Abstract
Protoplast swelling was used to investigate auxin signaling in the growth-limiting stem epidermis. The protoplasts of epidermal cells were isolated from elongating internodes of pea (Pisum sativum). These protoplasts swelled in response to auxin, providing the clearest evidence that the epidermis can directly perceive auxin. The swelling response to the natural auxin IAA showed a biphasic dose response curve but that to the synthetic auxin 1-naphthalene acetic acid (NAA) showed a simple bell-shaped dose response curve. The responses to IAA and NAA were further analyzed using antibodies raised against ABP1 (auxin-binding protein 1), and their dependency on extracellular ions was investigated. Two signaling pathways were resolved for IAA, an ABP1-dependent pathway and an ABP1-independent pathway that is much more sensitive to IAA than the former. The response by the ABP1 pathway was eliminated by anti-ABP1 antibodies, had a higher sensitivity to NAA, and did not depend on extracellular Ca2+. In contrast, the response by the non-ABP1 pathway was not affected by anti-ABP1 antibodies, had no sensitivity to NAA, and depended on extracellular Ca2+. The swelling by either pathway required extracellular K+ and Cl–. The auxin-induced growth of pea internode segments showed similar response patterns, including the occurrence of two peaks in the dose response curve for IAA and the difference in Ca2+ requirements. It is suggested that two signaling pathways participate in auxin-induced internode growth and that the non-ABP1 pathway is more likely to be involved in the control of growth by constitutive concentrations of endogenous auxin.
Since its discovery by Went (1928), auxin has been studied extensively as a major plant hormone, and yet controversies remain over many of the basic issues of its physiology (Iino, 2001). This is even true for the simple classical response, the stimulation of elongation growth observed in segments of coleoptiles and stems (Lüthen et al., 1999).
A large body of evidence indicates that auxin controls ion transport through the plasma membrane. It has been shown that auxin affects either the activity or the abundance of plasma membrane-located H+-ATPase (Senn and Goldsmith, 1988; Hager et al., 1991; Rück et al., 1993; Frías et al., 1996), K+ channels (Blatt and Thiel, 1994; Philippar et al., 1999), and Cl– channels (Marten et al., 1991; Zimmermann et al., 1994). It probably also affects the activity of Cl– uptake transporters (Babourina et al., 1998). Although the causal relationships among these electrical responses have not been resolved, many of them are in line with the idea that auxin stimulates ion uptake to sustain turgor, a condition of cell growth. The question, then, is whether any of the responses is more directly involved in the growth-limiting step, cell wall loosening (Cleland, 1995). Although its applicability has sometimes been questioned, the acid growth theory provides the most successful model for auxin-induced growth; namely, acidification of the apoplast by an enhanced H+-ATPase activity results in cell wall loosening (Hager et al., 1971; for review, see Lüthen et al., 1999).
ABP1 (auxin-binding protein 1) has been studied most extensively as a candidate auxin receptor for growth responses (Jones, 1994; Napier et al., 2002). When supplied to the bathing medium, antibodies raised against ABP1 inhibited auxin-induced plasma membrane hyperpolarization in tobacco (Nicotiana tabacum) mesophyll protoplasts (Barbier-Brygoo et al., 1989, 1991; Leblanc et al., 1999a) and auxin-induced enhancement of H+-ATPase activity in maize coleoptile protoplasts (Rück et al., 1993). The antipeptide D16 antibodies, raised against the putative auxin-binding site, were shown to have an auxin agonist activity in the hyperpolarization response (Rück et al., 1993). Although ABP1 is targeted to the endoplasmic reticulum, it also has been identified on the outer surface of the plasma membrane of maize coleoptile protoplasts (Diekmann et al., 1995). More recently, Jones et al. (Jones et al., 1998; Chen et al., 2001a, 2001b) obtained molecular genetic evidence that ABP1 mediates auxin-induced growth. In particular, Arabidopsis embryos were found to require ABP1 to progress through development (Chen et al., 2001b). Together, these results have indicated that ABP1, or most probably a fraction of ABP1 located on the outer surface of the plasma membrane, functions as the auxin receptor for the control of elongation growth. Despite these lines of evidence, however, the receptor role of ABP1 in growth responses has been controversial. Hertel (1995) argued that ABP1 is not the auxin receptor for elongation growth. An alternative candidate discussed by Hertel et al. was the plasma membrane-located auxin efflux carrier (Zhao et al., 2002). Recent studies indicated that soluble auxin receptors distinct from ABP1 participate in auxin-induced responses, at least at the plasma membrane (Kim et al., 1998, 2000, 2001) and for transcriptional regulation (Dharmasiri et al., 2003). The cellular location of the auxin receptor also has been an issue of controversy. In contrast to the conclusion obtained for ABP1, it was suggested that the auxin receptor for elongation growth is likely to be located inside the cell (Vesper and Kuss, 1990; Claussen et al., 1996). In fact, the non-ABP1 receptor candidates mentioned above could be considered to function inside the cell.
Early studies by Cocking et al. indicated that protoplasts isolated from roots, leaves, and coleoptiles swell and eventually burst when treated with auxin (Cocking, 1962; Power and Cocking, 1970; Hall and Cocking, 1974). It would be anticipated that such swelling responses involve the responses in ion transporters summarized above. In fact, later investigations with the protoplasts of oat (Avena sativa) coleoptiles (Keller and Van Volkenburgh, 1996) and Phaseolus vulgaris pulvini (Iino et al., 2001) indicated that the swelling response requires the presence of K+ and Cl– in the bathing medium. Furthermore, Steffens et al. (2001) used anti-ABP1 antibodies to demonstrate that ABP1 located on the outer surface of the plasma membrane is the auxin receptor for the swelling response of the protoplasts of maize coleoptiles and Arabidopsis hypocotyls.
To understand the growth of coleoptiles and stems at the organ level, we must resolve which cells perceive auxin and which cells respond with cell wall loosening. In these organs, the epidermis is under tension, and the extensibility of epidermal cell walls limits their growth (Kutschera, 1987; Peters and Tomos, 1996). In relation to this mechanical condition for organ growth, it has been hypothesized that the epidermis is the target tissue for auxin (Kutschera, 1987). Attempts to prove this hypothesis have not yielded clear results (e.g. Masuda and Yamamoto, 1972), and, with apparent contradiction to the hypothesis, nonepidermal inner tissues have been shown to perceive auxin (for review, see Peters and Tomos, 1996). However, if epidermal cells were found to respond directly to auxin, such a response would be more relevant to the auxin-induced organ growth, although auxin responses need not be unique to the epidermis. Thus far, electrophysiological studies, including those cited above, have not focused on the epidermis. An exception was the work of Felle (1988), who found with maize coleoptile segments that cytosolic Ca2+, cytosolic pH, and the plasma membrane potential of epidermal cells oscillated in response to auxin. This result, however, does not prove that the epidermal cells perceive auxin directly.
The protoplast swelling response offers a single-cell system with which the overall effects of auxin on plasma membrane ion transporters can be studied quantitatively. In the present study, we have employed protoplasts of pea (Pisum sativum) stem epidermis (Long and Iino, 2001) to investigate auxin signaling in growth-limiting cells. In the red light-grown pea seedlings used, the elongation growth of internodes is limited by endogenous auxin (Haga and Iino, 1998). We found that epidermal protoplasts respond directly to auxin and swell. The study was extended to find that the swelling response involves two distinct signaling pathways, one of which is unrelated to ABP1.
