Abstract
Ectopic expression in Arabidopsis of a pea (Pisum sativum) cDNA (2ox2) encoding a gibberellin (GA) 2-oxidase (PsGA2ox2), involved in the deactivation of biologically active GAs, has been used to establish a role for GAs in promoting pollen tube growth. One line, 35S:2ox2/28c, when homozygous for the transgene, exhibits a novel small fruit phenotype. The 28c transgene reduces pollen tube growth, and this results in a reduced number of fertilized seeds that are only present at the end of the silique nearest the stigma. To confirm that the 28c pollen tube phenotype is due to sense expression of the 2ox2 mRNA, a “hairpin” RNA interface silencing construct, designed to silence 2ox2 expression, has been used to restore pollen tube growth and fruit development. The interaction between 28c and other mutants with increased GA response has also been examined to provide further evidence that GAs play an important role in pollen tube growth. Based on the ability of mutant alleles to suppress the 35S:2ox2/28c phenotype, we define new roles for the gar2-1 and rga alleles in GA signaling during pollen tube elongation in addition to their previously established roles in vegetative tissues. In contrast to the constitutive GA response observed in internodes and leaves lacking RGA and GAI, the rga-2 gai-d5 mutant combination is only a partial suppressor of the 28c phenotype. Because the dominant dwarfing gai-1 allele reduces GA response in vegetative tissues, its effect on plant fertility has been examined. Although gai-1 reduces seed set, this appears to reflect defects in reproductive development other than pollen tube function. Finally, we show that the genetic background (Landsberg erecta or Columbia) modifies the 28c phenotype and that this effect is not due to the ER/er difference between these two ecotypes.
Gibberellins (GAs) are a class of plant hormones with a range of important functions in plant growth and development, including seed germination, trichome development, stem and leaf elongation, flower induction, anther development, and fruit and seed development (Langridge, 1957; Pharis and King, 1985; Ross et al., 1997; Yamaguchi et al., 1998; Kamiya and Garcia-Martinez, 1999; Hedden and Phillips, 2000; Richards et al., 2001). Much of this knowledge has come from the identification and analysis of mutants, particularly in Arabidopsis, pea (Pisum sativum), maize (Zea mays), rice (Oryza sativa), and tomato (Lycopersicon esculentum), with defects in GA biosynthesis or GA response (for review, see Ross et al., 1997).
GAs are known to have various roles in reproductive development, depending on the species examined (Pharis and King, 1985). One role of GAs is the promotion of normal anther development: Too much or not enough GA can prevent the formation and release of viable mature pollen (Nester and Zeevaart, 1988; Jacobsen and Olszewski, 1993; Goto and Pharis, 1999). GAs are also present in developing pollen postanthesis (Barendse et al., 1970; Mander et al., 1996), and numerous studies have reported an effect of GA application on pollen tube growth in vivo or in vitro (Bhandal and Malik, 1979; Viti et al., 1990; Setia et al., 1994; Kimura et al., 1996). Recently, a physiological role of GAs in pollen tube growth has been established using intact plants expressing a construct in which the cauliflower mosaic virus 35S promoter drives expression of a cDNA encoding a GA-degrading enzyme (Singh et al., 2002). This cDNA represents a pea gene known as PsGA2ox2 (2ox2) and encodes a member of a recently characterized class of GA-catabolizing enzymes, the GA 2-oxidases (Lester et al., 1999; Martin et al., 1999). These two oxoglutarate-dependent dioxygenases are involved in the irreversible conversion of active GAs and their precursors into inactive forms and can potentially be used to reduce the endogenous levels of active GAs. Other cDNAs encoding 2-oxidases have also been isolated from runner bean (Phaseolus coccineus), Arabidopsis, rice, and poplar (Populus spp.; Thomas et al., 1999; Sakamoto et al., 2001; Busov et al., 2003; Schomberg et al., 2003) and have shown to perform oxidation reactions at carbon 2 of various biologically active and inactive GAs in vitro. Several independent 35S:2ox2 lines have been characterized, and for all lines the growth of pollen tubes carrying the transgene is impaired relative to wild-type (WT) pollen tubes. For one line, 35S:2ox2/28c (hereafter referred to as 28c), homozygous plants were obtained that exhibited a novel fruit phenotype in which fertilized seeds were only found at the end of the ovary nearest the stigma. Physiological analysis of 28c plants, combined with in vitro culture of pollen tubes, suggests that reduced pollen tube growth caused by GA deficiency prevents pollen tubes reaching, and successfully fertilizing, ovules at the distant end of the ovary. Therefore, this effect on fruit development is distinct from mutations such as spatula that reduce fertilization by preventing the normal formation of female tissues such as the transmitting tract (Alvarez and Smyth, 1999). Further support for a role of GAs in pollen tube growth was obtained by demonstrating that the spy-5 allele, which increases GA response, can partially rescue the 28c pollen tube phenotype.
In addition to SPY, other loci known to be involved in GA signaling include GAR2, RGA, GAI, and RGL2. Two of the best-characterized GA-signaling genes are loss-of-function gai and rga alleles (Silverstone et al., 2001; Fleck and Harberd, 2002). RGA and GAI are closely related members of the GRAS family (Pysh et al., 1999) that function, at least in vegetative tissues, as partially redundant negative regulators of GA response (Dill and Sun, 2001; King et al., 2001). RGL2 is also a GRAS protein and is required for normal GA signaling in germinating seeds (Lee et al., 2002). The dominant gar2-1 allele was isolated as a suppressor of the dominant gai-1 dwarf phenotype (Wilson and Somerville, 1995), but also confers resistance to the effects of paclobutrazol on seed germination and vegetative growth (Peng et al., 1997). Thus, the gar2-1 allele is thought to increase GA response, but the dominant nature of the single known gar2 allele limits conclusions regarding the role of the WT GAR2 gene product in GA signaling.
In this paper, we examine the interaction between 28c and mutants with increased GA response to provide further evidence that GAs play an important role in pollen tube growth. Based on the ability of mutant alleles to suppress the 28c phenotype, we define new roles for the GAR2 and RGA proteins in GA signaling during pollen tube elongation.
