Abstract
Multiple gibberellins (GAs) were quantified in the stems of intact, decapitated, and decapitated auxin-treated barley (Hordeum vulgare) plants. Removal of the developing inflorescence reduced the endogenous levels of indole-3-acetic acid (IAA), GA1, and GA3 and increased the level of GA29 in internodal and nodal tissues below the site of excision. Application of IAA to the excised stump restored GA levels to normal in almost all cases. The conversion of [14C]GA20 to bioactive [14C]GA1 and of [14C]GA5 to bioactive [14C]GA3 was reduced by decapitation, and IAA application was able to restore conversion rates back to the levels found in intact plants. The amount of mRNA for the principal vegetative 3-oxidase (converting GA20 to GA1, and GA5 to GA3) was decreased in decapitated plants and restored by IAA application. The results indicate that the inflorescence of barley is a source of IAA that is transported basipetally into the internodes and nodes where bioactive GA1 and GA3 are biosynthesized. Thus, IAA is required for normal GA biosynthesis in stems, acting at multiple steps in the latter part of the pathway.
The classical plant hormones auxin and GA, which are responsible for elongation and various other processes, have been investigated extensively both individually and simultaneously, the latter in an effort to elucidate the relationship between them. In the past, many conflicting theories have been put forward concerning their interactions (Brian and Hemming, 1958; Kurashi and Muir, 1962; Law and Davies, 1990; van Huizen et al., 1997), but recently, it was discovered that in pea (Pisum sativum) stems the auxin indole-3-acetic acid (IAA) promotes GA biosynthesis. Auxin was found to increase the transcript level of a GA 3-oxidase, PsGA3ox1, encoded by Mendel's LE gene, and to reduce that of a GA 2-oxidase, PsGA2ox1, which encodes an enzyme for GA deactivation (Ross et al., 2000). Later, this model was extended into another dicot species, tobacco (Nicotiana tabacum), where IAA predominantly promotes a different biosynthetic step, GA 20-oxidation (Wolbang and Ross, 2001), highlighting the comparable yet distinct nature of the auxin-GA relationship in that species.
The aim of the present research was to determine whether auxin promotes GA biosynthesis in monocotyledons, in particular the grasses. Previous studies on GAs at the seedling and successive stages of grass development are abundant (Fujioka et al., 1988a, 1988b; Croker et al., 1990; Phinney et al., 1991; Kobayashi et al., 1996; Spray et al., 1996), as are studies on the auxin physiology of the coleoptile and etiolated mesocotyl (Weiler et al., 1981; Koshiba et al., 1995; Barker-Bridgers et al., 1998; Haga and Iino, 1998; Parsons et al., 1988). However, there is a lack of auxin studies using developmental stages successive to the coleoptile/mesocotyl. Therefore, despite the important role that some grass species play in agriculture, little has been reported about the role of auxin as the grass plant matures. There have been even fewer studies involving both auxins and GAs, leaving unanswered key questions concerning their interaction(s).
Koning et al. (1977) experimented with removal of the inflorescence of oat (Avena sativa) plants, a method that could be likened to the decapitation of pea plants. Growth of the p-1 internode (the internode below the peduncular internode), which occurs at a rapid rate during inflorescence development, was diminished with the removal of the inflorescence and abolished when the nodes above and below it were also removed. The p-1 node situated at the base of the p-1 internode was regarded as vital for growth of this internode because GA3 could only restore growth fully when it was present. GA levels for the relevant tissues had been published in a previous paper (Kaufman et al., 1976) identifying the inflorescence and nodes as a rich source of GA, with GA3 as the predominant form in the inflorescence. Koning et al. (1977) concluded that the inflorescence was the major source of GA, which facilitated the elongation of the p-1 internode. Considering the findings of Ross et al. (2000) and Wolbang and Ross (2001), where removal of the apical bud led to the reduction in the levels of auxin and GA1 (and, therefore, internode elongation), the present paper tests the possibility that the inflorescence is a source of auxin for the p-1 internode.
We focus on the maturing reproductive plant, specifically the p-1 internode and the nodes above and below it. We employ a number of techniques to investigate the auxin-GA relationship including decapitation and IAA application, GA quantification and metabolism studies, and determination of mRNA levels by quantitative PCR. In addition, analyses of specific areas of growth within the p-1 internode of barley (Hordeum vulgare) are undertaken.
Evidence is presented that the auxin IAA promotes GA biosynthesis in barley, and the role of the developing inflorescence is discussed in relation to this auxin/GA interaction.
RESULTS
Effects of Decapitation and IAA Application on Endogenous GA Levels
Experiments were conducted with oat and barley. Oat plants were included to enable comparison with previous reports (Kaufman et al., 1976; Koning et al., 1977). Plants were either left intact, decapitated, or decapitated and treated with IAA. The point of decapitation and the location of relevant parts are shown in Figure 1. Decapitation involved the excision of the developing inflorescence. When oat plants were decapitated, there were reductions in the levels of IAA, GA1, and GA3 in internode tissue, compared with intact plants (Table I). All three reductions were statistically significant. IAA application restored IAA levels to those observed in intact plants and also restored the levels of GA1 and GA3. The oat inflorescence contained high levels of IAA (6-fold greater than that of the p-1 internode from the intact plant).