RESULTS
Epidermal peels of the rapidly elongating stem zone of red light-grown pea seedlings were used to isolate protoplasts. In our routine procedure, the final protoplast preparation was obtained within 3 h after the start of peeling. The standard protoplast bathing medium was composed of 0.5 m sorbitol, 10 mm KCl, 1 mm CaCl2, 20 mm Glc, and 10 mm MES-Tris (pH 6.0). In all experiments, the freshly isolated protoplasts were immediately incubated under red light on the sample stage of an inverted microscope, and, after 30 min of adaptation, they were subjected to photographic recording and experimental treatment.
The purity of epidermal protoplasts was examined under a fluorescence microscope by counting parenchyma protoplasts that showed red fluorescent chloroplasts under blue light excitation. The protoplasts of guard cells would also have been included in this count. Independent preparations indicated that about 6% of the protoplasts derived from parenchyma or guard cells (the mean from six preparations was 6.1% with sd of 0.6%; about 200 protoplasts were counted for each preparation). The protoplasts showing fluorescent chloroplasts generally had a much higher density of organelles as compared with epidermal protoplasts. Although fluorescence examination of protoplasts was not possible with the inverted microscope used for actual analysis, the nonepidermal protoplasts could easily be identified in the photographic images. The protoplasts suspected to be of nonepidermal origin were not used for analysis.
Epidermal Protoplasts Swell in Response to Auxin. Time Courses and Dose Response Curves
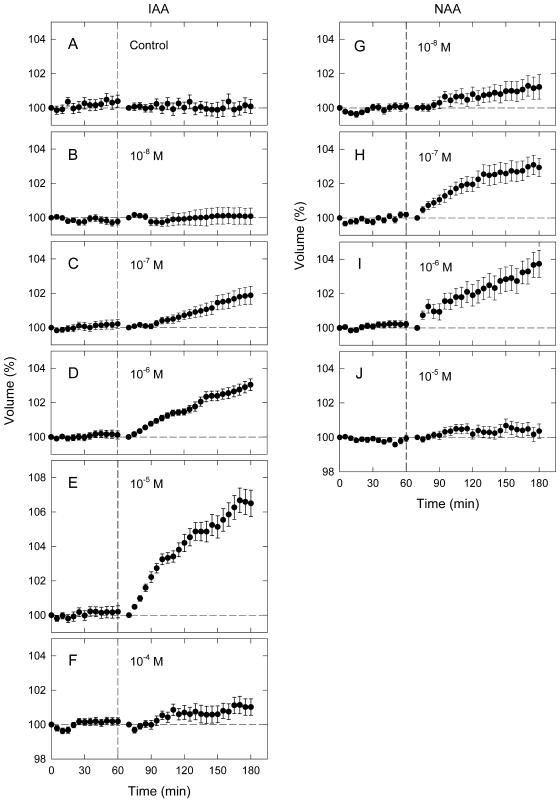
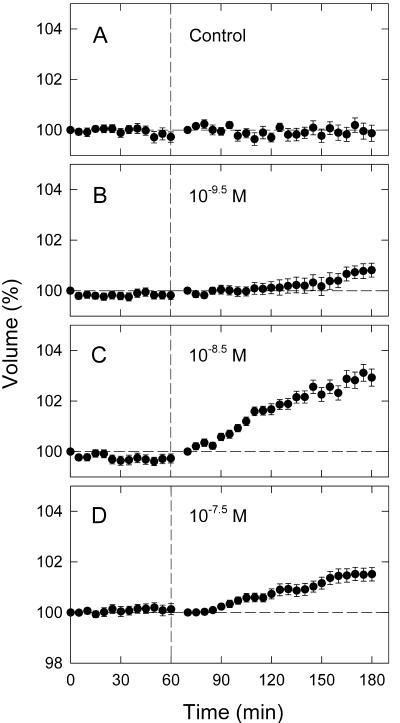
The time course data shown in Figure 1 demonstrated that protoplasts swell in response to IAA and NAA. In all cases, the protoplasts maintained a nearly constant volume before auxin application. Also, those not treated with auxin retained constant volume in the subsequent period of 120 min (Fig. 1A; see also Long and Iino, 2001). Therefore, the swelling was induced in the protoplasts maintaining constant volume. For either IAA or NAA, the rate and the extent of swelling increased with concentration up to 10–5 m but was reduced again at the highest concentration (10–4 m).
Figure 1.
Auxin-induced swelling of pea epidermal protoplasts. Photographic recording of protoplast images was initiated at time zero, which was 30 min after the onset of incubation on the microscope stage. At the vertical dashed line (immediately after obtaining protoplast images at 60 min) a solution containing indole-3-acetic acid (IAA; B–F) or 1-naphthalene acetic acid (NAA; G–J) in bathing medium was added to the concentrations indicated. The control (A) was obtained by adding bathing medium alone. The relative volume of each protoplast was calculated as the percentage of the initial volume, which was the volume at time zero (the time course before auxin application) or 70 min (the time course after auxin application). The means from 98 to 144 protoplasts (A–F; three experiments) or 65 to 82 protoplasts (G–J; two experiments) are shown. Vertical bar = se.
Protoplast volume during the first 10 min after auxin application was not determined because this period was required before all the protoplasts settled to the bottom after mixing and could be focused for photography. With either IAA or NAA, a high rate of swelling was already detected in the first 5-min interval of measurements (Fig. 1). Therefore, the lag time for the response appeared to be 10 min or shorter. The swelling continued for the measurement period of 2 h. Generally, the rate of swelling decreased after the highest rate was established within the first 1 h.
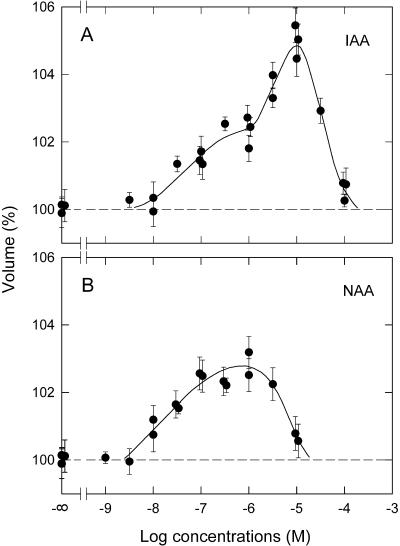
Next, we obtained dose response curves by determining the swelling response 90 min after auxin application. For either IAA (Fig. 2A) or NAA (Fig. 2B), a bell-shaped curve was identified with a threshold at 10–8.5 to 10–8 m. However, the shape of the curves was different between IAA and NAA. The curve for IAA spanned a wider concentration range, with the peak occurring at 10–5 m (IAA) or 10–6 m (NAA). A shoulder was found on the response curve for IAA (at about 10–6 m) but not for NAA. These results indicated that the response to IAA is biphasic. This feature was not apparent with NAA.
Figure 2.
Dose response curves for the protoplast swelling induced by IAA (A) and NAA (B). Experiments were carried out as described for Figure 1. The relative protoplast volume at 90 min after auxin application was plotted. The means from 30 to 55 protoplasts, each obtained from a single experiment, are shown (vertical bar = ±se). Although the auxin concentrations were separated by 0.5 log units, some of the points are displaced laterally to avoid their overlapping. The curves were fitted by eye.
