Abstract
Lipoic acid-dependent pathways of α-keto acid oxidation by mitochondria were investigated in pea (Pisum sativum), rice (Oryza sativa), and Arabidopsis. Proteins containing covalently bound lipoic acid were identified on isoelectric focusing/sodium dodecyl sulfate-polyacrylamide gel electrophoresis separations of mitochondrial proteins by the use of antibodies raised to this cofactor. All these proteins were identified by tandem mass spectrometry. Lipoic acid-containing acyltransferases from pyruvate dehydrogenase complex and α-ketoglutarate dehydrogenase complex were identified from all three species. In addition, acyltransferases from the branched-chain dehydrogenase complex were identified in both Arabidopsis and rice mitochondria. The substrate-dependent reduction of NAD+ was analyzed by spectrophotometry using specific α-keto acids. Pyruvate- and α-ketoglutarate-dependent reactions were measured in all three species. Activity of the branched-chain dehydrogenase complex was only measurable in Arabidopsis mitochondria using substrates that represented the α-keto acids derived by deamination of branched-chain amino acids (Val [valine], leucine, and isoleucine). The rate of branched-chain amino acid- and α-keto acid-dependent oxygen consumption by intact Arabidopsis mitochondria was highest with Val and the Val-derived α-keto acid, α-ketoisovaleric acid. Sequencing of peptides derived from trypsination of Arabidopsis mitochondrial proteins revealed the presence of many of the enzymes required for the oxidation of all three branched-chain amino acids. The potential role of branched-chain amino acid catabolism as an oxidative phosphorylation energy source or as a detoxification pathway during plant stress is discussed.
The enzymatic decarboxylation of α-keto organic acids in cells is often facilitated by the cofactor thiamine pyrophosphate (TPP), which acts as a strong nucleophile to attack the carbonyl carbon of α-keto acids. The remaining aldehyde can be simply protonated; for example, during acetaldehyde formation by pyruvate decarboxylase (EC 4.1.1.1) or, alternatively, the energy released by the decarboxylation can be coupled to the generation of NADH. The α-keto acid decarboxylating dehydrogenase complexes encapsulate this alternative reaction series. These complex structures use E1 (α-keto acid dehydrogenase), E2 (acyltransferase), and E3 (lipoamide dehydrogenase) enzymes and five cofactors (TPP, CoA, lipoic acid, FAD+, and NAD+) in their catalytic cycle (Reed, 1974; Mooney et al., 2002). Active-site coupling is used to catalyze decarboxylation of α-keto acids (E1 enzyme), esterification of aldehydes to CoA (E2 enzyme), and reduction of NAD+ to NADH (E3 enzyme; Reed, 1981; Perham et al., 2002). The most widely studied α-keto acid enzyme complexes are the pyruvate dehydrogenase complex (PDC) that regulates the entry of carbon into the tricarboxylic acid (TCA) cycle by the provision of acetyl-CoA and the α-ketoglutarate dehydrogenase complex (KGDC) that acts within the TCA cycle to synthesize succinyl-CoA. Both of these enzyme complexes contain lipoic acid covalently bound to Lys residues of E2 enzymes. These lipoate moieties facilitate active site coupling between E1 and E3 enzymes by providing a means of acyl group movement for esterification to CoA and a mechanism of electron transfer to synthesize NADH (Reed, 1974; Perham et al., 2002). The further metabolism of these CoA esters has been investigated extensively through the reactions of the TCA cycle in mitochondria from many eukaryotes. The branched-chain keto-acid dehydrogenase complex (BCKDC) operates in a very similar fashion to the pyruvate and α-ketoglutarate complexes. It contains lipoic acid bound to its E2 enzyme and appears to share the same E3 enzymes as the other lipoic acid-containing α-keto acid complexes (Yeaman, 1989; Lutziger and Oliver, 2001). This BCKDC is involved as an early step in the complex catabolism of the branched-chain amino acids, Val, Ile, and Leu (Yeaman, 1989; Diebold et al., 2002).
In mammals, pyruvate and KGDCs are found exclusively in the mitochondrial matrix. In plants, their distribution is more complicated. Mitochondria contain pyruvate and α-ketoglutarate complexes (Mooney et al., 2002), but the plastid also contains a distinct pyruvate dehydrogenase involved in acetyl-CoA provision for lipid synthesis (Johnston et al., 1997). The ambiguity surrounding the site of branched-chain amino acid metabolism in plants has been part of the controversy surrounding the site of β-oxidation of fatty acids in plants. In mammals, β-oxidation, the successive removal of C-2 units from fatty acids and certain branched-chain α-keto acids, occurs in mitochondria where the branched-chain dehydrogenase complex is also located (Yeaman, 1989). However, in plants, the subcellular localization of β-oxidation is controversial. Over the past 3 decades, biochemical evidence has been presented to place both straight-chain fatty acid and branched-chain α-keto acid oxidation in the peroxisomes of plants (Gerhardt, 1992), whereas more controversial evidence also has been presented for both processes to occur in mitochondria (Dieuaide et al., 1993; Masterson and Wood, 2001). Molecular and genetic evidence over the last 10 years increasingly has placed straight-chain fatty acid β-oxidation in peroxisomes (Germain et al., 2001; Graham and Eastmond, 2002), whereas the case for branched-chain α-keto acid oxidation in mitochondria has been strengthened considerably (Fujiki et al., 2000; Daschner et al., 2001; Diebold et al., 2002).
In the branched-chain α-keto acid catabolism pathway, the three branched-chain amino acids, Val, Leu, and Ile, are initially transaminated to their respective branched-chain α-keto acid by the branched-chain amino acid transaminase (BCAT; EC 2.6.1.42). This reversible reaction is also the final step of the biosynthesis of these amino acids. Plants contain a small family of BCAT genes. Green fluorescent protein (GFP)-targeting studies in Arabidopsis show that some BCATs are chloroplastic, and at least one is localized in mitochondria (Diebold et al., 2002). After the transamination step, the α-keto acids are decarboxylated and esterified to CoA by the BCKDC. Early reports have suggested that BCKDC activity was localized to the peroxisomes (Gerhardt, 1992). However, when genes for the plant BCKDC were sequenced, each was found to have N-terminal extensions used for mitochondrial targeting (Fujiki et al., 2000). Despite these studies, to date, no direct evidence for a mitochondrial localization of the proteins or their activity has been presented for plants.
The CoA esters generated by BCKDC are then oxidized by an acyl-CoA dehydrogenase delivering electrons to the electron transfer flavoprotein (ETF) that directly donates electrons into the respiratory chain at ubiquinone. An Arabidopsis mitochondrial acyl-CoA dehydrogenase (isolvaleryl-CoA dehydrogenase [IVD]; EC 1.3.99.10) with activity toward both isovaleryl-CoA (from Leu) and isobutyryl-CoA (from Val) has been identified using gene cloning, in vivo targeting studies, and biochemical analysis in mitochondria (Daschner et al., 2001). After this step, the three pathways diverge to a series of separate reactions leading to propionyl-CoA in the case of Val metabolism, propionyl-CoA, and acetyl-CoA in the case of Ile metabolism and to acetyl-CoA and acetoacetate in the case of Leu metabolism (Graham and Eastmond, 2002). In the Leu pathway, the next enzyme after IVD is 3-methlycrotonyl-CoA carboxylase (MCCase; EC 6.4.1.4). This biotin-containing enzyme has been studied in potato (Solanum tuberosum) and soybean (Glycine max; Aubert et al., 1996; Anderson et al., 1998). In isolated pea (Pisum sativum) mitochondria, 14C-Leu tracer experiments have chased radiolabel to acetoacetate and acetyl-CoA, confirming the presence of the whole-Leu catabolism pathway in plant mitochondria (Anderson et al., 1998).