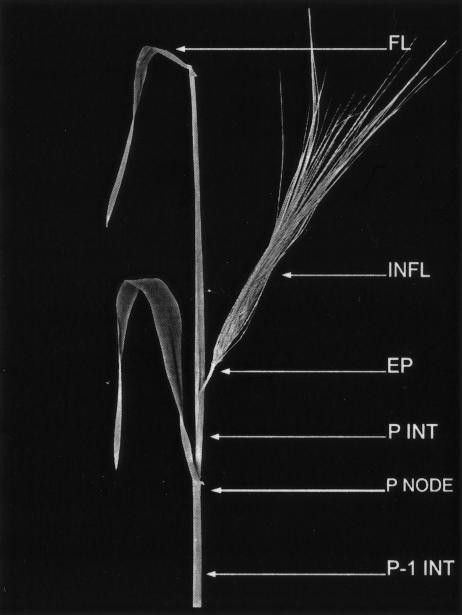
Figure 1.
Representative barley plant at the time of treatment. FL, Flag leaf; INFL, inflorescence; EP, excision point; P INT, peduncular internode; P NODE, peduncular node; P-1 INT, peduncular –1 internode. The inflorescence has been teased out of its enveloping sheath (the flag leaf sheath) to enable decapitation (at EP) and auxin treatment.
Table I.
Effects of decapitation and IAA application on GA1, GA3, and IAA levels of the p-1 internode of oat 48 h after decapitation
Data are also shown for the removed inflorescence and are means ± se (n = 2).
| Hormone | Intact | Decapitated | IAA Treated | Inflorescence |
|---|---|---|---|---|
| ng g fresh wt-1 | ||||
| IAA | 12.3 ± 1.0 | 1.7 ± 0.1 | 12.6 ± 0.8 | 77.3 ± 1.7 |
| GA1 | 0.14 ± 0.005 | 0.023 ± 0.0045 | 0.17 ± 0.045 | 0.26 ± 0.005 |
| GA3 | 0.049 ± 0.006 | n.d.a | 0.082 ± 0.009 | 0.11 ± 0.01 |
n.d., No dilution of internal standard.
Decapitation of barley plants also reduced IAA, GA1, and GA3 levels in the p-1 internode, in this case harvested with the intercalary meristem at the base of the internode (Table II). Similar results were obtained in a separate experiment (Fig. 2), in which the p-1 internode and the p node above it (including the intercalary meristem of the p internode) were harvested separately. Application of IAA again restored IAA, GA1, and GA3 levels to at least those found in intact internodes. The GA3, GA1, and IAA data in Table II were obtained after purification of the samples by HPLC as methyl esters. This was necessary to remove an impurity that co-eluted with GA3 as free acids on HPLC and that produced a 504 mass-to-charge ratio ion that eluted very close to GA3 on gas chromatography-mass spectrometry (GC-MS) with the nonpolar column used in this work. This compound may correspond to that previously reported by Croker et al. (1990).
Table II.
Effects of decapitation and IAA application on GA1, GA3, and IAA levels of the p-1 internode of barley 48 h after decapitation
Data are means ± se (n = 2).
| Hormone | Intact | Decapitated | IAA-treated |
|---|---|---|---|
| ng g fresh wt-1 | |||
| IAA | 14.2 ± 0.7 | 4.0 ± 0.7 | 36.1 ± 0.3 |
| GA1 | 0.12 ± 0.032 | 0.016 ± 0.0015 | 0.44 ± 0.035 |
| GA3 | 0.064 ± 0.012 | 0.0027 ± 0.0027 | 0.15 ± 0.015 |
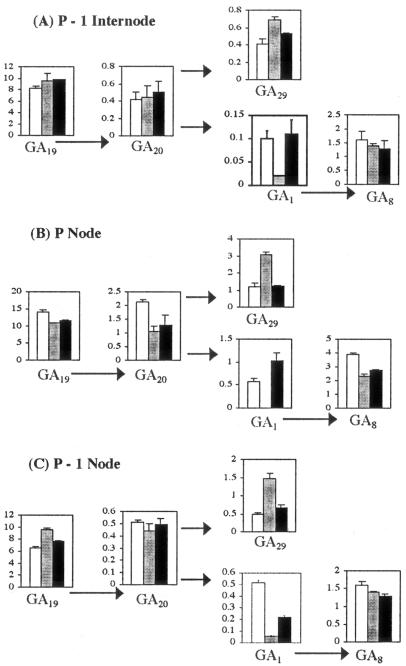
Figure 2.
Effects of decapitation and IAA on endogenous GA levels in p-1 internode (A), p node (B), and p-1 node (C) of barley. White columns, Intact; stippled columns, decapitated; black columns, decapitated + IAA. Metabolic relationships are indicated by arrows. ses are shown except where too small to be visible (n = 2).
The level of GA29 was increased by decapitation, whereas those of GA20 and GA19 were largely unaffected or reduced slightly (Fig. 2). GA5 was not successfully quantified. GA levels were generally greater in the nodal zone than in the p-1 internode (Fig. 2). This is consistent with the localization of growth in the basal portion of the internode (Table III). In intact internodes, the level of GA1 was approximately 2-fold greater than that of GA3 (Table II). Metabolic relationships between the GAs are shown in Figure 3.
Table III.