RESULTS
Exogenous GA Restores Fruit Size on 35S:2ox2/28c Plants
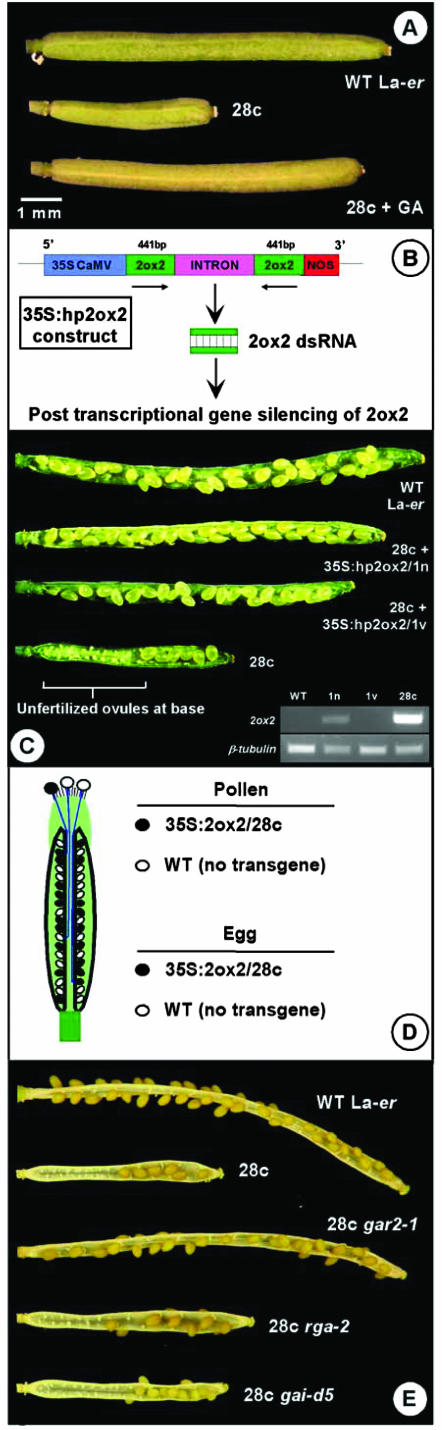
Plants homozygous for the 28c transgene possess small fruit with the majority of seeds at the end nearest the stigma. Extensive characterization of this line supports the conclusion that this fruit phenotype is an indirect consequence of reduced pollen tube growth (Singh et al., 2002): Impaired pollen tube function leads to reduced seed set, and this in turn reduces fruit growth. To further test this hypothesis, individual self-pollinated 28c flowers were treated just before anthesis with 2.5 μg of GA3 in 0.5 μL of ethanol. As expected, GA3 promoted the growth of 28c fruit (Fig. 1A). However, in contrast to self-pollinated WT fruit, and despite the fact that exogenous GAs can rescue 28c pollen tube growth in vitro, seeds were still only present at the end of the ovary nearest the stigma, similar to untreated 28c control fruit (data not shown). This effect on silique size is consistent with the ability of exogenous GA to stimulate fruit growth when seeds are absent in Arabidopsis (Vivian-Smith and Koltunow, 1999) and other species (e.g. Eeuwens and Schwabe, 1975), and supports the conclusion that the reduced size of 28c fruit is entirely due to reduced seed fertilization rather than to a direct effect on silique growth (Singh et al., 2002).
Figure 1.
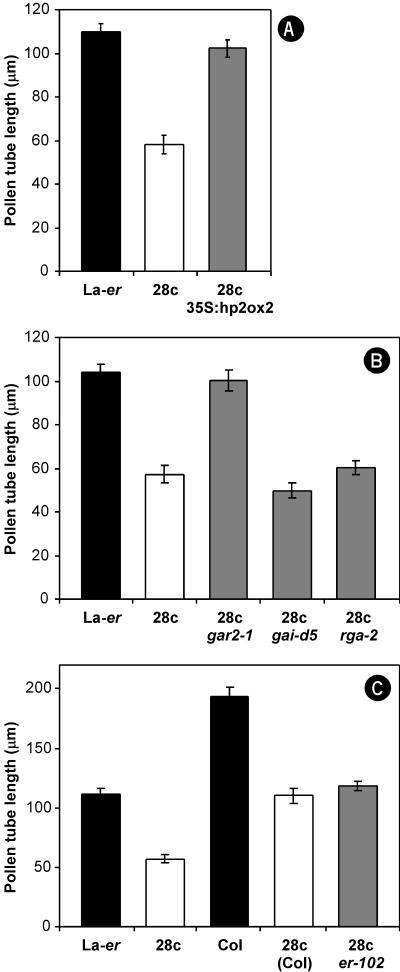
Suppression of the small fruit phenotype exhibited by homozygous 35S:2ox2/28 plants. A, Treatment of 28c flowers with GA3 restores fruit size. B, Schematic representation of the construct designed to silence the 35S:2ox2/28c transgene by posttranscriptional gene silencing/RNA interference (RNAi). C, Fruit from self-pollinated WT and 28c plants and from plants homozygous for 28c and the 35S:hp2ox2 construct. The inset shows 2ox2 mRNA levels, measured by semi-quantitative reverse transcriptase-PCR with β-tubulin as a control, in rosettes of WT, 28c+35S:hp2ox2/1n, 28c+35S:hp2ox2/1v, and 28c plants. D, Schematic representation of a self-pollinated hemizygous 35S:2ox2/28c flower at anthesis, indicating the growth of haploid pollen tubes through the transmitting tract to fertilize ovules containing the female gametophyte (egg). WT pollen tubes can fertilize ovules throughout the ovary, whereas 28c pollen tubes can generally only reach ovules near the stigma. E, Suppression of the 28c fruit phenotype by gar2-1 and rga-2, alleles known to increase GA response in other organs.
The 28c Fruit Phenotype Is Consistent throughout Plant Development
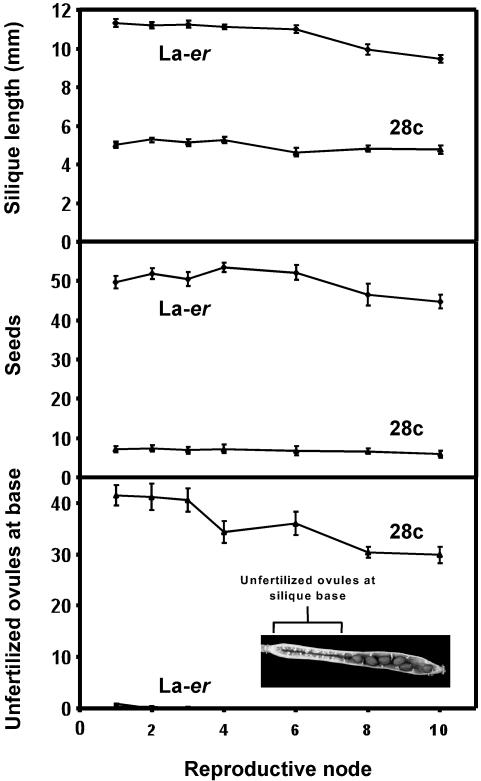
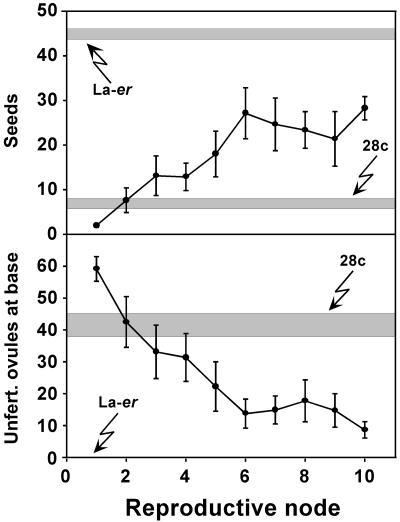
To determine if the 28c fruit phenotype changes dramatically as the plant continues to produce flowers, fruit development was carefully examined over the first 10 reproductive nodes (i.e. the first 10 consecutive nodes at which flowers were produced on the main inflorescence stem). Although external examination of self-pollinated WT and 28c plants did not uncover any obvious developmental variation, the data in Figure 2 reveal subtle changes during ontogeny. For example, WT fruit at later nodes were slightly shorter than at early nodes, possibly because these plants were beginning to senesce by the time nine to 10 siliques were set. This effect was not observed in 28c plants, presumably because the reduced seed set delays apical senescence (Singh et al., 2002). For line 28c, silique length and seed number were consistent throughout the period examined. By contrast, the number of unfertilized ovules at the silique base (Fig. 2, Inset) decreased slightly in 28c fruit at later nodes. Because this decrease was not associated with an increase in seed numbers, it may be due to a reduction in the number of ovules present in older ovaries. Nevertheless, by all three criteria examined (silique length, seed number, and the number of unfertilized ovules at the silique base), the 28c fruit phenotype is consistent and clearly different from WT throughout reproductive development.