Involvement of ABP1. Examinations with Antibodies
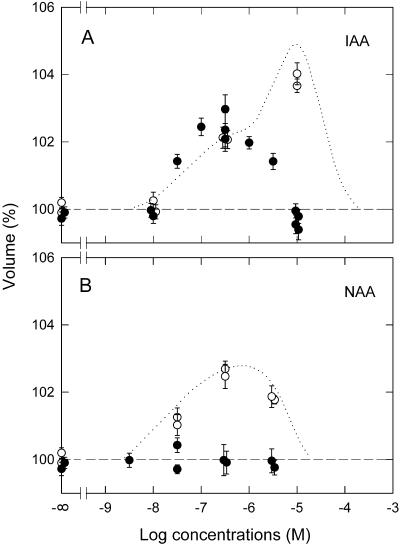
Antibodies prepared against maize ABP1 were used to examine the possible participation of ABP1 in the swelling response. Freshly isolated protoplasts were bathed in a medium containing antibodies, and the responses to IAA (Fig. 3A, solid circles) and NAA (Fig. 3B, solid circles) were examined as above. At representative concentrations, controls were obtained by using pre-immune IgG in place of the antibodies (white circles). The dose response curves in Figure 2 are reproduced for comparison.
Figure 3.
Effects of anti-ABP1 antibodies on IAA- and NAA-induced protoplast swelling: dose response investigation. Protoplasts, suspended in bathing medium containing anti-ABP1 antibodies (10–7.5 m), were treated with IAA (A, solid circles) or NAA (B, solid circles). White circles, Controls obtained by using pre-immune IgG (10–7.5 m) in place of anti-ABP1 IgG. The dotted lines reproduce the curves in Figure 2. The means from 25 to 60 protoplasts are shown. Other details were as described for Figure 2.
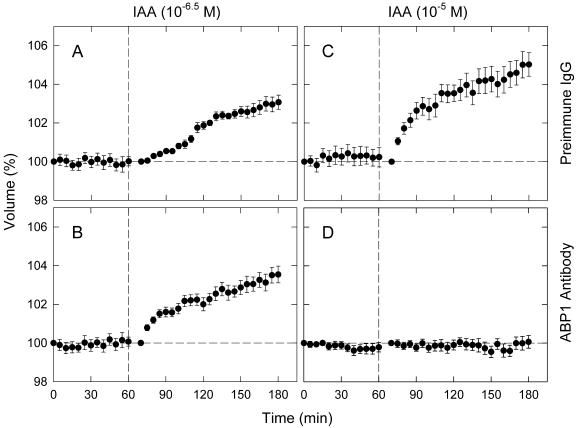
The swelling response at 10–5 m IAA was totally inhibited by the anti-ABP1 antibodies; the response at lower concentrations (10–8–10–6 m) was not affected (Fig. 3A). The resulting dose response curve was bell shaped, showing a maximal response at 10–6 m. In the case of NAA, the response was almost totally inhibited at all concentrations (Fig. 3B). The control measurements obtained using pre-immune IgG were similar to those obtained without IgG. Figure 4 shows time courses obtained in the presence of ABP1 antibodies at two representative concentrations of IAA (10–6.5 and 10–5 m). Control time courses were obtained by using pre-immune IgG. At 10–6.5 m IAA, the time course of antibody-treated protoplasts was similar to the control time course. At 10–5 m IAA, antibody-treated protoplasts showed no swelling throughout the 2-h period after IAA application, whereas the control protoplasts showed a normal response.
Figure 4.
The effect of anti-ABP1 antibodies on IAA-induced protoplast swelling: time course investigation. Protoplasts were suspended in bathing medium containing pre-immune IgG (A and C) or anti-ABP1 antibodies (B and D). At the vertical dashed line, IAA was added to the concentration of 10–6.5 m (A and B) or 10–5 m (C and D). The means from 30 to 44 protoplasts (two experiments) are shown. Other details were as described for Figures 1 and 3.
These results indicated that ABP1 located on the outer surface of the plasma membrane mediated the response to NAA at all concentrations, whereas it mediated the response to IAA only at high IAA concentrations. The response at low concentrations of IAA might be caused by the ABP1 located inside the protoplast. However, this possibility was regarded as very unlikely because no comparable response was found with NAA, for which ABP1 has a high affinity (note also that all the responses demonstrated to be ABP1 mediated could be induced effectively by NAA). It seemed likely that a distinct receptor protein mediated the response not inhibited by anti-ABP1 antibodies.
As evident from Figure 3A, the ABP1-independent response to IAA formed a bell-shaped optimum curve (solid circles). The ABP1-dependent response, evaluated in Figure 3A by subtracting the ABP1-independent response from the response found without the antibody treatment (dotted line and white circles), also appeared to form a bell-shaped optimal curve with a peak activity at higher IAA concentration. Comparison of this curve with the one for NAA (dotted line and white circles in Fig. 3B) indicated that the ABP1-dependent response is more sensitive to NAA than IAA. The difference in sensitivity was estimated to be between 1 and 2 orders of magnitude.
Antipeptide D16 antibodies, shown previously to have an auxin agonist activity in ABP1-mediated responses, were also found to induce an auxin-like swelling response on pea epidermal protoplasts (Fig. 5). The effective concentration range appeared to be as narrow as that for NAA, in agreement with the result that the response to NAA is mediated principally by ABP1.
Figure 5.
Protoplast swelling induced by D16 antibodies. A solution of D16 antibodies was applied to the protoplast suspension to the concentrations indicated. The control (A) was obtained by applying a solution containing 0.05-strength phosphate-buffered saline (PBS) and bathing medium components. This control investigated the possible effect of the highest concentrations of PBS components, derived from the antibody stock solution. The means from 38 to 47 protoplasts (two experiments) are shown. Other details were as described for Figure 1.
Dependence of the Swelling Response on External K+ and Cl–
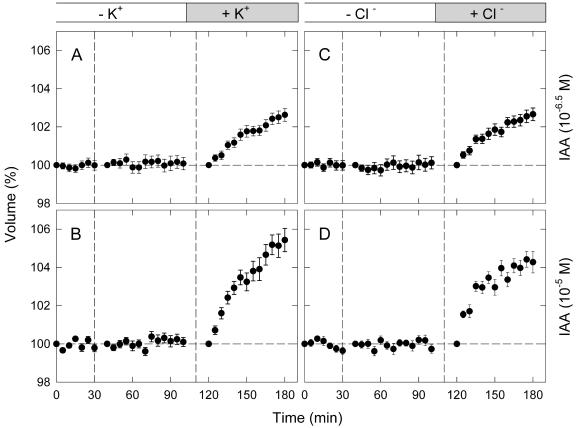
It was anticipated that the swelling response was caused by an enhanced influx of ions (most probably of K+ and Cl–), rather than a reduced efflux of ions or a metabolic release of osmotic substances (Iino et al., 2001). In accordance, we investigated whether the protoplasts could swell in response to IAA when bathed in a medium free of K+ or Cl–. The K+-free medium was prepared by replacing K+ with the membrane-impermeant tetraethylammonium ion, which also acts as a specific K+ channel inhibitor (Brown, 1993). The Cl–-free medium was prepared by replacing Cl– with membrane-impermeant iminodiacetic acid. Investigation was made at two concentrations of IAA, one representing the ABP1-independent response (10–6.5 m) and the other representing the ABP1-dependent response (10–5 m).
At either concentration, IAA could not induce any detectable swelling response in the protoplasts bathed in K+-free medium (Fig. 6, A and B) or Cl–-free medium (Fig. 6, C and D). When the same protoplasts were washed with and suspended in the standard medium containing both ions, they showed a clear swelling response to the second application of IAA (Fig. 6; the data after the second vertical dashed line). These results demonstrated that the ABP1-dependent and -independent responses both require the presence of extracellular K+ and Cl–. Therefore, it could be concluded that, in either response, protoplasts swell by taking up K+ and Cl– from the bathing medium. The results also indicated that K+ cannot be taken up when Cl– is absent or vice versa.