Growth of the p-1 internode of barley at the stage used throughout this study
The p-1 internode was divided into three sections: uppermost 20 mm, middle, and lowermost 20 mm. These three sections were measured every day for 5 d, along with the total length of the p-1 internode. Data are shown as means ± se (n = 9).
| Time | Upper 20 | Middle | Lower 20 | Total length |
|---|---|---|---|---|
| mm | ||||
| Day 1 | 20.00 ± 0.00 | 62.23 ± 6.98 | 20.00 ± 0.00 | 102.23 ± 6.98 |
| Day 2 | 20.00 ± 0.00 | 63.38 ± 7.05 | 32.86 ± 1.30 | 116.25 ± 7.29 |
| Day 3 | 20.00 ± 0.00 | 63.89 ± 6.92 | 39.56 ± 1.43 | 123.45 ± 7.29 |
| Day 4 | 20.00 ± 0.00 | 63.70 ± 7.04 | 46.31 ± 2.10 | 130.00 ± 6.98 |
| Day 5 | 20.00 ± 0.00 | 63.39 ± 7.19 | 46.22 ± 2.17 | 130.06 ± 7.21 |
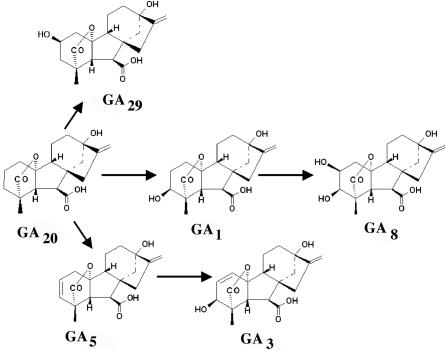
Figure 3.
Final steps leading to bioactive GAs and deactivation products in the early 13-hydroxylation pathway of barley. GA1 and GA3 are bioactive per se.
The inflorescence of barley was found to contain approximately 3-fold more IAA than, and comparable levels of GA1 and GA3 to, the p-1 internode (data not shown). Other auxins (4Cl-IAA and indolebutyric acid [IBA]) were not detectable in the p-1 internodal tissue, although internal standards for both were recovered by GC-MS (data not shown).
Decapitation generally reduced the elongation of the p-1 internode in both barley and oat (data not shown). Application of IAA did not consistently restore growth to that of intact plants. However, in the experiment reported in Table I, IAA significantly stimulated elongation of oat internodes (mean elongation values for decapitated and auxin-treated decapitated internodes were 6.0 ± 1.1 and 15.3 ± 2.5 mm, respectively).
Quantification of GA1 levels and measuring internode lengths of the grd2-463 3-oxidase mutant (Chandler and Robertson, 1999) and the respective wild type evaluated the effect of GA1 reduction on the growth of internodal tissue. The p-1 internode of the grd2-463 3-oxidase mutant exhibited a 77% reduction in the GA1 level, and GA3 levels were too low to quantify (Table IV). These reductions were associated with a 25% and a 35% reduction in the elongation of the p-1 and p-2 internodes, respectively, compared with the wild type (Table IV). Other independent alleles at the Grd2 locus (grd2-430 and grd2-489) result in extreme dwarfism (data not shown), indicating that Grd2 encodes the principal 3-oxidase in barley stems.
Table IV.
Levels of GA1 and GA3 in the p-1 internode of wild-type and grd2-463 plants
Data are the mean levels ± se (nanograms per gram fresh wt; n = 2). Also shown is the length of the p-1 and p-2 internodes of wild-type and grd2 plants. Data are the mean length ± se (in millimeters; n = 20).
| Genotype | GA1 | GA3 | p-1 Internode | p-2 Internode |
|---|---|---|---|---|
| ng g fresh wt-1 | mm | |||
| Wild type | 0.13 ± 0.015 | 0.083 ± 0 | 193.26 ± 5.45 | 173.15 ± 2.66 |
| grd2-463 | 0.043 ± 0.0075 | n.d.a | 146.26 ± 3.42 | 112.13 ± 1.57 |
n.d., No dilution of internal standard.
Effects of Decapitation and IAA Application on the Metabolism of [14C]GA20 and [14C]GA5
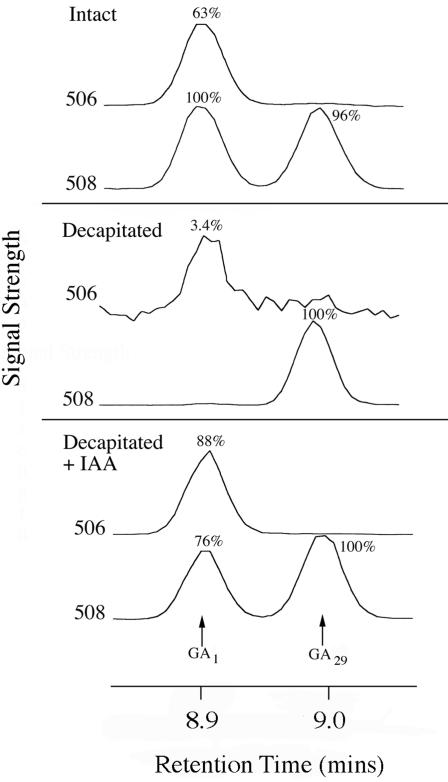
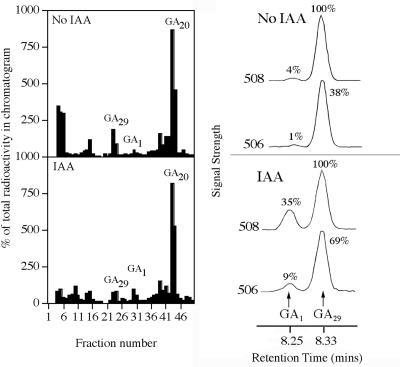
[14C]GA20 and [14C]GA5 were fed to barley stems to clarify which steps leading to bioactive GA1 and GA3 were affected by decapitation and subsequent IAA application. After feeds of [14C]GA20 to the p-1 internode of intact, decapitated, and decapitated plants treated with IAA, HPLC chromatograms contained peaks corresponding to GA29, as shown by GC-MS, and the un-metabolized GA20. Further large peaks eluted in fractions 12 to 14 and 41 to 42 (data not shown). There was a peak eluting near, but slightly earlier than, GA1 (data not shown), but GC-MS analysis of the fractions from the authentic GA1 zone confirmed that this peak was not GA1. Figure 4 shows GC-MS traces confirming the presence of GA1 in both the intact and IAA-treated cases and its virtual absence in the decapitated case. The other peak in the GC-MS traces (mass-to-charge ratio 508) corresponds to 1 ng of [2H2] GA29 added as an “internal standard” after HPLC. The other unknown peaks were also analyzed by GC-MS. The substance eluting close to GA1 was not epi-GA1. Furthermore, GA29-catabolite, GA5, and GA6, were eliminated as candidates for the unidentified peak eluting in fractions 41 and 42. Similarly, the peak in fraction 12 to 14 was found not to correspond to either GA8 or epi-GA8.
