler et al., 2002; Gong et al., 2004; Luerssen et al., 1988; Sutherland et al., 1996; Teasdale et al., 1979; Vollmer and Dacey, 1991). Immature animals recover more rapidly and to a greater degree following ischemic brain injury as compared to older animals (Yager et al., 2006). Levels of lactate dehydrogenase and free radicals are significantly greater in the oxygen- and glucose-deprived hippocampal slices of 24 month old rats as compared to slices from young and middle aged rats (Siqueira et al., 2004). Taken together, these studies lend strong support to our conclusion that myelin pathogenesis and functional deficit following SCI are age-associated.
The literature supports a role for myelin pathogenesis in the functional outcome of SCI. Closed contusion injuries spare axons in the subpial white matter in the vicinity of the impact site (Bunge et al., 1993; Kakulas, 1999), and some of these spared axons lose their myelin sheaths (Totoiu and Keirstead, 2005) as a result of the death of oligodendrocytes (Kakulas, 1999). As a result, action potential propagation is disrupted because of the exposure of voltage-gated potassium channels at the internodes (Nashmi and Fehlings, 2001). Oligodendrocytes also undergo apoptosis at considerable distances from the lesion, which disrupt myelin and action potential propagation by surviving axons. Hence, remyelination enhances action potential conduction (Utzschneider et al., 1994; Waxman et al., 1994) and restores functional deficits (Jeffery and Blakemore, 1997; Jeffery et al., 1999).
The age-associated loss in neuroprotection following injury is accompanied by a more severe inflammatory response (Genovese et al., 2006; Kyrkanides et al., 2001; Streit, 2006; Zhao et al., 2006). The inflammatory response is a major component of secondary degeneration that follows the acute stages of SCI (Hausmann, 2003). Several studies support the notion that the inflammatory response is detrimental following injury, causing further loss of tissue that was spared by the initial mechanical insult (Glaser et al., 2004; Glaser et al., 2006; Gonzalez et al., 2003; Popovich et al., 1997; Popovich et al., 1999; Popovich et al., 2002; Taoka et al., 1997). This age-associated loss in neuroprotection and regulation of inflammation may drive the age-associated increases in pathology and demyelination that we observed.
Age-associated decreases in growth factors and neurosteroids, such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), and progesterone, may play a role in the marked decrease in repair mechanisms following injury, such as remyelination (Arsenijevic and Weiss, 1998; Cheng and Mattson, 1994; De Nicola et al., 2006; Du et al., 2003; Ibanez et al., 2003; Koenig et al., 1995; Kokaia et al., 1994; Larsson et al., 1999; Lindvall et al., 1994; Mason et al., 2000; Mason et al., 2003; McTigue et al., 1998; Nakao et al., 1995). BDNF and IGF-1 both promote cell survival of neurons and oligodendrocytes, in addition to promoting progenitor cell proliferation and remyelination following demyelination (Arsenijevic and Weiss, 1998; Cheng and Mattson, 1994; Du et al., 2003; Kokaia et al., 1994; Larsson et al., 1999; Lindvall et al., 1994; Mason et al., 2000; Mason et al., 2003; McTigue et al., 1998; Nakao et al., 1995). Progesterone and its derivatives, dihydroprogesterone (DHP) and tetrahydroprogesterone (THP), are not only neuroprotective, but also increase the expression of myelin proteins and stimulate remyelination following demyelination (Ciriza et al., 2004a; Ghoumari et al., 2003; Ibanez et al., 2003; Ibanez et al., 2004; Schumacher et al., 2004). Therefore, the decrease of growth factors and neurosteroids that naturally occurs with age contribute substantially to the age-associated impairment of remyelination following SCI.
The age-associated decline in CNS integrity, increased susceptibility to injury, and attenuated repair mechanisms are particularly concerning in light of our increasingly active aging population. Our findings clearly indicate that myelin pathogenesis, general pathology, bladder and locomotor deficits following SCI are age-associated. These features represent outcome measures for interventional strategies that may limit damage after injury, and underscore the need for prophylactic measures that maintain CNS integrity with aging.
