Abstract
Calluses from two ecotypes of reed (Phragmites communis Trin.) plant (dune reed [DR] and swamp reed [SR]), which show different sensitivity to salinity, were used to study plant adaptations to salt stress. Under 200 mm NaCl treatment, the sodium (Na) percentage decreased, but the calcium percentage and the potassium (K) to Na ratio increased in the DR callus, whereas an opposite changing pattern was observed in the SR callus. Application of sodium nitroprusside (SNP), as a nitric oxide (NO) donor, revealed that NO affected element ratios in both DR and SR calluses in a concentration-dependent manner. Nω-nitro-l-arginine (an NO synthase inhibitor) and 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxyde (a specific NO scavenger) counteracted NO effect by increasing the Na percentage, decreasing the calcium percentage and the K to Na ratio. The increased activity of plasma membrane (PM) H+-ATPase caused by NaCl treatment in the DR callus was reversed by treatment with Nω-nitro-l-arginine and 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxyde. Western-blot analysis demonstrated that NO stimulated the expression of PM H+-ATPase in both DR and SR calluses. These results indicate that NO serves as a signal in inducing salt resistance by increasing the K to Na ratio, which is dependent on the increased PM H+-ATPase activity.
When plants are exposed to NaCl, cellular ion homeostasis may be impaired. Under salinity conditions, tolerant plants typically maintain high potassium (K+) and low sodium (Na+) in the cytosol of cells (Greenway and Munns, 1980; Jeschke, 1984). Such mechanisms involve Na+ compartmentalization into vacuoles and/or extrusion to the external medium and K+ accumulation in the cytoplasm. These processes appear to be mediated by several transport systems, such as H+-ATPase, carriers (symporters and antiporters), and channels associated with plasma membranes (PMs) and tonoplasts (Niu et al., 1995; Rausch et al., 1996).
Control of Na+ movement across the PM and tonoplast to maintain a low Na+ concentration in the cytoplasm is a key factor of cellular adaptation to salt stress (Niu et al., 1995; Rausch et al., 1996). Na+ transport across the PM is dependent on the electrochemical gradient created by the PM H+-ATPase (Serrano, 1996). PM H+-ATPase belongs to a family of P-type ATPase, which has a catalytic subunit of approximately 100 kD. This enzyme is a proton pump, whose major role couples ATP hydrolysis to proton transport and creates electrochemical gradient across the PM used by secondary transporters (Serrano, 1989). In addition, this membrane protein is involved in many physiological processes, including salt tolerance, intracellular pH regulation, stomatal opening, and cell elongation (Rayle and Cleland, 1992; Niu et al., 1993; Michelet and Boutry, 1995; Cosgrove, 1997; Kerkeb et al., 2001; Yang et al., 2003). The PM H+-ATPase is encoded by a multigene family, and at least 10 isoforms of the H+-ATPase exist in plants. Krysan et al. (1996) analyzed T-DNA knockout Arabidopsis mutants of H+-ATPase isoforms and demonstrated that at least one H+-ATPase isoform is involved in NaCl tolerance. The expression of the PM H+-ATPase seems to be dependent on the plant species, developmental stage, and environmental stimuli. Previous studies showed that the PM H+-ATPase activity was affected by salt treatment, including a partial inhibition in the roots of tomato (Lycopersicon esculentum; Ballesteros et al., 1998), a stimulation in mung bean (Vigna radiata) roots (Raven, 1990), and no effect in cotton (Gossypium hirsutum) roots (Kochian, 2000). Therefore, regulation of the expression and activity of the PM H+-ATPase may represent an important cellular mechanism for salt tolerance. It remains unclear the precise mechanism that the expression and metabolic regulation of the PM ATPase is affected by salinity.
Nitric oxide (NO) is a bioactive molecule that is involved in many physiological processes in animals, such as vasorelaxation, platelet inhibition, neurotransmission, cytotoxicity, and immunoregulation (Anbar, 1995). NO has also been suggested to act as a signal molecular mediating responses to biotic and abiotic stresses in the plant kingdom. It could induce germination instead of red right (Giba et al., 1998; Beligni and Lamattina, 2000), affect growth and development of plant tissue (Leshem and Haramaty, 1996; Gouvêa et al., 1997; Durner and Klessing, 1999), and enhance plant cell senescence (Pedroso and Durzan, 2000; Pedroso et al., 2000a, 2000b). Also, NO was suggested to be involved in the responses to drought stress, heat stress, disease resistance, and apoptosis (Delledonne et al., 1998; Leshem et al., 1998; Durner and Klessing, 1999; Ribeiro et al., 1999; Beligni and Lamattina, 2000; Pedroso et al., 2000b; Mata and Lamattina, 2001). However, the experimental evidence for NO as a signal molecular in plants under salt stress remains elusive.
NO is synthesized by NO synthase (NOS) in animal cells. Immunological analysis with antibodies raised against mammalian NOS enzyme suggested that NOS-like proteins did exist in plants (Pedroso and Durzan, 2000). However, proteomic analysis revealed that plant proteins that cross-react with mammalian NOS antibodies are not related to NOS (Butt et al., 2003). NOS activity was first determined in plants by Ninnemann and Maier (1996) and could be inhibited by Nω-nitro-l-Arg (l-NNA) and NG-monomethyl-l-Arg, both of which are known inhibitors of mammalian NOS enzymes. Recently, a pathogen-inducible plant NOS has been identified as a variant P protein of the Gly decarboxylase complex (Chandok et al., 2003).