Figure 4.
Effects of decapitation and IAA-application on the metabolism of [14C]GA20 in the p-1 internode of barley. The substrate was injected into the p-1 internode and that internode was harvested after 48 h. The GA1 HPLC zone was grouped and 1 ng of methylated [2H2]GA29 was added before further derivatization. Shown are the resulting GC-MS (selected ion monitoring) mass chromatograms corresponding to unlabeled GA1 (506), 14C-labeled GA1 (508), and [2H2] GA29 (508). The peak areas are expressed as a percentage of the most abundant peak.
[14C]GA20 was also fed to pseudostem segments from 2-week-old seedlings (two leaves expanded). Excised pseudostem segments consisted of blade and sheath and were floated in Murashige and Skoog medium with or without IAA. Harvests were made after incubation periods of 6, 12, and 24 h. Metabolites were separated by HPLC as methyl esters. Figure 5 shows HPLC chromatograms and corresponding GC-MS traces for tissue harvested at 6 h. Aliquots from the GA29 and GA1 zones, containing radioactive peaks, were combined and subjected to selected ion monitoring GC-MS. At all time points, the ratio of GA1 to GA29 was greater in the IAA-treated case.
Figure 5.
A, Effects of IAA on [14C]GA20 metabolism in excised pseudostem segments of 2-week-old barley. Excised pseudostem segments consisted of blade and sheath and were floated for 6 h in Murashige and Skoog media with or without IAA. B, Metabolite identity was confirmed by GC-MS selected ion monitoring. Equal aliquots from the GA29 and GA1 zones, containing radioactive peaks, were combined and should trimethylsilylated. Shown are the resulting chromatograms corresponding to unlabeled GA29 and GA1 (506) and 14C-labeled GA29 and GA1 (508). The peak areas are expressed as a percentage of the most abundant peak.
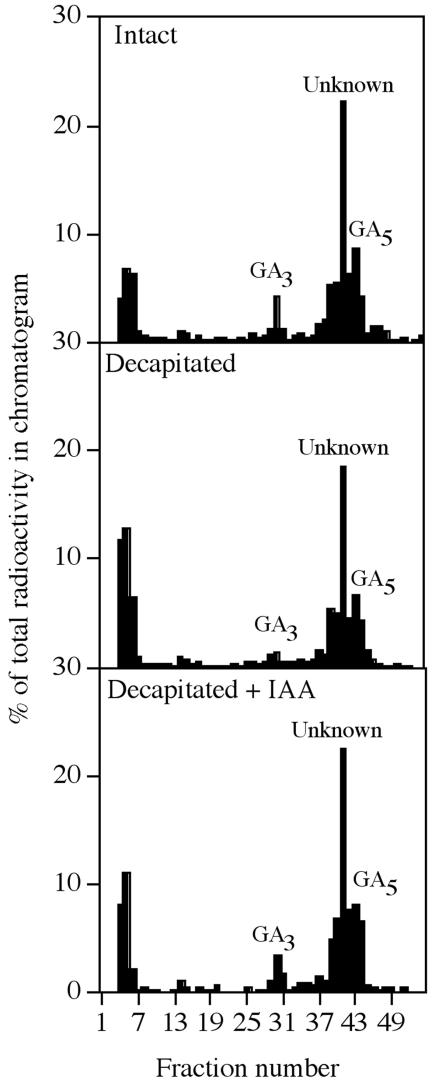
The HPLC chromatograms after feeds of [14C]GA5 showed four major radioactive peaks corresponding to GA3, GA5, and two unknown peaks (Fig. 6). [14C]GA5 was converted to [14C]GA3 in the intact and IAA-treated case but to a lesser extent in the decapitated case (Fig. 6). Analysis by GC-MS confirmed that decapitation reduced the conversion of [14C]GA5 to [14C]GA3.
Figure 6.