In this report, we present an analysis of the lipoic acid-containing proteins in mitochondria isolated from pea, rice (Oryza sativa), and Arabidopsis. We show direct experimental evidence that the E2 subunits of α-keto acid dehydrogenase complexes are localized in mitochondria. We also show the presence of BCKDC in Arabidopsis and rice mitochondria but were unable to find evidence for this protein in pea mitochondria. The activity of BCKDC in Arabidopsis mitochondria was readily detected spectrophotometrically. Branched-chain amino acids and some branched-chain α-keto acids stimulated mitochondrial O2 consumption, suggesting that at least part of the branched-chain amino acid oxidation pathway was functional. Analysis of the mitochondrial proteome by liquid chromatography (LC)-tandem mass spectrometry (MS/MS) provided direct evidence for enzyme components of the Val, Leu, and Ile pathways and identified members of gene families that putatively encode mitochondrial enzymes in Arabidopsis.
RESULTS
Identification of Lipoic Acid-Containing Proteins in Plant Mitochondria
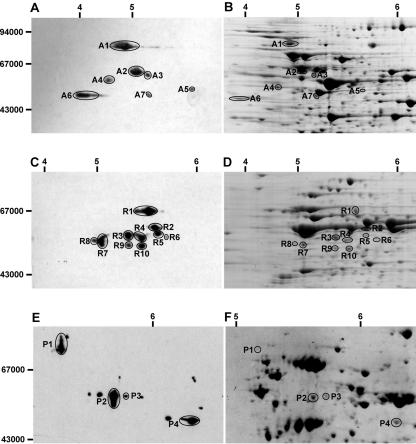
A number of the putative lipoyl-containing proteins predicted from plant genome sequencing have very similar molecular masses. As a consequence, their presence could not be differentiated readily by one-dimensional PAGE using antibodies raised against lipoic acid (data not shown). However, after two-dimensional isoelectric focusing (IEF)/SDS-PAGE, a large series of protein spots immunoreactive to antilipoic acid antibodies were detected in mitochondrial samples from pea, rice, and Arabidopsis (Fig. 1). These proteins were excised from gels and identified by MS/MS (Table I).
Figure 1.
Identification of proteins that react with antilipoic acid antibodies in Arabidopsis, rice, and pea mitochondria. A, Western blot of two-dimensional IEF/SDS-PAGE separation of Arabidopsis mitochondrial proteins probed with antilipoic acid antibodies. B, Two-dimensional IEF/SDS-PAGE separation of Arabidopsis mitochondrial proteins stained with colloidal Coomassie solution. C, Western blot of two-dimensional IEF/SDS-PAGE separation of rice mitochondrial proteins probed with antilipoic acid antibodies. D, Two-dimensional IEF/SDS-PAGE separation of rice mitochondrial proteins stained with colloidal Coomassie solution. E, Western blot of two-dimensional IEF/SDS-PAGE separation of pea mitochondrial proteins probed with antilipoic acid antibodies. F, Two-dimensional IEF/SDS-PAGE separation of pea mitochondrial proteins stained with colloidal Coomassie solution. Numbers on left of A, C, and E represent the apparent molecular masses. Numbers above A to F represent pI of separated protein spots.
Table I.
Identification of lipoic acid-containing proteins in plant mitochondria
Protein spots from Figure 1 were identified by MS/MS Spot no., accession no. of matching gene, description of matching gene, and the apparent molecular masses of the match and the experimental proteins are shown. The quality of the MS match is shown by the MOWSE score from Mascot matching (typically >40 is P < 0.05), percentage coverage of the match protein sequence by the peptides identified and the number of matching peptides (MP). Searches were performed with MS tolerance of 1.2 D and MS/MS tolerance of 0.6 D. Arabidopsis and rice proteins were all matched to genes from the same species. Pea peptides were matched with identical peptides in several other species.
| Species | Spot No. | Accession No. | Description | MM Gel | MM Match | MOWSE | Coverage | MP |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| Arabidopsis | A1 | At3g52200 | Pyruvate dehydrogenase E2 subunit | 82 | 69 | 369 | 24 | 15 |
| A2 | At3g13930 | Pyruvate dehydrogenase E2 subunit | 65 | 58 | 358 | 28 | 14 | |
| A3 | At1g54220 | Pyruvate dehydrogenase E2 subunit | 63 | 58 | 636 | 26 | 14 | |
| A4 | At3g17240 | E3 subunit, dihydrolipoamide dehydrogenase | 59 | 54 | 365 | 26 | 12 | |
| A5 | At3g25860 | Pyruvate dehydrogenase E2 subunit | 55 | 50 | 280 | 18 | 7 | |
| A6 | At5g55070 | α-Ketoglutarate dehydrogenase E2 subunit | 52 | 50 | 85 | 8 | 3 | |
| A7 | At3g06850 | Branched-chain acid dehydrogenase E2 subunit | 51 | 53 | 137 | 20 | 10 | |
| Rice | R1 | BAC20732 | Pyruvate dehydrogenase E2 subunit | 68 | 58 | 48 | 3 | 2 |
| R2 | BAB92783 | Branched-chain acid dehydrogenase E2 subunit | 61 | 55 | 707 | 33 | 19 | |
| R3 | BAA90623 | Pyruvate dehydrogenase E2 subunit | 58 | 55 | 328 | 13 | 10 | |
| R4 | BAC20732 | Pyruvate dehydrogenase E2 subunit | 57 | 58 | 309 | 15 | 10 | |
| R5 | 6021 .m00139 | Pyruvate dehydrogenase E2 subunit | 60 | 45 | -a | 16 | 6 | |
| R6 | BAC20732 | Pyruvate dehydrogenase E2 subunit | 57 | 58 | 296 | 17 | 9 | |
| R7 | CAD40552 | α-Ketoglutarate dehydrogenase E2 subunit | 56 | 48 | 156 | 17 | 6 | |
| R8 | CAD40552 | α-Ketoglutarate dehydrogenase E2 subunit | 54 | 48 | 420 | 28 | 15 | |
| R9 | BAC20732 | Pyruvate dehydrogenase E2 subunit | 52 | 58 | 207 | 14 | 6 | |
| R10 | BAC20732 | Pyruvate dehydrogenase E2 subunit | 53 | 58 | 389 | 21 | 13 | |
| Pea | P1 | BAA77024, At3g52200 | Pyruvate dehydrogenase E2 subunit (Lithospermum erythromizon) | 78 | - | 43 | 15 | 4 |
| P2 | BAB02323, At3g13930 | Pyruvate dehydrogenase E2 subunit (Arabidopsis) | 67 | - | 139 | 5 | 3 | |
| P3 | BAB02323, At3g13930 | Pyruvate dehydrogenase E2 subunit (Arabidopsis) | 67 | - | 92 | 5 | 3 | |
| P4 | AW257475, At5g55070 | α-Ketoglutarate dehydrogenase E2 subunit (Medicago truncatula) | 49 | - | 42 | 5 | 1b |
R5 was not present in the search database Mascot but was matched to a predicted rice PDC E2 subunit from genome sequencing program (6021 .m00139) using ProlD software on an in-house database using The Institute for Genomic Research rice genome open reading frames. b De novo sequencing of P4 was required to confirm it was α-ketoglutarate dehydrogenase, three additional de novo sequences matched with 70% to 90% identity to α-ketoglutarate from Arabidopsis (At5g55070).