Dune reed (DR), which grows in the desert and sand dune region of northwestern China, is an important ecotype of reed (Phragmites communis Trin.). It is exposed frequently to a combination of stresses. DR vegetates and develops normally and forms some quite large populations under such harsh conditions (Wang et al., 1995). DR had proved to retain some stable variations of morphological, physiological, and genetic characteristics in response to external stresses (Wang et al., 1995). It is an ideal material for studies on the adaptations of plant to various environmental conditions. Instead, swamp reed (SR), another ecotype of reed, grows in ponds that are full of water all year round (Wang et al., 1995). Recently, it was shown that regenerated plantlets from embryogenic DR and SR calluses retained the same genetic characteristics as the wild plants (Cui et al., 2002). In this study, we used the calluses from DR and SR to study the adaptation to salt stress and investigated the role of NO as a second messenger to induce adaptive responses.
RESULTS
Effects of NO on Relative Water Content (RWC) and Membrane Permeability (MP) under Salt Stress
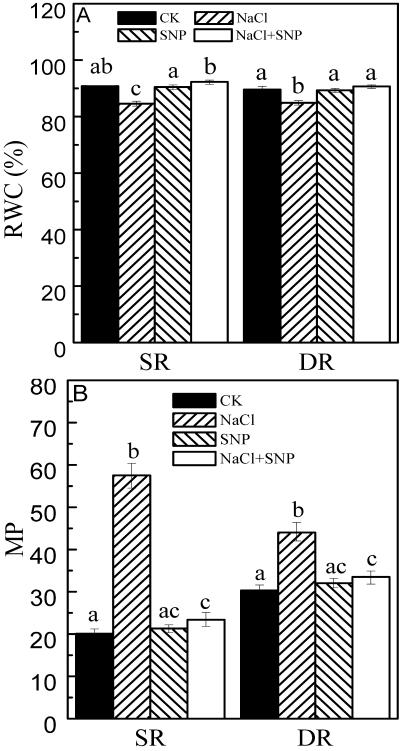
It has been demonstrated that NO could counteract oxidative damage and had protective effect against water stress (Beligni and Lamattina, 1999; Mata and Lamattina, 2001). Because salt stress induces the generation of oxidative stress, we examined if NO had capacity to protect reed calluses from salt stress. Thus, we measured RWC and MP of calluses under salt stress. RWCs in both DR and SR calluses decreased from 90% to 85%, and from 91% to 85%, respectively under NaCl treatment for 48 h. Treatment with 0.2 mm sodium nitroprusside (SNP; an NO donor) restored RWCs in both DR and SR calluses under salt stress to the normal status (Fig. 1A). MP reflects the membrane injury as a result of oxidative damage induced by salt stress. As shown in Figure 1B, MP increased by 185% in the SR callus, whereas it only increased by 45% in the DR callus under the same salt stress. Upon NaCl treatment, 0.2 mm SNP reduced MPs close to the control level in both calluses (Fig. 1B).
Figure 1.
Effects of NO on RWC (A) and MP (B) in the calluses from DR and SR. The calluses from DR and SR were cultured on the medium (as control). NaCl (200 mm) was contained in the medium for salt stress. Two micromolar SNP (1.5 mL), as NO donor, was added on the surface of the medium. After 48 h, the calluses were collected. Mean values and se were calculated from three independent experiments. Within each set of experiment, bars with different letters were significantly different at the 0.05 level. Statistic analysis showed that the difference of RWCs between DR and SR was not significant.
Changes in Elements Ratios in the Calluses under Salt Stress
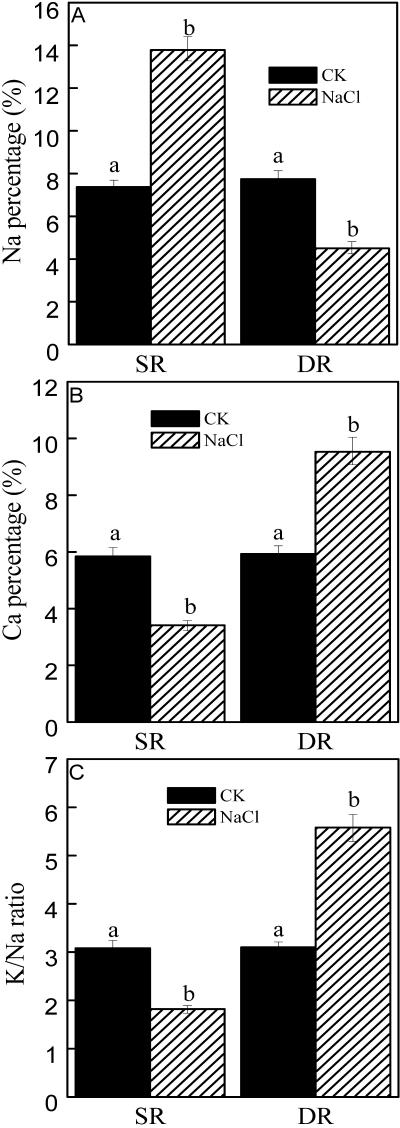
Ionic balance inside the cell is closely related to plant adaptation to salt stress, so we compared elements ratios under salt stress in both DR and SR calluses. Figure 2 showed that the Na percentage decreased by 42% in the DR callus after NaCl treatment for 48 h, whereas the Ca percentage and the K to Na ratio increased by 61% and 80%, respectively. In contrast, in the SR callus the Na percentage increased by 87%, whereas the Ca percentage and the K to Na ratio decreased to 59% of that of control after NaCl treatment (Fig. 2).