Figure 6.
Dependence of IAA-induced protoplast swelling on external K+ and Cl–. Protoplasts were washed with and suspended in a K+-free (A and B) or a Cl–-free (C and D) medium and incubated as described for Figure 1. Time zero corresponded to 30 min after the onset of incubation. At the first vertical dashed line, IAA was applied to the bathing medium to the concentration of 10–6.5 m (A and C) or 10–5.0 m (B and D). Immediately after obtaining protoplast images at 100 min, the protoplasts were washed with and suspended in the standard medium. At 110 min (the second vertical line), IAA was applied again to the same concentration. The means obtained from 32 to 40 protoplasts (two experiments) are shown.
Dependence of the Swelling Response on External Ca2+
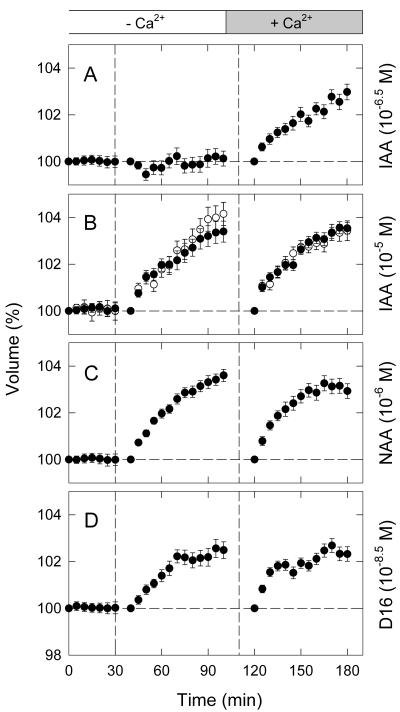
Although the Ca2+ present in the bathing medium does not contribute to the swelling response as osmotic ions, as evident from the results shown above, the external Ca2+ might be taken up as a second messenger. This possibility was investigated next.
As shown in Figure 7A, protoplasts washed with and suspended in a Ca2+-free medium did not swell in response to 10–6.5 m IAA. The same protoplasts showed a clear swelling response when resuspended in the Ca2+-containing standard medium and treated again with IAA (the data after the second vertical dashed line). The results demonstrated that the ABP1-independent response requires the presence of extracellular Ca2+.
Figure 7.
Contribution of external Ca2+ to the protoplast swelling responses induced by IAA, NAA, and D16 antibodies. Experiments were conducted as described for Figure 6 using a Ca2+-free medium and including the resuspension in the standard medium. Protoplasts were treated at vertical dashed lines with IAA (A and B), NAA (C), or D16 antibodies (D) at the concentration indicated in blankets. The means obtained from 17 to 30 protoplasts (two experiments) are shown.
In sharp contrast, the protoplasts treated with IAA at a concentration of 10–5 m showed a clear swelling response in the Ca2+-free medium (Fig. 7B, black circles). The same protoplasts washed with and suspended in the Ca2+-containing medium showed another rapid response that was comparable with the initial response in the Ca2+-free medium. The second swelling response observed in the Ca2+-containing medium might be seen to be too large to be regarded as a response that continued after the swelling observed in the Ca2+-free medium (compare with the time course shown in Fig. 1E). In response, it might be suggested that the ABP1-mediated response partially depended on external Ca2+ and that the Ca2+-dependent portion became inducible after resuspension of the protoplasts in the Ca2+-containing medium. However, when the protoplasts incubated in the Ca2+-containing medium from the beginning were treated similarly, these protoplasts showed a swelling response to the second application of IAA that was comparable with the response to the first application (Fig. 7B, white circles). Therefore, it appeared that, although the protoplasts were made free of IAA only about 10 min after the first treatment period, they become fully responsive to the second IAA treatment. The results shown in Figure 7B also indicated that the response to the first IAA application recorded without Ca2+ was similar to that recorded with Ca2+. Taken together, these results indicated that external Ca2+ did not make any appreciable contribution to the ABP1-mediated response.
Similar experiments were carried out with NAA (10–6 m) and D16 antibodies (10–8.5 m). As shown for 10–5 m IAA, the protoplasts suspended in the Ca2+-free medium showed a clear swelling response to NAA (Fig. 7C) and D16 antibodies (Fig. 7D). As also found with 10–5 m IAA, the same protoplasts showed another comparable response after resuspension in the Ca2+-containing medium. The results substantiated the conclusion that the ABP1-mediated response does not depend on external Ca2+.
Auxin-Induced Internode Segment Growth. Dose Response Curves and Dependence on Ca2+
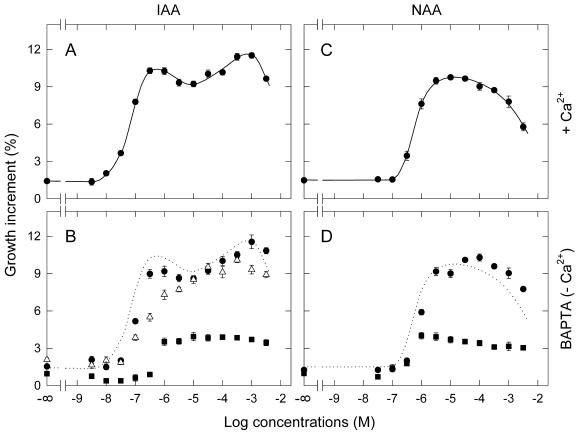
To compare the results described above with the growth response, we measured dose response curves for both IAA and NAA from pea internode segments. The effects of apoplastic Ca2+ on the dose response relationship were also investigated. Experiments were designed so as to obtain the growth data under comparable experimental conditions. The segments (5 mm long) were isolated from the third internode of red light-grown seedlings and incubated for 2 h, without applied auxin, to deplete endogenous auxin. They were subsequently incubated for 2 h with auxin to determine auxin-induced growth. The incubation took place under red light.
Dose response curves were obtained using an incubation medium containing 10 mm KCl, 1 mm CaCl2, 10 mm Suc, and 5 mm MES-KOH (pH 6.0). The curve for IAA revealed two peaks with a shallow trough (Fig. 8A). On the other hand, the curve for NAA was bell shaped with a single peak (Fig. 8C). It also was noted that the effective concentration range was much wider with IAA than NAA.
Figure 8.
Dose response curves for IAA- and NAA-induced growth of pea internode segments. A and C, Dose response curves for the growth induced by IAA and NAA, respectively, in the presence of 1 mm Ca2+. The curves were fitted by eye. B and D, Dose response curves for the growth induced by IAA and NAA, respectively, in the absence of Ca2+ and in the presence of 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,M′-tetraacetic acid (BAPTA) at 1 (•), 3 (▵), and 10 (▪) mm. The dotted lines reproduce the curves in A and C. Segments were treated with IAA or NAA for 2 h after 2-h pre-incubation without auxin. The ordinate indicates the increment in length, calculated as the percent of the initial length. Each point is the mean from eight to 16 segments (vertical bar = ±se). The se is not seen when smaller than the symbol size. The initial lengths in millimeters (mean ± sd) of all analyzed segments were 5.131 ± 0.086 (with Ca2+), 5.191 ± 0.065 (1 mm BAPTA), 5.164 ± 0.086 (3 mm BAPTA), and 5.122 ± 0.068 (10 mm BAPTA).