The complement of prominent lipoic acid proteins in pea mitochondria has been well studied and includes an 80-kD E2 subunit of PDC (Fig. 1, P1), a 50-kD E2 subunit of PDC (Fig. 1, P2/P3), and a 55-kD E2 subunit of KGDC (Fig. 1, P4). In pea, the extra spots on either side of the main identified proteins are probably urea-induced modifications of the major proteins (based on charge separations, molecular mass, and the abundance distributions of the spot string features). We could not identify these addition spots because of their low abundance. The other small protein spots shown but not identified were not able to be located on the paired colloidal Coomassie-stained gel.
In rice, no high-molecular mass lipoyl-containing protein of 70 to 80 kD was present, in agreement with the absence of this two-lipoyl domain subunit in other monocots including maize (Zea mays), wheat (Triticum aestivum), and barley (Hordeum vulgare; Millar et al., 1999b; Thelen et al., 1999). The various 50- to 60-kD proteins detected by the antibodies in rice included three different isoforms of the one-lipoyl domain PDC E2 subunit and a single KGDC E2 subunit. Five of the protein spots in this size range (Fig. 1, R1, R4, R6, R9, and R10) appeared to have been generated from a single PDC E2 subunit gene, suggesting that posttranslational modification of this gene product beyond simple lipoic acid attachment occurs. In Arabidopsis mitochondria, the two-lipoyl domain 80-kD subunit of PDC was readily detected, and mass spectrometry (MS) revealed that it was the product of At3g52200. A number of subunits of approximately 50 kD were also analyzed and found to include two separate, one-lipoyl domain PDC E2 subunits (A2 and A3) and the KGDC E2 subunit (A6). The A5 spot was encoded by At3g25860, putatively a plastid PDC E2. Given the very low abundance of this protein, it seems likely that it may represent plastid contamination (approximately 1.5% of the protein in our Arabidopsis mitochondrial samples is plastidic in origin; Millar et al., 2001; Heazlewood et al., 2004). In both rice and Arabidopsis, one of the immunoreactive 50-kD proteins was identified as the E2 subunit of BCKDC (A7 and R2). This suggested that, in contrast to pea shoot mitochondria, Arabidopsis cell culture mitochondria and rice shoot mitochondria contain appreciable amounts of BCKDC. The apparent absence of BCKDC in pea leaf mitochondria may help to explain previous failures to detect BCKDC activity in this tissue (Mooney et al., 2002) but is in conflict with the radiolabeled substrate tracer data from pea shoot mitochondria (Anderson et al., 1998). Consensus between these three reports may be reached by simply suggesting BCKDC in pea mitochondria is very low in abundance.
BCKDC Activity Detected in Plant Mitochondria
To further investigate the presence of BCKDC in plant mitochondria, we measured α-keto acid-dependent NAD+ reduction by mitochondrial samples from the three species. Samples from all three species exhibited significant rates of pyruvate- and α-ketoglutarate-dependent NAD+ reduction. However, using three substrates for BDKDC commonly used in mammalian mitochondria (α-ketoisovaleric acid, α-ketoisocaproic acid, and α-keto-β-methylvaleric acid), only Arabidopsis mitochondria catalyzed significant rates of NAD+ reduction (Table II). These α-keto-acids are the products of transamination reactions from the branched-chain amino acids, Val, Leu, and Ile, respectively. We also observed that BCKDC activity was inhibited by inclusion of 0.05% (w/v) Triton X-100 in assays (data not shown), suggesting that there is a detergent-labile step in the reaction. In contrast, PDC and KGDC reactions tolerate high Triton X-100 concentrations (Randall et al., 1977; Dry and Wiskich, 1987). Provision of exogenous pig E3 to the activity assay in the presence of Triton X-100 did not recover BCKDC activity, suggesting that the lability was not simply caused by loss of E3. Loss of this subunit can be a problem with assays of E1:E2:E3 complexes from some organs (Poulsen and Wedding, 1970; Millar et al., 1999a). As a consequence, BCKDC activity was measured in freeze-thawed mitochondrial samples in the presence of respiratory inhibitors to prevent NADH oxidation.
Table II.
Spectrophotometric assays of PDC, KGDC, and BCKDC in three species
Data represent the mean ± se of at least three mitochondria preparations and are expressed as nanomoles NADH per minute per milligram of protein.
| Substrate | Pea | Rice | Arabidopsis | |
|---|---|---|---|---|
| PDC | Pyruvate | 67 ± 5.5 | 83 ± 3.1 | 185 ± 12.5 |
| KGDC | α-Ketoglutarate | 40 ± 0.8 | 99 ± 6.7 | 139 ± 9.8 |
| BCKDC | α-Ketoisocaproic acid (Leu) | 0 ± 0.4 | 4.5 ± 1.8 | 25 ± 3.1 |
| α-Keto-β-methylvaleric acid (Ile) | 0 ± 0.4 | 1.6 ± 0.2 | 21 ± 1.0 | |
| α-Ketoisovaleric acid (Val) | 1 ± 0.9 | 0 ± 2.0 | 34 ± 3.2 |
Branched-Chain Amino Acids and Branched α-Keto Acids as Respiratory Substrates for Arabidopsis Mitochondria
Branched-chain amino acid catabolism by Arabidopsis mitochondria was investigated further by measuring O2 consumption in the presence of selected substrates. The TCA cycle substrates pyruvate and α-ketoglutarate both initiated substantial rates of O2 consumption as expected (Table III). Simple addition of other α-keto acids to mitochondria did not trigger any O2 consumption. This could have been because of either the lack of transporters for these compounds or because the lack of a membrane potential in de-energized mitochondria did not allow uptake by available transporter routes. Energization of the membrane by adding a pulse of exogenous NADH (that was rapidly consumed) allowed subsequent assessment of branched-chain α-keto acid-dependent O2 consumption after substrate entry to the mitochondria. Only the α-keto acid derived from Val (α-ketoisovaleric acid) supported oxygen consumption in this assay (Table III). To assess the oxidation pathway from branched-chain amino acids directly, aliquots of Val, Leu, and Ile were added to mitochondria during α-ketoglutarate oxidation by mitochondria. The availability of α-ketoglutarate as an amino acceptor is essential for BCAT transamination of the branched-chain amino acids in the first step of the pathway. We consistently observed a stimulation of α-ketoglutarate-dependent O2 consumption in the presence of Val and Leu (Table III). Together, these results indicate that a low but significant rate of branched-chain amino acid catabolism can occur in Arabidopsis mitochondria.
Table III.