Figure 2.
Comparison of fruit development on self-pollinated WT (La-er) and 28c plants over the first 10 reproductive nodes on the main stem. n = 8 for each genotype. The inset shows a typical 28c fruit developing from a self-pollinated flower.
Targeted Silencing of the 35S:2ox2/28c Transgene
Although a number of independent 35S:2ox2 lines with defective pollen tube growth were characterized in Singh et al. (2002), we sought to confirm that the pollen tube phenotype of line 28c is due to expression of the 2ox2 cDNA rather than the formal possibility of a mutation in, or reduced activity of, an Arabidopsis gene required for normal pollen tube growth. We used the recently developed hairpin silencing technology (Smith et al., 2000) in an attempt to specifically silence the 28c transgene by posttranscriptional gene silencing (PTGS)/RNAi and restore pollen tube growth. In this experiment, homozygous 28c plants were supertransformed with a 35S:hp2ox2 construct (Fig. 1B) designed to silence expression of the pea 2ox2 gene without effecting expression of any of the known Arabidopsis GA 2-oxidase genes (Thomas et al., 1999; Schomberg et al., 2003).
Consistent with the observed silencing of 2ox2 expression by the 35S:hp2ox2 transgene (Fig. 1C, inset), plants homozygous for the 28c and 35S:hp2ox2 constructs had fruit development partially restored to that of WT plants (Fig. 1C; Table I). Controlled reciprocal crosses involving 28c/35S:hp2ox2 and 28c plants demonstrated that the 35S:hp2ox2 construct acts within the developing pollen grain and/or pollen tube to suppress the 28c phenotype. No evidence for an effect of RNAi in the pistil (against the 2ox2 message) on 28c pollen tubes was observed, suggesting that the approximately 21-nucleotide signal thought to be involved in RNAi (Carthew, 2001; Waterhouse et al., 2001) cannot move into pollen tubes from the transmitting tract (data not shown). Fruit development of self-pollinated 28c hemizygous plants is very similar to that observed in self-pollinated WT plants (Fig. 3) because the approximately 50% of WT pollen produced in these plants can fertilize ovules throughout the ovary (Fig. 1D; Singh et al., 2002). As expected from this result, one copy of the 35S:hp2ox2/1v transgene was also sufficient to increase seed numbers and fruit size in a homozygous 28c background (data not shown).
Table I.
The 35S:hp2ox2 transgene partially suppresses the 35S:2ox2/28c fruit phenotype. All plants were allowed to self-pollinate n = 20 siliques for each genotype.
| Genotype | Silique Length | Seeds | Unfertilized Ovules at Silique Base |
|---|---|---|---|
| mm | |||
| WT La-er | 11.4 ± 0.1 | 50.1 ± 1.4 | 2.0 ± 0.5 |
| 35S:2ox2/28c | 5.2 ± 0.1 | 8.8 ± 0.8 | 34.7 ± 1.9 |
| 28c + 35S:hp2ox2/1i | 6.8 ± 0.2 | 17.6 ± 1.3 | 25.7 ± 2.0 |
| 28c + 35S:hp2ox2/1p | 6.0 ± 0.3 | 14.9 ± 1.3 | 26.5 ± 3.7 |
| 28c + 35S:hp2ox2/1n | 8.9 ± 0.2 | 32.7 ± 1.6 | 10.0 ± 1.3 |
| 28c + 35S:hp2ox2/1v | 7.3 ± 0.2 | 21.7 ± 1.0 | 9.2 ± 1.4 |
Figure 3.
Fruit development at node 3 for self-pollinated plants of various genotypes. n ≥ 8 for each genotype. The inset shows a typical 28c fruit developing from a self-pollinated flower.
Interaction of 28c with GA-Signaling Mutants
Although we have previously shown that the GA-response mutant, spy-5, can partially suppress the 28c pollen tube phenotype (Singh et al., 2002), the interaction between 28c and other mutants known to increase GA response has not been examined.
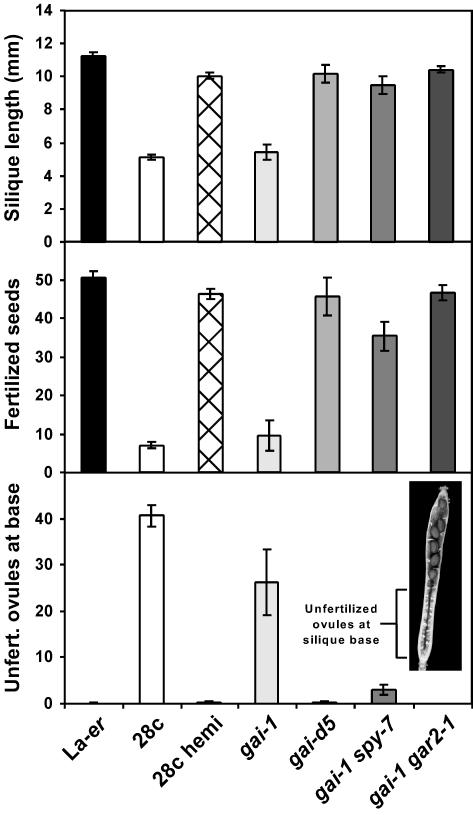
Self-pollinated 28c gar2-1 plants possessed siliques very similar to WT fruit in terms of length and seed numbers (Fig. 1E; data not shown). Similar results were observed in 28c gai-1 gar2-1 plants in which gar2-1 was able to suppress the gai-1 and 28c phenotypes in the same plant (data not shown). To determine if RGA and GAI are also required for normal GA signaling in pollen tubes, fruit growth was examined in 28c rga-2 and 28c gai-d5 double mutants (Fig. 1E). 28c rga-2 siliques were slightly longer than 28c fruit, and contained more seeds and fewer unfertilized ovules at the base of the silique. By contrast, fruit development was very similar in 28c and 28c gai-d5 plants (Fig. 1E; data not shown).
