Abstract
Candida albicans is the most common human fungal pathogen. Recent evidence has revealed the occurrence of apoptosis in C. albicans that is inducible by environmental stresses such as hydrogen peroxide, acetic acid, and amphotericin B. Apoptosis is regulated by the calcineurin-caspase pathway in C. albicans, and calcineurin is under the control of Hsp90 in echinocandin resistance. However, the role of Hsp90 in apoptosis of C. albicans remains unclear. In this study, we investigated the role of Hsp90 in apoptosis of C. albicans by using an Hsp90-compromised strain tetO-HSP90/hsp90 and found that upon apoptotic stimuli, including hydrogen peroxide, acetic acid or amphotericin B treatment, less apoptosis occurred, less ROS was produced, and more cells survived in the Hsp90-compromised strain compared with the Hsp90/Hsp90 wild-type strain. In addition, Hsp90-compromised cells were defective in up-regulating caspase-encoding gene CaMCA1 expression and activating caspase activity upon the apoptotic stimuli. Investigations on the relationship between Hsp90 and calcineurin revealed that activation of calcineurin could up-regulate apoptosis but could not further down-regulate apoptosis in Hsp90-compromised cells, indicating that calcineurin was downstream of Hsp90. Hsp90 inhibitor geldanamycin (GdA) could further decrease the apoptosis in calcineurin-pathway-defect strains, indicating that compromising Hsp90 function had a stronger effect than compromising calcineurin function on apoptosis. Collectively, this study demonstrated that compromised Hsp90 reduced apoptosis in C. albicans, partially through downregulating the calcineurin-caspase pathway.
Introduction
Candida albicans is the leading fungal pathogen and may cause a variety of infections in immunocompromised individuals. The frequency of fungal infections continues to increase in concert with the growing immunocompromised patient population, including individuals infected with HIV and those undergoing chemotherapy, major surgery, or solid organ transplantation [1]–[3]. In addition, the many antifungal drugs in clinical use target ergosterol or its biosynthesis due to the limited number of drug targets available to exploit in fungal pathogens that are absent or sufficiently divergent in human hosts. Thus, many current antifungal therapies have unfortunate clinical side effects. Besides, more clinical isolates are resistant to traditional antifungal drugs [4]. Therefore it is necessary to develop new antifungal strategies. Uncovering the mechanistic basis of cell death decisions in fungi may well provide new development in the search for novel antifungal agents.
Recent evidence has revealed the occurrence of apoptosis in C. albicans which can be induced by various environmental stimuli such as hydrogen peroxide (H2O2), acetic acid (AA) and amphotericin B (AMB) [5]–[8]. C. albicans cells undergo a series of physiological changes during apoptosis, including chromatin condensation, nuclear fragmentation and increased production of reactive oxygen species (ROS). Now, ROS production is regarded as one of the typical hallmarks of apoptosis [7]–[9].
Apoptosis is a complex process involving multiple factors [9]–[16], including YCA1 [9] and calcineurin [14]. YCA1 encodes a metacaspase in Saccharomyces cerevisiae, which is functionally similar to mammalian caspase [9], [11], [13]. Upon apoptotic stimulation, metacaspase is activated, leading to the occurrence of apoptosis. We have reported previously that H2O2-induced apoptosis in C. albicans was accompanied by activation of CaMCA1, the ortholog of YCA1 [13]. Calcineurin, an important protein in Ca2+-dependent signal transduction pathway [15]–[17], is also related to apoptosis [14], [18], [19]. It consists of a catalytic subunit A (encoded by CNA1) and a regulatory subunit B (encoded by CNB1) [20], and acts on transcriptional factor Crz1p [21]–[23]. Its function can be compromised by Cyclosporin A pharmacologically. Recently, we reported that calcineurin and Crz1p are also required for H2O2-induced apoptosis in C. albicans through regulating CaMCA1 expression and caspase activity [14].
Hsp90 is a crucial molecular chaperone in stress response of C. albicans [24]–[29] and has been reported to orchestrate echinocandin resistance via regulating calcineurin pathway [30]. Calcineurin pathway is involved in apoptosis [14], [18], [19], while the role of Hsp90 in apoptosis remains unclear. In this study, we investigated the role of Hsp90 in apoptosis of C. albicans and revealed that compromised Hsp90 attenuated apoptosis through regulating calcineurin pathway and caspase activity.