O2 consumption by amino acids and branched-chain acids in Arabidopsis
Data represent the mean ± se of at least three mitochondria preparations and are expressed as nanomoles O2 per minute per milligram of protein.
| Substrate | O2 Consumption Rate |
|---|---|
| Pyruvate | 152 ± 10.0 |
| α-Ketoglutarate | 125 ± 15.7 |
| Leu | 10 ± 3.8 |
| Ile | 2.1 ± 1.0 |
| Val | 17 ± 5.2 |
| α-Ketoisovaleric acid (Leu) | 0.3 ± 2.0 |
| α-Keto-β-methylvaleric acid (Ile) | 0 ± 1.2 |
| α-Ketoisovaleric acid (Val) | 3.4 ± 1.1 |
Elucidating the Genes Responsible for Branched-Chain Amino Acid Metabolism by Proteomic Analysis
The experimental data presented in Tables II and III demonstrate some level of amino acid catabolism in isolated mitochondria, but they do not show that this process continues to completion to yield acetyl-CoA or propionyl-CoA. Convincing evidence that a Leu pathway exists in vivo has been presented previously in 14C tracer experiments (Anderson et al., 1998); however, similar experiments have not been carried out examining the Val or Ile pathways in plant mitochondria. The most pressing issue now is to identify the genes encoding the proteins involved in these pathways in plant mitochondria and to assess the presence of components unique to the Val and Ile catabolism. In total, 13 different enzymes or enzyme complexes are involved in the pathways for Val, Leu, and Ile metabolism (Fig. 2), and sequence similarity searches of the Arabidopsis genome revealed that multigene families exist for most enzymes. A total of 51 Arabidopsis genes encoding proteins with similarity to these enzymes or subunits were found (data not shown). Proteomic analysis of Arabidopsis mitochondria by LC-MS/MS showed that only 11 of these genes encode the enzymes present in mitochondrial preparations from Arabidopsis cell cultures (Table IV). As noted earlier, these Arabidopsis mitochondrial preparations are known to contain a small amount of plastid and peroxisomal contamination (Heazlewood et al., 2004), and the possibility that some of these identifications might result from the presence of abundant proteins from other cellular locations cannot be ruled out.
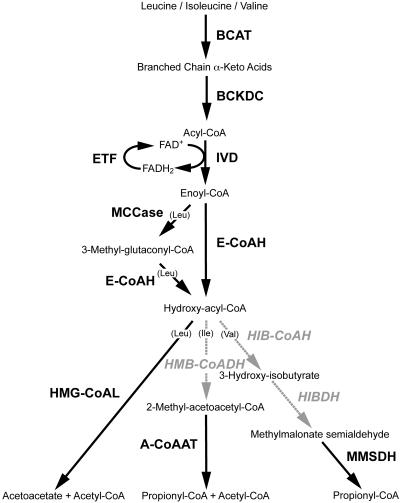
Figure 2.
Arabidopsis mitochondrial localized components involved in the catabolism of branched amino acids. Steps in black involve enzymes that have been localized to Arabidopsis mitochondria and include BCAT, BCKDC, E-CoAH, ETF, hydroxymethylglutaryl-CoA lyase (HMG-CoAL), IVD, acetyl-CoA C-acetyltransferase (A-CoAAT), MCCase, and methylmalonate-semialdehyde dehydrogenase (MMSDH). Steps in gray show enzymes that may be localized elsewhere and include 3-hydroxyisobutyryl-CoA hydrolase (HIB-CoAH), 3-hydroxybutyryl dehydrogenase (HIBDH), and 3-hydroxy-2-methylbutyryl-CoA dehydrogenase (HMB-CoADH). Bracketed amino acids indicate divergent pathways specific to those amino acids.
Table IV.
Identification of Leu, Ile, and Val catabolism enzymes in Arabidopsis mitochondria
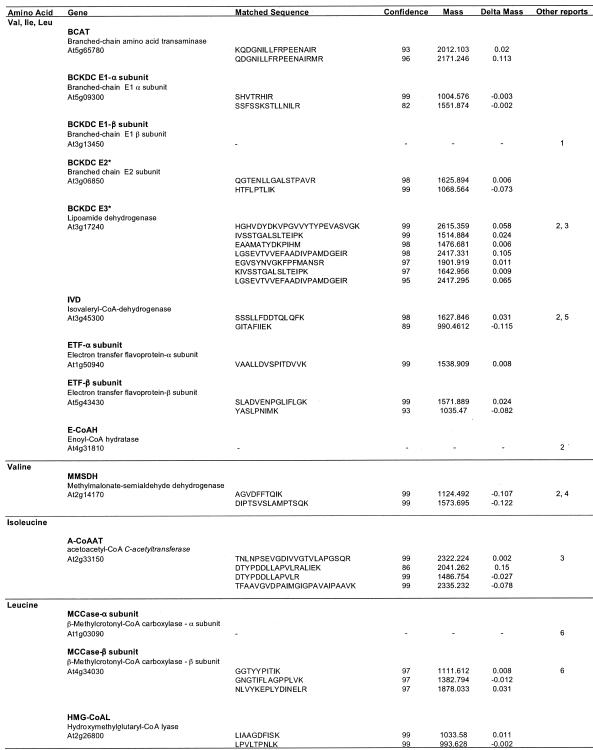
LC-MS/MS analysis of peptides derived from trypsination of total mitochondrial proteins. Gene name and description are shown along with the matched sequence, the ProID score of the match (confidence), the mass of the experimental identified peptides (mass), and the difference between this mass and the matched peptide theoretical mass (delta mass). Other reports of these proteins in mitochondria from Arabidopsis are: 1, Fujiki et al. (2000); 2, Millar et al. (2001); 3, Kruft et al. (2001); 4, Sweetlove et al. (2002); 5, Daschner et al. (2001); and 6, Che et al. (2002).
There are five enzymes common to the initial stages of catabolism of all three branched-chain amino acids: the transaminase (BCAT), BCKDC, IVD, enoyl-CoA hydratase (E-CoAH; EC 4.2.1.18), and the ETF accepting electrons from IVD. Peptide sequences corresponding to each of these proteins were obtained from the cell culture mitochondria (Table IV). We have reported sequences for some of these proteins previously from two-dimensional gel separations of Arabidopsis mitochondria (Millar et al., 2001), and some have been found in mitochondria from other plant species (Miernyk et al., 1991; Daschner et al., 2001), whereas six represent novel identifications of proteins localized to mitochondria. The BCAT identified here as mitochondrial (At5g65780) has been ascribed to the plastid previously, on the basis of GFP tagging of the presequence, and it is possible that plastid contamination of the mitochondrial samples we used for proteomic analysis accounts for our identification of it. However, this protein may represent a genuinely dual-targeted protein localized to both organelles by an ambiguous targeting presequence or, as shown for other dual-localized proteins, alternative transcription start sites (Peeters and Small, 2001). However, the different results may also reflect the different tissue types used or the involvement of the passenger protein (removed during GFP fusion construction) in protein targeting (Chew and Whelan, 2003). Further experimentation is required to resolve the in vivo location of this gene product.
Leu metabolism proceeds by an intermediary step, catalyzed by MCCase, before E-CoAH formation, and we were able to identify the β-subunit of this enzyme in Arabidopsis mitochondria from an IEF/SDS-PAGE-separated gel spot (Table IV). The presence of both the β- and α-subunit for the mitochondrial MCCase in Arabidopsis also has been reported previously by Che et al.(2002).
After E-CoAH formation, the hydroxy-acyl-CoAs branch to form separate catabolic pathways for the three branched-chain amino acids (Fig. 2). A single lyase step splits hydroxylmethylglutaryl-CoA to yield acetoacetate and acetyl-CoA in the final step of Leu catabolism. Notably, a single copy of this enzyme (corresponding to At2g26800) is encoded in Arabidopsis and is predicted to be mitochondrial targeted by TargetP, Predotar, and MitoProtII (data not shown). We identified peptides matching At2g26800 in our LC-MS/MS experiments (Table IV). Although we have no convincing MS evidence for the presence of the next step in Ile metabolism or the next two steps in Val metabolism in mitochondria, we were able to obtain MS matches corresponding to the enzymes catalyzing the final step in each pathway (Table IV).
