Abstract
The temporal dynamics of partitioning and rhizodeposition of recent photosynthate in wheat (Triticum aestivum) roots were quantified in situ in solution culture. After a 30-min pulse of 14CO2 to a single intact leaf, 14C activities of individual carbon fluxes in the root, including exudation, respiration, and root content, were measured continuously over the next 20 h concurrently with 14C efflux from the leaf. Immediately after the end of the 14CO2 pulse, 14C activity was detected in the root, the hydroponic solution, and in root respiration. The rate of 14C exudation from the root was maximal after 2 to 3 h, and declined to one-third of maximum after a further 5 h. Completion of the rapid phase of 14C efflux from the leaf coincided with peak 14C exudation rate. Thus, exudation flux is much more rapidly and dynamically coupled to current photosynthesis than has been appreciated. Careful cross-calibration of 14C counting methods allowed a dynamic 14C budget to be constructed for the root. Cumulative 14C exudation after 20 h was around 3% of 14C fixed in photosynthesis. Partitioning of photosynthate between shoot and root was manipulated by partial defoliation before applying the 14CO2 pulse to the remaining intact leaf. Although the rate of photosynthesis was largely unaffected by partial defoliation, the proportion of new photosynthate subsequently partitioned to and exuded from the root was substantially reduced. This clearly indicates that exudation depends more on the rate of carbon import into the root than on the rate of photosynthesis.
Root exudation of soluble organic carbon is a key process that drives microbial activity in the soil, but it is often omitted from considerations of plant carbon balance as being a minor component (Grayston et al., 1996; Kuzyakov and Cheng, 2001). However, it has been estimated that between 1% and 40% of net photosynthate is lost by this pathway (Whipps, 1990; Meharg, 1994). Exudation has a prime role in influencing nutrient availability in the rhizosphere and thus plant growth itself. Although translocation of photosynthate from the shoot to the root and consequently to the rhizosphere is thought to be a rapid process (Meharg and Killham, 1988; Rattray et al., 1995; Ekblad and Högberg, 2001; Högberg et al., 2001), the dynamics of the exudation of current photosynthates are not understood.
Soil provides a formidable technical barrier to measuring individual fluxes, and experimental approaches have relied on a few techniques. In the first, carbon partitioning in the plant and rhizosphere has been examined by destructive sampling, typically on sets of plants harvested at intervals of days or weeks. Although subsequent analyses may be fairly detailed (Kraffczyk et al., 1984), this approach only provides a snapshot of the outcome of many concurrent processes without providing an understanding of how those processes unfold in an individual plant and its rhizosphere (Jones and Darrah, 1993). Not only this, but if the export of fixed carbon from leaves occurs over a timescale of minutes to hours (Farrar and Farrar, 1985), isolated or intermittent sampling times may not be the most appropriate for observing carbon fluxes in roots and the rhizosphere. A second complementary and noninvasive approach is the collection of fractions of respired CO2 from the soil-root continuum as an estimate of the magnitude of below-ground processes (Högberg et al., 2001). This can only provide a composite picture of root-plus-microbial respiration rather than differentiating individual fluxes.
The aim of this study was to resolve the short-term dynamics of carbon transfer between leaf, root, and rhizosphere using noninvasive 14C-isotopic labeling. The experiments were designed to address the following questions: How much of the carbon exported from the leaf enters the root? How much of the carbon imported into the root is lost by exudation? Is carbon from recent photosynthesis a source of exudate? How much recent photosynthate is lost in exudation? Does exudation depend on the rate of photosynthesis?
RESULTS
Carbon Export from the Leaf
The export of 14C from the source leaf was strongly biphasic (Figs. 1 and 2). The percentage of 14C remaining in the leaf over time conformed well (r2 > 0.99) to a double-exponential decay function:
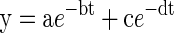 |
where t is time (in hours) after the end of the 14CO2 pulse feed to the leaf, y is the amount of 14C activity remaining in the leaf (as a percentage of the activity detected in the leaf immediately at the end of the pulse), and a, b, c, and d are constants. Adding a third, asymptotic, term to the equation did not improve the fit. Constants a and c describe the proportionate contribution of each of the simultaneous decays to the total, and constants b and d can be used to calculate the half-lives of the two contributory fluxes. The export of 14C described by the first term was largely complete within 3 h of the end of the pulse, having a half-time of approximately 0.5 h (the transport pool). The second term had a half-time of around 20 h (the storage pool). The relative proportion described by the first term varied between 0.50 and 0.71 of the total. In this and other variables, the coefficient of variation was about 15%.
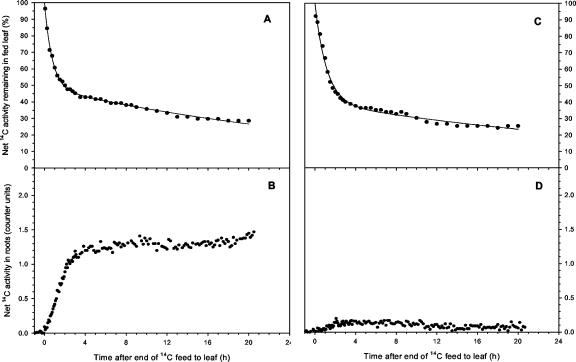
Figure 1.
Examples of simultaneous measurements of 14C activity in leaf 2 of wheat (A and C, expressed as a percentage of activity in leaf 2 at the end of the pulse feed) and in the root system plus exudates (B and D, expressed in radiation counter units) after a 30-min pulse feed to leaf 2 on 15-d-old plants. This compares a control plant (A and B) with one partially defoliated 5 h before the end of the pulse feed (C and D). The fitted line (y = ae–bt + ce–dt) has the following values for the constants: (A) a = 51.5 ± 1.0, b = 1.33 ± 0.05, c = 48.5 ± 0.5, d = 0.030 ± 0.001 (r2 > 0.99); (C) a = 60.3 ± 1.8, b = 0.87 ± 0.06, c = 39.6 ± 1.3, d = 0.026 ± 0.003 (r2 > 0.99). The traces shown are representative of at least three independently replicated experiments.