When IAA-induced growth was determined after 4 h of treatment (instead of 2 h), a bell-shaped dose response curve with only a single major peak (at about 10–5 m) was generated, although the curve for IAA was still wider than that for NAA (data not shown). Therefore, the timing of growth measurement appeared to be important to resolve the two peaks.
To obtain dose response curves with reduced levels of apoplastic Ca2+, segments were incubated in a medium containing BAPTA, a membrane-impermeant Ca2+ chelator (CaCl2 was omitted from the medium); BAPTA was present in both the pre-incubation and treatment mediums. When segments were treated with 1 mm BAPTA, the dose response curve for IAA (Fig. 8B) or NAA (Fig. 8D) was similar to that obtained without BAPTA. At 10 mm BAPTA, the growth was substantially reduced over the entire range of auxin concentrations (Fig. 8, B and D). A clear difference was noted, however, between IAA and NAA. For NAA, the dose-dependent increase in growth occurred in the same concentration range as that found in the Ca2+-containing medium. For IAA, the dose-dependent increase occurred at concentrations higher than that in the Ca2+-containing medium. In fact, no IAA-induced increase in growth could be detected in the range from 10–8 to 10–7 m, in which the segments incubated in the Ca2+-containing medium showed a sharp increase in growth. Therefore, in contrast to the case for NAA, the response to low concentrations of IAA was selectively reduced when the apoplastic Ca2+ level was reduced by BAPTA.
When segments were treated with an intermediate concentration (3 mm) of BAPTA, the response at low concentrations of IAA was not eliminated but was reduced more than that at high concentrations (Fig. 8B). As a result, the peak of response at low concentrations became less apparent.
These results obtained by measuring growth supported the idea that both the ABP1-dependent and -independent signaling pathways contribute to the growth response, as will be discussed below.
DISCUSSION
We have demonstrated that protoplasts of pea stem epidermis undergo auxin-induced swelling (Fig. 1). This result provides the clearest evidence that epidermal cells respond directly to auxin. Furthermore, we have demonstrated that two distinct signaling pathways participate in the protoplast swelling response. The auxin receptor in one pathway is ABP1 located on the outer surface of the plasma membrane (Figs. 3 and 4D). In this pathway, NAA is more effective than IAA, as deduced from the results shown in Figure 3, and external Ca2+ is not required (Fig. 7, B–D). The auxin receptor for the other pathway is most probably not ABP1 (see “Results”). In sharp contrast to the ABP1 pathway, this pathway (non-ABP1 pathway) is insensitive to NAA (Figs. 3 and 4B) and depends strictly on external Ca2+ (Fig. 7A). The swelling induced by either pathway results from uptake of K+ and Cl– (Fig. 6).
ABP1 Pathway
Using the protoplasts isolated from maize coleoptiles and Arabidopsis hypocotyls, Steffens et al. (2001) demonstrated that ABP1 mediates auxin-induced swelling. In line with these results, we were able to show that the ABP-1-mediated swelling response also takes place in pea epidermal protoplasts. Early results from biochemical studies indicated that ABP1 has a greater affinity for NAA than for IAA, the difference being at least 1 order of magnitude (Löbler and Klämbt, 1985, and refs. therein). In agreement with this difference, our results indicated that the ABP1-mediated swelling response is similarly more sensitive to NAA than to IAA.
The use of anti-ABP1 antibodies has linked ABP1 located on the outer surface of the plasma membrane to the following responses: plasma membrane hyperpolarization (Leblanc et al., 1999a), activation of H+ efflux through the plasma membrane (Rück et al., 1993), and swelling of protoplasts (Steffens et al., 2001; present study). Together with our conclusion that ABP1-mediated protoplast swelling results from uptake of K+ and Cl–, these findings lead to the following general model: Auxin interacts with ABP1 to activate plasma membrane H+-ATPases and to hyperpolarize the plasma membrane. As a consequence of membrane hyperpolarization, uptake of K+ and Cl– is induced, leading in turn to osmoregulated water uptake and, thus, protoplast swelling. The K+ uptake is mediated by inward-rectifying K+ channels (Becker and Hedrich, 2002), whereas the Cl– uptake is probably mediated by Cl–/H+ symporters (Iino et al., 2001).
Non-ABP1 Pathway
In previous studies, anti-ABP1 antibodies were shown to inhibit responses in membrane potential, H+ efflux, and protoplast volume (Barbier-Brygoo et al., 1991; Rück et al., 1993; Steffens et al., 2001) with little sign of a second, ABP1-independent component. The use of NAA alone (e.g. Barbier-Brygoo et al., 1991) could account for the failure in detecting its occurrence (Fig. 3B). However, an ABP1-independent response was not identified even when IAA was used (Rück et al., 1993; Steffens et al., 2001). The non-ABP1 pathway might be specific to epidermal cells. It is also possible that the concentrations of IAA used were too high to detect the ABP1-independent response (Fig. 3A, solid circles).
Our finding that the non-ABP1 pathway is insensitive to NAA might provide a clue to the nature of the second auxin receptor. The auxin uptake carrier located in the plasma membrane was reported to have a much reduced affinity for NAA (Delbarre et al., 1996; Marchant et al., 1999). In fact, the auxin uptake carrier was once suggested to act as a receptor for a gene expression response (Boot et al., 1996), but characterization of the mutant phenotype makes this possibility unlikely (Marchant et al., 1999). Kim et al. (1998, 2000, 2001) isolated a soluble auxin-binding protein from rice (Oryza sativa) that is distinct from ABP1. This protein, named ABP57, enhanced H+-ATPase activity in an IAA-dependent manner when added to plasma membrane preparations. In this in vitro assay system, NAA had no activity (Kim et al., 2000). The insensitivity to NAA, together with its function to activate H+-ATPase, makes ABP57 an attractive candidate receptor for the non-ABP1 pathway.
Involvement of Ca2+ as a Second Messenger
Calcium ions have been implicated as second messengers in various plant signal transduction processes (White, 2000). The idea that Ca2+ plays a second messenger role in auxin signaling has been supported by the finding that cytosolic Ca2+ increases in response to auxin (Felle, 1988; Gehring et al., 1990). Our result that the ABP1-independent response requires external Ca2+ (Fig. 7A) suggests that, in this response, apoplastic Ca2+ plays a role as a second messenger. The result also indicates that the Ca2+ stored inside the cell cannot make any appreciable contribution to the response. In sharp contrast, the ABP1-mediated response was found not to require external Ca2+ (Fig. 7B). This result, however, does not exclude a possible contribution by the Ca2+ stored inside the cell. It will be interesting to extend the protoplast swelling data with measurements of cytosolic Ca2+ and relate the changes in Ca2+ to intra- and extracellular pools.
Our results might provide an explanation for the contradictory results regarding whether or not apoplastic Ca2+ is required for the auxin-induced medium acidification by coleoptile segments. Cohen and Nadler (1976) found that the acidification response critically depends on the presence of apoplastic Ca2+. On the other hand, Claussen et al. (1997) found no such Ca2+ requirement in their acidification or growth response. The former authors used IAA (10–5 m) and the latter authors used NAA (typically 5 × 10–6 m). It is possible that Cohen and Nadler investigated the non-ABP1 pathway, whereas Claussen et al. investigated the ABP1 pathway.
Relationship with Auxin-Induced Internode Growth
As mentioned above, the binding affinity of ABP1 is at least 10 times higher for NAA than IAA. However, the dose response curves for growth have indicated that segments of coleoptiles or stems are at least 10 times more sensitive to IAA (e.g. Shinkle and Briggs, 1984; see also Fig. 8). The applied NAA may be less effectively transported in the segments, but the relationship described above is perhaps too large to be explained by the difference in transport efficiency. As already pointed out by Hertel (1995), the disagreement in ligand specificity challenges the role of ABP1 as a receptor in growth responses. On the other hand, the non-ABP1 pathway alone cannot account for growth response simply because NAA is an active auxin for the latter.