Acknowledgements
We thank Dave Ferguson, Brandon Shepard, and David Wharton for assistance with animal care and locomotor testing, and Michelle Tu and Bita Alaghebandan for assistance with tissue processing. This project was supported by Research for Cure, and individual donations to the Reeve-Irvine Research Center. Monica Siegenthaler was supported by a pre-doctoral NIH training grant (#AG00096-22) and the Bill and Joan Jackson Scholars Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arsenijevic Y, Weiss S. Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor. J Neurosci. 1998;18:2118–2128. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-associated myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Blight AR. Remyelination, revascularization, and recovery of function in experimental spinal cord injury. Adv Neurol. 1993;59:91–104. [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- Chari DM, Crang AJ, Blakemore WF. Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age. J Neuropathol Exp Neurol. 2003;62:908–916. doi: 10.1093/jnen/62.9.908. [DOI] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004a;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Azcoitia I, Lundeen SG, Garcia-Segura LM. Selective estrogen receptor modulators protect hippocampal neurons from kainic acid excitotoxicity: differences with the effect of estradiol. J Neurobiol. 2004b;61:209–221. doi: 10.1002/neu.20043. [DOI] [PubMed] [Google Scholar]
- Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Gonzalez SL, Labombarda F, Deniselle MC, Garay L, Guennoun R, Schumacher M. Progesterone treatment of spinal cord injury: Effects on receptors, neurotrophins, and myelination. J Mol Neurosci. 2006;28:3–15. doi: 10.1385/jmn:28:1:3. [DOI] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25:116–126. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- Erdincler P, Tuzgen S, Erdincler UD, Oguz E, Korpinar A, Ciplak N, Kuday C. Influence of aging on blood-brain barrier permeability and free radical formation following experimental brain cold injury. Acta Neurochir (Wien) 2002;144:195–199. doi: 10.1007/s007010200024. discussion 199–200. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Di Paola R, Crisafulli C, Muia C, Bramanti P, Cuzzocrea S. Increased oxidative-related mechanisms in the spinal cord injury in old rats. Neurosci Lett. 2006;393:141–146. doi: 10.1016/j.neulet.2005.09.060. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O'Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- Gilson JM, Blakemore WF. Schwann cell remyelination is not replaced by oligodendrocyte remyelination following ethidium bromide induced demyelination. Neuroreport. 2002;13:1205–1208. doi: 10.1097/00001756-200207020-00027. [DOI] [PubMed] [Google Scholar]
- Glaser J, Gonzalez R, Perreau VM, Cotman CW, Keirstead HS. Neutralization of the chemokine CXCL10 enhances tissue sparing and angiogenesis following spinal cord injury. J Neurosci Res. 2004;77:701–708. doi: 10.1002/jnr.20204. [DOI] [PubMed] [Google Scholar]
- Glaser J, Gonzalez R, Sadr E, Keirstead HS. Neutralization of the chemokine CXCL10 reduces apoptosis and increases axon sprouting after spinal cord injury. J Neurosci Res. 2006;84:724–734. doi: 10.1002/jnr.20982. [DOI] [PubMed] [Google Scholar]
- Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- Guy J, Ellis EA, Kelley K, Hope GM. Spectra of G ratio, myelin sheath thickness, and axon and fiber diameter in the guinea pig optic nerve. J Comp Neurol. 1989;287:446–454. doi: 10.1002/cne.902870404. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hains BC, Johnson KM, Hulsebosch CE. Effect of age at time of spinal cord injury on behavioral outcomes in rat. J Neurotrauma. 2004;21:983–993. doi: 10.1089/0897715041650999. [DOI] [PubMed] [Google Scholar]
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamashita A, Shimizu K. Somatostatin and brain-derived neurotrophic factor mRNA expression in the primate brain: decreased levels of mRNAs during aging. Brain Res. 1997;749:283–289. doi: 10.1016/S0006-8993(96)01317-0. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Hahn R. Relation between myelin sheath thickness and axon size in spinal cord white matter of some vertebrate species. J Neurol Sci. 1978;38:421–434. doi: 10.1016/0022-510x(78)90147-8. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJ. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol Cell Neurosci. 2000;16:542–556. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- Hoffman AR, Lieberman SA, Ceda GP. Growth hormone therapy in the elderly: implications for the aging brain. Psychoneuroendocrinology. 1992;17:327–333. doi: 10.1016/0306-4530(92)90038-9. [DOI] [PubMed] [Google Scholar]