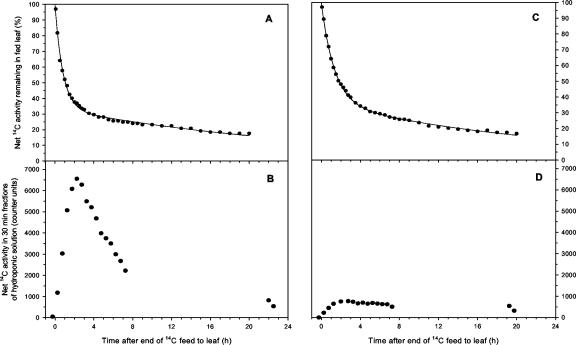
Figure 2.
Examples of simultaneous measurements of 14C activity in leaf 2 of wheat (A and C, expressed as a percentage of activity in leaf 2 at the end of the pulse feed) and in the 30-min fractions of hydroponic solution (B and D, expressed in radiation counter units) after a 30-min pulse feed to leaf 2 on 15- and 16-d-old plants. This compares a control plant (A and B) with one partially defoliated 5 h before the end of the pulse-feed (C and D). The fitted line (y = ae–bt + ce–dt) has the following values for the constants: (A) a = 66.4 ± 1.3, b = 1.24 ± 0.05, c = 33.7 ± 0.8, d = 0.037 ± 0.003 (r2 > 0.99); (C) a = 63.4 ± 0.7, b = 0.75 ± 0.02, c = 36.6 ± 0.7, d = 0.043 ± 0.002 (r2 > 0.99). The traces shown are representative of at least three independently replicated experiments.
In partially defoliated plants, the amount of 14C fixed was not significantly different from that in controls (P > 0.05), confirming that the removal of the rest of the leaf blades had little effect on the photosynthetic rate of the remaining leaf exposed to 14CO2 in the pulse feed. The dynamics of 14C export were also similar between treatments (Figs. 1 and 2), therefore any subsequent differences between control and partially defoliated plants in root and rhizosphere 14C activity (see below) were the consequence of changes in carbon partitioning between shoot and root rather than of changes in leaf export.
We have previously shown that respiration of the leaf blade inside the cuvette is negligible (Farrar, 1985). Losses are due to export. Consequently, at the end of the 20-h chase period, export from this portion of the leaf amounted to between 78% and 86% of the net 14C fixed (Tables I and II).
Table I.
14C activity in the root 20 h after the end of the pulse feed to leaf 2 of intact and partially defoliated plants of wheat
Values represent means ± sem (n = 3). Significant differences (P < 0.05) as determined by two-tailed unpaired t test are indicated by different postscripts.
| Treatment | Cumulative 14C Export from Fed Leaf 2 | 14C Activity in Root | 14C Activity in Root |
|---|---|---|---|
| % of total 14C fixed | % of 14C export from fed leaf 2 | ||
| Intact | 77.8 ± 2.1a | 15.5 ± 2.5a | 20.1 ± 3.7a |
| Defoliated | 79.4 ± 1.9a | 0.8 ± 0.2b | 1.0 ± 0.3b |
Table II.
Cumulative 14C activity lost from root to solution 4.5 h (measured) and 20 h (estimated) after the end of the pulse feed to leaf 2 of intact and partially defoliated plants of wheat
Values represent means ± sem (n = 3). Significant differences (P < 0.05) as determined by two-tailed unpaired t test are indicated by different postscripts.
| Treatment
|
Cumulative 14C Export from Fed Leaf 2 at 20 h
|
Cumulative 14C Root Exudation
|
Cumulative 14C Root Exudation
|
|
|---|---|---|---|---|
| 4.5 h | 20 h | |||
| % of total 14C fixed | % of 14C export | |||
| Intact | 82.4 ± 1.7a | 1.8 ± 0.3a | 3.1 ± 0.7 | 3.8 ± 0.8a |
| Defoliated | 86.2 ± 0.6a | 0.3 ± 0.1b | 0.9 ± 0.1 | 1.1 ± 0.1a |
Carbon Import into the Root
14C activity was detected in the root system plus exudate almost immediately after the end of the pulse to the leaf (Fig. 1). In roots of control plants, 14C activity increased rapidly for the following 2 h, reaching a maximum approximately 3 h after the end of the pulse. Thereafter, in most replicates, it declined to a slightly lower value and remained fairly constant over the remainder of the 20-h chase period. In contrast, in partially defoliated plants, 14C activity increased only slightly above background, remaining at a similar value throughout the 20-h chase period, and with a final value an order of magnitude lower than in control plants (Table I).
Carbon Exudation from the Root
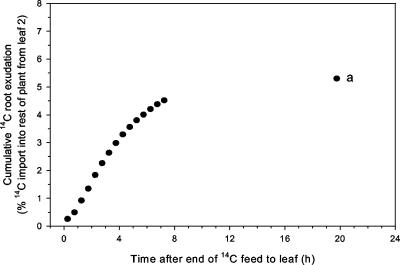
14C activity was detected in the nutrient solution immediately after the end of the pulse feed to the leaf (Fig. 2). In control plants, the rate of 14C exudation increased to a maximum 2 to 3 h after the end of the pulse feed. Thereafter, it declined almost as rapidly to approximately one-third of the maximum value at 7.5 h, but exudation was still occurring shortly after the start of the subsequent photoperiod (19–23 h). Mean total loss of 14C-labeled products from the root to the rhizosphere was 3.0% of the cumulative export from the leaf at 7.5 h. In partially defoliated plants, the rate of 14C loss via exudation was significantly reduced compared with control plants at 3 h but was fairly similar to control plants at the end of the chase period. Mean total loss of 14C-labeled products to the rhizosphere in these plants was 0.6% of cumulative leaf export at 7.5 h. Control and defoliated plants differed significantly (P < 0.05) in the measured values for cumulative 14C exudation at 4.5 h (Table II), that is, shortly after the point of maximal exudation rate, and the estimated cumulative value at 20 h is one-third as a result of defoliation. Of that 14C that had been imported into parts of the plant other than the source leaf, the proportion lost as root exudation stabilized within the first few hours of fixation (Fig. 3).