Results
Compromised Hsp90 reduces apoptosis upon apoptotic stimulation
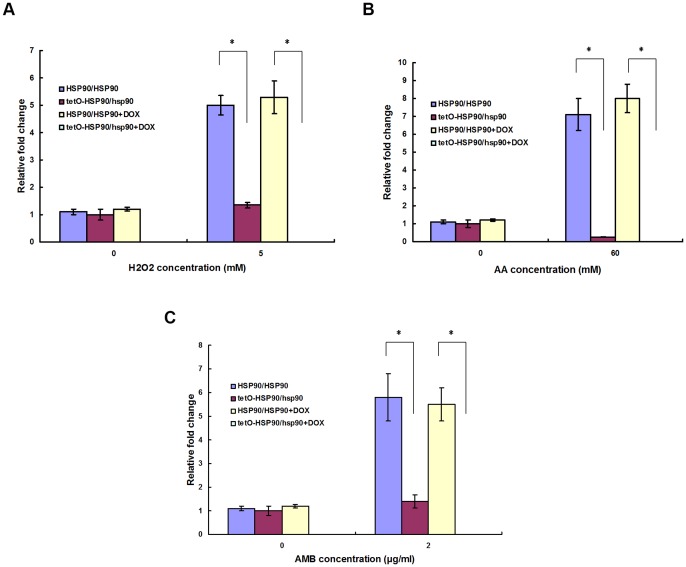
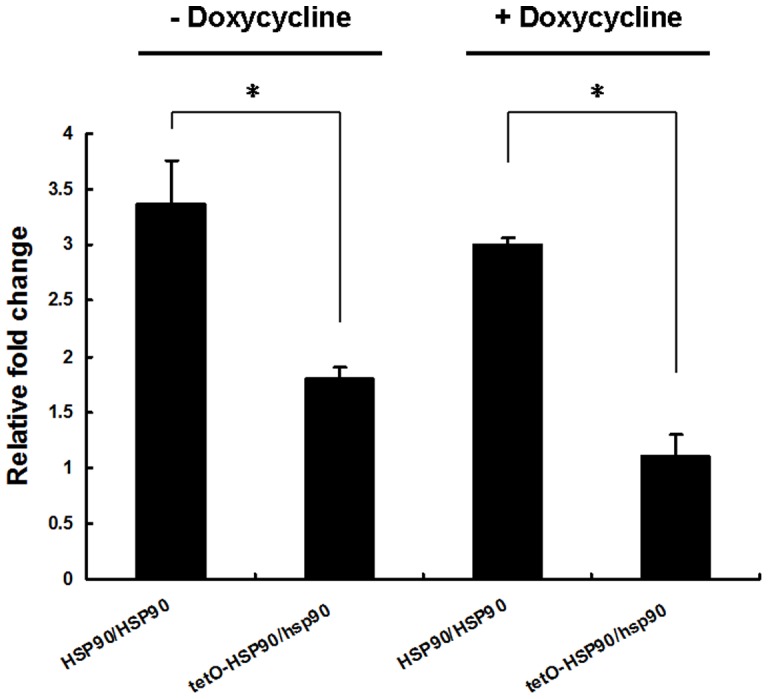
To investigate the role of Hsp90 in apoptosis of C. albicans, we compared the difference in apoptosis between wild-type HSP90/HSP90 strain and Hsp90-compromised strain upon three apoptotic stimuli. The Hsp90-compromised strain used in this study was an engineered strain tetO-HSP90/hsp90 with one copy of HSP90 knocked out, while the other copy was inserted under a tetracycline promoter Tet-off [30]. In the tetO-HSP90/hsp90 strain, the expression of tetO-HSP90 was inhibited in the presence of tetracycline or analog doxycycline (DOX), while it was induced when they were absent. Transcriptional repression of HSP90 from tetO promoter in apoptosis was confirmed by real-time PCR detection (Fig. 1). After treatment with 5 mM H2O2 for 3 h, a 5-fold increase in HSP90 expression was observed in wild-type strain. However, no change in HSP90 expression was observed in the Hsp90-compromised tetO-HSP90/hsp90 strain in the presence of 20 μg/ml DOX (Fig. 1A). Similar results were also obtained from other apoptotic stimulations including AA or AMB treatment. After exposure to 60 mM AA for 3 h, a 7-fold increase in HSP90 expression was observed in wild-type strain. In contrast, no change in HSP90 expression was seen in tetO-HSP90/hsp90 strain in the presence of 20 μg/ml DOX (Fig. 1B). After treatment with AMB, a 5.8-fold increase in HSP90 expression was observed in wild-type strain, while no change in HSP90 expression was shown in tetO-HSP90/hsp90 strain in the presence of 20 μg/ml DOX (Fig. 1C).
Figure 1. HSP90 expression after apoptotic stimulus treatment.
C. albicans cells were treated with or without apoptotic stimuli for 3 h in the absence or presence of 20 μg/ml DOX. (A) Cells treated with H2O2. (B) Cells treated with AA. (C) Cells treated with AMB. Transcriptional level of HSP90 was detected through real-time RT-PCR and normalized on the basis of the 18S level. Data are shown as mean ± SD from three independent experiments.
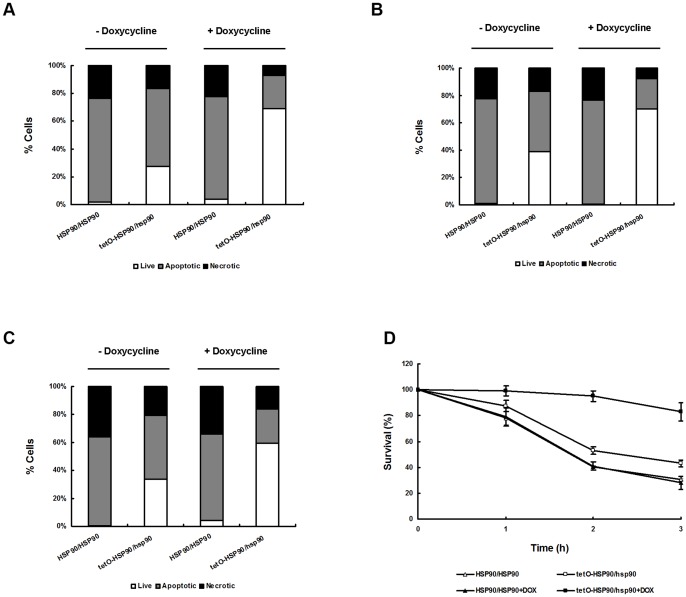
Given the different expressions of HSP90, we further investigated the impact of the apoptotic stimuli on the fate of the two strain cells using TUNEL assay to determine apoptotic cells and PI uptake assay to determine necrotic cells. After H2O2 treatment, a smaller proportion of cells were apoptotic or necrotic in the Hsp90-compromised tetO-HSP90/hsp90 strain compared to the wild-type strain, as shown in Fig. 2A and Table 1. Similar results were obtained from other apoptotic stimulations including AA (Fig. 2B and Table 2) or AMB (Fig. 2C and Table 3) treatment. These data indicated that compromised Hsp90 decreased apoptosis in C. albicans.
Figure 2. The impact of compromised Hsp90 on the fate of C. albicans cells after apoptotic stimuli.