Figure 2.
Ratios of elements in the calluses from DR and SR under salt stress. A, Na percentage. B, Calcium (Ca) percentage. C, K to Na ratio. The calluses from DR and SR were cultured on the Murashige and Skoog solid medium. NaCl (200 mm) was contained in the medium for salt stress. After 48 h, the calluses were collected for determination of ratios of elements with x-ray microanalysis. The results were calculated by expressing the atomic number for a particular element in a given cell as a percentage of the total atomic number for all the elements measured (K, Na, Ca, magnesium, phosphorus, sulfur, and chlorine) in the cell. Mean values and se were calculated from three independent experiments. Within each set of experiment, bars with different letters were significantly different at the 0.05 level.
Effects of NO on Element Ratios
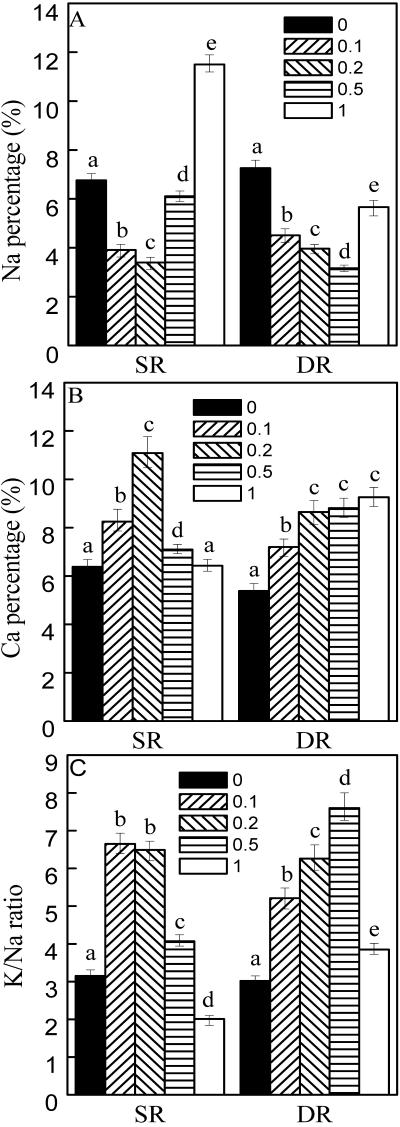
Because NO could confer resistance to environmental stresses, we next examined the effects of NO on ion distribution and elements ratio in both DR and SR calluses. To this purpose, exogenous SNP was applied to examine the relationship between NO and elements ratios. In the DR callus, the Na percentage was lowered, and the Ca percentage and the K to Na ratio were increased under SNP treatments (Fig. 3). In the SR callus, Na percentage reached its minimum (50% of the control) and Ca percentage reached the maximum (74% higher than the control) under 0.2 mm SNP treatment (Fig. 3). SNP (0.2 mm) was used in the following experiments.
Figure 3.
Effects of NO on elements ratio in the calluses from DR and SR. A, Na percentage. B, Ca percentage. C, K to Na ratio. The calluses from DR and SR were cultured on the Murashige and Skoog solid medium. One and one-half milliliters of 0.1, 0.2, 0.5, and 1.0 mm SNP, as NO donor, was added to the surface of the medium. After 48 h, the calluses were collected for determination as described in Figure 2. Mean values and se were calculated from three independent experiments. Within each set of experiment, bars with different letters were significantly different at the 0.05 level.
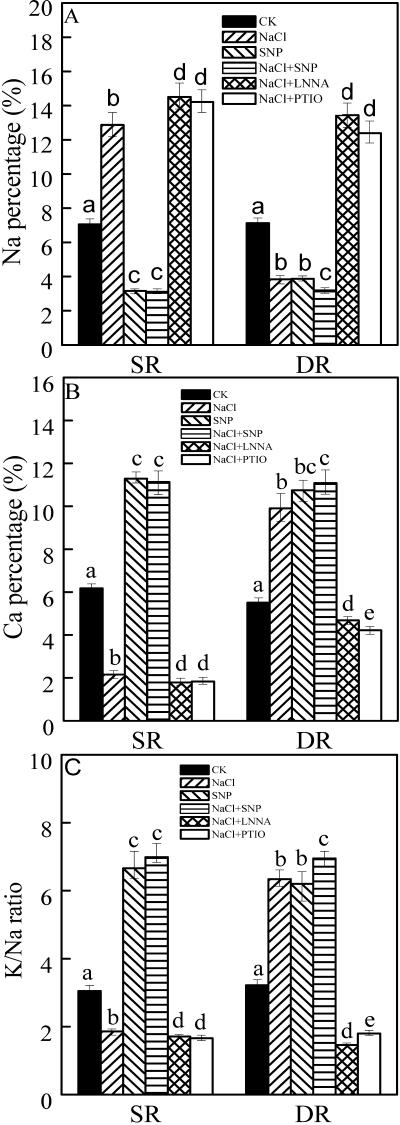
As stated above, in the DR callus, the Na percentage decreased, whereas the Ca percentage and K to Na ratio increased under NaCl treatment (Figs. 2 and 4). In contrast, in the SR callus, the Na percentage increased, whereas the Ca percentage and the K to Na ratio decreased under NaCl treatment (Figs. 2 and 4). Treatment with SNP induced the decrease of the Na percentage and the increase of the Ca and K to Na in both DR and SR calluses (Fig. 4). NaCl + SNP treatment slightly enhanced this trend. To attribute the role of NO in inducing high K to Na, specific inhibitors (an NOS inhibitor, l-NNA, and an NO scavenger, PTIO) were used. Both l-NNA and PTIO increased the Na percentage but decreased the Ca percentage and the K to Na ratio in both DR and SR calluses under salt stress (Fig. 4). These results suggest the possible involvement of NO in regulating ion homeostasis.