Val metabolism proceeds by HIB-CoAH (EC 3.1.2.4) and HIBDH (EC 1.1.1.31). The first of these enzymes is probably peroxisomal in Arabidopsis, and mutation of the primary isoform (chy1 mutant) leads to both impaired Val catabolism and fatty acid β-oxidation (Zolman et al., 2001). The HIB-CoAH protein sequences in Arabidopsis all have PTS1-like peroxisomal targeting sequences at the C termini. The localization of HIBDH in Arabidopsis is less clear, and three members of the HIB-CoAH gene family in Arabidopsis encode proteins strongly predicted to be mitochondrial by multiple targeting prediction programs (data not shown), but we were not able to experimentally confirm this localization. We found sequences corresponding to methylmalonate semialdehyde dehydrogenase (EC 1.2.1.27), which catalyzes the final step of Val conversion to propionyl-CoA, in our mitochondrial preparations, confirming previous results (Sweetlove et al., 2002). Propionyl-CoA is subsequently converted to succinyl-CoA, which can then enter the TCA cycle (Graham and Eastmond, 2002).
Ile metabolism proceeds from hydroxy-acyl-CoAs via β-oxidation catalyzed by 3-hydroxy-2-methylbutyryl-CoA dehydrogenase (HMB-CoADH, EC 1.1.1.178), which is incorporated into the fatty acid multifunctional protein. In Arabidopsis, two isoforms of multifunctional protein exist (At3g29010, At3g06860) as well as a shortened form, predicted by sequence similarity to only have HMB-CoADH activity (At3g15290). All three predicted proteins contain PTS1-like C termini strongly suggesting peroxisomal localization. The final step of Ile pathway is catalyzed by acetyl-CoA C-acetyltransferase (EC 2.3.1.9). This ketoacyl-CoA thiolase has PTS2-like domains at the N terminus, also suggesting a possible peroxisomal location, but we found peptides from this protein in mitochondria (Table IV). This enzyme was also identified as a very significant protein in Arabidopsis mitochondrial preparations by Kruft et al. (2001). Thus, this enzyme may also be dual targeted to both organelles, or there may be a mitochondrial-specific isoform among the small ketoacyl-CoA thiolase family. The isoform found is predicted to be mitochondrial by several targeting prediction programs (data not shown). Interestingly, single-amino acid alterations of the rat peroxisomal ketoacyl-CoA thiolase presequence leads to dual targeting to peroxisomes and mitochondria (Tsukamoto et al., 1994). This suggests that dual targeting of proteins containing apparently PTS-2-like sequences is possible.
DISCUSSION
Lipoic Acid-Containing Mitochondrial Proteins
We have shown that multiple PDC E2 subunit proteins in Arabidopsis and rice exist as functional proteins in mitochondria with bound lipoyl-cofactors. In contrast, only single isoforms of KGDC and BCKDC E2s have been identified in these plants. Heterogeneity of protein subunits within PDC E2 core structures has not been explored widely in plants, and its functional significance is unknown. Previously, E2 subunits with single or double lipoyl domains in dicot plants were identified, and the potential for mixed E2 core complexes in PDC was proposed (Millar et al., 1999b). In contrast, only single lipoyl domains have been found in monocots (Millar et al., 1999b; Thelen et al., 1999). We have noted here that, even within the clearly mitochondrial 1-lipoyl domain E2 proteins of monocot and dicot plants, there are two apparent classes. Arabidopsis At3g13930 and rice BAC20732 make an orthologous pair based on sequence comparison, whereas Arabidopsis At1g54220 and rice BAA90623 are likewise related sequences. Interestingly, in both rice and Arabidopsis, the former pair is the highly abundant isoforms, whereas the latter pair is low-abundance isoforms. In mammals and yeast, a low-abundance protein, the E3-binding protein (E3-BP), that has single lipoic acid attached and is closely related to the E2 subunit has been identified. This protein cannot catalyze the formation of CoA-esters but binds to E3 of the mitochondrial PDC (Lawson et al., 1991). E3-BP plays a structural role in PDC but is also involved in lipoyl-to-lipoyl transfer of acetyl groups with the catalytically active E2 pool. No evidence for this protein has been found in plants to date, but the lower abundance E2s found in rice and Arabidopsis (At1g54220 and rice BAA90623) may play an analogous role. Several lower abundance E2 proteins in Arabidopsis and rice are likely to be plastid E2 isoforms (A5, At3g25860; and R5, 6021.m00139) based on sequence similarity and targeting prediction and are probably present in our mitochondrial preparations because of low-level plastidic contamination.
Intriguingly, the spot designated A4 in Table I that reacts with the lipoic acid antibody was identified as an E3 subunit (lipoamide dehydrogenase) rather than an E2 subunit (acyltransferase). It is unclear what this means because E3 is not known to contain lipoic acid as a covalent modification. The substrate for E3 is lipoic acid, but, under the stringent denaturing conditions used, it is very unlikely that non-covalent binding of lipoic acid could explain the immunoreaction. Spot A4 has a pI of approximately 5, which is much more acidic than the bulk of the E3 proteins identified previously in Arabidopsis mitochondria, which were found at a pI of 6.3 to 6.7 (Millar et al., 2001). Thus, the protein spot represented by A4 is only a small fraction of the total E3 in Arabidopsis mitochondria. A survey of potential lipoyl domains (Prosite PS00189) among Arabidopsis protein sequences identified only annotated E2 subunits of KGDC, PDC, BCKDC, and the H protein of GDC (data not shown). Therefore, if this E3 lipoamide dehydrogenase does contain a covalent lipoylbinding domain, it is not identical to the defined lipoyl domain of the other proteins. As expected, the E2 subunits for KGDC were identified in all species, whereas E2 subunits if BCKDC were only found in rice and Arabidopsis. The BCKDC E2 gene has been cloned by several groups (Mooney et al., 1998; Fujiki et al., 2000). An antibody raised against the predicted product detected E2 only in dark-adapted plant leaves and BCKDC activity was also only detected in dark-adapted whole-leaf extracts with α-keto-β-methylvaleric acid (Ile derivative) as substrate (Fujiki et al., 2000). This dark-dependent expression of BCKDC may explain the apparent absence of BCKDC in our mitochondrial samples from light-grown pea leaves and the difficulties others have had measuring the activity of BCKDC from similarly grown pea plants (Mooney et al., 2002).
Branched-Chain Amino Acid Metabolism in Plant Mitochondria
The BCKDC E2 from Arabidopsis has been overexpressed and shown to form a 24-mer core complex in Escherichia coli (Mooney et al., 2000). However, this is the first direct analysis of this subunit in planta (to our knowledge) defining its activity and substrate specificity. Our analysis reveals that all three BCKDC substrates tested (representing the deaminated branched-chain amino acids Val, Leu, and Ile) were able to catalyze NAD+ reduction with a slight preference for the Val and Leu derivatives (Table II). The mammalian BCKDC also has a preference for these α-keto acids (Jones and Yeaman, 1986). However, Val and Leu supported the highest oxidation rates in intact Arabidopsis mitochondria, with no measurable rates observed with Ile or its derivative α-keto-β-methylvaleric acid (Table III). This preference is consistent with the known substrate specificities of the acyl-CoA dehydrogenase that we detected, known as IVD (Table IV). Previously, this enzyme has been shown to have highest maximum velocity with Leu- and Val-derived substrates (Daschner et al., 2001). Our inability to measure BCKDC activity in dark-grown rice mitochondria after detecting the presence of the protein may be a result of either the growth or isolation conditions used. Mammalian BCKDC activity, like plant mitochondrial PDC, is regulated by phosphorylation of a conserved Ser residue located on the E1 subunit (Mooney et al., 2002). No clear evidence for phosphorylation of plant BCKDC has been reported, and no clear ortholog of the mammalian BCKDC kinase is present in plant sequence databases. However, if this regulatory mechanism is conserved in plants, the mitochondria isolated from rice in this work may contain BCKDC in an inactive state, explaining our ability to detect the protein but not to measure its activity.