Figure 3.
Root 14C exudation as a percentage of 14C export from leaf 2. Cumulative data for the control wheat plant in Figure 2 (a = estimated value for cumulative exudation at 20 h). The traces shown are representative of at least three independently replicated experiments.
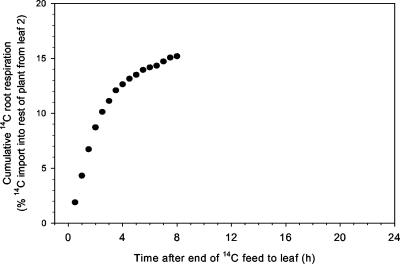
Carbon Loss via Root Respiration
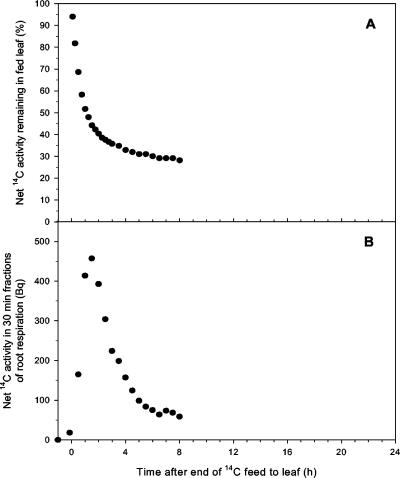
14C activity was detected in the air bubbled through the nutrient solution immediately after the end of the pulse feed to the leaf (Fig. 4). The rate of 14C respiration had similar dynamics to those for root 14C exudation over the 7.5-h period after the pulse feed to the leaf, except that the peak rate occurred earlier at 1.5 h, and the rate at 7.5 h was approximately one-tenth of maximum. When root-respired 14C was expressed as a proportion of total respired C, these dynamics were substantially unchanged, thus root respiration rate was not changing. The proportion of cumulative 14C export from the fed leaf lost as root respiration stabilized within the first few hours of fixation (Fig. 5). The proportional values for root respiration in Figure 5 were then used to validate the estimated dynamics of gross import of 14C into the root; both methods of estimation were in close agreement with each other (see Fig. 6).
Figure 4.
Simultaneous measurements of 14C activity in leaf 2 of wheat (A, expressed as a percentage of activity in leaf 2 at the end of the pulse feed) and in the 30-min fractions of root respiration (B, becquerel per 30-min fraction) after a 30-min pulse feed to leaf 2 on a 17-d-old plant. The traces shown are representative of at least three independently replicated experiments.
Figure 5.
Root 14C respiration as a percentage of 14C export from leaf 2. Cumulative data for the plant in Figure 4.
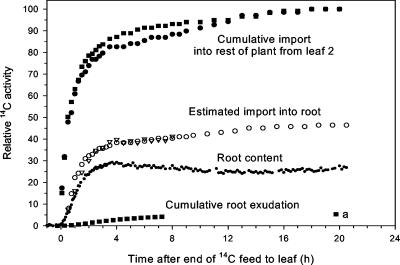
Figure 6.
Dynamic carbon budget using combined data for control wheat plants in Figures 1 and 2; all data were converted to bequerels and were then scaled so that for each plant cumulative 14C import into rest of plant from leaf 2 at 20 h = 100 {▪, data for control plant in Figs. 2 and 3; a,= estimated value for cumulative exudation at 20 h; the figure shows the two estimates of cumulative gross 14C import into the root (○, fixed proportion of 14C import into rest of plant from leaf 2 with 0.5-h time displacement; ▵, sum of root 14C content and estimated root 14C respiration derived from dynamics in Fig. 3}. The total cumulative import into the rest of the plant from leaf 2 at 20 h was 16,000 Bq.
Dynamic Carbon Budget
Figure 6 presents the dynamic carbon budget from a single pulse-feed experiment. By expressing the data in relative terms and by using the very careful calibrations for each 14C counting method, a comprehensive budget for 14C was constructed in which individual below-ground components are represented (Fig. 6).
DISCUSSION
The principal conclusions drawn from this study are that exudation is maximal 2 to 3 h after fixation in photosynthesis, that exudation is, after 20 h, around 3% of the 14C fixed in photosynthesis, and that exudation depends more on the rate of carbon import into the root than on the rate of photosynthesis. The proportion of photosynthetic fixed C exuded from the roots reported in this study is lower than frequently published elsewhere; however, many previous studies have tended to overestimate rhizodeposition due to the use of inappropriate methodology (Meharg, 1994).
The techniques presented here demonstrate the ability to obtain measurements on the principal C fluxes in the plant-rhizosphere continuum on intact plants with fine temporal resolution and with simultaneous measurement of C sources and sinks; the key element is very careful cross-calibration of radiation detectors. This has enabled, first, the characterization of the dynamics of below-ground carbon fluxes, which were previously hinted at by piecing together data from serial harvesting events or implied from respiration trapping; second, has allowed direct relationships between individual carbon fluxes to be established; and, third, has permitted a dynamic carbon budget of key root processes to be constructed.
Carbon Dynamics
The dynamics of 14C efflux from the leaf were described by a double-exponential equation. The biphasic pattern of efflux is consistent with the two-compartment model originally put forward by Moorby and Jarman (1975), and developed by Farrar and coworkers for cereal and grass leaves (Farrar and Farrar, 1985; Baxter and Farrar, 1999; Grantz and Farrar, 2000). The two phases describe the fractions of fixed 14C entering the pool of soluble sugars in the leaf that are exported immediately (transport pool) or incorporated into the storage pool before being re-mobilized. The 14C is partitioned directly to root and shoot apices, but not to mature leaves; although we fed only one leaf blade, the anastomosing of vasculature in the nodal plexus between shoot and root means that 14C is not preferentially allocated to any particular root (Williams et al., 1991).