Figure 4.
Effects of NO on elements ratio in the calluses from DR and SR under salt stress. A, Na percentage. B, Ca percentage. C, K to Na ratio. The calluses from DR and SR were cultured on the solid Murashige and Skoog medium (as control). NaCl (200 mm) was contained in the medium for salt stress. One and one-half milliliters of 0.2 mm SNP, 0.3 mm l-NNA, or 0.4 mm 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxyde (PTIO) was added on the surface of the medium. After 48 h, the calluses were collected. Elements ratio was measured as described in Figure 2. Mean values and se were calculated from three independent experiments. Within each set of experiment, bars with different letters were significantly different at the 0.05 level.
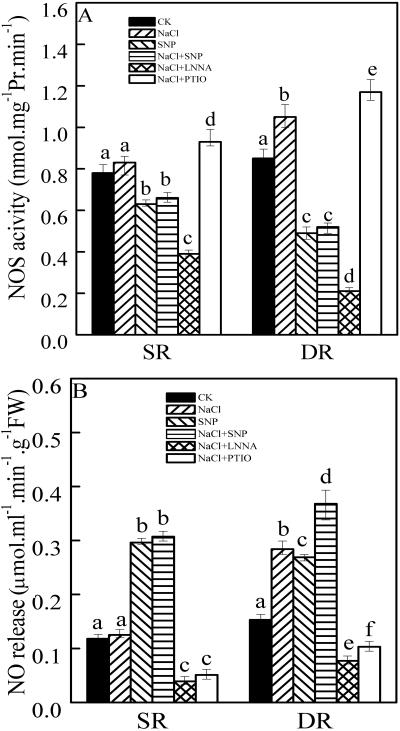
NOS Activity and NO Production under Salt Stress
To further elucidate the correlation between NO accumulation and salt tolerance, we measured NOS and NO production under salt stress. Figure 5A showed that the NOS activity remained almost unchanged under NaCl treatment in the SR callus but increased by 22% in the DR callus (Fig. 5A). The rate of NO release increased by 84% in the DR callus but stayed stable in the SR callus (Fig. 5B). Interestingly, the NOS activity was inhibited by about 60% in the presence of 0.2 mm SNP treatment alone or in combination with NaCl treatment in the DR callus, whereas the rates of NO release increased to 176% and 239%, respectively. In the SR callus, about 20% of the NOS activity was reduced after treatment with SNP or SNP + NaCl, whereas the rates of NO release increased by about 2.5-fold. l-NNA inhibited the NOS activity, whereas PTIO slightly enhanced the activity in both DR and SR calluses under salt stress though they inhibited the rates of NO release (Fig. 5B).
Figure 5.
NOS activities (A) and NO productions (B) in the calluses from DR and SR under salt stress. The calluses from DR and SR were treated as in Figure 4. Mean values and se were calculated from three independent experiments. Within each set of experiment, bars with different letters were significantly different at the 0.05 level.
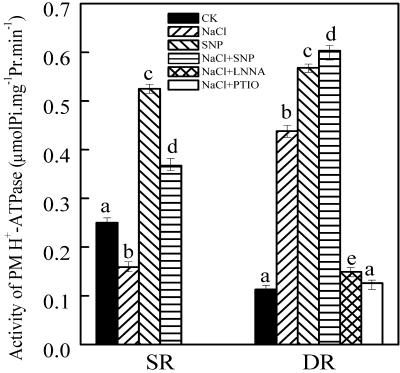
PM H+-ATPase Activity
Because PM H+-ATPase could generate a proton electrochemical gradient driving active ions transport (Michelet and Boutry, 1995), the regulation of PM H+-ATPase activity was examined. The activity of PM H+-ATPase increased by 288% in the DR callus, but it decreased by 36% in the SR callus under NaCl stress (Fig. 6). Because NO affects element ratios, we examined the relationship between NO and PM H+-ATPase activity. SNP (0.2 mm) enhanced PM H+-ATPase activities by 403% and 110% in the calluses from DR and SR, respectively. SNP + NaCl intensified SNP effect on the PM H+-ATPase activity in the DR callus. Treatment with l-NNA and PTIO reduced PM H+-ATPase activity in the DR callus under salt stress (Fig. 6).