Our inability to find key enzymes of Ile and Val metabolism in our mitochondria (Table IV, Fig. 2), together with their apparent presence in peroxisomes, suggests a further possible complexity in these catabolic pathways involving transport of hydroxy-acyl-CoA compounds out of mitochondria to peroxisomes, followed by transport of products back to mitochondria for the final steps of Val and Ile metabolism. Trafficking of CoA esters between organelles is not well understood in plants, and evidence for carnitine shuttles is limited (Masterson and Wood, 2001). Searches of the Arabidopsis genome failed to identify candidate carnitine acyl transferases based on sequence similarity to mammalian carnitine acyl transferase sequences (data not shown). Recently, the bou mutation of an Arabidopsis mitochondrial carrier, which is most similar to the yeast and mammalian carnitine-acetylcarnitine carrier, has been shown to strongly inhibit plant cellular fatty acid catabolism (Lawand et al., 2002). This result has been interpreted in terms of a direct role for mitochondria in fatty acid catabolism (Lawand et al., 2002). We have found this same carrier in our Arabidopsis mitochondrial samples (data not shown). Interestingly, mutants in Val metabolism also appear to inhibit fatty acid metabolism in plants, putatively through the accumulation of toxic intermediates that inhibit β-oxidation (Zolman et al., 2001). Thus, although the products that bou carries in vivo are yet to be defined, an indirect link with fatty acid metabolism via the transport of carnitine or other derivatives of branched-chain CoA esters, to maintain Val and Ile metabolism, should not be overlooked.
Control and Purpose of Branched-Chain Amino Acid Catabolism in Plants
Expression of the BCKDC E2, E1α, and E1β genes is induced by darkness, mannitol, photosynthetic inhibitors, and sugar starvation and is also elevated during leaf senescence (Fujiki et al., 2000, 2001). Further work on the E1β gene induction showed that although low sugar levels enhanced expression, the addition of Leu or Leu-derived α-keto acids enhanced message levels even more, but only under low sugar conditions (Fujiki et al., 2001). Similar starvation induction of the Leu pathway component, MCCase, has been reported in potato (Aubert et al., 1996) and Arabidopsis (Fujiki et al., 2001; Che et al., 2002). LUC reporter constructs of the Arabidopsis BCKDC E2 and E1β drove sugar starvation-induced LUC expression in tobacco (Nicotiana tabacum) cell cultures (Fujiki et al., 2002). In this system, induction of the BCKDC gene promoters could be influenced by protein kinase and phosphatase inhibitors, indicating the presence of a phosphorylation-based signal transduction pathway in the perception and transduction of the sugar starvation trigger (Fujiki et al., 2002). Overall, these data suggest that branched-chain amino acids promote their own catabolism but only when plant cells are sugar starved and they may be alternative sources of respiratory substrates for the TCA cycle during severe plant stress. However, branched-chain amino acids and α-keto acids are also considered to be cytotoxic (Ogier de Baulny and Saudubray, 2002) and in mammals can induce apoptosis (Schuldiner et al., 1996; Eden and Benvenisty, 1999). Thus, their removal via respiratory oxidation could also be viewed as a cell detoxification mechanism, maintaining a pool of branched-chain amino acid for protein synthesis while preventing their build-up to toxic levels. Under conditions promoting high rates of branched-chain amino acid production or rapid protein turnover (such as in our rapidly growing cell cultures), branched-chain amino acid catabolism may be an important detoxification mechanism.
MATERIALS AND METHODS
Cell Culture and Plant Material
A heterotrophic Arabidopsis cell culture, established from callus of ecotype Landsberg erecta stem explants, was cultured in Murashige and Skoog basal media supplemented with 3% (w/v) Suc, 0.5 mg L–1 naphthaleneacetic acid, and 0.05 mg L–1 kinetin, and maintained in 250-mL conical flasks in the dark at 22°C in an orbital shaker (150 rpm). At 6 to 7 d, each flask contained 8 to 10 g fresh weight cells, and growth was approximately in the middle of the log phase. Batches of 200 g of rice (Oryza sativa cv Amaroo) seed were washed in 1% (w/v) bleach for 10 min, rinsed in distilled water, and grown in the dark in vermiculite trays (30 × 40 cm) at a constant 30°C, watered daily, and coleoptile tissue harvested at 7 d for mitochondrial isolation. Pea (Pisum sativum L. cv Green Feast) plants were germinated in vermiculite and grown in controlled environment chambers with a light intensity of 700 μmol m–2 s–1 at 24°C and 65% humidity for 10 d on a 16-/8-h day/night cycle.
Mitochondrial Isolation
Mitochondria were isolated according to published methods for Arabidopsis (Millar et al., 2001), pea (Taylor et al., 2002), and rice (Heazlewood et al., 2003). Marker enzymes studies of Arabidopsis mitochondria according to this method reveal approximately 1.5% contamination by plastid proteins and approximately 0.2% contamination by peroxisomal proteins (Millar et al., 2001; Heazlewood et al., 2004).
Respiratory and Spectrophotometric Measurements
O2 consumption by isolated mitochondria was measured in a Clark-type O2 electrode (Rank Bros., Cambridge, UK) in 1-mL volume. For mitochondria, the reaction medium contained: 0.3 m mannitol, 10 mm TES-KOH (pH 7.5), 5 mm KH2PO4, 10 mm NaCl, 2 mm MgSO4, and 0.1% (w/v) bovine serum albumin. Pyruvate (10 mm), α-ketoglutarate (10 mm), malate (0.5 mm), Leu (10 mm), Ile (10 mm), Val (10 mm), α-ketoisocaproic acid (10 mm), α-keto-β-methylvaleric acid (10 mm), α-ketoisovaleric acid (10 mm) ADP (0.1–1 mm), ATP (0.5 mm), NAD (0.5 mm), TPP (0.05 mm), and CoA (0.06 mm) were added as indicated to modulate O2 consumption rates of mitochondria. The activity of PDC, KGDC, and BCKDC was measured as the formation of NADH at 340 nm and 25°C using a Cary 300Bio spectrophotometer (Varian, Palo Alto, CA). The reaction medium contained 70 mm TES-KOH (pH 7.5 for PDC and BCKDC and pH 7.0 for KGDC), MgCl2 (2 mm), TPP (0.2 mm), NAD (2 mm), CoA (0.12 mm), and Cys (20 mm). When assaying PDC activity, 0.05% (w/v) Triton X-100 and pyruvate (1 mm) were added. To measure KGDC activity, 0.05% (w/v) Triton X-100, α-ketoglutarate (1 mm), AMP (0.5 mm), and 2 units of pig lipoamide dehydrogenase (EC 1.8.1.4) were also included. The reactions to measure BCKDC activity also included KCN (0.5 mm), n-propylgallate (0.05 mm), and myxothiazol (2.5 μm), and as substrate α-ketoisocaproic acid (1 mm), α-keto-β-methylvaleric acid (1 mm), or α-ketoisovaleric acid (1 mm). Kinetic values are presented as mean ± se (n = 3).