The number of live cells (white bars), apoptotic cells (grey bars) and necrotic cells (black bars) were assessed after apoptotic stimulus treatment for 3 h in the absence or presence of 20 μg/ml DOX. (A) Cells treated with 5 mM H2O2. (B) Cells treated with 60 mM AA. (C) Cells treated with 2 μg/ml AMB. (D) After 1.25 mM H2O2 treatment for 3 h, overall viability was determined by clonogentic assays. Data are shown as mean ± SD from three independent experiments.
Table 1. The percentage of cells in C. albicans after H2O2 treatment with or without DOX1.
| Strains | Cells (%) | ||
| Live | Apoptotic | Necrotic | |
| HSP90/HSP90 | 1.88±0.01 | 74.31±0.71 | 23.81±2.57 |
| tetO-HSP90/hsp90 | 27.26±0.02* | 56.13±0.51* | 16.61±1.13 |
| HSP90/HSP90+ DOX | 3.69±0.33 | 74.10±1.34 | 22.21±1.92 |
| tetO-HSP90/hsp90+DOX | 69.12±5.13* | 23.64±0.74* | 7.24±0.97* |
Three independent experiments were carried out and the results were presented as the mean ± SD. * indicated p<0.05 compared with the wild-type strain in the same DOX concentration.
Table 2. The percentage of cells in C. albicans after AA treatment with or without DOX2.
| Strains | Cells (%) | ||
| Live | Apoptotic | Necrotic | |
| HSP90/HSP90 | 0.73±0.01 | 77.00±2.01 | 22.27±1.88 |
| tetO-HSP90/hsp90 | 39.01±1.45* | 44.00±0.76* | 16.99±1.58 |
| 0.44±0.01 | 76.02±1.09 | 23.54±0.97 | |
| tetO-HSP90/hsp90+DOX | 70.21±2.33* | 22.01±0.04* | 7.78±0.21* |
Three independent experiments were carried out and the results were presented as the mean ± SD. * indicated p<0.05 compared with the wild-type strain in the same DOX concentration.
Table 3. The percentage of cells in C. albicans after AMB treatment with or without DOX3.
| Strains | Cells (%) | ||
| Live | Apoptotic | Necrotic | |
| HSP90/HSP90 | 0.47±0.01 | 63.58±0.77 | 35.95±1.66 |
| tetO-HSP90/hsp90 | 33.67±3.11* | 45.44±0.94* ` | 20.89±0.46* |
| HSP90/HSP90+ DOX | 4.01±0.37 | 61.99±0.31 | 34.00±0.11 |
| tetO-HSP90/hsp90+DOX | 59.52±0.37* | 24.88±0.23* | 15.60±0.97* |
Three independent experiments were carried out and the results were presented as the mean ± SD. * indicated p<0.05 compared with the wild-type strain in the same DOX concentration.
The overall viability experiment was carried out to analyze the fate of fungal cells after apoptotic stimulation. After treatment with H2O2, tetO-HSP90/hsp90 cells survived longer than wild-type cells (Fig. 2D). After exposure to 1.25 mM H2O2 for 3 h, a higher survival percentage was observed in tetO-HSP90/hsp90 cells as compared with wild-type strain cells. These data indicated that tetO-HSP90/hsp90 cells had more probability to survive upon apoptotic stimulation, which is consistent with the results of TUNEL and PI uptake assays.
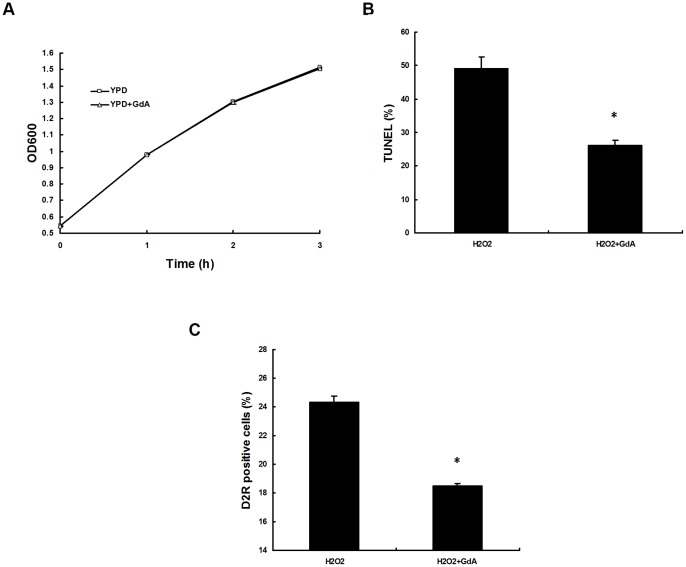
The role of Hsp90 in apoptosis was further investigated using an Hsp90 inhibitor, geldanamycin (GdA) (Fig. 3). The GdA concentration used in the experiment did not affect the growth of the normal strain without addition of the apoptotic stimuli, and there was no significant difference in OD600 between the blank control group and GdA group (Fig. 3A). However, the addition of GdA significantly decreased the proportion of apoptotic cells after 1.25 mM H2O2 treatment for 3 h (P<0.05, Fig. 3B), which is consistent with the results obtained from genetic depletion of HSP90.
Figure 3. Pharmacological inhibition of Hsp90 with GdA decreased apoptosis and caspase activity of C. albicans.
Wild-type HSP90/HSP90 strain cells were exposed to 1.25 mM H2O2 for 3 h in the absence or presence of GdA (0.5 μM). (A) The effect of 0.5 μM GdA on growth by OD600. C. albicans cells were grown in YPD to exponential phase (OD600 = 0.5) and then treated with or without 0.5 μM GdA. OD600 was detected at each time point. (B) Apoptotic cells were classified by TUNEL using flow cytometry. (C) Caspase activity was determined by D2R dye by flow cytometry. Results are shown as mean ± SD from three independent experiments. * indicates p<0.05 compared to the wild-type cells.