Figure 6.
Effects of NO on PM H+-ATPase in the calluses from DR and SR. The calluses from DR and SR were treated as described in Figure 4. Mean values and se were calculated from three independent experiments. Bars with different letters were significantly different at the 0.05 level.
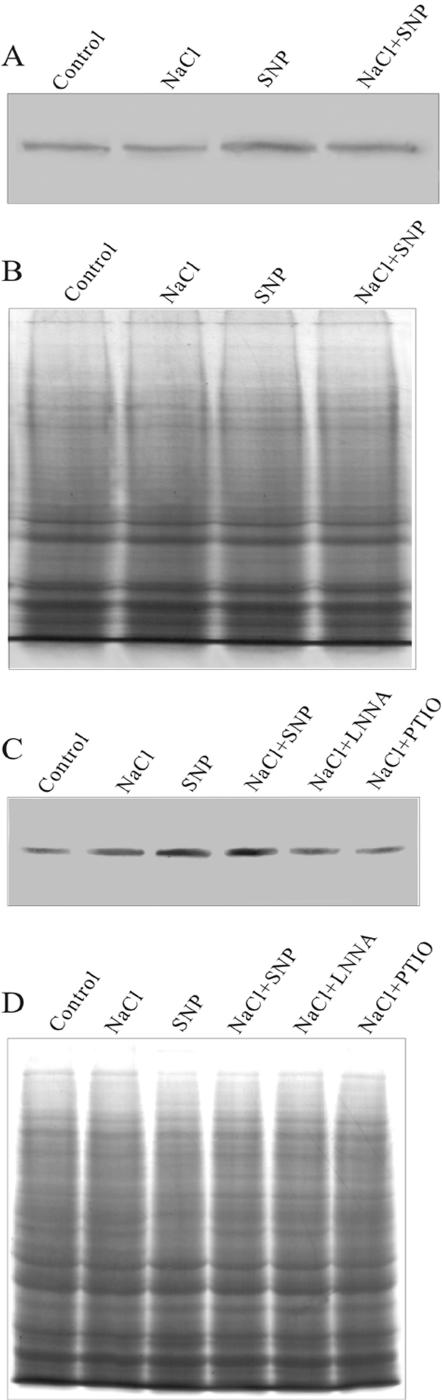
Western-Blot Analysis of PM H+-ATPase Expression
Western-blot analysis showed that the steady-state protein level of PM H+-ATPase increased in the DR callus and decreased in the SR callus under NaCl treatment. Two Coomassie Bright Blue-stained acrylamide gels were shown to indicate that equal amounts of proteins were loaded for western-blot analysis (Fig. 7). Application of the NO donor, SNP, induced the expression of PM H+-ATPase in both DR and SR calluses (Fig. 7). The changes of protein quantity were correlated with those of the activity of PM H+-ATPase. Treatment with l-NNA and PTIO repressed the expression of PM H+-ATPase (Fig. 7).
Figure 7.
Western-blot analysis of PM H+-ATPase expression (A, SR; and C, DR). The Coomassie Bright Blue-stained gels (B, SR; and D, DR) are present to show that equal amounts of proteins were loaded. The calluses from DR and SR were treated as described in Figure 4. Three independent experiments were performed, and the immunoblotting results showed the similar trends of protein expression.
DISCUSSION
In the present work, we provide evidence for the involvement of NO in salt tolerance. NO, produced under salt stress, could serve as a second messenger for the induction of PM H+-ATPase expression; thus, high K to Na was maintained to confer salt resistance.
Ion homeostasis is essential for reed plant to resist salt stress. Flowers (1985) believed that reed plants belonged to halophyte. Its salt resistance mainly depended on its discharging Na and high K to Na ratio in plant cell (Matoh et al., 1988). Two ecotypes of reeds (DR and SR) showed different responses in their elements ratios to salt stress (Fig. 2). The Na percentage decreased and the K to Na ratio increased in the DR callus under salt stress, which showed that DR callus was salt resistant.
NO, as a signaling molecular, is involved in multiple plant-resistant reactions against environmental stresses (Delledonne et al., 1998; Durner and Klessing, 1999; Ribeiro et al., 1999; Beligni and Lamattina, 2000; Mata and Lamattina, 2001). To elucidate the role of NO production under salt stress, we used NO donor and inhibitors in this paper. A close relation between NO accumulation and elements ratio was observed in the plant cell (Fig. 3). NaCl treatment of the DR callus resulted in the generation of NO (Fig. 5), which reduced the Na percentage and increased the K to Na ratio (Figs. 2 and 4). Application of the NO donor, SNP, produced similar effects (Fig. 4). However, inhibition of NO accumulation by l-NNA and PTIO reversed the effects of NaCl on element ratios in the DR callus (Fig. 4). Measurement of the NOS activity and the rate of NO release showed that they both increased in the DR callus under salt stress (Fig. 5). Inhibition of the NO production by specific inhibitors indicated that NO was produced from NOS-like activity and not as a by-product of NR, which was inconsistent with previous reports (Ribeiro et al., 1999; Garces et al., 2001). Also, NO could enhance RWC and reduce MP under salt stress, which was a protective action of NO to plant cell under salt stress (Fig. 1). From these results, we concluded that NO production induced salt resistance by influencing element ratios in the DR callus.