Our root and rhizosphere data confirm previous findings (Minchin et al., 1994; Rattray et al., 1995) that, in vegetative cereal and grass crops, fixed organic carbon is transported very rapidly below ground and can be detected in the environment external to the root in less than 1 h from photosynthetic fixation. In our experiments, the exudation flux was quantified directly, and at sufficiently fine temporal resolution, so that the magnitude and shape of the exudation peak were clearly discernable to give the novel finding that maximal exudation of recent photosynthate occurs within 3 h of fixation and thereafter declines rapidly.
A peak of exudation has been inferred from indirect measurements. First, in serial harvests of nonsterile soil microcosms at 0.5, 3, and 24 h after a 2-h 14C pulse feed to the shoot, 14C incorporation into the rhizosphere microbial biomass was greatest 5 h after the start of the pulse feed (Rattray et al., 1995), although it was not possible to determine the precise timing, magnitude, or shape of the peak.
Second, the measurement of below-ground CO2 efflux has previously served as a proxy for monitoring root C fluxes in nonsterile soil. A standard method is to collect respired 14CO2 in a NaOH trap after a pulse feed to the shoot. Exchanging the trap at a 1-h frequency allowed Kuzyakov and Cheng (2001) to obtain a fairly detailed quantification of the dynamics of below-ground respiration on intact wheat plants, showing a maximum of root-derived 14CO2 efflux at 6 h after 14C pulse labeling. Nguyen et al. (1999) achieved an even higher degree of resolution using a radioactivity flow detector with a count time of 2 min, showing two maxima of root-derived 14CO2 efflux at 8 and 21 h, declining to background levels by 78 h after labeling. However, neither of these approaches gets close to a description of exudation dynamics. Even if microbial respiration of 14C exudate is taken as a crude measure of exudation flux, these experiments do not separate microbial from root respiration, particularly when 14C root respiration is dominant. Nor can these experiments quantify the unrespired fraction of exudate, or the time delays inherent in microbial acquisition and metabolism of the various exudate components. Much effort has been applied to solving this problem in soil using various approaches (Swinnen et al., 1995b; Kuzyakov and Cheng, 2001), but it still remains an elusive goal of rhizosphere research.
The value of the hydroponic techniques used here is that they provide the first step in the characterization of individual constituent below-ground processes, including root import and retention, root respiration, and root exudation. This has shown very clearly that current photosynthate is partitioned to roots within a time frame of a few hours from fixation, and that this gives rise to time-constrained maxima in root respiration rate and exudation flux.
An additional aspect of monitoring 14C flux dynamics concerns the protocol used for the pulse feed itself. In many radiotracer experiments, the full dose of 14CO2 is released instantaneously and the shoot then depletes the CO2 concentration in the feed chamber. Together with the increased partial pressure of water vapor in the chamber, this will affect assimilation and transpiration rates, and will perturb the dynamics of carbon partitioning (Minchin et al., 1994). In our experiments, such perturbations are eliminated by maintaining an air flow through the stirred chamber enclosing the leaf and by constantly adding 14CO2 to the air stream during the pulse feed. The increase of CO2 concentration of the air in the chamber was minimal (1.2 μmol mol–1), therefore 14C flux dynamics can be reliably quantified even during the pulse feed itself.
Interrelationships of Carbon Fluxes
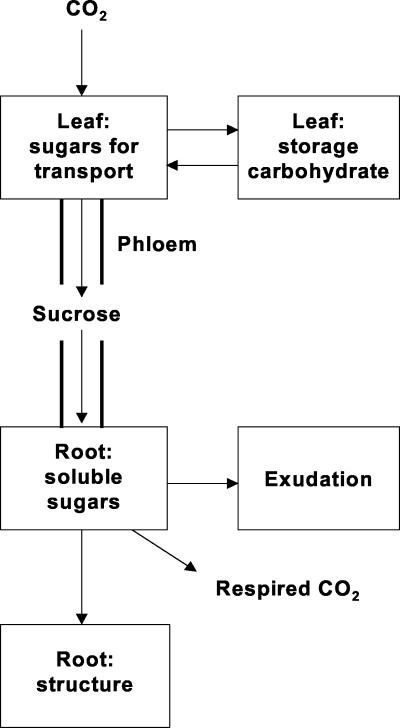
The speed with which recent photosynthate was lost to the rhizosphere indicates a very close link between current photosynthesis and exudation. There was close correspondence between the timing of the transport flux of 14C from the leaf and that of 14C exudation; when the amount of 14C in the transport pool had fallen to a low value, exudation was rapidly attenuated. This coupling is important for understanding rhizosphere processes, especially the driving force for microbial mineralization of and competition for key nutrients (Grayston et al., 1996). Furthermore, the partial defoliation demonstrated that exudation flux is not solely dependent on the rate of photosynthesis. Short-term modulation of the partitioning of carbon between shoot and root after defoliation substantially reduced the exudation flux of recent photosynthate in the absence of a corresponding change in photosynthetic rate or export from the leaf. Exudation is more directly linked to the rate of carbon import into the root than to the rate of photosynthesis itself. Root C fluxes can be viewed in terms of the simple model presented in Figure 7, where Suc exported from the shoot enters the root and becomes incorporated into structure or is respired in growth and maintenance processes. Exudation is probably the loss of soluble sugars from the same pool that provides substrates for growth and respiration, especially as respiration has a similar time course to exudation. However, the peaks do not occur at identical times, thus there is a physical difference in the time taken for CO2 and exudates to leave the root, or a more complex pool needs to be identified.
Figure 7.
Simple model showing the fate of carbon from recent photosynthesis imported into the root of wheat. Exudation is consistent with the loss of soluble sugars from the same pool that provides substrates for growth and respiration. The phloem branches to supply sinks other than roots, notably the shoot, but this is omitted for clarity.