In fact, both the two signaling systems might be responsible for the auxin-induced growth of segments. The data shown in Figure 8 provide correlative evidence for this hypothesis. The dose response curve for IAA-induced growth showed two peaks (Fig. 8A), in agreement with the participation of ABP1-indepdendent and -dependent responses that have different dose requirements. On the other hand, the dose response curve for NAA-induced growth showed only one peak (Fig. 8C), in agreement with the participation of only the ABP1-dependent response. The growth response to IAA at low doses depended more critically on the presence of apoplastic Ca2+ than the response at high doses (Fig. 8B); the growth response to NAA showed no such dose-dependent difference (Fig. 8D). These results agree with the different Ca2+ requirements of the two pathways. The growth response to high IAA concentrations was also inhibited by a high concentration of BAPTA (10 mm). Therefore, the IAA-dose dependent difference in Ca2+ requirement was not as sharp as that for the protoplast swelling response (Fig. 7). Reducing apoplastic Ca2+ probably caused other less specific effects on growth.
Dose response curves for IAA-induced segment growth have been reported by a number of workers. Two distinct peaks (Fig. 8A) have not been reported previously, but biphasic dose response curves have been described. Working with segments of dark-grown oat coleoptiles, Shinkle and Briggs (1984) found a small shoulder on the ascending part of the dose response curve for IAA-induced growth. This shoulder was not evident with NAA. Nissen (1985) analyzed earlier dose response data for IAA-induced growth of coleoptile segments to conclude that some of them show a biphasic feature. The results reported by Karcz et al. (1990) for maize coleoptile segments should also be mentioned. These authors obtained dose response curves for IAA-induced medium acidification by the segments, together with those for segment growth, at 30-min intervals over a period of 3 h and reported normalized curves. The curves for medium acidification (but not for segment growth) indicated two peaks, although the authors did not draw attention to it. The IAA concentrations causing the two peaks were in excellent agreement with those found in pea segment growth.
When the level of apoplastic Ca2+ was reduced extensively by treatment with a high-concentration BAPTA (10 mm), the dose response curve for segment growth became very similar between IAA and NAA (Fig. 8, B and D). In view of the explanations given above, this dose response curve may be attributed almost solely to the ABP1 pathway. It is interesting to note that, in this dose response curve, the response stayed more or less constant over a wide range of concentrations (i.e. the curve was not bell shaped). The mechanism underlying the descending arm of ABP1-mediated response, being distinct from that for the ascending arm, might involve apoplastic Ca2+. Our results may be related to those of Leblanc et al. (1999b), who found that applied ABP1 and C-terminal peptides can mimic the NAA-induced hyperpolarization response but cannot produce the descending arm at higher doses.
Concluding Remarks
Based on the discussion above, we hypothesize that the two signaling pathways uncovered in pea epidermal protoplasts participate in the auxin-induced growth of pea internodes. In the frame of the acid growth theory, the activation of plasma membrane H+-ATPase by the ABP1 pathway (Rück et al., 1993) could be linked to the loosening of epidermal cell walls and internode growth. Considering the nature of the response investigated (i.e. swelling of protoplasts), it is probable that the non-ABP1 pathway also involves the activation of plasma membrane H+-ATPase.
It might be argued that the non-ABP1 pathway, which is more sensitive to IAA than the ABP1 pathway, is more critically involved in the control of internode growth by constitutive concentrations of endogenous auxin. Many of the results reported by Jones et al. (Jones et al., 1998; Chen et al., 2001a, 2001b) indicated that changes in ABP1 expression are associated with corresponding changes in the response to applied auxin. However, tobacco plants overexpressing ABP1 were found to grow with no obvious phenotype, although isolated leaf protoplasts were larger in size, and excised leaf tissues had greater responsiveness to applied NAA (Jones et al., 1998). These results are compliant with the idea that the non-ABP1 pathway plays a primary role for the control of growth by endogenous auxin, at least under normal growth conditions. The insensitivity to NAA and the requirement for extracellular Ca2+ of the non-ABP1 pathway now provide physiological tools for further elucidation of the roles played by two signaling pathways in organ growth.
Although ABP1 has been shown to function on the outer surface of the plasma membrane, there is some evidence that the site of receptor action for the control of growth is intracellular (Claussen et al., 1996). Our finding of the occurrence of the non-ABP1 pathway is relevant to this controversial issue. It would be of interest to resolve whether the receptor for this pathway functions inside the cell.
MATERIALS AND METHODS
Plant Material
Seedlings of peas (Pisum sativum L. cv Alaska) were raised as described by Haga and Iino (1997). In brief, surface-sterilized seeds were sown on moist paper towels and incubated at 25°C under red light (2.5–3.0 μmol m–2 s–1) in a light-tight growth room. The seedlings were used for the experiments 5 d after sowing, when the third internode was the top elongating internode. The seedlings were selected for the length of the third internode (20–25 mm).
Isolation of Epidermal Protoplasts
Epidermal peels were obtained from the upper part (approximately 15 mm long) of the third internode. Maximal and uniform elongation took place in this part (Haga and Iino, 1997). The peels were immersed in enzyme solution, vacuum infiltrated, and incubated for 1.5 h on a rotating shaker (60 rpm). The enzyme solution consisted of 1.7% (w/v) cellulase RS (Yakult, Tokyo), 0.1% (w/v) pectolyase Y-23 (Seishin Pharmaceutical, Tokyo), 0.5 m sorbitol, 10 mm KCl, 1 mm CaCl2, 20 mm Glc, and 10 mm MES (adjusted to pH 6.0 with Tris).
After the enzyme treatment, the mixture was filtered through a nylon mesh and centrifuged at 110g for 10 min. The pellet was suspended in a 2-mL bathing medium that contained 0.5 m sorbitol, 10 mm KCl, 1 mm CaCl2, 20 mm Glc, and 10 mm MES (adjusted to pH 6.0 with Tris). The suspension was loaded on an 18% (v/v) Percoll (Sigma, St. Louis) solution also containing the bathing medium components and centrifuged for 5 min at 110g. The protoplasts collected from the interface between the Percoll solution and the loaded medium were washed twice by suspending them in a 5-mL bathing medium and centrifuging them at 110g for 5 min. The protoplast pellet was suspended in a small amount of the bathing medium to obtain the final preparation. All steps of protoplast preparation were carried out in the growth room and under the red light condition used to raise seedlings. More details not described here can be found in Long and Iino (2001).
Incubation of Protoplasts for Experimental Treatments
A suspension (typically 225 μL) of freshly prepared protoplasts was added to an all-side clear quartz cuvette (base area 10 × 10 mm, height 45 mm). The cuvette was set on the sample stage of an inverted microscope (IMT-1, Olympus, Tokyo), and the protoplasts were incubated at 25°C ± 0.5°C under red light (50 μmol m–2 s–1); for details of the system, see Wang and Iino (1997). During incubation, protoplasts were subjected to experimental treatments and photography.
Auxin Treatment
A 25-μL solution of IAA or NAA, made up in the bathing medium, was added to a 225-μL protoplast suspension. This addition was generally made after a period of incubation on the microscope stage. After a gentle agitation, the suspension was set again on the microscope stage to investigate the effect of auxin on the protoplast volume.