In contrast, in the poor salt-resistant SR callus, NO was not produced much under salt stress, so Na could not be discharged from the plant cell, and the K to Na ratio was relatively low (Figs. 4 and 5). Application of the NO donor, SNP, to the SR callus decreased Na percentage and increased the K to Na ratio (Fig. 4), thus conferring salt resistance.
Our work also implicated that NO influenced Ca absorption (Fig. 4). Ca is involved in cell wall structure and MP and considered to be one of the second messengers in plant responses to the stresses (Gong et al., 1998; Monroy et al., 1998). The increased Ca level could improve plant salt tolerance (Geisler et al., 2000). Therefore, the increased Ca percentage in the DR callus is important for salt resistance. This reveals the protective role of NO in plant under salt stress.
PM H+-ATPase was a P-type proton pump in plant. The transmembrane electrochemical gradient generated by the enzyme is the primary force for ions' cross-membrane transports (Michelet and Boutry, 1995). It has been proven that the activity of the enzyme is a key index of plant adaptation to salt stress (Niu et al. 1993). Under salt stress, the activity of PM H+-ATPase increased in the salt-resistant DR callus but decreased in the salt-sensitive SR callus. Exogenous application of SNP increased the PM H+-ATPase activity in both calluses, whereas l-NNA and PTIO inhibited its activity in the DR callus under salt stress (Fig. 6), further suggesting that NO is involved in the regulation of PM H+-ATPase activity. Western-blot analysis further confirmed that NO resulted in an increase in the amount of ATPase protein in both calluses (Fig. 7).
Our results give the first indication, to our knowledge, that NO functions as a second messenger in inducing expression of PM H+-ATPase, which may account for the enhanced PM H+-ATPase activity. Thus, ion homeostasis is reestablished so as to adapt to salt stress.
MATERIALS AND METHODS
Plant Material
Embryogenic calluses, derived from mature seeds of two ecotypes of reed (Phragmites communis Trin.; DR and SR), were obtained as described by Cui et al. (2002). After 4-month subcultures, 0.65 ± 0.05 g of embryogenic callus was maintained on the 30 mL of Murashige and Skoog solid medium (Murashige and Skoog, 1962). Sodium chloride was added in the medium for salt stress. Different concentrations of SNP, l-NNA, or PTIO (these reagents were prepared with sterilized water) were added on the surface of the solid Murashige and Skoog medium after filter sterilization. After 48 h of treatment, the callus was collected, washed for 2 min by distilled water (Heyser and Nabors, 1981), and the excess water was blotted with filter paper. The samples were used immediately or stored at –80°C rapidly for later use.
RWC Determination
Dry material of callus was obtained after being revised and heated at 80°C for 48 h. RWC was measured as the formula: RWC (%) = (fresh weight – dry weight)/fresh weight.
MP Measurement
MP was determined according to Sairam and Srivastava (2002) with some modifications. The callus (0.5 g) was placed in petri dishes with 10 mL of de-ionized water at 25°C for 2 h. After the incubation, the conductivity in the bathing solution was determined (C1). Then, the samples were heated at 80°C for 2 h, and conductivity was read again in the bathing solution (C2). Electrolyte leakage was expressed as a percentage of the total conductivity after heating at 80°C (MP = C1/C2 × 100).
X-Ray Microanalysis
Element ratio measurement was performed using a scanning electron microscope (Phillips Electronics N.V., Eindhalen, The Netherlands) fitted with a Kevex energy-dispersive x-ray detector (Kenex, Valencia, CA) as described by Vázquez et al. (1999) with some modifications. The callus was placed directly on the aluminum stage and quickly frozen under vacuum. The examination time of each sample was less than 10 min to avoid cell distortion. At least four to five cells per sample were examined. The results were calculated by expressing the atomic number for a particular element in a given cell as a percentage of the total atomic number for all the elements measured (K, Na, Ca, magnesium, phosphorus, sulfur, and chlorine) in the cell.
NOS Activity Determination
NOS activity determination was performed according to Murphy and Noack (1994) with some modifications. About 5 g of callus was homogenized in 10 mL of homogenization buffer (50 mm triethanolamine hydrochloride [pH 7.5] containing 0.5 mm EDTA, 1 μm leupeptin, 1 μm pepstatin, 7 mm gluathione, and 0.2 mm phenylmethylsulfonyl fluoride). After centrifuging at 10,000g for 20 min (4°C), the supernatant was collected and recentrifuged at 100,000g for 45 min. The supernatant was used for NOS determination. NOS activity was analyzed by hemoglobin assay as previously described (Murphy and Noack, 1994). Protein concentration was determined as described by Bradford (1976).
NO Content Determination
NO content was determined as described by Murphy and Noack (1994) with some modifications. Fresh calluses (5 g) from two reed ecotypes were incubated with 100 units of catalase and 100 units of superoxide dismutase for 5 min to remove endogenous ROS before addition of 10 mL of oxyhemoglobin (5 mm). After 2 min of incubation, NO was measured spectrophotometrically by measuring the conversion of oxyhemoglobin to methemoglobin.