The reduction in exudation with partial defoliation apparently contradicts the findings of other studies. Defoliation of Lolium perenne resulted in an increase in exudation of soluble organic carbon for 3 to 5 d (Paterson and Sim, 1999); the mechanism underlying the increase in exudation might be the consequence of remobilization of root carbohydrate reserves. Our data clearly show that the flux of recent photosynthate to the root is attenuated in the 24 h after partial defoliation, and that exudation accounts for a higher proportion of imported 14C. The specific activity of exudates may be higher after defoliation, as respiration is reduced but cytosolic sugar pools are maintained shortly after defoliation (Williams and Farrar, 1990).
Dynamic Carbon Budget
A critical step in constructing the carbon budget has been the careful cross-calibration of measurements from the various radiation detectors. As well as differing in sensitivity and linearity, the individual detectors needed calibration for geometry and quench factors, which vary with the material being monitored, the nature of the environment in which it is monitored (e.g. excitation effect of illumination on the detectors and quench effect of water), and the precise positioning of material in relation to detector windows lacking in uniform efficiency.
The simultaneous measurement of 14C in the leaf and the root, and the leaf and the rhizosphere, was key to relating nonconcurrent measurements of individual below-ground fluxes. Because leaf export dynamics are quantified in every experiment, other fluxes can be characterized by their direct relationship to leaf export, expressed as its inverse, rest-of-plant import. Consequently, if rest-of-plant import is expressed in relative units scaled to a defined end-point (here, 20 h), proportional relationships between individual below-ground fluxes can be established. The extent to which individual fluxes may be directly comparable can be observed by the degree of congruence between rest-of-plant import dynamics from different experiments.
We used two approaches to estimate the gross 14C import into the root. First, we assumed that the partitioning ratio between shoot and root remained constant over time, so that estimated gross root import was plotted as a fixed proportion of empirical values for rest-of-plant import, with a 0.5-h offset to allow for translocation time. The partitioning ratio was calculated separately for each plant using the empirical values for root 14C content 20 h after the pulse feed, on the assumption that cumulative root respiration and root content would then have reached steady state (Farrar, 1985). In the second approach, the empirical values for cumulative 14C root respiration were expressed as proportions of the concurrent values for rest-of-plant import. These proportional values were then used to derive estimated values for cumulative root respiration in the root content experiment; finally, estimated respiration was added to measured root content to provide estimates of root import. Both estimates were in substantial agreement, thus we believe that dynamic relationships between individual below-ground fluxes can be established. When cumulative values for one flux are expressed as a proportion of those for another flux, the proportion stabilizes within the first few hours; for example, cumulative exudation as a proportion of rest-of-plant import has largely stabilized by 7.5 h after the end of the pulse feed.
Quantifying Exudation
The rate of exudation should be nearly that for gross outwardly directed carbon flux because exudates were removed from the vicinity of the root every 30 min. Criticisms of experimental quantification of exudation focus on two aspects of root-rhizosphere carbon exchange. First, soluble organic carbon is lost from roots by passive diffusion in response to a concentration gradient that is driven by the concentration of individual exudates in the cytosol and soil solution (Jones and Darrah, 1996) and that is comediated by microbial acquisition of the respective compounds. If microbial activity is suppressed and exudates are allowed to accumulate, gross flux out of the root may be underestimated by a substantial margin (Jones and Darrah, 1992, 1993, 1996). Second, roots are capable of recapturing significant amounts of organic carbon from the rhizosphere by constitutively expressed active transport systems (Jones and Darrah, 1996; Owen and Jones, 2001). Consequently, nonremoval of exudates from the rhizosphere would similarly result in an underestimate of outward flux. The current protocol ensures that the concentration gradient acting as the driving force for the outward flux is maximized and the carbon substrates for active reuptake are sequestered away from the root within a short time.
We estimate the exudation of total C, rather than 14C, by two methods. Both are based on the assumption that exudation occurs from cytosolic pools with the same specific activity as cytosolic sugars and respired CO2. In the first approach, we estimate that root respiration uses 6 mg of carbohydrate g–1 root dry weight h–1 (Bingham and Farrar, 1988), and that the amount of 14C allocated to exudation and respiration measured in our experiments is in the ratio 0.21:1; therefore, we calculate that root exudation occurs at a rate of 1.26 mg of carbohydrate equivalents g–1 root dry weight h–1. In a second approach, we can use data on time courses of the specific activity of sugars in roots after feeding 14CO2 (T. Abebe and J. Farrar, unpublished data) to show that at 3 h, the root sugar pools have a specific activity of 80 kBq mg–1. Applying this value to the rate of exudation in bequerels per hour, the rate of exudation of carbohydrate equivalents is then 0.25 mg g–1 root dry weight h–1. However, this value will be too low, as 3 h after feeding 14CO2 to the leaf, the specific activity of cytosolic sugars in the root (38% of total root pool; Williams and Farrar, 1990) will be much greater than that of vacuolar sugars (62% of total root pool). If we assume that all the 14C is in the cytosolic pool, the exudation rate can be corrected to 0.66 mg of carbohydrate equivalent g–1 dry weight h–1.
A comparison of exudation values with those reported in the literature is complicated by a number of issues. First, in many studies, there is little idea of what percentage exudation is of total fixed C because there is often no quantification of the amount of radiotracer taken up in photosynthesis or subsequent respiration rates. Exudation is then reported as a proportion of radiotracer recovered at harvest or of plant dry weight, which can lead to large variability in reported values. Second, in soil, exudation is notoriously difficult to quantify on account of the difficulty in separating root tips and hairs from soil to determine soil organic-C residue, the fact that soil residue does not equate with exudation anyway because there will have been root reuptake and microbial respiration of exudates, and the further difficulty in separating microbial respiration from root respiration. Consequently, many previous experiments have only provided qualitative information; that is, that exudation varies with nutritional (Hodge et al., 1996) and environmental conditions (Meharg and Killham, 1989; Hodge et al., 1998), defoliation (Paterson and Sim, 1999), growth stage of plant (Swinnen et al., 1995a), and rhizosphere inocula (Meharg and Killham, 1995). Third, exudation is measured over different experimental periods so that different quantities are reported with little idea of how these have accumulated over time.