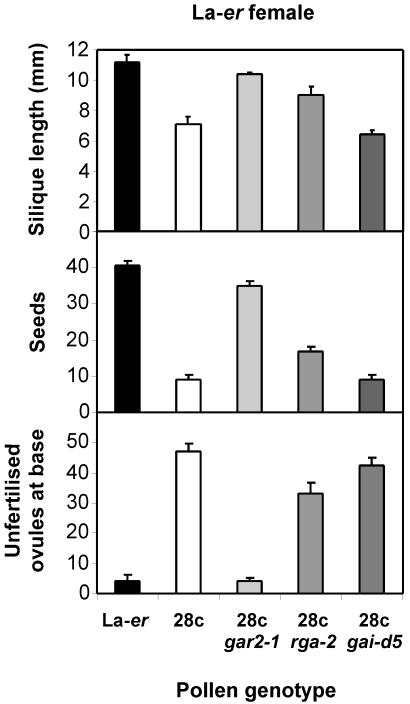
An important issue is whether the effects of the gar2-1 and rga-2 alleles on seed fertilization represent direct changes in the growth of 28c pollen tubes, rather than indirect effects on other tissues such as the transmitting tract. To address this question, two types of experiments were conducted. In the first, controlled crosses were carried out using pollen of different genotypes and (Landsberg erecta) La-er pistils because we have previously established that this approach can be used to characterize pollen tube growth (Singh et al., 2002). The results obtained (Fig. 4) confirm that gar2-1 and rga-2 are partial suppressors of 28c that act in pollen tubes, whereas gai-d5 does not have a detectable effect on the 28c phenotype. Thus, the fruit phenotypes shown in Figure 1E can be attributed to increased growth of 28c gar2-1 and 28c rga-2 pollen tubes compared with 28c pollen tubes.
Figure 4.
The rga-2 and gar2-1 alleles suppress the 28c phenotype by altering pollen function. Data represent fruit development on WT (La-er) ♀ plants pollinated with pollen of different genotypes. n ≥ 6 for each genotype.
In the second experiment, pollen tube length in vitro was compared between different genotypes (Fig. 5). Initially, we confirmed that 28c pollen tubes are shorter than WT pollen tubes (Singh et al., 2002), and that this phenotype is suppressed by silencing of the 28c transgene (Figs. 1C and 5A). Consistent with the results in Figures 1E and 4, gar2-1 was a very effective 28c suppressor, whereas gai-d5 did not suppress the 28c pollen tube phenotype in vitro. In contrast to the mild suppression of 28c by rga-2 in planta, rga-2 did not detectably suppress the 28c pollen tube phenotype in vitro, presumably due to a lack of sensitivity of this assay (Fig. 5B).
Figure 5.
Pollen tube growth in vitro. Pollen grains were collected from flowers at anthesis, allowed to grow for 24 h, and pollen tubes were measured. n ≥ 25 pollen tubes for each genotype.
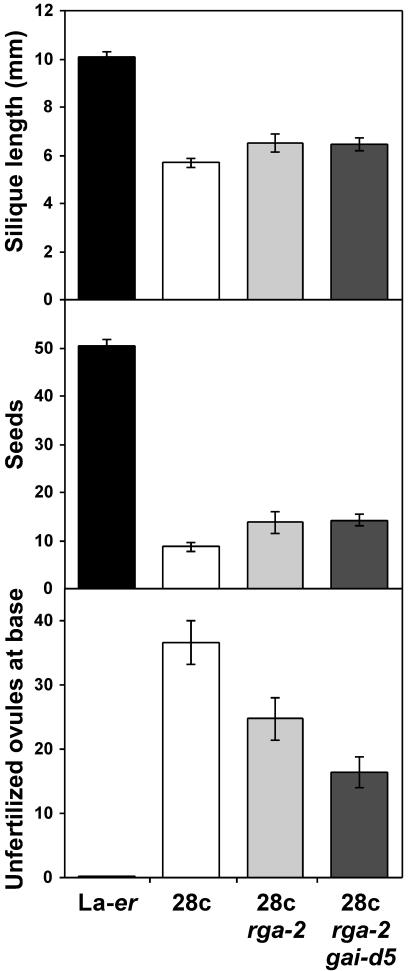
In vegetative tissues, severely GA-deficient ga1 plants lacking RGA and GAI are able to grow normally due to constitutive derepression of the GA signal transduction pathway (Dill and Sun, 2001; King et al., 2001). To determine if a similar situation exists in pollen tubes, 28c rga-2 gai-d5 plants were examined, and the development of 28c rga-2 and 28c rga-2 gai-d5 fruit was found to be very similar to each other and clearly different from the development of WT fruit (Fig. 6). A possible problem with the interpretation of this experiment is that loss of RGA and GAI reduces male fertility by affecting anther development (Dill and Sun, 2001; King et al., 2001), and reduced pollen production/viability might partially mask the 28c rga-2 gai-d5 pollen tube phenotype. To eliminate this explanation, the following experiment was conducted. An F2 population of 36 plants (all homozygous for the 28c transgene), derived from a cross between 28c rga-2 and 28c gai-d5, was examined in terms of fruit size, and no plants were found to have siliques obviously larger than 28c rga-2 or 28c rga-2 gai-d5 plants. This F2 population would have included, among other genotypes, plants homozygous for gai-d5 and heterozygous for RGA/rga-2, or homozygous for rga-2 and heterozygous for GAI/gai-d5 (approximately 25% of progeny). Because plants of these two genotypes would not be expected to have defects in male fertility (due to the presence of one WT GAI or RGA allele), but would produce 50% pollen of genotype 28c rga-2 gai-d5, the failure to observe fruit similar in size to WT fruit suggests that 28c pollen tubes lacking rga and gai are still significantly impaired in growth. Taken together, these results suggest that unlike the situation in vegetative tissues, pollen tubes lacking RGA and GAI still require significant levels of active GAs for growth.
Figure 6.
Effect of the rga-2, gai-d5, and rga-2 gai-d5 mutant combinations on 28c fruit development. All plants were allowed to self-pollinate. Data is shown for reproductive node 8 because the flowers at the first several nodes of rga gai-d5 plants are often male sterile. n ≥ 8 for each genotype.
In summary, like spy-5, the gar2-1 and rga-2 alleles, and the rga-2 gai-d5 mutant combination suppress to various degrees the pollen tube, and hence the fruit size, phenotype of homozygous 28c plants. Pollen tubes grown in vitro that lack 28c but carry the spy-5, gar2-1, rga-2 or rga-2 gai-t6 alleles are also resistant to the inhibitory effects of uniconazole (Singh et al., 2002), a chemical inhibitor of GA biosynthesis (data not shown).
Does the Gain-of Function gai-1 Mutation Mimic the Effect of 28c on Pollen Tube Growth?
The GAI locus encodes a GRAS family member (Pysh et al., 1999) that acts as a negative regulator of GA signaling (Peng et al., 1997). GAI is expressed in most plant organs, including inflorescences (Wen and Chang, 2002). The original gain-of-function gai-1 allele causes reduced response to endogenous GAs, and this leads to a range of defects in plant development, the most obvious of which is reduced vegetative growth, but more subtle phenotypes include reduced/delayed seed germination, reduced male fertility (Wilson and Somerville, 1995), and changes in fruit anatomy (Vivian-Smith and Koltunow, 1999). To determine if gai-1 also alters pollen tube growth, fruit growth was examined in detail on self-pollinated plants. Compared with self-pollinated WT La-er plants, gai-1 fruit were shorter, and contained fewer fertilized seeds (particularly at early reproductive nodes) and more unfertilized ovules at the base of the silique (Fig. 7). In fact, some gai-1 siliques resemble fruit developing on self-pollinated 28c plants.
Figure 7.
The dominant dwarfing gai-1 allele reduces plant fertility. All plants were allowed to self-pollinate. Horizontal gray bars represent the mean ± 1 se for the indicated genotype at node 3. La-er plants did not contain unfertilized ovules at the silique base in this experiment. n ≥ 8 for each genotype.