Compromised Hsp90 reduces ROS production upon apoptotic stimulation
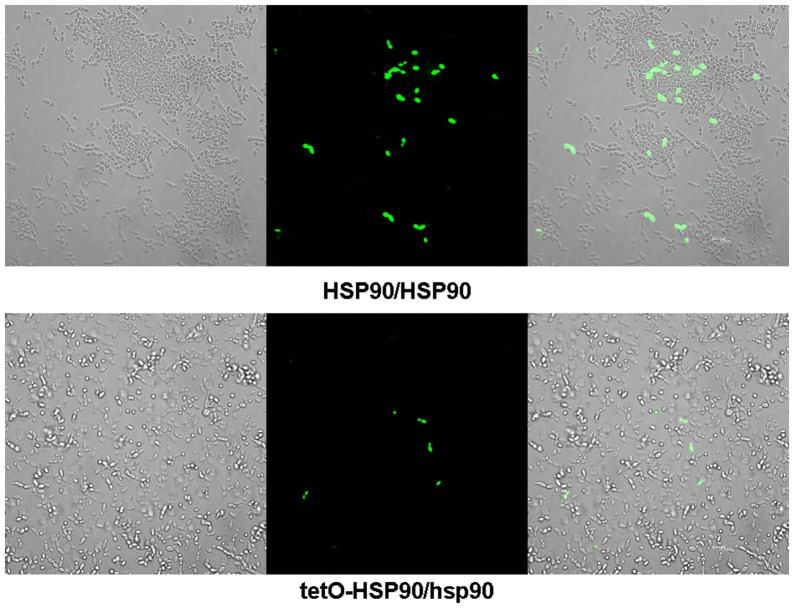
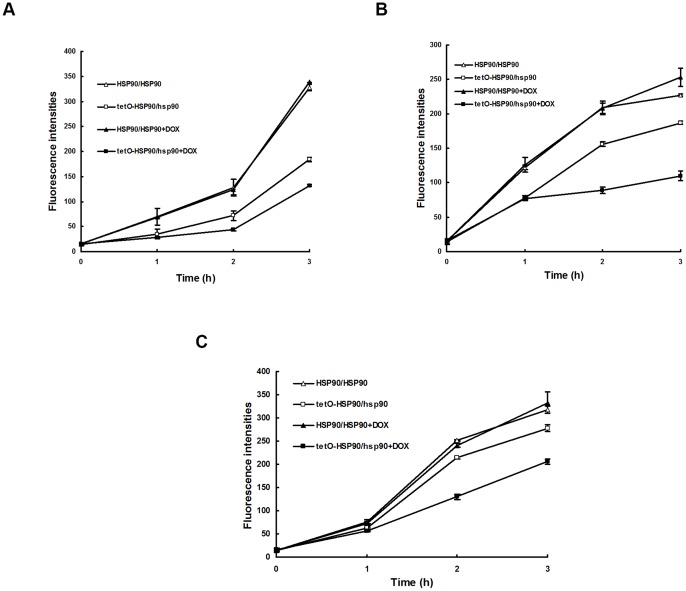
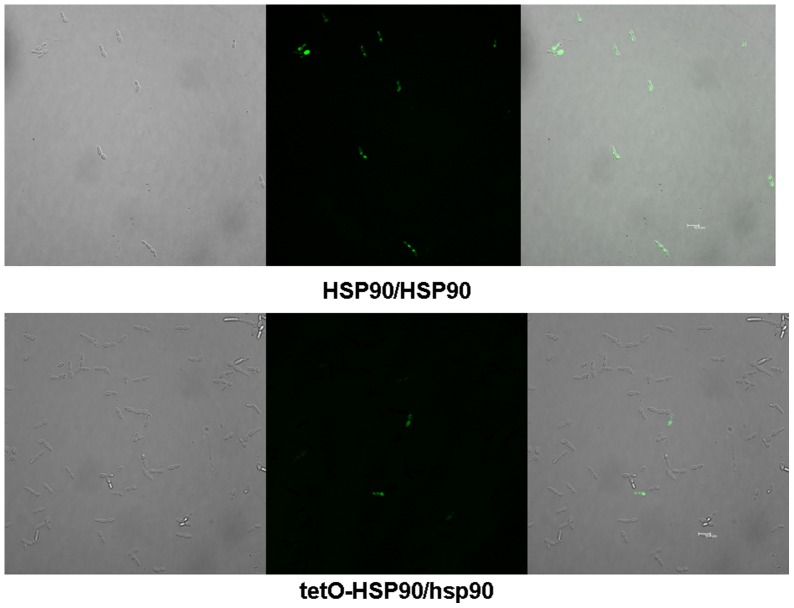
We further investigated the impact of Hsp90 compromise on ROS production, knowing that ROS is regarded as one of the typical hallmarks of apoptosis. The results indicated that compromised Hsp90 reduced ROS production upon various apoptotic stimulations. The result of confocal laser microscopy showed that the fluorescence signal indicating intracellular ROS was obviously strong in HSP90/HSP90 cells after 5 mM H2O2 treatment for 3 h, while it was relatively weak in HSP90 depleted tetO-HSP90/hsp90 cells in the presence of 20 μg/ml DOX (Fig. 4). This result was also confirmed by flow cytometry. When genetic depletion of HSP90 was done by adding doxycycline, intracellular ROS in H2O2-treated tetO-HSP90/hsp90 cells decreased to about 50% of that in HSP90/HSP90 cells (Fig. 5A). Similar results were obtained by using 60 mM AA (Fig. 5B) or 2 μg/ml AMB (Fig. 5C) as the apoptotic stimuli. So after genetic depletion of HSP90, intracellular ROS production was seen to significantly decrease in tetO-HSP90/hsp90 cells compared with that in wild-type cells upon the same apoptotic stimulation, which is consistent with the results shown above.