Our study demonstrates that an understanding of the dynamics of exudation is important in the quantification of exudation. We report that the quantity exuded is closely coupled to root import of soluble sugars and, by inference, to the concentration of soluble sugars in the root. The dynamics suggest that exudation is dominated by the loss of soluble sugars from the root because the highest rate of loss occurs concurrently with their import into the root. A notable difference between controls and partially defoliated plants is in the exudation rate during the first few hours after fixation, but not in the exudation rate at 20 h.
Rhizodeposition rate is acknowledged to be one of the most difficult parameters to quantify under field conditions (Swinnen et al., 1994; Grayston et al., 1996). The piecing together of information from destructive harvesting or cumulative sampling methods has provided initial insights into carbon partitioning in the rhizosphere. However, there has been a need for understanding partitioning in terms of interrelated processes unfolding over time. The data presented here provide the first step in the sequential movement toward this goal. Hydroponic systems cannot be expected to correspond to the field environment, yet they allow access for measurement of individual fluxes that are difficult to differentiate in the field. Our use of in situ measurement techniques has enabled specific fluxes to be characterized at fine temporal resolution, making it possible to determine relationships between key processes. This has revealed that exudation flux is much more rapidly and dynamically coupled to current photosynthesis than had been appreciated. At the same time, changes induced in the partitioning ratio of fixed carbon between shoot and root have revealed that root exudation is not solely dependent on the rate of photosynthesis but is closely coupled to the rate of carbon import into the root.
This process of quantifying the magnitude and dynamics of exudation flux now needs to be carried forward into the soil environment, initially in the semicontrolled conditions of microcosms, as the next step in the sequential approach to understanding processes in the field. In parallel with this, a spatial characterization of root exudation needs to be achieved so that microscale fluxes can be examined.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Plants were grown in controlled environment cabinets (Sanyo-Gallenkamp; Fi-totron PG660/C/RO/HQI, Loughborough, UK) illuminated with three sodium halide lamps (Powertone 250W MHN/TD; Phillips, Eindhoven, The Netherlands) supplemented by two 60-W tungsten filament lamps, which provided 500 μmol m–2 s–1 photosynthetically active radiation at shoot base level over a 14-h photoperiod. The cabinet was maintained at 20°C and a vapor pressure deficit of 0.7 kPa and was supplied with external air at true ambient CO2 concentration (360–400 μmol mol–1). Seeds of winter wheat (Triticum aestivum cv Consort) were soaked overnight in aerated deionized water and were then transferred to moist filter paper and placed in the climate-controlled cabinet (day 1). After 96 h, seedlings were transferred to one-half-strength Long Ashton solution (Hewitt, 1966) modified with the addition of 10 mg L–1 sodium metasilicate. For all except the root efflux experiments, 10 plants were suspended in a trough containing 3.6 L of solution aerated at 0.7 L min–1. The solution was changed at least twice per week. For the root efflux experiments, single plants were grown in cylindrical flexible plastic tubes with an internal cross-sectional area of 0.5 cm2 and a liquid column height of 48 cm. In all experiments, the roots were maintained in the dark. Each tube was connected via a multichannel peri-staltic pump to a reservoir of nutrient solution, which was supplied to the bottom of each tube at 0.2 mL min–1. Aeration was similarly supplied via the bottom of each tube at 0.6 mL min–1. The pump controlling solution and aeration flows operated on an intermittent cycle of 10 min on and 10 min off to avoid reducing root mineral uptake by overaeration. All experiments were conducted when leaf 2 was fully expanded and plants were 15 to 19 d old.
In each type of experiment, a partial defoliation treatment was applied. At 4.5 h before the start of the 14C pulse feed (see below), the blade of leaf 1 was removed, together with the part of the shoot immediately above the ligule of leaf 2 (i.e. the emerging parts of leaves 3 and 4), but ensuring that leaf 2 was left intact.
Pulse Feed of 14CO2 to Leaves
Approximately 3 h into the photoperiod, a single plant was transferred to a controlled-temperature room at 20°C, but with variable humidity. The middle portion of the second oldest leaf (leaf 2) was enclosed in a 14CO2 fumigation chamber (internal of width 56 mm) having a clear perspex top window for external illumination. Inside the chamber, the leaf was held closely over the circular end-window (diameter of 25 mm) of a Geiger-Muller tube (type ZP1410; Centronic Ltd., New Addington, Surrey, UK) connected to an external rate-meter (Scaler Ratemeter SR7; Nuclear Enterprises, Edinburgh, UK). Air at ambient CO2 concentration was supplied to the leaf chamber at 1 L min–1, and was mixed well inside the chamber by a small fan. Illumination of the whole plant and leaf chamber was provided by two sodium halide lamps supplying 270 μmol m–2 s–1 photosynthetically active radiation to the top of the leaf chamber (with the loss of approximately 5% through the window), and operating on the same photoperiod as for plant growth. After 1 h in the chamber, the leaf was exposed to a 30-min pulse of 14CO2 (3.7 MBq total 14C activity) supplied in the incoming air stream by the continuous addition of a NaH14CO3 solution (Amersham Pharmacia Biotech, Little Chalfont, UK) from a perfusor pump to an excess of lactic acid. Excess 14CO2 was captured on exit from the leaf chamber in a column of soda lime. Measurements of 14C activity in the leaf were recorded continuously from 1 h before the end of the pulse feed and usually until at least 20 h after the end of the pulse feed.
14C Import into the Root
Before enclosing the leaf in the 14CO2 fumigation chamber, the entire root system (except a very small region adjacent to the shoot) was spread carefully over a double layer of chromatography paper (3MM; Whatman, Clifton, NJ) drawing one-half-strength nutrient solution from a reservoir. A radiation monitor (butane-filled proportional counter, Universal Monitor 123; EG&G Berthold, Bad Wildbad, Germany) with a rectangular counting window (dimensions of 18 × 12 cm) covered by a single layer of non-PVC plastic wrap was placed on top in direct contact with the tissue paper and root, these being arranged so that they lay completely within the frame of the counting window. Measurements of 14C activity in the root were recorded continuously, as for the leaf.