Two-Dimensional IEF/SDS-PAGE Gels and Western Blotting
IEF sample buffer consisted of 6 m urea, 2 m thiourea, 2% (w/v) CHAPS, 2% (v/v) ampholytes (pH 3–10), 2 mm tributylphosphine, and 0.001% (w/v) bromphenol blue. Aliquots of 330 μL were used to reswell dried 180-mm (pH 3–10) nonlinear immobilized ph gradient strips (Immobiline DryStrips, APBiotech, Sydney) overnight, and then IEF was performed for 19.5 h reaching a total of 49 kV h–1 at 20°C on a flat-bed electrophoresis unit (Multiphor II, APBiotech). Immobilized ph gradient strips were then transferred to an equilibration buffer consisting of 50 mm Tris-HCl (pH 6.8), 4 m urea, 2% (w/v) SDS, 0.001% (w/v) bromphenol blue, and 100 mm β-mecaptoethanol and incubated for 20 min at room temperature with rocking. The equilibrated strips were then slotted into central single wells of 4% (w/v) acrylamide stacking gels above 0.1 × 18.5 × 20 cm, 12% (w/v) acrylamide, and 0.1% (w/v) SDS-polyacrylamide gels. Gel electrophoresis was performed at 100 V with circulating cooling (4°C) and completed in 5 h. Proteins were visualized by colloidal Coomassie (G250) staining. For immunodectection, separated proteins were electroblotted onto a nitrocellulose membrane and incubated with primary antibodies to lipoic acid. A chemiluminescence detection system linked to horseradish peroxidase was used as a secondary antibody, and quantitative light emission was recorded using a Luminescent Image Analyser (LAS 100, Fuji, Tokyo).
Quadrupole Time-of-Flight (Q-TOF) MS
Q-TOF MS/MS was performed on an Applied Biosystems Q-STAR Pulsar (Q-TOF MS) using an IonSpray source (Applied Biosystems, Foster City, CA). Protein spots to be analyzed were cut from the two-dimensional PAGE gel, dried at 50°C in a dry block heater, and stored at –70°C. Protein spots were harvested and prepared for analysis by MS according to Sweetlove et al. (2002). The MS/MS collision data derived from samples were analyzed with Mascot (http://www.matrixscience.com) and the Pro ID MS/MS analysis component of Analyst QS (Applied Biosystems). An in-house database comprising The Institute for Genomic Research and National Center for Biotechnology Information Arabidopsis proteins sets was searched with resulting MS/MS data at error tolerances of MS ± 0.15 D and MS/MS ± 0.05 D for ProID and at the default settings of MS ± 1.2 D and MS/MS ± 0.6 D for Mascot. Where MS/MS peptide matches were less than 98% confidence, manual interpretations of the spectra were undertaken to confirm the protein match. For LC-MS/MS, mitochondrial samples of approximately 500 μg were acetone precipitated at –20°C over night. The resulting precipitated protein pellet was resuspended in 100 mm Tris-HCl (pH 8.5). The protein lysate was digested overnight at 37°C with 1/10 (w/w) trypsin and insoluble material removed by centrifugation. Digested samples of 15 to 20 μg were analyzed on a Q-STAR Pulsar (Q-TOF MS) system (Applied Biosystems) utilizing an inline Agilent 1100 capillary LC system incorporating a Zorbax C18 reverse-phase column (Agilent, Palo Alto, CA) for peptide separations. Peptides were analyzed by the MS over a 10-h elution period with increasing acetonitrile concentrations from 2% to 80% (v/v) in water and 0.1% (v/v) formic acid. Ions were automatically selected for the N2 collision cell by the Analyst QS software package (Applied Biosystems). MS/MS-derived spectra were analyzed using the procedures outlined above.
Acknowledgments
We thank Dr. Kenneth M. Humphries and Prof. Luke Szweda (Department of Physiology and Biophysics, School of Medicine, Case Western Reserve University, Cleveland) for the gift of antilipoic acid antibodies, Jasun Ito (University of Western Australia, Crawley) for technical assistance, and Dr. Steve Smith (University of Edinburgh, UK) critical reading of this manuscript.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.035675.
This work was supported by the Australian Research Council Discovery Programme (to A.H.M. and D.A.D.) and by the University of Western Australia (University Postgraduate Scholarship to N.L.T.).
References
- Anderson MD, Che P, Song J, Nikolau BJ, Wurtele ES (1998) 3-Methylcrotonyl-coenzyme A carboxylase is a component of the mitochondrial leucine catabolic pathway in plants. Plant Physiol 118: 1127–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert S, Alban C, Bligny R, Douce R (1996) Induction of beta-methylcrotonyl-coenzyme A carboxylase in higher plant cells during carbohydrate starvation: evidence for a role of MCCase in leucine catabolism. FEBS Lett 383: 175–180 [DOI] [PubMed] [Google Scholar]
- Che P, Wurtele ES, Nikolau BJ (2002) Metabolic and environmental regulation of 3-methylcrotonyl-coenzyme A carboxylase expression in Arabidopsis. Plant Physiol 129: 625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew O, Whelan J (2003) Dual targeting ability of targeting signals is dependent on the nature of the mature protein. Funct Plant Biol 30: 805–812 [DOI] [PubMed] [Google Scholar]
- Daschner K, Couee I, Binder S (2001) The mitochondrial isovaleryl-coenzyme A dehydrogenase of Arabidopsis oxidizes intermediates of leucine and valine catabolism. Plant Physiol 126: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold R, Schuster J, Daschner K, Binder S (2002) The branched-chain amino acid transaminase gene family in Arabidopsis encodes plastid and mitochondrial proteins. Plant Physiol 129: 540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide M, Couee I, Pradet A, Raymond P (1993) Effects of glucose starvation on the oxidation of fatty acids by maize root tip mitochondria and peroxisomes: evidence for mitochondrial fatty acid beta-oxidation and acyl-CoA dehydrogenase activity in a higher plant. Biochem J 296: 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry IB, Wiskich JT (1987) 2-Oxoglutarate dehydrogenase and pyruvate dehydrogenase activities in plant mitochondria interaction via a common coenzyme A pool. Arch Biochem Biophys 257: 92–99 [DOI] [PubMed] [Google Scholar]
- Eden A, Benvenisty N (1999) Involvement of branched-chain amino acid aminotransferase (Bcat1/Eca39) in apoptosis. FEBS Lett 457: 255–261 [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Ito M, Itoh T, Nishida I, Watanabe A (2002) Activation of the promoters of Arabidopsis genes for the branched-chain alpha-keto acid dehydrogenase complex in transgenic tobacco BY-2 cells under sugar starvation. Plant Cell Physiol 43: 275–280 [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Ito M, Nishida I, Watanabe A (2001) Leucine and its keto acid enhance the coordinated expression of genes for branched-chain amino acid catabolism in Arabidopsis under sugar starvation. FEBS Lett 499: 161–165 [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Sato T, Ito M, Watanabe A (2000) Isolation and characterization of cDNA clones for the E1β and E2 subunits of the branched-chain α-ketoacid dehydrogenase complex in Arabidopsis. J Biol Chem 275: 6007–6013 [DOI] [PubMed] [Google Scholar]