Effects of IAA on the metabolism of [14C]GA5 in the p-1 internode of intact, decapitated, and decapitated, IAA-treated barley stems. The substrate was injected into the p-1 internode and the p-1 node, and this unit was harvested 48 h later. Metabolites were separated by HPLC as the methyl esters. The retention times of GA5 and GA3 are shown. One-minute fractions were collected.
Effects of Decapitation and IAA Application on the mRNA Levels of Hv3ox2
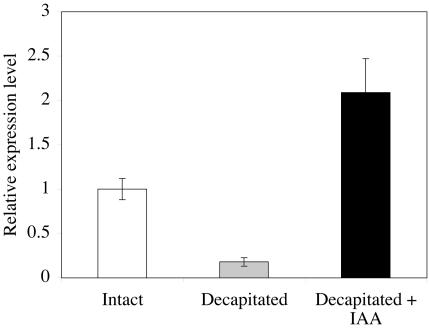
To examine the molecular basis of auxin effects on the GA biosynthetic pathway, we monitored the mRNA levels of the Hv3ox2 gene. This gene encodes the main enzyme for the steps GA20 to GA1 and GA5 to GA3 (data not shown). Barley 3ox2 forward and reverse primers produced a single product of the expected size of 139 bp when completed SYBR Green PCR reactions were analyzed by agarose gel electrophoresis. Sequencing of the PCR product further confirmed that the correct target sequence was being amplified (data not shown). The results demonstrated a decrease in the amount of 3ox2 mRNA in decapitated plants and a restoration by IAA (Fig. 7).
Figure 7.
Effects of decapitation and IAA application on 3ox2 mRNA levels in the p node of barley, monitored by quantitative PCR. Shown are the relative expression levels of 3ox2 mRNA in intact, decapitated, and decapitated plants treated with IAA; the level in intact plants is assigned the value 1. Data are the mean levels ± se (n = 2).
DISCUSSION
Removal of the developing inflorescence of barley and oat reduced the IAA content of the stem. This appears to be the first direct evidence, to our knowledge, that the inflorescence acts as a source of IAA for the stems of these agronomically important species. The high level of IAA in the inflorescence is consistent with that evidence.
In barley and oat, the levels of the bioactive GAs, GA1 and GA3, in stems were also reduced by inflorescence excision. However, exogenous IAA was able to restore the content of these GAs to at least the levels found in intact plants. Obtaining this result in oats allows a direct comparison with the findings of Koning et al. (1977) and Kaufman et al. (1976). Koning et al. (1977) suggested that GA3 is produced in the inflorescence and transported into the stem, and Kaufman et al. (1976) suggested that GA3 is the predominant GA in the inflorescence, nodes, and p-1 internode. Our results indicate that the transported hormone is not a GA but rather the auxin IAA. The stem itself can synthesize bioactive GAs, provided that auxin is present. We have also found, in contrast to Kaufman et al. (1976), that GA1, not GA3, is the major bioactive GA in oat stems.
The importance of GA1 and GA3 for stem growth in barley is demonstrated by the effects of the mutation to the Grd2 locus, which specifically reduces the content of these GAs and, as a consequence, stem elongation (Chandler and Robertson, 1999). The effect of decapitation on GA1 levels was typically as least as great as that of the grd2-463 mutant allele (Table III). Thus, in intact plants, bioactive GAs produced in response to auxin from the inflorescence would be expected to play a role in internode extension.
A range of other GAs was also quantified in barley stems, including GA19, GA20, and GA8 (Fig. 2). There was no evidence from the levels of these GAs that the effect of decapitation on GA1 and/or GA3 content was primarily because of decreased synthesis of GA20 or increased deactivation of GA1. Therefore, we investigated the effects of decapitation and auxin application on the steps immediately after GA20.
Metabolism studies with [14C]GA20 revealed that IAA promotes the 3-oxidation of GA20 to GA1, as in pea (Ross et al., 2000). The observation that endogenous GA29 levels were generally elevated in decapitated plants, an effect reversed by IAA application (Fig. 2), indicates that the 2-oxidation of GA20 to GA29 is inhibited by auxin, again as in pea (Ross et al., 2000). Furthermore, decapitation reduced and IAA application restored the conversion of [14C]GA5 to [14C]GA3 in stems (Fig. 6). The results of metabolism of [14C]GA20 in pseudostem segments, consisting of leaf sheath and blade, suggests that IAA plays an important role in GA biosynthesis and, therefore, elongation within the leaf as well as the stem (Fig. 5).
Quantitative PCR demonstrated the relationship between IAA content and GA 3-oxidase (Hv3ox2) mRNA levels in barley stems. mRNA levels of Hv3ox2 were reduced in decapitated plants, compared with intact plants, whereas IAA application reversed this effect (Fig. 7).
The present results extend the effects of auxin to the pathway GA20 to GA5 to GA3, an important branch commonly found in grasses. The metabolism of GA5 to GA3 has been observed in rice (Oryza sativa; Kobayashi et al., 1991) and in maize (Zea mays; Fujioka et al., 1990). Itoh et al. (2001) and Spray et al. (1996) suggested that a multifunctional enzyme is responsible for the steps GA20 to GA1, GA20 to GA5, and GA5 to GA3 in rice and maize, respectively. It seems probable that auxin plays a major role in regulating this multifunctional enzyme in grasses.