To convert radiation count rates on fresh leaf and root material into absolute values of 14C activity, plants from similar experiments were harvested at several time intervals after the end of the 14C pulse feed (e.g. 5 min, and 3 and 20 h). Once the radiation count rates on the intact leaf and root had been recorded, the plants were immediately cut up into component parts, weighed, dried (at 60°C for 3 d), and reweighed. Their 14C content was then determined with a Biological Sample Oxidizer OX400 (RJ Harvey Instrument Corp., Hillsdale, NJ) with 14CO2 capture by bubbling through 15 mL of Oxosol-14C scintillation fluid (14CO2 capture efficiency > 98%) followed by liquid scintillation counting for 600 s (Wallac Winspectral 1414; EG&G Berthold). The data from the harvest at 5 min also enabled the total 14C uptake of the plant during the pulse feed to be calculated.
14C Efflux from the Root
To minimize the microbial capture of 14C root exudates, a broad-spectrum anti-microbial solution (Stabilized Antibiotic Antimycotic Solution; Sigma-Aldrich, Poole, UK) was added to the nutrient solution at a rate one-half that recommended for cell culture (5 mL L–1) approximately 40 h before the 14C pulse feed to the leaf. On the day of the experiment, the plant tubes were transferred to the temperature-controlled room and 14CO2 was fed to leaf 2 as described above. In these experiments, the nutrient solution was vigorously aerated in the reservoir instead of directly aerating the solution in the plant tube. The nutrient solution flow rate into the plant tube was 12 mL min–1, allowing the entire volume of liquid in the plant tube to be replaced rapidly and recirculated over the root. At intervals of 30 min, fresh solution was pumped into the plant tube for 2 min. Each 30-min fraction of solution flushed out of the plant tube was collected in a separate sample tube and was immediately frozen. Samples were collected from 1 h before the end of until 7.5 h after the end of the pulse feed. After this, a continuous flow of nutrient solution was provided to the plant tube at a rate of approximately 0.5 mL min–1. At the start of the next photoperiod, the plant tube was flushed through twice with nutrient solution, and then two additional 30-min fractions were collected. Cumulative 14C exudation during the dark period was estimated by assuming that exudation rate for this period was the same as that recorded at 20 h. Consequently, the estimated values would slightly underestimate the actual cumulative values. The 14C activity of solution fractions was determined by liquid scintillation counting using Optiphase HiSafe 2 scintillation fluid (EG&G Berthold) and a count time of 10 min. The potential error arising from dissolved 14CO2 in the solution fractions was assessed by the addition of dilute HCl to a few subsamples of differing 14C activities to drive off any dissolved CO2, and then determining the change in 14C activity by liquid scintillation counting. After acidification to below pH 4, the remaining 14C activity was 89% to 96% of the initial values. In view of the relatively small proportion of 14C activity not attributable to exudates, data were not adjusted for this error.
Root Respiration
Buffered nutrient solution (130 mL; pH 5.5; 5 mm MES) containing antibiotics was placed in an empty plant root chamber (volume of 180 cm3) and CO2-free air (0.8 L min–1) bubbled through the nutrient solution overnight. On the day of the experiment, the roots of an intact plant were placed in the chamber and the top was hermetically sealed. Two and one-half hours after transfer to the root chamber, the plants were labeled with 14CO2 as described above. The air outflow from the root chamber was dehumidified with Drierite and respired CO2 was measured with an infrared gas analyzer (CIRAS-I; PP Systems, Hitchin, UK) and 14CO2 was captured by bubbling through Oxosol-14C scintillation fluid as described above. The scintillant was replaced every 30 min, and the 14C activity of each fraction was determined. At the end of the experiment, the root was excised and its fresh and dry weights were determined as described previously.
Cross-Calibration of Radiation Counters
To relate all the measurements of 14C activity in leaves, roots, and respired fractions to each other, it was necessary to cross-calibrate the various radiation counters using solid and liquid standards. All data were corrected for counting efficiencies and for background 14C activity.
The linearity of individual radiation counters (Geiger-Muller tube and universal monitor) was assessed by two methods. In the first, solid standards (CFR2,3,4; Amersham Pharmacia Biotech) were covered with successive layers of non-PVC plastic wrap to proportionally reduce particle emission. This produced a linear relationship between counts and the relative reduction in particle emission. In the second, small pieces of dried leaf (e.g. 12- × 6-mm) covering a range of 14C activities were counted using each radiation counter, and then the leaf material was oxidized and the 14C activity was determined by liquid scintillation counting. The counts from the Geiger-Muller tube were in constant ratio to those from the Universal monitor, but the ratio of each to the liquid scintillation counter declined slightly at high activity. Nevertheless, this indicated that counting efficiencies were very close to linear over the range encountered in experiments.
Geometric aspects of counting were also investigated. Radiation counter windows were not of uniform efficiency, with a quantifiable reduction in efficiency toward the margins. The conversion of counts to bequerels did not pose a problem when the portion of plant material being counted was of uniform activity. To determine whether any spatial heterogeneity in activity existed, leaf or root material was subdivided and the 14C activity of each smaller portion was measured by oxidizing and liquid scintillation counting. In mid-time course of the experiment, a linear gradient of activity could be detected in the fed leaf segment, but because the mid-point of the gradient coincided with the center of the Geiger-Muller tube, no adjustment needed to be made to the counts. The activity in the root was higher toward the main apices, but the main part of the root was fairly uniform; a small upper portion of root immediately adjoining the shoot was not directly under the counting window, but a proportional correction of about 2% was able to be made in respect of this.
Statistical Analysis
Differences between intact and defoliated plants in respect of 14C contents or losses were tested for using a two-tailed unpaired t test.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.032045.
This work was supported by the United Kingdom Biotechnology and Biological Sciences Research Council (to D.L.J and J.F.).