Antibody Treatment
The antibodies used in the present study were those described by Venis et al. (1992). The stock solution of anti-ABP1 IgG and that of pre-immune IgG (used as a control) were prepared in 0.1-strength PBS at a concentration of 2.3 μm. The stock solution of D16 antibodies (3.6 μm) was prepared in 0.5-strength PBS. These solutions were used after dilution to appropriate concentrations with bathing medium.
To investigate the effect of anti-ABP1 antibodies on auxin-induced swelling (Figs. 3 and 4), a 25-μL solution of the antibodies was added to a 200-μL suspension of protoplasts (to allow 25 μL for auxin solution). The final antibody concentration indicated was the concentration after addition of the auxin solution. To investigate the effect of D16 antibodies on the protoplast volume (Figs. 5 and 7D), a 25-μL solution of the antibodies was added to the protoplast suspension (225 μL).
Experiments Using Modified Bathing Mediums
The bathing medium components were modified as follows to investigate the roles for ions. The K+-free medium was prepared by using 10 mm TEA-Cl in place of 10 mm KCl. The Cl–-free medium was prepared by using 12 mm iminodiacetic acid, 10 mm KOH, and 1 mm Ca(OH)2 in place of 10 mm KCl and 1 mm CaCl2. To prepare the Ca2+-free medium, CaCl2 was omitted from and 1 mm EGTA was added to the bathing medium. The modified medium was used from the step of suspending the protoplast pellet after enzyme treatment.
The protoplasts treated with auxin in a modified medium were resuspended in the standard medium and treated again with auxin. The change in mediums was achieved by mixing the protoplast suspension with the standard medium (about 5 mL), centrifuging the mixture for 5 min at 110g, and resuspending the pellet in a 225-μL standard medium.
Protoplast Volume Determination
Protoplast volume was determined as described previously (Wang and Iino, 1997; see also Iino et al., 2001). In brief, the diameter of individual protoplasts was measured from their magnified photographic images, and the protoplast volume was calculated from the diameter. For the volume analysis, protoplasts were selected with regard to roundness and clarity of the margin but not for their size. Protoplasts that were suspected to be of nonepidermal origin were also not used for analysis (see “Results”). The volume of each protoplast at a given time in a time course series of photographs (in time course studies) or at a fixed time after auxin treatment (in dose response studies) was expressed as the percentage of the initial volume. The initial volume of auxin-treated protoplasts was the volume determined 10 min after auxin application.
Measurement of Segment Growth
Internode segments (5 mm long) were excided from the upper part of the third internode (approximately 5–10 mm below the hook) with a pair of razor blades attached to an acrylic holder. The segments were first incubated for 2 h in a solution containing 10 mm KCl, 1 mm CaCl2, 10 mm Suc, and 5 mm MES (adjusted to pH 6.0 with KOH) and then for 2 h in a solution containing IAA and these components. Incubation was carried out at 25°C under red light (2.5–2.7 μmol m–2 s–1). For incubation, multiwell polystyrene containers (Nalge Nunc, Rochester, NY) were used. Each well (16-mm i.d.) contained a 0.4-mL incubation medium and one segment. The containers were shaken at 70 rpm during incubation. When the segments were treated with BAPTA, CaCl2 was omitted from and BAPTA was added to the mediums for both pre-incubation and auxin treatment.
Segments were photographed on technical pan film (Eastman-Kodak, Rochester, NY) just before the onset of incubation in IAA-containing medium and at the end of 2 h of incubation. The negative film was expanded by means of a slide projector. The segment length, traced on paper, was determined using a computer-interfaced digitizer. Growth increment during the 2-h treatment period was calculated as the percentage of the initial length (the length at the onset of treatment).
No light other than the red light (2.5–3.0 μmol m–2 s–1) was used to handle seedlings, to excise seedlings, and to obtain photographs.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.031294.
References
- Barbier-Brygoo H, Ephritikhine G, Klämbt D, Ghislain M, Guern J (1989) Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci USA 86: 891–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Brygoo H, Ephritikhine G, Klämbt D, Maurel C, Palme K, Schell J, Guern J (1991) Perception of the auxin signal at the plasma membrane of tobacco mesophyll protoplasts. Plant J 1: 83–93 [DOI] [PubMed] [Google Scholar]
- Babourina O, Shabala S, Newman I (1998) Auxin stimulates Cl– uptake by oat coleoptiles. Ann Bot 82: 331–336 [Google Scholar]
- Becker D, Hedrich R (2002) Channelling auxin action: modulation of ion transport by indole-3-acetic acid. Plant Mol Biol 49: 349–356 [PubMed] [Google Scholar]
- Blatt MR, Thiel G (1994) K+ channels of stomatal guard cells: bimodal control of the K+ inward-rectifier evoked by auxin. Plant J 5: 55–68 [DOI] [PubMed] [Google Scholar]
- Boot CJM, van Duijn B, Mennes AM, Libbenga KR (1996) Regulation of a class of auxin-induced genes in cell-suspension cultures from Nicotiana tabacum. In AR Smith, AW Berry, NW Harpham, IE Moshkov, GV Novikova, ON Kulaeva, MA Hall, Plant Hormone Signal Perception and Transduction. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 41–48
- Brown AM (1993) Functional bases for interpreting amino acid sequences of voltage-dependent K+ channels. Annu Rev Biophys Biomol Struct 22: 173–198 [DOI] [PubMed] [Google Scholar]
- Chen J-G, Shimomura S, Sitbon F, Sandberg G, Jones AM (2001a) The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. Plant J 28: 607–617 [DOI] [PubMed] [Google Scholar]
- Chen J-G, Ullah H, Young JC, Sussman MR, Jones AM (2001b) ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev 15: 902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen M, Lüthen H, Böttger M (1996) Inside or outside? Localization of the receptor relevant to auxin-induced growth. Physiol Plant 98: 861–867 [Google Scholar]
- Claussen M, Lüthen H, Blatt M, Böttger M (1997) Auxin-induced growth and its linkage to potassium channels. Planta 201: 227–234 [Google Scholar]
- Cleland RE (1995) Auxin and cell elongation. In PJ Davies, ed, Plant Hormones. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 214–227
- Cocking EC (1962) Action of growth substances, chelating agents and antibiotics on isolated root protoplasts. Nature 193: 998–999 [DOI] [PubMed] [Google Scholar]
- Cohen JD, Nadler KD (1976) Calcium requirement for indole acetic acid-induced acidification by Avena coleoptiles. Plant Physiol 57: 347–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Imhoff V, Guern J (1996) Comparison of mechanisms controlling uptake and accumulation of 2, 4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198: 532–541 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Jones AM, Estelle M (2003) Auxin action in a cell-free system. Curr Biol 13: 1418–1422 [DOI] [PubMed] [Google Scholar]
- Diekmann W, Venis MR, Robinson DG (1995) Auxins induce clustering of the auxin-binding protein at the surface of maize coleoptile protoplasts. Proc Natl Acad Sci USA 92: 3425–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H (1988) Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta 174: 495–499 [DOI] [PubMed] [Google Scholar]