- Ibanez C, Shields SA, El-Etr M, Leonelli E, Magnaghi V, Li WW, Sim FJ, Baulieu EE, Melcangi RC, Schumacher M, Franklin RJ. Steroids and the reversal of age-associated changes in myelination and remyelination. Prog Neurobiol. 2003;71:49–56. doi: 10.1016/j.pneurobio.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Ibanez C, Shields SA, El-Etr M, Baulieu EE, Schumacher M, Franklin RJ. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol Appl Neurobiol. 2004;30:80–89. doi: 10.1046/j.0305-1846.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Blakemore WF. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain. 1997;120:27–37. doi: 10.1093/brain/120.1.27. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Crang AJ, O'Leary MT, Hodge SJ, Blakemore WF. Behavioural consequences of oligodendrocyte progenitor cell transplantation into experimental demyelinating lesions in the rat spinal cord. Eur J Neurosci. 1999;11:1508–1514. doi: 10.1046/j.1460-9568.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. The applied neuropathology of human spinal cord injury. Spinal Cord. 1999;37:79–88. doi: 10.1038/sj.sc.3100807. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Markesbery WR. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience. 2000;98:149–156. doi: 10.1016/s0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu EE. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268:1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Othberg A, Kokaia M, Lindvall O. BDNF makes cultured dentate granule cells more resistant to hypoglycaemic damage. Neuroreport. 1994;5:1241–1244. doi: 10.1097/00001756-199406020-00021. [DOI] [PubMed] [Google Scholar]
- Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- Kullberg S, Ramirez-Leon V, Johnson H, Ulfhake B. Decreased axosomatic input to motoneurons and astrogliosis in the spinal cord of aged rats. J Gerontol A Biol Sci Med Sci. 1998;53:B369–B379. doi: 10.1093/gerona/53a.5.b369. [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, O'Banion MK, Whiteley PE, Daeschner JC, Olschowka JA. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J Neuroimmunol. 2001;119:269–277. doi: 10.1016/s0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- Larsson E, Nanobashvili A, Kokaia Z, Lindvall O. Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1220–1228. doi: 10.1097/00004647-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient's age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68:409–416. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- Mason JL, Ye P, Suzuki K, D'Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J Neurosci. 2000;20:5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23:7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi RC, Magnaghi V, Cavarretta I, Martini L, Piva F. Age-induced decrease of glycoprotein Po and myelin basic protein gene expression in the rat sciatic nerve. Repair by steroid derivatives. Neuroscience. 1998;85:569–578. doi: 10.1016/s0306-4522(97)00628-3. [DOI] [PubMed] [Google Scholar]
- Moorthy K, Yadav UC, Siddiqui MR, Sharma D, Basir SF, Baquer NZ. Effect of estradiol and progesterone treatment on carbohydrate metabolizing enzymes in tissues of aging female rats. Biogerontology. 2004;5:249–259. doi: 10.1023/B:BGEN.0000038026.89337.02. [DOI] [PubMed] [Google Scholar]
- Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf) 1998;49:421–432. doi: 10.1046/j.1365-2265.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- Nakao N, Kokaia Z, Odin P, Lindvall O. Protective effects of BDNF and NT-3 but not PDGF against hypoglycemic injury to cultured striatal neurons. Exp Neurol. 1995;131:1–10. doi: 10.1016/0014-4886(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Brain Res Rev. 2001;38:165–191. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007a;292:C670–C686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Brain mitochondrial dysfunction in aging: conditions that improve survival, neurological performance and mitochondrial function. 2007b;12:1154–1163. doi: 10.2741/2133. [DOI] [PubMed] [Google Scholar]
- NSCISC (National Spinal Cord Injury Statistical Center) [accessed February 23, 2006];Facts and Figures at a Glance- August 2006. 2006 [Online]. Available: http://www.spinalcord.uab.edu/show.asp?durki=21446.