Figure 4. Representative confocal scanning laser fluorescence images of HSP90/HSP90 and tetO-HSP90/hsp90 cells stained for ROS accumulation following exposure to 5 mM H2O2 for 3 h in the presence of DOX.
Bar represents 10.8 μm.
Figure 5. Intracellular ROS production after apoptotic stimuli.
Cells were exposed to apoptotic stimuli for 3 h in the absence or presence of 20 μg/ml DOX, and intracellular ROS was detected by DCFH-DA. (A) Quantitative assay of ROS generation after 5 mM H2O2 treatment. (B) Quantitative assay of ROS generation after 60 mM AA treatment. (C) Quantitative assay of ROS generation after 2 μg/ml AMB treatment. Data shown are mean ± SD from three independent experiments.
Compromised Hsp90 leads to defects in caspase activation upon apoptotic stimuli
To determine whether Hsp90 contributed to apoptosis through regulating CaMCA1/caspase pathway, we investigated the impact of Hsp90 on H2O2-induced CaMCA1 expression and caspase activity by using real-time PCR and confocal laser microscopy.
After 5 mM H2O2 treatment for 3 h, a 3-fold increase in CaMCA1 expression was observed in the wild-type strain, while, only a 1.1-fold increase was observed in tetO-HSP90/hsp90 strain after compromising HSP90 expression (Fig. 6), indicating that the Hsp90-compromised strain was defected and unable to activate CaMCA1 expression upon H2O2 stimulus.
Figure 6. CaMCA1 expression after H2O2 treatment.
C. albicans cells were treated with 5 mM H2O2 for 3 h in the absence or presence of 20 μg/ml DOX. Transcriptional level of CaMCA1 was detected by real-time RT-PCR and normalized on the basis of the 18S level. Data are shown as mean ± SD from three independent experiments. * indicates p<0.05 compared to the wild-type cells.
Similar results were obtained from the caspase activity detection by using D2R staining. After 5 mM H2O2 treatment for 3 h in the presence of 20 μg/ml DOX, the percentage of wild-type cells stainable by D2R was 71.99%, while the percentage of tetO-HSP90/hsp90 cells was 35.42% (p<0.05, Table 4), indicating that caspase activity was decreased in tetO-HSP90/hsp90 cells. The results obtained from confocal laser microscopy confirmed the above result: the number of D2R staining positive cells from HSP90/HSP90 cells was significantly larger than that from HSP90 depleted tetO-HSP90/hsp90 cells after the same treatment (Fig. 7).
Table 4. Caspase activity of C. albicans cells after H2O2 treatment4.
| Strains | 5 mM H2O2 |
| HSP90/HSP90 | 72.81±1.32 |
| tetO-HSP90/hsp90 | 57.92±1.37* |
| HSP90/HSP90+DOX | 71.99±0.48 |
| tetO-HSP90/hsp90+DOX | 35.42±1.49* |
After treatment with or without 20 μg/ml DOX for 3 h, caspase activity of C. albicans cells was detected flow cytometry after D2R staining. Three independent experiments were carried out and the results were presented as the mean ± SD of D2R positive cells percentage. * indicated p<0.05 compared with the wild-type strain group.
Figure 7. Representative confocal scanning laser fluorescence images of HSP90/HSP90 and tetO-HSP90/hsp90 cells stained for caspase activity following exposure to 5 mM H2O2 for 3 h in the presence of DOX.
Bar represents 10.8 μm.
Consistent results were obtained by using 60 mM AA (Table 5) and 2 μg/ml AMB (Table 6) as the apoptotic stimuli. After 60 mM AA treatment for 3 h with DOX, the percentage of D2R stainable cells was 42.43% in the wild-type strain, while it significantly decreased to 18.59% in the tetO-HSP90/hsp90 strain (p<0.05). After 2 μg/ml AMB treatment for 3 h by given DOX, the percentage of D2R stainable cells were 51.92% in the wild-type strain, while it significantly decreased to 22.83% in the tetO-HSP90/hsp90 strain (p<0.05), indicating that caspase activation was defective in HSP90 depleted tetO-HSP90/hsp90 cells.
Table 5. Caspase activity of C. albicans cells after AA treatment5.
| Strains | 60 mM AA |
| HSP90/HSP90 | 43.61±0.40 |
| tetO-HSP90/hsp90 | 27.90±1.51* |
| HSP90/HSP90+DOX | 42.43±3.38 |
| tetO-HSP90/hsp90+DOX | 18.59±0.16* |
After treatment with or without 20 μg/ml DOX for 3 h, caspase activity of C. albicans cells was detected flow cytometry after D2R staining. Three independent experiments were carried out and the results were presented as the mean ± SD of D2R positive cells percentage. * indicated p<0.05 compared with the wild-type strain group.
Table 6. Caspase activity of C. albicans cells after AMB treatment6.
| Strains | 2 μg/ml AMB |
| HSP90/HSP90 | 50.72±0.49 |
| tetO-HSP90/hsp90 | 32.22±0.45* |
| HSP90/HSP90+DOX | 51.92±2.34 |
| tetO-HSP90/hsp90+DOX | 22.83±0.46* |
After treatment with or without 20 μg/ml DOX for 3 h, caspase activity of C. albicans cells was detected flow cytometry after D2R staining. Three independent experiments were carried out and the results were presented as the mean ± SD of D2R positive cells percentage. * indicated p<0.05 compared with the wild-type strain group.
To further reveal the impact of Hsp90 on caspase activity, Hsp90 inhibitor geldanamycin was also exploited (Fig. 3C). As shown in Fig. 3C, addition of GdA significantly decreased the proportion of D2R stainable cells upon H2O2 treatment (P<0.05, Fig. 3C), which is consistent with the result obtained from genetic depletion of HSP90.