It appears, therefore, that IAA is required for the normal biosynthesis of active GAs in stems of barley and oats. The inflorescence is a major contributor to the auxin pool of elongating stems, in which the bioactive GAs GA1 and GA3 are produced. In this way, the inflorescence potentially plays a critical role in controlling the levels of key GAs in the stem and, therefore, stem elongation. Furthermore, IAA appears to be needed for GA biosynthesis in barley leaves. Our results provide the first evidence, to our knowledge, that the auxin-GA relationship recently discovered in pea and tobacco also exists in monocotyledonous plants, despite their radically different growth habit. This finding indicates the evolutionary antiquity of the auxin-GA interaction.
MATERIALS AND METHODS
Plant Material and Growing Conditions
Barley (Hordeum vulgare var. Himalaya), a GA 3oxidase mutant of this line (grd2-463), and oat (Avena sativa) seeds were sown in 10-cm-diameter pots, and plants were grown at a density of one plant per pot. The growing medium was a 6:4 (w/v) mixture of composted pine bark:sand (pH 6). Plants were watered daily, and a complete nutrient solution containing N:P:K at 23:4:13 (v/v) was provided weekly. Plants were grown in a heated greenhouse with a photoperiod of 18 h obtained by extending the natural light as described previously (Beveridge and Murfet, 1996). The daytime temperature was typically 23°C to 28°C.
Measurements
The rapidly expanding p-1 internode of barley was divided into three segments. A small window in the sheath was removed, and a correction pen was used to mark the lowermost and the uppermost 20 mm of the internode. Sections were measured until fully expanded. The final lengths of the p-1 and p-2 internodes were recorded for both the wild type and the grd2-463 mutant in a separate experiment.
Treatments and Application of Chemicals
Plants were either left intact or decapitated. Decapitation involved excising the developing inflorescence (which at this stage was enclosed within the sheath of the flag leaf) at the top of the peduncular internode using a razor blade (Fig. 1). One-half of the decapitated plants were treated with IAA in hydrous lanolin (3 mg g–1); the remainder received lanolin alone. The auxin/lanolin or lanolin paste was re-applied 8, 20, 32, and 44 h after decapitation; on each occasion, the previous lanolin was removed.
Plant portions harvested for hormone metabolism and quantification studies included the p-1 internode and, in some cases, the nodes on either side (p and p-1 nodes). Sheath material was removed from these portions. Nodal segments included the node itself and approximately 10 mm of internodal tissue on either side. The p-1 internode was young and still actively elongating. At the time of experimentation, plants were approximately 5 to 6 weeks old, and the p-1 internode was 70 to 100 mm in length. The potential final length of this internode was greater than 200 mm in our conditions. Two replicates were harvested for each type of segment and treatment. Number of plants per replicate varied from three to 10.
For metabolism studies, [14C]GA5 or [14C]GA20 (Prof. Lewis N. Mander, Australian National University, Canberra) was injected into the p-1 internode in 10 μL of 1:1 (v/v) distilled water:methanol solution at a rate of 10,000 dpm plant–1. In [14C]GA5 experiments, substrate was also injected into the p-1 node. Substrate was injected concurrently with decapitation and initial auxin treatment. Tissue was harvested 48 h after injection and immersed in cold (–20°C) 80% (v/v) methanol containing butylated hydroxyltoluene.
We also studied the metabolism of [14C]GA20 by pseudostem segments from 2-week-old seedlings (two leaves expanded). The expanded leaf blades were excised at the ligules, and the pseudostem consisted of sheath and immature leaves encased within. Five pseudostem segments were placed in each petri dish, and segments were approximately 6 to 7 cm long. Segments were incubated at 24°C in 6-cm petri dishes containing 10 mL of sterile liquid Murashige and Skoog medium, with or without added IAA (5 μg mL–1). Substrate was added at a rate of 500,000 dpm per petri dish. Segments were harvested at three time points: 6, 12, and 24 h after the beginning of the incubation period. Tissue was removed from the incubation medium, washed with distilled water, and placed in cold (–20°C) 80% (v/v) methanol containing butylated hydroxyltoluene.
Hormone Extraction and Purification
For the extraction of GAs and auxins, tissue was homogenized, and the extracts were held at 4°C for 24 h, before filtering (Whatman no. 1, Whatman, Clifton, NJ). Endogenous levels of GA1, GA19, GA20, GA3, GA5, GA29, and GA8 were quantified using deuterated internal standards as described previously (Lawrence et al., 1992). IAA was quantified using [13C6] IAA as an internal standard (Cohen et al., 1986), 4Cl-IAA using [2H4] 4Cl-IAA, and IBA using [13C1] IBA (synthesized by Cornelius Moorhoff, University of Tasmania, Australia). Sep-Pak C18 cartridges were used to purify all samples, as before (Ross et al., 1995), and extracts were then dried for HPLC.
HPLC
For quantification of endogenous GAs samples were subjected to HPLC as free acids (Ross, 1998). The solvent program ran from 20% to 75% (v/v) methanol in 0.4% (v/v) acetic acid over 25 min with a linear gradient, followed by an isocratic (75% [v/v]) elution. The flow rate was 2 mL min–1, and 1-min fractions were collected. Fractions were grouped according to the retention time of a tritiated GA20 tracer, dried, methylated, and then partitioned against ether as described previously (Wolbang and Ross, 2001; with the exception of the zone containing GA29 and GA8). For quantification of endogenous GA3, samples were subjected to HPLC as methyl esters to separate GA3 from interfering impurities.