PM H+-ATPase Activity Determination
PM was extracted by the method of Qiu and Su (1998) with some modifications. Five grams of callus was homogenized in 10 mL of homogenization buffer, containing 0.25 m Suc, 10% (w/v) glycerol, 0.5% (w/v) polyvinylpyrrolidone, 3 mm EDTA, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 15 mm mercaptoethanol, and 25 mm Tris-MES (pH 7.6). The homogenate was filtered through two layers of cotton gauze and centrifuged at 13,000g for 20 min (4°C). The supernatant was recentrifuged at 80,000g for 30 min to obtain a microsomal pellet (microsomal membranes), which was resuspended in a buffer containing 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, and 5 mm Tris-MES (pH 6.5). The microsomal membranes were used for PM H+-ATPase activity determination and western-blot analysis. The activity of PM H+-ATPase was determined as described by Qiu and Su (1998).
Western-Blot Analysis
SDS-PAGE was performed as described by Laemmli (1970). Fifty micrograms of membrane proteins was solubilized and separated on a 7.5% (w/v) acrylamide gel. After electrophoresis, the separated proteins were electrotransferred to a nitrocellulose membrane. The membrane was blocked for 60 min with 5% (w/v) nonfat milk in 0.05% (w/v) Tween 20, 10 mm Tris (pH 8.0), and 150 mm NaCl. A polyclonal antibody raised against PM H+-ATPase was added and incubated with the membrane overnight, then alkaline phosphatase-coupled secondary antibody was added and incubated for 1.5 h. The color was developed with a solution containing nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate.
Statistical Analysis
Each experiment was repeated at least three times. Values were expressed as means ± se. All means comparisons were done using Student's t test for independent sample. In all cases, the confidence coefficient was set at 0.05.
Acknowledgments
We are grateful to Dr. Ramon Serrano for his generosity in providing the antibody against PM H+-ATPase. We thank Dr. Jiangqi Wen and Ms. Carol Eames for their help in writing the manuscript.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.030023.
This work was supported by the Excellent PhD Thesis Foundation (grant no. 199924), by the Key Natural Science Foundation of Gansu Province (grant no. ZS031–A25–034–D), by the Trans-Century Training Program Foundation for Talents by the Ministry of Education, by the One Hundred Talent Project, and by the Innovation Project Institute.
References
- Anbar M (1995) Nitric oxide: a synchronizing chemical messenger. Experientia 51: 481–490 [DOI] [PubMed] [Google Scholar]
- Ballesteros E, Kerkeb B, Donaire JP, Belver A (1998) Effects of salt stress on H+-ATPase activity of plasma membrane-enriched vesicles isolated from sunflower roots. Plant Sci 134: 181–190 [Google Scholar]
- Beligni MV, Lamattina L (1999) Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 208: 337–344 [Google Scholar]
- Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210: 215–221 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Butt YK, Lum JH, Lo SC (2003) Proteomics identification of plant proteins by mammalian nitric oxide synthase antibodies. Planta 216: 762–771 [DOI] [PubMed] [Google Scholar]
- Chandok MR, Ytterberg AJ, van Wijk KJ, Klessig DF (2003) The pathogen-inducible nitric oxide synthase (iNOS) in plants is a variant of the P protein of the glycine decarboxylase complex. Cell 113: 469–482 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (1997) Relaxation in a high-stress environment: the molecular bases of extensible cell walls and cell enlargement. Plant Cell 9: 1031–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui SX, Wang W, Zhang CL (2002) Plant generation from callus cultures in two ecotypes of reed (Phramites communis Trinius). In Vitro Cell Dev Biol Plant 38: 325–329 [Google Scholar]
- Delledonne M, Xia YJ, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394: 585–588 [DOI] [PubMed] [Google Scholar]
- Durner J, Klessing DF (1999) Nitric oxide as a signal in plants. Curr Opin Plant Biol 2: 369–374 [DOI] [PubMed] [Google Scholar]
- Flowers TJ (1985) Physiology of halophytes. Plant Soil 89: 41–56 [Google Scholar]
- Garces H, Durzan D, Pedroso MC (2001) Mechanical stress elicits nitric oxide formation and DNA fragmentation in Arabidopsis thaliana. Ann Bot 87: 567–574 [Google Scholar]
- Geisler M, Frangne N, Gomès E, Martinoia E, Palmgren MG (2000) The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol 124: 1814–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giba Z, Grubišiæ D, Todoroviæ S, Sajc L, Stojakoviæ Đ, Konjeciæ R (1998) Effects of nitric oxide-releasing compounds on phytochrome-controlled germination of Empress tree seeds. Plant Growth Regul 26: 175–181 [Google Scholar]
- Gong M, Luit AH, Knight MR (1998) Heat-shock-induced changes intracellular Ca2+ level in tobacco seedlings in relation to thermo-tolerance. Plant Physiol 116: 429–437 [Google Scholar]
- Gouvêa CMCP, Souza JF, Magalhães CAN, Martins IS (1997) NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regul 21: 183–187 [Google Scholar]
- Greenway H, Munns R (1980) Mechanism of salt-tolerance in nonhalophytes. Annu Rev Plant Physiol 31: 149–190 [Google Scholar]