- Peters A. Age-associated changes in oligodendrocytes in monkey cerebral cortex. J Comp Neurol. 1996;371:153–163. doi: 10.1002/(SICI)1096-9861(19960715)371:1<153::AID-CNE9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Peters A, Moss MB, Sethares C. Effects of aging on myelinated nerve fibers in monkey primary visual cortex. J Comp Neurol. 2000;419:364–376. doi: 10.1002/(sici)1096-9861(20000410)419:3<364::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Killiany RJ. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J Comp Neurol. 2001;435:241–248. doi: 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Is there remyelination during aging of the primate central nervous system? J Comp Neurol. 2003;460:238–254. doi: 10.1002/cne.10639. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, Gonzalez Deniselle MC, Gonzalez SL, Ibanez C, Labombarda F, Coirini H, Baulieu EE, De Nicola AF. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm IGF Res. 2004;14(Suppl A):S18–S33. doi: 10.1016/j.ghir.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Scivoletto G, Romanelli A, Mariotti A, Marinucci D, Tamburella F, Mammone A, Cosentino E, Sterzi S, Molinari M. Clinical factors that affect walking level and performance in chronic spinal cord lesion patients. Spine. 2008;33:259–264. doi: 10.1097/BRS.0b013e3181626ab0. [DOI] [PubMed] [Google Scholar]
- Scivoletto G, Morganti B, Ditunno P, Ditunno JF, Molinari M. Effects on age on spinal cord lesion pateients' rehabilitation. Spinal Cord. 2003;41:457–464. doi: 10.1038/sj.sc.3101489. [DOI] [PubMed] [Google Scholar]
- Shields SA, Gilson JM, Blakemore WF, Franklin RJ. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia. 1999;28:77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Hinks GL, Franklin RJ. The re-expression of the homeodomain transcription factor Gtx during remyelination of experimentally induced demyelinating lesions in young and old rat brain. Neuroscience. 2000;100:131–139. doi: 10.1016/s0306-4522(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Siqueira IR, Cimarosti H, Fochesatto C, Salbego C, Netto CA. Age-associated susceptibility to oxygen and glucose deprivation damage in rat hippocampal slices. Brain Res. 2004;1025:226–230. doi: 10.1016/j.brainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. Changes in neurologia and myelination in the white matter of aging mice. J Gerontol. 1976;31:513–522. doi: 10.1093/geronj/31.5.513. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke. 1996;27:1663–1667. doi: 10.1161/01.str.27.9.1663. discussion 1668. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Okuma Y, Tomobe K, Nomura Y. The age-associated degeneration of oligodendrocytes in the hippocampus of the senescence-accelerated mouse (SAM) P8: a quantitative immunohistochemical study. Biol Pharm Bull. 2005;28:615–618. doi: 10.1248/bpb.28.615. [DOI] [PubMed] [Google Scholar]
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H, Takatsuki K. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Skene A, Parker L, Jennett B. Age and outcome of severe head injury. Acta Neurochir Suppl (Wien) 1979;28:140–143. doi: 10.1007/978-3-7091-4088-8_33. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Utzschneider DA, Archer DR, Kocsis JD, Waxman SG, Duncan ID. Transplantation of glial cells enhances action potential conduction of amyelinated spinal cord axons in the myelin-deficient rat. Proc Natl Acad Sci USA. 1994;91:53–57. doi: 10.1073/pnas.91.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga S, Garcia-Segura LM, Azcoitia I. Neuroprotection by the steroids pregnenolone and dehydroepiandrosterone is mediated by the enzyme aromatase. J Neurobiol. 2003;56:398–406. doi: 10.1002/neu.10249. [DOI] [PubMed] [Google Scholar]
- Vollmer DG, Dacey RG., Jr The management of mild and moderate head injuries. Neurosurg Clin N Am. 1991;2:437–455. [PubMed] [Google Scholar]
- Waxman SG, Utzschneider DA, Kocsis JD. Enhancement of action potential conduction following demyelination: experimental approaches to restoration of function in multiple sclerosis and spinal cord injury. Prog Brain Res. 1994;100:233–243. doi: 10.1016/s0079-6123(08)60790-6. [DOI] [PubMed] [Google Scholar]
- Yager JY, Wright S, Armstrong EA, Jahraus CM, Saucier DM. The influence of aging on recovery following ischemic brain damage. Behav Brain Res. 2006;173:171–180. doi: 10.1016/j.bbr.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Zhao C, Li WW, Franklin RJ. Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-associated decline in CNS remyelination. Neurobiol Aging. 2006;27:1298–1307. doi: 10.1016/j.neurobiolaging.2005.06.008. [DOI] [PubMed] [Google Scholar]