[14C]GA5 and [14C]GA20 metabolites were subjected to HPLC radiocounting as methyl esters. The solvent program ran from 30% to 60% (v/v) methanol in distilled water during 35 min, using an exponential program, followed by an isocratic (60% [v/v]) elution. The flow rate was 1.6 mL min–1, and 1-min fractions were collected and assayed for radioactivity as before (Ross et al., 1995). In some cases, aliquots were taken for radiocounting and the remaining portion dried for identification by GC-MS.
GC-MS
GA and auxin samples were derivatized for GC-MS by first adding 10 μL of pyridine and 40 μL of bis-trimethylsilyltrifluoroacetamide with 1% (v/v) trimethylchlorosilane, followed by heating at 80°C for 20 min. Samples were then dried, and a further 15 μL of bis-trimethylsilyltrifluoroacetamide with 1% (v/v) trimethylchlorosilane was added, followed by heating at 80°C for 15 min. GC-MS was performed as described previously (Ross, 1998). In the case of the [14C]GA20 feeding experiment where the substrate was injected into the stem, GC-MS was carried out on the grouped GA1 zone, and 1 ng of [2H2]GA29 was added as an internal standard. In the case of the [14C]GA20 feeding to pseudostem segments in Murashige and Skoog medium, the GA1 and the GA29 zones were grouped for GC-MS. In the case of the [14C]GA5 feeding, the GA3 zone was grouped, and 1 ng of [2H2]GA1 was added as an internal standard before GC-MS analysis.
Quantitative PCR
The nodal portion was harvested from intact, decapitated, and decapitated IAA-treated plants. Samples were ground into a powder with a mortar and pestle in liquid N2 and approximately 100 mg (fresh weight) of tissue was used for RNA extraction. Total RNA was extracted using the Qiagen RNA easy kit (Qiagen, Hilden, Germany) with an on column DNase digestion. The RNA was eluted in 50 μL of RNase-free water. The RNA concentration was determined by measuring the absorption at 260 nm (A260). Reverse transcription was carried out on 5 μg of RNA, before quantitative PCR, using first strand cDNA synthesis (Invitrogen, Carlsbad, CA) using SuperScript III and 250 ng of random hexamers.
For Hv3ox2 quantification, primers were designed by Primer3 (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi), to flank an intron. The following primers were used to produce a 139-bp amplicon: forward primer, 5′-TCCTCCTTCTTCTCCAAGTG-3′; and reverse primer, 5′-TGTGGAACTCCTCCATCAC-3′. Primers and probe for the 18S amplicon were as designed by Ozga et al. (2003): forward primer, 5′-ACGTCCCTGCCCTTTGTACA-3′; reverse primer 5′-CACTTCACCGGACCATTCAAT-3′; and Taq-Man fluorescent dye-labeled probe (Biosearch Technologies, Novato, CA), 5′-FAM-ACCGCCCGTCGCTCCTACCG-BHQ-3′. All primers were acquired from Geneworks (Adelaide, SA, Australia).
For quantification of Hv3ox2, the QuantiTect SYBR Green PCR kit (Qiagen) was used to make up a 20-μL reaction for a Rotorgene 2000 (Corbett Research, Mortlake, NSW, Australia). The PCR mixture consisted of 10 μL of 2× Qiagen QuantiTect SYBR Green PCR Master Mix and 400 nm of both the forward and reverse primer. The template was cDNA generated from 50 ng of total RNA as the starting material. Thermal cycling conditions for SYBR Green PCR were 95°C for 15 min and 60 cycles of 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s. The SYBR Green PCR product was then sequenced to confirm that the correct fragment was being amplified.
18S was selected to correct for variations in input template quantity. For the quantification of 18S, the QuantiTect Probe PCR Kit (Qiagen) was used to make up a 20-μL reaction. The PCR mixture consisted of 10 μL of Taq-Man Probe PCR Mastermix with HotStarTaq DNA polymerase, 400 nm of both the forward and the reverse primer, and 0.1 to 0.2 μm probe. The template was cDNA generated from 50 ng of total RNA as the starting material. Thermal cycling conditions were 95°C for 15 min followed by 45 cycles of 94°C for 15 s and 60°C for 60 s.
Standard curves were generated for both Hv3ox2 and 18S. A standard curve was generated for Hv3ox2 from a plasmid carrying the complete cDNA of Hv3ox2 of known quantity. A standard curve for 18S was generated from one of the samples (from intact nodal tissue).
Acknowledgments
We thank Dr. Noel Davies (Central Science Laboratory, University of Tasmania, Australia) and Tracey Jackson, Ian Cummings, and Professor Lewis Mander (Australian National University, Canberra) for labeled GAs.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.030460.
This work was supported by the University of Tasmania Institutional Research Grants Scheme.