The role of calcineurin in apoptosis of Hsp90 compromised cells
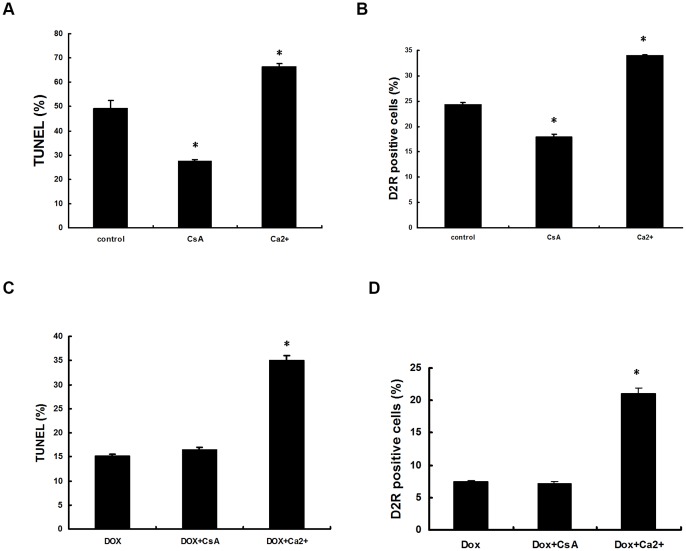
We reported in our previous study that calcineurin pathway was required for H2O2-induced C. albicans apoptosis through regulating CaMCA1 expression and caspase activity. Our interest in this study was the relationship between calcineurin and Hsp90 in apoptosis. Based on previous publications [27], [28], [30], calcineurin was assumed to be downstream of Hsp90 and regulated by Hsp90. In this work, we confirmed that calcineurin was downstream of Hsp90 in apoptosis: activated calcineurin still up-regulated apoptosis in Hsp90-compromised cells, and inactivated calcineurin was unable to further down-regulate apoptosis in Hsp90-compromised cells. Our previous study found that cyclosporin A (CsA) at the concentration of 0.08 μM decrease apoptosis by inhibiting calcineurin [14], and therefore we used this concentration in this study. Briefly, compromising the calcineurin function by CsA administration significantly decreased apoptosis in wild-type HSP90/HSP90 strain cells (Fig. 8A), while it was unable to further decrease apoptosis in Hsp90-compromised strain cells (Fig. 8C). However, activating calcineurin function by giving 1mmol Ca2+ significantly increased apoptosis in both wild-type HSP90/HSP90 and Hsp90-compromised strain cells after 1.25 mM H2O2 treatment through TUNEL assay (Fig. 8A and Fig. 8C). The similar result was also obtained from caspase activity detection using D2R dye (Fig. 8B and Fig. 8D). These results indicate that calcineurin was downstream of Hsp90 in the apoptosis-regulating pathway.
Figure 8. Calcineurin is downstream of Hsp90 in apoptosis.
C. albicans cells were exposed to 1.25 mM H2O2 for 3 h in the absence or presence of CaCl2 (1 mM) or CysA (0.08 μM). (A) and (B) are wild-type HSP90/HSP90 strain cells. (C) and (D) are tetO-HSP90/hsp90 cells in the presence of 20 μg/ml DOX. Results are shown as mean ± SD from three independent experiments. * indicates p<0.05 compared to the wild-type cells.
Compromising Hsp90 function has a stronger effect than compromising calcineurin function on apoptosis
To investigate the calcineurin function in Hsp90-mediated apoptosis, further experiments were carried out. Hsp90 inhibitor GdA was used on both wild-type strain (CAF2-1) and the calcineurin-pathway-defect strain, including crz1Δ mutant (DSY2195) and cna1Δ mutant (DSY2091). The calcineurin-pathway-defect genotype was confirmed because the addition of Ca2+ was unable to up-regulate apoptosis in DSY2195 and DSY2091 upon H2O2 treatment. Addition of Hsp90 inhibitor GdA also significantly decreased apoptosis of the two calcineurin-pathway-defect strains, DSY2195 and DSY2091 (P<0.05, Fig. 9) upon H2O2 treatment, indicating that compromising Hsp90 function had a stronger effect than compromising calcineurin function on apoptosis.
Figure 9. The impact of compromised Hsp90 on calcinerin-pathway-defect cells.
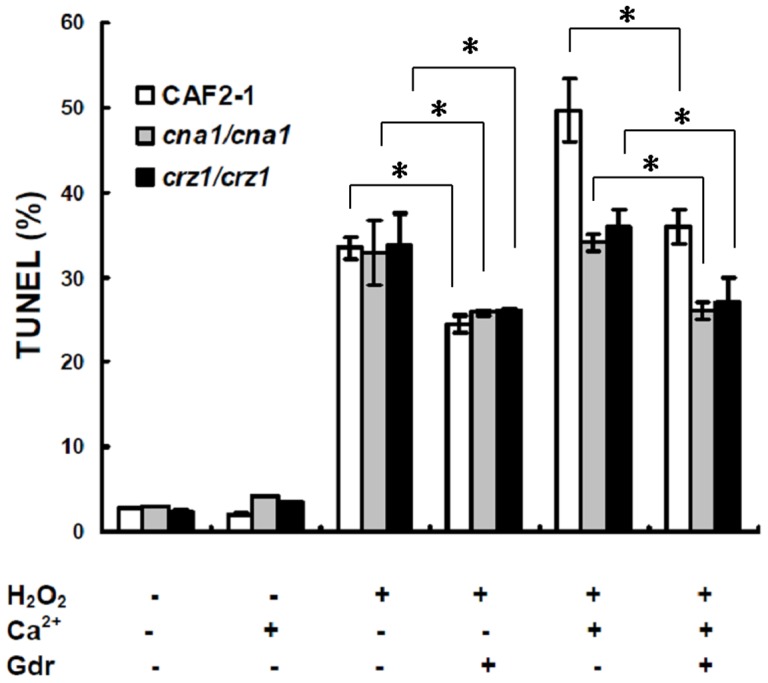
C. albicans cells were treated with 1.25 mM H2O2 for 3 h in the absence or presence of CaCl2 (1 mM) or GdA (0.5 μM). Data are shown as mean ± SD from three independent experiments. * indicates p<0.05 compared to the same strain, same H2O2 and CaCl2 treatment, and without GdA treatment.