- Gerhardt B (1992) Fatty acid degradation in plants. Prog Lipid Res 31: 417–446 [DOI] [PubMed] [Google Scholar]
- Germain V, Rylott EL, Larson TR, Sherson SM, Bechtold N, Carde JP, Bryce JH, Graham IA, Smith SM (2001) Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J 28: 1–12 [DOI] [PubMed] [Google Scholar]
- Graham IA, Eastmond PJ (2002) Pathways of straight and branched chain fatty acid catabolism in higher plants. Prog Lipid Res 41: 156–181 [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Howell KA, Whelan J, Millar AH (2003) Towards the analysis of the rice mitochondrial proteome. Plant Physiol 132: 230–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazlewood JL, Tonti-Filippini JS, Gout AM, Day DA, Whelan JM, Millar AH (2004) Experimental analysis of the Arabidopsis mitochondrial proteome highlights signalling and regulatory components, provides assessment of targeting prediction programs and points to plant specific mitochondrial proteins. Plant Cell 16: 241–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston ML, Luethy MH, Miernyk JA, Randall DD (1997) Cloning and molecular analyses of the Arabidopsis thaliana plastid pyruvate dehydrogenase subunits. Biochim Biophys Acta 1321: 200–206 [DOI] [PubMed] [Google Scholar]
- Jones SM, Yeaman SJ (1986) Oxidative decarboxylation of 4-methylthio-2-oxobutyrate by branched-chain 2-oxo acid dehydrogenase complex. Biochem J 237: 621–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruft V, Eubel H, Jansch L, Werhahn W, Braun HP (2001) Proteomic approach to identify novel mitochondrial proteins in Arabidopsis. Plant Physiol 127: 1694–1710 [PMC free article] [PubMed] [Google Scholar]
- Lawand S, Dorne AJ, Long D, Coupland G, Mache R, Carol P (2002) Arabidopsis A BOUT DE SOUFFLE, which is homologous with mammalian carnitine acyl carrier, is required for postembryonic growth in the light. Plant Cell 14: 2161–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JE, Behal RH, Reed LJ (1991) Disruption and mutagenesis of the Saccharomyces cerevisiae PDX1 gene encoding the protein X component of the pyruvate dehydrogenase complex. Biochemistry 30: 2834–2839 [DOI] [PubMed] [Google Scholar]
- Lutziger I, Oliver DJ (2001) Characterization of two cDNAs encoding mitochondrial lipoamide dehydrogenase from Arabidopsis. Plant Physiol 127: 615–623 [PMC free article] [PubMed] [Google Scholar]
- Masterson C, Wood C (2001) Mitochondrial and peroxisomal beta-oxidation capacities of organs from a non-oilseed plant. Proc R Soc Lond B Biol Sci 268: 1949–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk JA, Thomas DR, Wood C (1991) Partial purification and characterisation of the mitochondrial and peroxisomal isozymes of enoyl-coenzyme A hydratase from germinating pea seedlings. Plant Physiol 95: 564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Hill SA, Leaver CJ (1999a) Plant mitochondrial 2-oxoglutarate dehydrogenase complex: purification and characterization in potato. Biochem J 343: 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Leaver CJ, Hill SA (1999b) Characterization of the dihydrolipoamide acetyltransferase of the mitochondrial pyruvate dehydrogenase complex from potato and comparisons with similar enzymes in diverse plant species. Eur J Biochem 264: 973–981 [DOI] [PubMed] [Google Scholar]
- Millar AH, Sweetlove LJ, Giege P, Leaver CJ (2001) Analysis of the Arabidopsis mitochondrial proteome. Plant Physiol 127: 1711–1727 [PMC free article] [PubMed] [Google Scholar]
- Mooney BP, Henzl MT, Miernyk JA, Randall DD (2000) The dihydrolipoyl acyltransferase (BCE2) subunit of the plant branched-chain alpha-ketoacid dehydrogenase complex forms a 24-mer core with octagonal symmetry. Protein Sci 9: 1334–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney BP, Miernyk JA, Randall DD (1998) Nucleotide sequence of a cDNA encoding the E1α subunit of the branched chain a-keto acid dehydrogenase complex from Arabidopsis thaliana (accession no. AF077955) (PGR98-168). Plant Physiol 118: 7119867602 [Google Scholar]
- Mooney BP, Miernyk JA, Randall DD (2002) The complex fate of alpha-ketoacids. Annu Rev Plant Biol 53: 357–375 [DOI] [PubMed] [Google Scholar]
- Ogier de Baulny H, Saudubray JM (2002) Branched-chain organic acidurias. Semin Nenatol 7: 65–74 [DOI] [PubMed] [Google Scholar]
- Peeters N, Small I (2001) Dual targeting to mitochondria and chloroplasts. Biochim Biophys Acta 1541: 54–63 [DOI] [PubMed] [Google Scholar]
- Perham RN, Jones DD, Chauhan HJ, Howard MJ (2002) Substrate channelling in 2-oxo acid dehydrogenase multienzyme complexes. Biochem Soc Trans 30: 47–51 [DOI] [PubMed] [Google Scholar]
- Poulsen LL, Wedding RT (1970) Purification and properties of the alpha-ketoglutarate dehydrogenase complex of cauliflower mitochondria. J Biol Chem 245: 5709–5717 [PubMed] [Google Scholar]
- Randall DD, Rubin PM, Fenko M (1977) Plant pyruvate dehydrogenase complex purification, characterization and regulation by metabolites and phosphorylation. Biochim Biophys Acta 485: 336–349 [DOI] [PubMed] [Google Scholar]
- Reed LJ (1974) Multienzyme complexes. Acc Chem Res 7: 40–46 [Google Scholar]
- Reed LJ (1981) Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Curr Top Cell Regul 18: 95–106 [DOI] [PubMed] [Google Scholar]
- Schuldiner O, Eden A, Ben-Yosef T, Yanuka O, Simchen G, Benvenisty N (1996) ECA39, a conserved gene regulated by c-Myc in mice, is involved in G1/S cell cycle regulation in yeast. Proc Natl Acad Sci USA 93: 7143–7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, Millar AH (2002) The impact of oxidative stress on Arabidopsis mitochondria. Plant J 32: 891–904 [DOI] [PubMed] [Google Scholar]
- Taylor NL, Day DA, Millar AH (2002) Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. J Biol Chem 277: 42663–42668 [DOI] [PubMed] [Google Scholar]
- Thelen JJ, Muszynski MG, David NR, Luethy MH, Elthon TE, Miernyk JA, Randall DD (1999) The dihydrolipoamide S-acetyltransferase subunit of the mitochondrial pyruvate dehydrogenase complex from maize contains a single lipoyl domain. J Biol Chem 274: 21769–21775 [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Hata S, Yokota S, Miura S, Fujiki Y, Hijikata M, Miyazawa S, Hashimoto T, Osumi T (1994) Characterization of the signal peptide at the amino terminus of the rat peroxisomal 3-ketoacyl-CoA thiolase precursor. J Biol Chem 269: 6001–6010 [PubMed] [Google Scholar]
- Yeaman SJ (1989) The 2-oxo acid dehydrogenase complexes: recent advances. Biochem J 257: 625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Monroe-Augustus M, Thompson B, Hawes JW, Krukenberg KA, Matsuda SP, Bartel B (2001) chy1, an Arabidopsis mutant with impaired β-oxidation, is defective in a peroxisomal beta-hydroxyisobutyryl-CoA hydrolase. J Biol Chem 276: 31037–31046 [DOI] [PubMed] [Google Scholar]