To confirm that the observed gai-1 phenotype is caused by defects in GA signaling, several additional mutants and mutant combinations were examined (Fig. 3). Fruit development was examined at the third reproductive node because this node is representative of WT and 28c plants as a whole, and reasonably representative for gai-1 plants (Figs. 2 and 7). First, we confirmed that fruit development was similar to WT in heterozygous GAI gai-1 plants (data not shown) and in gai-d5, a presumed loss-of-function derivative allele that contains the deletion present in gai-1 (Peng et al., 1997). Second, the spy-7 and gar2-1 alleles, identified based on their ability to suppress the gai-1 dwarf phenotype (Carol et al., 1995; Wilson and Somerville, 1995), also restore fruit development. Although the spy-7 mutation does not completely restore fruit growth in gai-1 plants, spy-7 (originally named gas1-1) is a relatively weak loss-of-function SPY allele that can only partially suppress the vegetative gai-1 dwarf phenotype (Carol et al., 1995; Peng et al., 1999).
One explanation for the observed gai-1 phenotype described above is impaired growth of gai-1 pollen tubes compared with WT pollen tubes, similar to the phenotypes described for 35S:2ox2 lines such as 28c. Alternatively, the gai-1 fruit phenotype could be explained by impaired fertilization caused by reduced amounts of pollen deposited on the stigma at the appropriate time, particularly at early reproductive nodes. This could be caused by reduced anther filament length or failure of the anther to develop properly, both of which represent known physiological roles of GAs. To determine if gai-1 is truly mimicking the 28c pollen tube phenotype, controlled crosses were conducted in the 28c background using gai-1 flowers pollinated with pollen from GAI gai-1 heterozygous plants. The observed numbers of progeny arising from GAI pollen (231 seeds; semidwarf plants) or gai-1 pollen (232 seeds; dwarf plants) did not differ significantly (P > 0.80) from the expected 1:1 ratio, suggesting that gai-1 does not alter pollen grain germination or pollen tube growth. This conclusion is also supported by the observation that self-pollinated gai-1 gai-1 SPY spy-7 plants, which should produce 50% gai-1 spy-7 pollen, possess fruit development similar to gai-1 SPY plants (data not shown).
Effect of Genetic Background on the 28c Fruit Phenotype
We have previously shown that when pollen carrying the 28c transgene is used to pollinate an La-er pistil, seeds are only observed at the stigmatic end of the ovary due to impaired elongation of the transgenic pollen tubes (Singh et al., 2002). By contrast, when plants of the Columbia ecotype were used as the female parent, 28c pollen (in the La-er background) was able to fertilize ovules farther from the stigma, resulting in larger fruit containing more seeds (data not shown). This result suggests that genetic differences exist between La-er and Columbia that act in maternal tissues to influence pollen tube growth and fertilization. Because this type of experiment involves hand pollination, and because the observed effect was relatively subtle, it was difficult to avoid some variation in the number of seeds fertilized for replicate crosses involving a given pollen genotype. To overcome the need for hand pollinations, the 28c transgene was backcrossed six times from La-er into the Columbia genetic background, and then made homozygous. The data in Table II demonstrates that although the homozygous 28c phenotype is qualitatively similar in Columbia, the fruit phenotype is more subtle than in the original La-er background. This result, obtained with self-pollinated plants, mirrors the result described above for hand pollinations. The simplest explanation for these results is that the Columbia ecotype carries a gene(s) that acts in the maternal tissues of the pistil to reduce the severity of the 28c pollen tube phenotype.
Table II.
Seed and fruit development at the third reproductive node on the main stem of self-pollinated plants
Values for seeds and unfertilized ovules are the sum from both carpels. n ≥ 8 plants for each genotype.
| Genotype | Silique Length | Seeds | Unfertilized Ovules at Silique Base |
|---|---|---|---|
| mm | |||
| WT La-er | 10.63 ± 0.16 | 44.00 ± 1.38 | 0.00 ± 0.00 |
| 28c | 5.19 ± 0.25 | 6.88 ± 1.06 | 41.50 ± 3.49 |
| WT Columbia | 13.05 ± 0.30 | 49.73 ± 1.43 | 0.00 ± 0.00 |
| 28c (Columbia) | 9.94 ± 0.32 | 20.61 ± 1.48 | 15.28 ± 3.10 |
| 28c er-102 | 8.08 ± 0.22 | 17.74 ± 1.05 | 12.84 ± 2.38 |
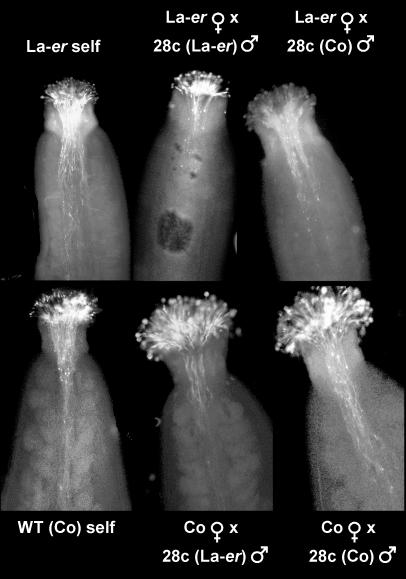
To characterize the effect of the La-er and Columbia genetic backgrounds in more detail, pollen tube growth was examined in planta and in vitro. For in planta analysis, aniline blue staining was used to visualize pollen tube growth in reciprocal crosses (Fig. 8). These experiments revealed that the genetic background of the pollen also influences the rate of pollen tube growth independently of the effect of the genetic background of the pistil. These results were confirmed when pollen tube growth was examined in vitro (Fig. 5C).
Figure 8.
Aniline blue staining of pollen tubes in vivo. Pistils were stained 20 h after hand pollination.
Because the er mutation present in La-er is known to influence the phenotype of several mutants with flower/fruit phenotypes, including spy (e.g. Lang et al., 1994; Swain et al., 2001), one possibility is that the ER/er difference between Columbia and La-er is responsible, at least in part, for the ecotype (i.e. genetic background) effects described above. To test this hypothesis, the 28c transgene (in Columbia) was combined with the strong loss-of-function er-102 allele in the Columbia background (Torii et al., 1996). Although, as expected, the er-102 mutation reduced silique length in a homozygous 28c background (P < 0.01), it did not have a significant effect on other aspects of the 28c fruit phenotype (Table II) or alter the growth of 28c pollen tubes in vitro (Fig. 6C). Thus, it is unlikely that the ER/er difference between Columbia and La-er contributes to the milder 28c phenotype observed in Columbia.
As an initial step toward characterizing the genetic basis of the observed difference between the La-er and Columbia ecotypes, a mapping population was constructed in a background homozygous for the 28c transgene and for the absence of the Columbia ER allele. An unambiguous segregation consistent with the action of a single allelic difference between La-er and Columbia was not observed (data not shown). Because of the high probability that plants would be incorrectly classified in a larger mapping population, no further attempts were made to identify the gene(s) responsible for this phenotype.