- Heyser JW, Nabors MW (1981) Osmotic adjustment of cultured tobacco cells (Nicotiana tabacum var. Samsun) grown on sodium chloride. Plant Physiol 67: 720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke WD (1984) K+-Na+ exchange at cellular membranes, intracellular compartmentation of cations, and salt tolerance. In Tolerance in Plants: Strategies for Crop Improvement. Wiley-Interscience, New York, pp 37–66
- Kerkeb L, Donaire JP, Rodriguez-Rosales MP (2001) Plasma membrane H+-ATPase activity is involved in adaptation of tomato calli to NaCl. Physiol Plant 111: 483–490 [DOI] [PubMed] [Google Scholar]
- Kochian LV (2000) Molecular physiology of mineral nutrient acquisition, transport and utilization. In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants, Chapter 2. American Society of Plant Physiologists, Rockville, MD, pp 1204–1249
- Krysan PJ, Young JC, Tax F, Sussman MR (1996) Identification of transferred DNA insertions within Arabidopsis genome is involved in signal transduction and ion transport. Proc Natl Acad Sci USA 93: 8145–8150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Leshem YY, Haramaty E (1996) Plant aging: the emission of NO and ethylene and the effect of NO-releasing compounds on growth of pea (Pisum sativum) foliage. J Plant Physiol 148: 258–263 [Google Scholar]
- Leshem YY, Wills RBH, Ku VV (1998) Evidence for the function of the free radical gas-nitric oxide (NO.) as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem 36: 825–833 [Google Scholar]
- Mata CG, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126: 1196–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoh T, Matsushita N, Takahashi E (1988) Salt tolerance of the reed plant Phragmites communis. Physiol Plant 72: 8–14 [Google Scholar]
- Michelet B, Boutry M (1995) The plasma membrane H+-ATPase: a highly regulated enzyme with multiple physiological functions. Plant Physiol 108: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy AF, Sangwan V, Dhindsa RS (1998) Low temperature signal transduction during cold acclimation: protein phosphates 2A as an early target for cold-inactivation. Plant J 13: 653–660 [Google Scholar]
- Murphy ME, Noack E (1994) Nitric oxide assay using hemoglobin method. Methods Enzymol 233: 240–250 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Ninnemann H, Maier J (1996) Indications for occurrence of nitric oxide synthases in fungi and plants and involvement in photoconidiation of Neurospora crassa. Photochem Photobiol 64: 393–398 [DOI] [PubMed] [Google Scholar]
- Niu X, Bressan R, Hasegawa P, Pardo J (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109: 735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Zhu JK, Narasimhan ML, Bressan RA, Haseqawa PM (1993) Plasma-membrane H+-ATPase gene expression is regulated by NaCl in cells of the halophyte Atriplex nummularia L. Planta 190: 433–438 [DOI] [PubMed] [Google Scholar]
- Pedroso MC, Durzan DJ (2000) Effects of different gravity environments on DNA fragmentation and cell death in Kalanchoe leaves. Ann Bot 86: 983–994 [DOI] [PubMed] [Google Scholar]
- Pedroso MC, Magalhaes JR, Durzan DJ (2000a) Nitric oxide induces cell death in Taxus cells. Plant Sci 157: 173–180 [DOI] [PubMed] [Google Scholar]
- Pedroso MC, Magalhaes JR, Durzan DJ (2000b) A nitric oxide burst precedes apoptosis in angiosperm and gymnosperm and foliar tissues. J Exp Bot 51: 1027–1036 [DOI] [PubMed] [Google Scholar]
- Qiu QS, Su XF (1998) The influence of extracellular-side Ca2+ on the activity of the plasma membrane H+-ATPase from wheat roots. Aust J Plant Physiol 25: 923–928 [Google Scholar]
- Rausch T, Kirsch M, Löw R, Lehr A, Vierck R, Zhigang A (1996) Salt stress response of higher plants: the role of proton pumps and Na+/H+ antiporters. J Plant Physiol 148: 425–433 [Google Scholar]
- Ribeiro EA, Cunha FQ, Tamashiro WMSC, Martins LS (1999) Growth phase dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett 445: 283–286 [DOI] [PubMed] [Google Scholar]
- Raven JA (1990) Predictions of Mn and Fe use efficiencies of plant growth with different energy, carbon and nitrogen sources. New Phytol 109: 279–287 [Google Scholar]
- Rayle DL, Cleland RE (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol 9: 1271–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam RK, Srivastava GC (2002) Changes in antioxidant activity in subcellular fraction of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162: 897–904 [Google Scholar]
- Serrano R (1989) Structure and function of plasma membrane ATPase. Annu Rev Plant Physiol Plant Mol 40: 61–94 [Google Scholar]
- Serrano R (1996) Salt tolerance in plants and microorganisms: toxicity targets and defence responses. Int Rev Cytol 165: 1–52 [DOI] [PubMed] [Google Scholar]
- Vázquez MD, Poschenrieder C, Corrales I, Barceló J (1999) Change in apoplastic aluminum during the initial growth response to aluminum by roots of a tolerant maize variety. Plant Physiol 119: 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Zhang CL, Liang HG (1995) Seasonal changes of polyamines in habitat adaptation of different ecotypes of reed plants. Oecologia 101: 119–123 [DOI] [PubMed] [Google Scholar]
- Yang YL, Zhang F, He WL, Wang XM, Zhang LX (2003) Iron-mediated inhibition of H+-ATPase in plasma membrane vesicles isolated from wheat roots. Cell Mol Life Sci 2003 60: 1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]