Discussion
In recent years, the role of Hsp90 in apoptosis has been extensively investigated in mammalian cells [31]–[34]. It was reported that Hsp90 expression increased apoptosis in monoblastoid cell line U937 [31], while other studies [32] reported that Hsp90 played a protective role against 3-hydroxykynurenine-induced apoptosis in neurons. Further investigations in mammalian cells demonstrated that Hsp90 was involved in apoptosis through regulating caspase-9 and caspase-3 [33]. In contrast to mammalian cells, the role of Hsp90 in C. albicans apoptosis remained unclear before this study. In this work, we demonstrated that compromised Hsp90 reduced apoptosis in C. albicans. We first investigated the impact of Hsp90 compromise on apoptosis and cell fate. Upon application of the three different apoptotic stimuli used in this study, Hsp90-compromised tetO-HSP90/hsp90 cells tended to develop less apoptosis, less necrosis, longer survival and less ROS production compared with HSP90/HSP90 wild-type cells. Similar results were obtained by using the Hsp90 inhibitor GdA. Secondly, we investigated the impact of Hsp90 compromise on CaMCA1 gene expression and caspase activity. Hsp90-compromised cells were defective in its ability to up-regulate CaMCA1 gene expression and to activate caspase activity upon all the apoptotic stimulations, which are consistent with the previous results. Thirdly, we investigated the relationship between calcineurin and Hsp90. Previous studies [24]–[30] demonstrated that calcineurin was regulated by Hsp90 in echinocandin resistance. In this study, we confirmed that calcineurin was downstream of Hsp90 in causing apoptosis because activated calcineurin could still up-regulate apoptosis in Hsp90-compromised cells while inactivated calcineurin could not further down-regulate apoptosis in Hsp90-compromised cells. Finally, we found that Hsp90 inhibitor GdA could further decrease apoptosis in calcineurin-pathway-defect strains, indicating that compromising Hsp90 function had a stronger effect than compromising calcineurin function on apoptosis.
The reduced C. albicans apoptosis in Hsp90-compromised cells was linked to the defect of CaMCA1 up-regulation and caspase activation. Caspase activation has been recognized as the most important process linked to apoptosis in mammalian cells [35]. Upon a death stimulus, the initiator caspases were activated, and then a second group of caspases were activated, leading to programmed cell death or apoptosis [36]–[40]. In yeasts, apoptosis is also associated with the activation of the caspase (or named metacaspase) [7]–[9]. The relationship between caspase activation and apoptosis in mammalian cells and in yeasts is consistent with our findings in this study.
Calcineurin was demonstrated to be regulated by Hsp90 in other biochemical processes [26]–[28]. Our findings confirmed the relationship between calcineurin and Hsp90 in apoptosis of C. albicans. In the presence of calcium ions, calcineurin can be activated, and regulate its target genes [15]–[17]. Here, we demonstrated that with calcium ions, the activated calcineurin could up-regulate apoptosis, while inactivated calcineurin could not further down-regulate apoptosis in Hsp90-compromised cells, indicating that calcineurin was downstream of Hsp90 in the apoptosis-regulating pathway (Fig. 10). Upon apoptotic stimuli, Hsp90 was activated and CaMCA1 expression was elevated. Elevation in CaMCA1 expression resulted in increased caspase activity, thus inducing apoptosis. Calcineurin inhibitor cyclosporin A or Hsp90 inhibitor geldanamycin could block this pathway.
Figure 10. A model for the role of Hsp90 in regulating apoptosis in C. albicans.
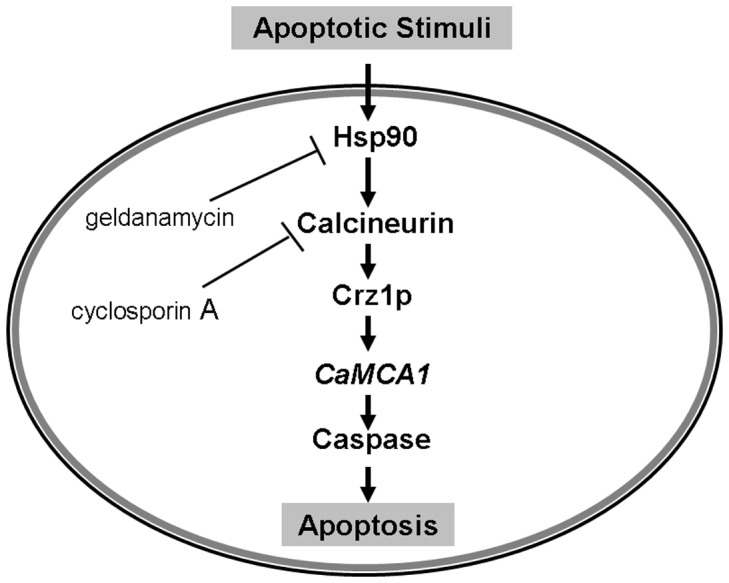
Upon apoptotic stimuli, Hsp90 was activated and CaMCA1 expression was elevated. Elevation in CaMCA1 expression resulted in increased caspase activity, thus inducing apoptosis. Calcineurin inhibitor cyclosporin A or Hsp90 inhibitor geldanamycin can block this pathway.