DISCUSSION
GA-Signaling Mutants and Pollen Tube Elongation
Extensive analysis of the 28c line (in the original La-er background) suggests that increased GA degradation impairs the growth of elongating pollen tubes, decreases the number of ovules fertilized, and, in almost all fruit on self-pollinated plants, restricts seed development to the region of the silique nearest the stigma. Reduced seed numbers in turn reduce fruit length to approximately one-half that observed for WT plants or self-pollinated plants hemizygous for the 28c transgene (Figs. 1 and 3). Qualitatively similar results, although more subtle, are also seen for the 28c transgene in the Columbia background. The conclusion described above is supported by the ability to restore fruit size by GA application (Fig. 1), targeted silencing (RNAi) of the 35S:2ox2/28c transgene (Fig. 1), replacing 1 35S:2ox2/28c chromosome with a WT chromosome (Fig. 3), and combining with the spy-5 (Singh et al., 2002), gar2-1, rga-2, or rga-2 gai-d5 alleles (Figs. 1, 4, 5, and 6).
GA applied to the pistil can increase fruit growth for 28c flowers (Fig. 1) and emasculated WT flowers (Vivian-Smith and Koltunow, 1999), demonstrating that this GA can penetrate the ovary tissues and elicit a response. Despite this result, and in contrast to the effect of applied GA on 28c pollen tubes in vitro (Singh et al., 2002), we have not been able to increase seed set in 28c plants treated with GA just before anthesis. The simplest explanation for these results is that GA applied to the pistil does not enter the pollen tubes. A corollary of this explanation, consistent with the reduced growth of 28c pollen tubes in vitro, is that GAs produced by pistil tissues do not play a significant role in pollen tube growth. In addition, Goto and Pharis (1999) have shown that applied GA can increase the length of the stigmatic papillae. Hence GA treatment may increase the distance between the site of pollen grain germination and the ovules, possibly masking a mild increase in the growth of 28c pollen tubes.
The generation of transgenic Arabidopsis plants via the Agrobacterium tumefaciens-mediated floral dip method (Clough and Bent, 1998) generates T-DNA insertions in apparently random positions throughout the genome. Consequently, endogenous genes are often disrupted by T-DNA inserts, potentially generating mutants with detectable phenotypes. Such mutants usually represent loss-of-function mutations, although gain-of-function alleles are also possible (e.g. Sheldon et al., 1999). This possibility can cause serious difficulties in the interpretation of phenotypes potentially resulting from transgene constructs designed to express various genes of interest. In plants, the solution to this problem is to generate and characterize multiple independent insertion events, and to exclude any lines that exhibit qualitatively divergent phenotypes. Although several independent 35S:2ox2 lines have been shown to alter pollen function, the reduced transmission of 35S:2ox2 has allowed only line 28c to be obtained in homozygous form (Singh et al., 2002). Because this one line is of vital importance to our work on GAs and pollen tube elongation, we have used a hairpin silencing construct (35S:hp2ox2) to confirm that the 28c pollen tube phenotype is due to expression of 2ox2, rather than impaired function of an Arabidopsis gene (Fig. 1). To the best of our knowledge, this represents the first use of the hairpin silencing technology for such a purpose, and it provides an important new tool for characterizing gene function using transgenic plants. Although it is formally possible that the observed suppression of the 28c pollen tube phenotype is due to silencing of an endogenous gene rather than the 35S:2ox2 transgene, this explanation appears unlikely because of the limited sequence identity between 2ox2 and GA 2-oxidases from Arabidopsis (data not shown), and because expression of the 35S:2ox2 transgene was successfully reduced (Fig. 1C).
The strong suppression of the 28c pollen tube by gar2-1 provides further evidence that this allele increases GA response in most, if not all, plant organs. The partial suppression of the 28c phenotype by rga-2 also reveals the requirement of the RGA protein in GA signaling in pollen tubes, a role not previously identified. A role for RGA in pollen tube elongation is consistent with the expression of RGA in developing flowers (Silverstone et al., 1998; Wen and Chang, 2002). These results also provide additional support for a physiological role of GAs in pollen tube growth. The similar suppression of the 28c pollen tube phenotype by rga-2 gai-d5, and by the rga-2 allele individually, contrasts with the situation in vegetative tissues. In rosettes and in inflorescence internodes, partial genetic redundancy between RGA and GAI occurs, and plants lacking both proteins exhibit constitutive GA response (Dill and Sun, 2001; King et al., 2001). Thus, other GRAS proteins potentially involved in GA response such as RGL1, RGL2, or RGL3 are not thought to have a significant role in these organs. By contrast, in elongating pollen tubes, RGL1, RGL2, or RGL3 may act with RGA, and possibly GAI, to negatively regulate GA response, consistent with the expression of all five genes in inflorescences (Silverstone et al., 1998; Lee et al., 2002; Wen and Chang, 2002).
The Reduced Fertility of gai-1 Is Not Caused by Reduced Pollen Tube Growth
Although the gai-1 allele causes reduced vegetative growth (Koornneef et al., 1985), decreased male fertility (Wilson and Somerville, 1995), and altered fruit anatomy (Vivian-Smith and Koltunow, 1999), and some gai-1 fruit resemble 28c fruit (Fig. 3), we have not found any evidence that gai-1 directly affects pollen tube growth. This conclusion is consistent with the inability of the derivative gai-d5 allele (containing a second-site intragenic suppressor mutation, Peng et al., 1997) to suppress the 28c pollen tube phenotype (Figs. 1E and 4). The gai-1 allele also fails to increase the severity of the 28c pollen tube phenotype in self-pollinated 28c gai-1 double mutants (data not shown).
Effect of Ecotype on Pollen Tube Growth
Backcrossing of the 28c transgene from La-er to the Columbia background and controlled pollinations involving pollen and pistils of different genotypes demonstrate that Columbia contains an allele of one or more genes involved directly in pollen tube growth and in the ability of 28c pollen tubes to grow through the transmitting tract and fertilize ovules. In fact, based on in vitro pollen tube assays, 28c (Columbia), pollen tubes grow as well as La-er pollen tubes, although not as well as WT Columbia pollen tubes (Fig. 5). Nevertheless, self-pollinated 28c fruit (in the Columbia background) possess, on average, approximately 15 unfertilized ovules at the base, whereas self-pollinated WT La-er siliques are fully fertile (Table II). This apparent contradiction may reflect limitations of the in vitro system used. Alternatively, Columbia pistils are receptive to fertilization for a shorter period than La-er pistils (Vivian-Smith and Koltunow, 1999), and this may partially counteract the effect of increased growth of 28c pollen tubes in the Columbia ecotype.
A New Approach for the Identification of Genes Altering GA Response and Fruit Development
The fruit phenotype of homozygous 28c plants provides the opportunity to use genetic screens to identify new GA-related genes based on their ability, when mutated, to suppress the 28c pollen tube phenotype and consequently increase fruit size. Analysis of the interaction between mutants with increased GA response and the 28c pollen tube phenotype demonstrates that mutations in the GAR2 locus that have an effect on GA signaling similar to that of gar2-1, and loss-of-function rga and spy alleles, may be identified in suppressor screens of the 28c pollen tube, and hence fruit size, phenotype. However, the limited suppression of 28c by rga-2 (Fig. 1E) suggests that new loss-of-function rga alleles will be relatively difficult to identify because of the similarity in fruit size of 28c and 28c rga-2 plants.