- Frías I, Caldeira MT, Pérex-Castiñeira JR, Navarro-Aviñó JP, Culiñez-Maciá FA, Kuppinger O, Stransky H, Pagés M, Hager A, Serrano R (1996) A major isoform of the maize plasma membrane H+-ATPase: characterization and induction by auxin in coleoptiles. Plant Cell 8: 1533–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW (1990) Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc Natl Acad Sci USA 87: 9645–9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K, Iino M (1997) The short-term growth stimulation induced by external supply of IAA in internodes of intact pea seedlings. Aust J Plant Physiol 24: 215–226 [Google Scholar]
- Haga K, Iino M (1998) Auxin-growth relationships in maize coleoptiles and pea internodes and control by auxin of the tissue sensitivity to auxin. Plant Physiol 117: 1473–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A, Debus G, Edel H-G, Stransky H, Serrano R (1991) Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase. Planta 185: 527–537 [DOI] [PubMed] [Google Scholar]
- Hager A, Menzel H, Krauss A (1971) Versuche und hypothese zur primärwirkung des auxins beim streckungswachstum. Planta 100: 47–75 [DOI] [PubMed] [Google Scholar]
- Hall MD, Cocking EC (1974) The response of isolated Avena coleoptile segments to indol-3-acetic acid. Protoplasma 79: 225–234 [DOI] [PubMed] [Google Scholar]
- Hertel R (1995) Auxin binding protein 1 is a red herring. J Exp Bot 46: 461–462 [Google Scholar]
- Iino M (2001) Phototropism in higher plants. In D-P Häder, M Lebert, eds, Photomovement. Elsevier Science, Amsterdam, pp 659–811
- Iino M, Long C, Wang X (2001) Auxin- and abscisic acid-dependent osmoregulation in protoplasts of Phaseolus vulgaris pulvini. Plant Cell Physiol 42: 1219–1227 [DOI] [PubMed] [Google Scholar]
- Jones AM (1994) Auxin-binding proteins. Annu Rev Plant Physiol Plant Mol Biol 45: 393–420 [Google Scholar]
- Jones AM, Im K-H, Savka MA, Wu M-J, DeWitt NG, Shillito R, Binns AN (1998) Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 282: 1114–1117 [DOI] [PubMed] [Google Scholar]
- Karcz W, Stolarek J, Pietruszka M, Malkowski E (1990) The dose-response curves for IAA induced elongation growth and acidification of their incubation medium of Zea mays coleoptile segments. Physiol Plant 80: 257–261 [Google Scholar]
- Keller CP, Van Volkenburgh E (1996) Osmoregulation by oat coleoptile protoplasts: effect of auxin. Plant Physiol 110: 1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-S, Min J-K, Kim D, Jung J (1998) Isolation of a novel auxin receptor from soluble fractions of rice (Oryza sativa L.) shoots. FEBS Lett 438: 241–244 [DOI] [PubMed] [Google Scholar]
- Kim Y-S, Min J-K, Kim D, Jung J (2000) Two isoforms of soluble auxin receptor in rice (Oryza sativa L.) plants: binding property for auxin and interaction with plasma membrane H+-ATPase. Plant Growth Regul 32: 143–150 [Google Scholar]
- Kim Y-S, Min J-K, Kim D, Jung J (2001) A soluble auxin-binding protein, ABP57. Purification with anti-bovine serum albumin antibody and characterization of its mechanistic role in the auxin effect on plasma membrane H+-ATPase. J Biol Chem 276: 10730–10736 [DOI] [PubMed] [Google Scholar]
- Kutschera U (1987) Cooperation between outer and inner tissues in auxin-mediated plant organ growth. In DJ Cosgrove, DP Knievel, eds, Physiology of Cell Expansion during Plant Growth. American Society of Plant Physiologists, Rockville, MD, pp 215–226
- Leblanc N, David K, Grosclaude J, Pradier JM, Barbier-Brygoo H, Labieu S, Perrot-Rechenmann C (1999a) A novel immunological approach establishes that the auxin-binding protein, Nt-abp1, is an element involved in auxin signaling at the plasma membrane. J Biol Chem 274: 28314–28320 [DOI] [PubMed] [Google Scholar]
- Leblanc N, Perrot-Rechenmann C, Barbier-Brygoo H (1999b) The auxin-binding protein Nt-Erabp1 alone activates an auxin-like transduction pathway. FEBS Lett 449: 57–60 [DOI] [PubMed] [Google Scholar]
- Löbler M, Klämbt D (1985) Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). I. Purification by immunological methods and characterization. J Biol Chem 260: 9848–9853 [PubMed] [Google Scholar]
- Long C, Iino M (2001) Light-dependent osmoregulation in pea stem protoplasts: photoreceptors, tissue specificity, ion relationships, and physiological implications. Plant Physiol 125: 1854–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthen H, Claussen M, Böttger M (1999) Growth: progress in auxin research. Prog Bot 60: 315–340 [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18: 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten I, Lohse G, Hedrich R (1991) Plant growth hormones control voltage-dependent activity of anion-channels in plasma membrane of guard cells. Nature 353: 758–762 [Google Scholar]
- Masuda Y, Yamamoto R (1972) Control of auxin-induced stem elongation by the epidermis. Physiol Plant 27: 109–115 [Google Scholar]
- Napier RM, David KM, Pettot-Rechenmann C (2002) A short history of auxin binding proteins. Plant Mol Biol 49: 339–348 [PubMed] [Google Scholar]
- Nissen P (1985) Dose responses of auxins. Physiol Plant 65: 357–374 [Google Scholar]
- Peters WS, Tomos AD (1996) The epidermis still in control? Bot Act 109: 264–267 [Google Scholar]
- Philippar K, Fuchs I, Lüthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Böttger M et al. (1999) Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA 96: 12186–12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JB, Cocking EC (1970) Isolation of leaf protoplasts: macromolecule uptake and growth substance response. J Exp Bot 21: 64–70 [Google Scholar]
- Rück A, Palme K, Venis MA, Napier RM, Felle HH (1993) Patch-clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant J 4: 41–46 [Google Scholar]
- Senn AP, Goldsmith MH (1988) Regulation of electrogenic proton pumping by auxin and fusicoccin as related to the growth of Avena coleoptiles. Plant Physiol 88: 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle J, Briggs WR (1984) Auxin concentration/growth relationship for Avena coleoptile sections from seedlings grown in complete darkness. Plant Physiol 74: 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Feckler C, Palme K, Christian M, Böttger M, Lüthen H (2001) The auxin signal for protoplast swelling is perceived by extracellular ABP1. Plant J 27: 591–599 [DOI] [PubMed] [Google Scholar]
- Venis MA, Napier RM, Barbier-Brygoo H, Maurel C, Perrot-Rechenmann C, Guern J (1992) Antibodies to a peptide from the maize auxin-binding protein have auxin agonist activity. Proc Natl Acad Sci USA 89: 7208–7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper MJ, Kuss CL (1990) Physiological evidence that the primary site of auxin action in maize coleoptiles is an intracellular site. Planta 182: 486–491 [DOI] [PubMed] [Google Scholar]
- Wang X, Iino M (1997) Blue-light-induced shrinking of protoplasts from maize coleoptiles and its relationship to coleoptile growth. Plant Physiol 114: 1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW (1928) Wuchsstoff und wachstum. Rec Trav Bot Neerl 25: 1–116 [Google Scholar]
- White PJ (2000) Calcium channels in higher plants. Biochem Biophys Acta 1465: 171–189 [DOI] [PubMed] [Google Scholar]
- Zhao H, Hertel R, Ishikawa H, Evans ML (2002) Species differences in ligand specificity of auxin-controlled elongation and auxin transport: comparing Zea and Vigna. Planta 216: 293–301 [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Thomine S, Guern J, Barbier-Brygoo H (1994) An anion current at the plasma membrane of tobacco protoplasts shows ATP-dependent voltage regulation and is modulated by auxin. Plant J 6: 707–716 [Google Scholar]