References
- Barker-Bridgers M, Ribnicky DM, Cohen JD, Jones AM (1998) Red light regulated growth: changes in the abundance of indoleacetic acid in the maize (Zea mays L.) mesocotyl. Planta 204: 207–211 [Google Scholar]
- Beveridge CA, Murfet IC (1996) The gigas mutant of pea is deficient in the floral stimulus. Physiol Plant 96: 637–645 [Google Scholar]
- Brian PW, Hemming HG (1958) Complementary action of gibberellic acid and auxin in pea internode extension. Ann Bot 22: 1–17 [Google Scholar]
- Chandler PM, Robertson M (1999) Gibberellin dose-response curves and the characterisation of dwarf mutants of barley. Plant Physiol 120: 623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Baldi BG, Slovin J (1986) 13C6-[Benzene ring]-indole-3-acetic acid. Plant Physiol 80: 14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker SJ, Hedden P, Lenton JR, Stoddart JL (1990) Comparison of gibberellins in normal and slender barley seedlings. Plant Physiol 94: 194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Gaskin P, MacMillan J, Phinney BO, Takahashi N (1988a) Qualitative and quantitative analyses of gibberellins in vegetative shoots of normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 seedlings of Zea mays L. Plant Physiol 88: 1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Katsumi M, Phinney BO, Gaskin P, MacMillan J, Takahashi N (1988b) The dominant non-gibberellin-responding dwarf mutant (D8) of maize accumulates native gibberellins. Proc Natl Acad Sci USA 85: 9031–9035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Phinney BO, Gaskin P, MacMillan J, Takahashi N (1990) Gibberellin A3 is biosynthesised from gibberellin A20 via gibberellin A5 in shoots of Zea mays L. Plant Physiol 94: 127–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K, Iino M (1998) Auxin-growth relationships in maize coleoptiles and pea internodes and control by auxin of the tissue sensitivity to auxin. Plant Physiol 117: 1473–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sentoku N, Kitano H, Matsuoka M, Kobayashi M (2001) Cloning and functional analysis of two gibberellin 3β-hydroxylase genes that are differently expressed during the growth of rice. Proc Natl Acad Sci USA 98: 8909–8914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PB, Ghosheh NS, Nakosteen L (1976) Analysis of native gibberellin in the internode, node, leaves and inflorescence of developing Avena plants. Plant Physiol 58: 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Sakurai A, Saka H, Takahashi N (1991) Quantitative analysis of endogenous gibberellins in normal and dwarf cultivars of rice. Plant Cell Physiol 30: 963–969 [Google Scholar]
- Kobayashi M, Spray CR, Phinney BO, Gaskin P, MacMillan J (1996) Gibberellin metabolism in maize. The stepwise conversion of gibberellin A12-aldehyde to gibberellin A20. Plant Physiol 110: 413–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning R, Tkaczyk A, Kaufman PB, Pharis RP, Morf W (1977) Regulation of internodal extension in Avena shoots by the inflorescence, nodes, leaves, and intercalary meristem. Physiol Plant 40: 119–124 [Google Scholar]
- Koshiba T, Kamiya Y, Iino M (1995) Biosynthesis of indole-3-acetic acid from l-tryptophan in coleoptile tips of maize (Zea mays L.) Plant Cell Physiol 36: 1503–1510 [Google Scholar]
- Kurashi S, Muir RM (1962) Increase in diffusible auxin after treatment with gibberellin. Science 137: 760–761 [DOI] [PubMed] [Google Scholar]
- Law DM, Davies PJ (1990) Comparative indole-3-acetic acid levels in the slender pea and other pea phenotypes. Plant Physiol 93: 1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NL, Ross JJ, Mander LN, Reid JB (1992) Internode length in Pisum: mutants lk, lka, and lkb do not accumulate gibberellins. J Plant Growth Regul 11: 35–37 [Google Scholar]
- Ozga JA, Yu j, Reinecke DM (2003) Pollination-, development-, and auxin-specific regulation of gibberellin 3β-hydroxylase gene expression in pea fruit and seed. Plant Physiol 131: 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A, Firn RD, Digby J (1988) The role of the coleoptile apex in controlling organ elongation: II. Effects of auxin substitution and auxin transport inhibitors on decapitated coleoptiles. J Exp Bot 39: 1343–1354 [Google Scholar]
- Phinney BO, Spray CR, Suzuki Y, Gaskin P (1991) Gibberellin metabolism in maize: tissue specificity. In N Takahashi, BO Phinney, J MacMillan, eds, Gibberellins. Springer-Verlag, New York, pp 22–31
- Ross JJ (1998) Effects of auxin transport inhibitors on gibberellins in pea. J Plant Growth Regul 17: 141–146 [Google Scholar]
- Ross JJ, O'Neill DP, Smith JJ, Kerckhoffs LHJ, Elliot RC (2000) Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J 21: 547–552 [DOI] [PubMed] [Google Scholar]
- Ross JJ, Reid JB, Swain SM, Hasan O, Poole AT, Hedden P, Willis CL (1995) Genetic regulation of gibberellin deactivation in Pisum. Plant J 7: 513–523 [Google Scholar]
- Spray CR, Kobayashi M, Suzuki Y, Phinney BO, Gaskin P, MacMillan J (1996) The dwarf-1 (d1) mutant of Zea mays blocks three steps in the gibberellin-biosynthetic pathway. Proc Natl Acad Sci USA 93: 10515–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huizen R, Ozga A, Reinecke DM (1997) Seed and hormonal regulation of gibberellin 20-oxidase expression in pea pericarp. Plant Physiol 115: 123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler EW, Jourdan PS, Conrad W (1981) levels of indole-3-actetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta 153: 561–571 [DOI] [PubMed] [Google Scholar]
- Wolbang CM, Ross JJ (2001) Auxin promotes gibberellin biosynthesis in decapitated tobacco plants. Planta 214: 153–157 [DOI] [PubMed] [Google Scholar]