Although the ability of known GA-response mutants to suppress the 28c fruit phenotype demonstrates that a 28c suppressor screen can potentially identify new GA-response mutants, other mutants, not directly related to GA action, may also be recovered. The most obvious false-positives would be mutations that inactivate one allele of the 28c locus (compare with 28c hemi in Fig. 3) or mutations that reduce expression of the 35S:2ox2 transgene (compare with 28c+35S:hp2ox2 in Fig. 1). Other types of mutants that could potentially be identified include those that increase fruit growth without increasing seed set. For example, increased GA levels or response in the maternal tissues of the silique would lead to larger 28c fruit (see Vivian-Smith and Koltunow, 1999), as evidenced by the ability of exogenous GA3 to increase the size of 28c fruit without increasing seed numbers (Fig. 1A). Mutants that exhibit parthenocarpy may also be identified, particularly because 28c fruit contain some fertilized seeds. Finally, the different behavior of 28c pollen in La-er and Columbia pistils (Figs. 5 and 8) demonstrates that mutations acting in maternal tissues, such as the transmitting tract, could also increase the size of 28c fruit. Suppression screens of the 28c small fruit phenotype have been conducted, and several mutants with larger fruit are currently being characterized.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis ecotypes used were La-er and Columbia. Seeds of gai-1 (gai-1 is the original gain-of-function gai allele that causes a dwarf phenotype), gai-d5, gai-1 spy-7, and gai-1 gar2-1 were supplied by Dr. Neil E. Olszewski (University of Minnesota, St. Paul). Growth conditions, in vitro pollen tube growth, and aniline blue staining were as described (Singh et al., 2002).
Double and triple mutants, in the 28c background, were generated by crossing, and were confirmed by resistance to kanamycin (for the 28c transgene, Singh et al., 2002), by phenotype, or by PCR. To follow the gai-1 and gai-d5 alleles, we used the primers described in Fridborg et al. (2001). The 28c gar2-1 and 28c gai-1 gar2-1 mutants were identified based on kanamycin resistance (28c), GAI/gai-1 (PCR), and the ability to suppress the gai-1 dwarf phenotype (gar2-1). Homozygous gar2-1 plants were identified by the absence of any phenotypically dwarf gai-1 progeny from a self-pollinated GAI gai-1 parent (homozygous for the 28c transgene). This population was then screened by PCR to identify individuals with the 28c gar2-1 and 28c gai-1 gar2-1 genotypes. To follow rga-2, we used a cleaved-amplified polymorphic sequence marker based on the primers 5′-GCTTCGAGTCAAACTCAGTG-3′ and 5′-GGTGATTTTCACGGTGGTTG-3′ and digested the 414-bp PCR product with Tru9I (T.p. Sun, Duke University, Durham, NC, personal communication). In addition, the genotype of 28c rga-2 gai-d5 plants was confirmed by growing seedlings on Growool (GroWool Horticultural Systems, Girraween, New South Wales, Australia) in the presence of 10–6 m paclobutrazol (see Peng et al., 1999).
All seeds were stratified for 3 d at 4°C under dim light to aid germination. Plants were grown in growth rooms with 18 h of white fluorescent light (22°C, 60–70 μmol photons m–2 s–1 at pot top) and 6 h of dark (20°C) on GroWool, 0.8% (w/v) agar (Spectrum, Gardena, CA) containing Murashige and Skoog salts (2.3 g L–1; Sigma-Aldrich, St. Louis), and 1% (w/v) Suc, or individually in a peat-based soil mixture from the Commonwealth Scientific and Industrial Research Organization Plant Industry (Canberra, Australia) in the Arasystem (BetaTech, Gent, Belgium). Data is shown as the mean ± se. Data for seed numbers, and the number of unfertilized ovules at the silique base, are the sum of the values for the two carpels for each silique.
Generation of the 35S:hp2ox2 Construct
Standard molecular biology techniques were used throughout (Sambrook et al., 1989). The pHannibal vector (Smith et al., 2000) was used to generate the hairpin (hp) 2ox2 construct. Primers F2ox2/5 (5′-TAATTCTCGAGGGATCCGATTGGATGAGGAAGGAATA-3′) and R2ox2/6 (5′-TATAGGTACCACCATGGTGAGTTGAAATCTGAAGAC-3′) were used to amplify 518 bp from the pea (Pisum sativa) 2ox2 cDNA. The PCR product was digested with XhoI and KpnI (Promega, Annadale, New South Wales, Australia) and ligated into the pHannibal vector to obtain pHan2ox2. This 518-bp region of pea 2ox2 was again amplified by using primers F2ox2/7 (5′-TATATCTAGAGATTGGATGAGGAAGGAATAGAGT-3′) and R2ox2/8 (5′-GTCTAATCGATCCATGGTGAGTTGAAATCT GAA-3′) to create ClaI and XbaI sites at the 5′ and 3′ ends, respectively. ClaI- and XbaI-digested PCR product was ligated into the pHan2ox2 construct to obtain the hp2ox2 construct. The NotI fragment from hp2ox2 was cloned into NotI-digested pBART (Dr. Peter Waterhouse, Commonwealth Scientific and Industrial Research Organization Plant Industry, Canberra, Australia) to generate the 35S:hp2ox2 construct (Fig. 1). To confirm that the 35S:hp2ox2 construct reduced 35S:2ox2/28c expression, superscript one-step reverse transcriptase-PCR for long templates (Invitrogen, Carlsbad, CA) was used on RNA extracted from 2-week-old rosettes according to the manufacturer's instructions, with the primers 5′-CGACGATCGACCTTTCTCTC-3′ and 5′-GGGGTGATCTTTTGCTATG-3′.
Plant Transformation
Agrobacterium tumefaciens GV3101 was transformed with the 35S:hp2ox2 construct by electroporation (Shen and Forde, 1989). The construct was supertransformed into homozygous 35S:2ox2/28c (kanR) plants using the A. tumefaciens-mediated vacuum infiltration method (Ye et al., 1999). Putative transformants containing the 35S:hp2ox2 construct were sprayed with 0.2 g L–1 basta (ammonium glufosinate; Hoechst Horticulture, Melbourne, Australia), and basta-resistant putative transgenic seedlings containing a single basta resistance locus, based on the ratio of bastaR:bastaS, were transferred to soil to obtain T2 seeds. The segregating populations of T2 plants were allowed to self-pollinate, and the T3 progeny sprayed with basta to identify homozygous T2 plants.
Acknowledgments
We thank Angelica Jermakow, Carol Sigston, and Warren Hudson-Taylor for technical assistance, Celia Miller for help with photography, and Dr. Judy Tisdal for supervising A.J.M.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.031666.
This work supported in part by Horticulture Australia Ltd. as part of the Aushort gene discovery project.