References
- Baxter R, Farrar JF (1999) Export of carbon from leaf blades of Poa alpina L. at elevated CO2 and two nutrient regimes. J Exp Bot 50: 1215–1221 [Google Scholar]
- Bingham IJ, Farrar JF (1988) Regulation of respiration in roots of barley. Physiol Plant 73: 278–285 [Google Scholar]
- Ekblad A, Högberg P (2001) Natural abundance of 13C in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia 127: 305–308 [DOI] [PubMed] [Google Scholar]
- Farrar JF (1985) Fluxes of carbon in roots of barley plants. New Phytol 99: 57–69 [Google Scholar]
- Farrar SC, Farrar JF (1985) Carbon fluxes in leaf blades of barley. New Phytol 100: 271–283 [Google Scholar]
- Grantz DA, Farrar JF (2000) Ozone inhibits phloem loading from a transport pool: compartmental efflux analysis in Pima cotton. Aust J Plant Physiol 27: 859–868 [Google Scholar]
- Grayston SJ, Vaughan D, Jones D (1996) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5: 29–56 [Google Scholar]
- Hewitt EJ (1966) Sand and Water Culture Methods Used in the Study of Plant Nutrition Technical Communication No. 22. Commonwealth Bureau of Horticultural and Plantation Crops, East Malling, Kent UK
- Hodge A, Grayston SJ, Ord BG (1996) A novel method for characterisation and quantification of plant root exudates. Plant Soil 184: 97–104 [Google Scholar]
- Hodge A, Paterson E, Grayston SJ, Campbell CD, Ord BG, Killham K (1998) Characterisation and microbial utilisation of exudate material from the rhizosphere of Lolium perenne grown under CO2 enrichment. Soil Biol Biochem 30: 1033–1043 [Google Scholar]
- Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411: 789–792 [DOI] [PubMed] [Google Scholar]
- Jones DL, Darrah PR (1992) Re-sorption of organic compounds by roots of Zea mays L. and its consequences in the rhizosphere: re-sorption of 14C-labelled glucose, mannose and citric acid. Plant Soil 143: 259–266 [Google Scholar]
- Jones DL, Darrah PR (1993) Re-sorption of organic compounds by roots of Zea mays L. and its consequences in the rhizosphere: experimental and model evidence for simultaneous exudation and re-sorption of soluble C compounds. Plant Soil 153: 47–59 [Google Scholar]
- Jones DL, Darrah PR (1996) Re-sorption of organic components by roots of Zea mays L. and its consequences in the rhizosphere: Characteristics of sugar influx and efflux. Plant Soil 178: 153–160 [Google Scholar]
- Kraffczyk I, Trolldenier G, Beringer H (1984) Soluble root exudates of maize: influence of potassium supply and rhizosphere microorganisms. Soil Biol Biochem 16: 315–322 [Google Scholar]
- Kuzyakov Y, Cheng W (2001) Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol Biochem 33: 1915–1925 [Google Scholar]
- Meharg AA (1994) A critical review of labeling techniques used to quantify rhizosphere carbon flow. Plant Soil 166: 55–62 [Google Scholar]
- Meharg AA, Killham K (1988) A comparison of carbon flow from pre-labeled and pulse-labeled plants. Plant Soil 112: 225–231 [Google Scholar]
- Meharg AA, Killham K (1989) Distribution of assimilated carbon within the plant and rhizosphere of Lolium perenne: influence of temperature. Soil Biol Biochem 21: 487–489 [Google Scholar]
- Meharg AA, Killham K (1995) Loss of exudates from the roots of perennial ryegrass inoculated with a range of microorganisms. Plant Soil 170: 345–349 [Google Scholar]
- Minchin PEH, Thorpe MR, Farrar JF (1994) Short-term control of root:shoot partitioning. J Exp Bot 45: 615–622 [Google Scholar]
- Moorby J, Jarman PD (1975) The use of compartmental analysis in the study of movement of carbon through leaves. Planta 122: 155–168 [DOI] [PubMed] [Google Scholar]
- Nguyen C, Todorovic C, Robin C, Christophe A, Guckert A (1999) Continuous monitoring of rhizosphere respiration after labeling of plant shoots with 14CO2. Plant Soil 212: 191–201 [Google Scholar]
- Owen AG, Jones DL (2001) Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol Biochem 33: 651–657 [Google Scholar]
- Paterson E, Sim A (1999) Rhizodeposition and C-partitioning of Lolium perenne in axenic culture affected by nitrogen supply and defoliation. Plant Soil 216: 155–164 [Google Scholar]
- Rattray EAS, Paterson E, Killham K (1995) Characterisation of the dynamics of C-partitioning within Lolium perenne and to the rhizosphere microbial biomass using 14C pulse chase. Biol Fert Soils 19: 280–286 [Google Scholar]
- Swinnen J, Van Veen JA, Merckx R (1994) 14C pulse-labeling of field-grown spring wheat: an evaluation of its use in rhizosphere carbon budget estimations. Soil Biol Biochem 26: 161–170 [Google Scholar]
- Swinnen J, Van Veen JA, Merckx R (1995a) Root decay and turnover of rhizodeposits in field-grown winter wheat and spring barley estimated by 14C pulse-labeling. Soil Biol Biochem 27: 211–217 [Google Scholar]
- Swinnen J, Van Veen JA, Merckx R (1995b) Carbon fluxes in the rhizosphere of winter wheat and spring barley with conventional vs. integrated farming. Soil Biol Biochem 27: 811–820 [Google Scholar]
- Whipps JM (1990) Carbon economy. In Lynch JM, Ed. The Rhizosphere. Wiley Interscience, New York, pp 59–98
- Williams JHH, Farrar JF (1990) Control of barley root respiration. Physiol Plant 79: 259–266 [Google Scholar]
- Williams JHH, Minchin PEH, Farrar JF (1991) Carbon partitioning in split root systems of barley: the effect of osmotica. J Exp Bot 42: 453–460 [Google Scholar]