References
- Alvarez J, Smyth DR (1999) CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development 126: 2377–2386 [DOI] [PubMed] [Google Scholar]
- Barendse GWM, Rodrigues-Pereira AJ, Berkers PA, Driessen FM, van Eyden-Emons A, Linskens HF (1970) Growth hormones in pollen, styles and ovaries of Petunia hybrida and Lilium species. Acta Bot Neerl 19: 175–186 [Google Scholar]
- Bhandal IS, Malik CP (1979) Effect of gibberellic acid, (2-chloroethyl), phosphoric acid, actinomycin-D and cycloheximide on the activity and leaching of some hydrolases in pollen suspension cultures of Crotalaria juncea. Physiol Plant 45: 297–300 [Google Scholar]
- Busov VB, Meilan R, Pearce DW, Ma C, Rood SB, Strauss SH (2003) Activation tagging of a dominant gibberellin catabolism gene (GA 2-oxidase) from poplar that regulates tree stature. Plant Physiol 132: 1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol P, Peng J, Harberd NP (1995) Isolation and preliminary characterisation of gas1-1, a mutation causing partial suppression of the phenotype conferred by the gibberellin-insensitive (gai) mutation in Arabidopsis thaliana (L.) Heyhn. Planta 197: 414–417 [DOI] [PubMed] [Google Scholar]
- Carthew RW (2001) Gene silencing by double-stranded RNA. Curr Opin Cell Biol 13: 244–248 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dill A, Sun, T-p (2001) Synergistic derepression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics 159: 777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeuwens CJ, Schwabe WW (1975) Seed and pod wall development in Pisum sativum L. in relation to extracted and applied hormones. J Exp Bot 26: 1–14 [Google Scholar]
- Fleck B, Harberd NP (2002) Evidence that the Arabidopsis nuclear gibberellin signalling protein GAI is not destabilised by gibberellin. Plant J 32: 935–947 [DOI] [PubMed] [Google Scholar]
- Fridborg I, Kuusk S, Robertson M, Sundberg E (2001) The Arabidopsis protein SHI represses gibberellin responses in Arabidopsis and barley. Plant Physiol 127: 937–948 [PMC free article] [PubMed] [Google Scholar]
- Goto N, Pharis RP (1999) Role of gibberellins in the development of floral organs of gibberellin-deficient mutant, ga1-1, of Arabidopsis thaliana. Can J Bot 77: 944–954 [Google Scholar]
- Hedden P, Phillips AL (2000) Manipulation of hormone biosynthetic genes in transgenic plants. Curr Opin Biotechnol 11: 130–137 [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE (1993) Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y, Garcia-Martinez JL (1999) Regulation of gibberellin biosynthesis by light. Curr Opin Plant Biol 2: 398–403 [DOI] [PubMed] [Google Scholar]
- Kimura PH, Okomoto G, Hirano K (1996) Effects of gibberellic acid and streptomycin on pollen germination and ovule and seed development in Muscat Bailey A. Am J Enol Viticulture 47: 152–156 [Google Scholar]
- King KE, Moritz T, Harberd NP (2001) Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics 159: 767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rijn L, Zeevaart JA (1985) A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant 65: 33–39 [Google Scholar]
- Lang JD, Ray S, Ray A (1994) sin1, a mutation affecting female fertility in Arabidopsis, interacts with mod1, its recessive modifier. Genetics 137: 1101–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J (1957) Effect of day-length and gibberellic acid on the flowering of Arabidopsis. Nature 180: 36–37 [Google Scholar]
- Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16: 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester DR, Ross JJ, Smith JJ, Elliott RC, Reid JB (1999) Gibberellin 2-oxidation and the SLN gene of Pisum sativum. Plant J 19: 1–9 [DOI] [PubMed] [Google Scholar]
- Mander LN, Owen DJ, Croker SJ, Gaskin P, Hedden P, Lewis MJ, Talon M, Gage DA, Zeevaart JA, Brenner ML et al. (1996) Identification of three C20-gibberellins: GA97 (2 β-hydroxy-GA53), GA98 (2 β-hydroxy-GA44) and GA99 (2 β-hydroxy-GA19). Phytochemistry 43: 23–28 [DOI] [PubMed] [Google Scholar]
- Martin DN, Proebsting WM, Hedden P (1999) The SLENDER gene of pea encodes a gibberellin 2-oxidase. Plant Physiol 121: 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester JE, Zeevaart JAD (1988) Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. Am J Bot 75: 45–55 [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Richards DE, Moritz T, Cano-Delgado A, Harberd NP (1999) Extragenic suppressors of the Arabidopsis gai mutation alter the dose-response relationship of diverse gibberellin responses. Plant Physiol 119: 1199–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharis RP, King RW (1985) Gibberellins and reproductive development in seed plants. Annu Rev Plant Physiol 36: 517–568 [Google Scholar]
- Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN (1999) The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J 18: 111–119 [DOI] [PubMed] [Google Scholar]
- Richards DE, King KE, Ait-Ali T, Harberd NP (2001) How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol 52: 67–88 [DOI] [PubMed] [Google Scholar]
- Ross JJ, Murfet IC, Reid JB (1997) Gibberellin mutants. Physiol Plant 100: 550–560 [Google Scholar]
- Sakamoto T, Kobayashi M, Hironori I, Tagiri A, Kayano T, Hiroshi T, Iwahori S, Matsuoka M (2001) Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol 125: 1508–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schomberg FM, Bizzell CM, Lee DJ, Zeevaart JAD, Amasino RM (2003) Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 15: 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setia N, Setia RC, Chhabra N (1994) Interactive effects of growth hormones and calcium antagonists on germination and tube elongation of groundnut pollen. Plant Cell Incompatibility Newslett 26: 70–80 [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES (1999) The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W-J, Forde BG (1989) Efficient transformation of Agrobacterium sp. by high voltage electroporation. Nucleic Acids Res 17: 8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun T-P (1998) The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal-transduction pathway. Plant Cell 10: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Jung H-S, Dill A, Kawaide H, Kamiya Y, Sun T-P (2001) Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13: 1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D-P, Jermakow AM, Swain SM (2002) Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell 14: 3133–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NA, Singh SP, Wang MB, Stoutjesdijk PA, Green AG, Waterhouse PM (2000) Total silencing by intron-spliced hairpin RNAs. Nature 407: 319–320 [DOI] [PubMed] [Google Scholar]
- Swain SM, Tseng T-S, Olszewski NE (2001) Altered expression of SPINDLY affects gibberellin response and plant development. Plant Physiol 126: 1174–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SG, Phillips AL, Hedden P (1999) Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci USA 87: 7983–7987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y (1996) The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8: 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viti R, Bartolini S, Vitagliano C (1990) Growth regulators on pollen germination in olive. Acta Hort 286: 227–230 [Google Scholar]
- Vivian-Smith A, Koltunow A (1999) Genetic analysis of growth regulator-induced parthenocarpy in Arabidopsis. Plant Physiol 121: 437–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse PM, Wang M-B, Finnegan EJ (2001) Role of short RNAs in gene silencing. Trends Plant Sci 6: 297–301 [DOI] [PubMed] [Google Scholar]
- Wen CK, Chang C (2002) Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Somerville CR (1995) Phenotypic suppression of the gibberellin-insensitive mutant (gai) of Arabidopsis. Plant Physiol 108: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Smith MW, Brown RG, Kamiya Y, Sun T-P (1998) Phytochrome regulation and differential expression of gibberellin 3 β-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell 10: 2115–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye GN, Stone D, Pang SZ, Creely W, Gonzalez K, Hinchee M (1999) Arabidopsis ovule is the target for Agrobacterium in planta vacuum infiltration transformation. Plant J 19: 249–257 [DOI] [PubMed] [Google Scholar]