Hsp90 inhibitor GdA could further decrease apoptosis in calcineurin-pathway-defect strains, including crz1Δ mutant and cna1Δ mutant, indicating that compromising Hsp90 function had a stronger effect than compromising calcineurin function on apoptosis. Compromising calcineurin function with cyclosporin A or compromising Hsp90 function (either with geldanamycin or genetically) has essentially identical effects on apoptosis. Consistent with this observation, inhibiting both pathways together has the same result as inhibiting either pathway alone. However, inhibition of Hsp90 has a stronger effect than genetic deletion of either CNA1 or CRZ1. In their study of exploring roles of Hsp90 and calcineurin in echinocandin responses, Singh et al [30] found that genetic deletion of CNA1 and CRZ1 had weaker effects on echinocandin tolerance than genetic deletion of CNB1 or treatment with cyclosporin A. Therefore, it is possible that combination of a CNB1 mutation with Hsp90 inhibition would have the same phenotype as deletion of CNB1 or Hsp90 inhibition. Therefore, further experiments are needed to investigate the underlying mechanism.
Hsp90 is known as an essential molecular chaperone that regulates the stability and function of a variety of proteins, many of which act as regulators of cellular signaling [24]–[26]. A recent proteomic and genomic study in yeast [41] revealed that 198 putative physical interactions and 451 putative genetic and chemical-genetic interactions were connected with Hsp90. Besides the calcineurin pathway, other pathways that are under the regulation of Hsp90 remain to be studied in future.
In summary, we have demonstrated that compromised Hsp90 reduced apoptosis in C. albicans partially through regulating the calcineurin-caspase apoptotic pathway. This work may open the door for the study of Hsp90 in apoptosis of C. albicans.
Materials and Methods
Strains and growth conditions
C. albicans strains used in this study are listed in Table 7. C. albicans cells were grown in yeast extract/peptone/dextrose (YPD) broth as described previously [7], [29], [30]. Briefly, cells were grown overnight in YPD at 30°C, diluted to OD600 of 0.2 with 20 μg/ml doxycycline as indicated, and grown overnight. Cells were then diluted to OD600 of 0.2 in the same conditions and grown to mid-log phase. For hydrogen peroxide, acetic acid or amphotericin B treatment, C. albicans cells were harvested and resuspended in the same conditions containing hydrogen peroxide, acetic acid or Amphotericin B.
Table 7. C. albicans strains used in this study.
| Strain | Parental strain | Genotype | Reference |
| HSP90/HSP90(SN95) | SC5314 | arg4Δ/arg4Δhis1Δ/his1Δ URA3/ura3::imm434 | Noble et al., 2005 |
| tetO-HSP90/hsp90 | SN95 | hsp90::CdHIS1/tetO-HSP90 | Singh et al., 2009 |
| CAF2-1 | SC5314 | ura3 Δ ::imm434/URA3 | Fonzi et al., 1993 |
| DSY2195 | DSY2188 | crz1Δ::hisG/crz1Δ::hisG::URA3::hisG | Karababa et al., 2006 |
| DSY2091 | CAF4-2 | cnaΔ::hisG/cnaΔ::hisG::URA3::hisG | Sanglard et al.,2003 |
Drugs
Hydrogen peroxide (H2O2), acetic acid (AA), amphotericin B (AMB), Geldanamycin (GdA), Doxycycline (DOX), Calcium chloride (CaCl2) and cyclosporin A (CsA) are purchased from Sigma-Aldrich.
Quantitative Real-time RT-PCR
RNA isolation, cDNA synthesis and real-time RT-PCR amplification were performed as described previously [6], [14]. Exponentially growing C. albicans cells were treated with H2O2 or sterile water as the control for 3 h before RNA isolation. Experiments were carried out using the Chromo 4 Real-Time PCR System (Bio-Rad, USA). SYBR Green I (Takara) was used to monitor the amplified products. Gene-specific primers were designed according to the manufacturer's protocol. Primers for HSP90 were 5′-GGGAATCTAACGCTGGTGGTAA-′3 and 5′- TTCGGTTTCTGGAACTTCTTTT-3′; primers for CaMCA1 were 5′-TATAATAGACCTTCTGGAC-3′ and 5′-TTGGTGGACGAGAATAATG -3′; and primers for 18S rRNA were 5′-TCTTTCTTGATTTTGTGGGTGG-3′ and 5′- TCGATAGTCCCTCTAAGAAGTG-3′. The C Τ value of 18S rRNA was subtracted from that of the gene of interest to obtain a ΔC Τ value. The ΔC Τ value of an arbitrary calibrator (e.g. an untreated control group) was subtracted from the ΔC Τ value for each sample to obtain a ΔΔC Τ value. The gene expression level relative to the calibrator was expressed as 2−ΔΔCΤ. Triplicate independent experiments were conducted to generate a mean value.
Apoptosis, necrosis and overall viability assays
C. albicans cells were grown in YPD to exponential phase and then treated with different apoptotic stimuli for 3 h. To investigate the occurrence of apoptosis, a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay by In Situ Cell Death Detection Kit (Roche Applied Sciences, Mannheim, Germany) was performed according to the manufacturer's instructions [7], [8]. In Brief, C. albicans cells were washed twice in PBS and then fixed with 3.6% paraformaldehyde. Cell samples were stored at 4°C until required. Cells were rinsed twice with PBS and then incubated with permeabilization solution for 2 minutes on ice. The cells were rinsed in PBS and labeled, using a solution of the label and enzyme solutions from In Situ Cell Death Detection Kit, appropriate controls labeled only with the label solution. The cells were incubated for 1 h at 37°C in a humidified atmosphere in the dark, rinsed in PBS. The number of cells determined to be positive by the TUNEL assay was quantified using a BD FACSCalibur flow cytometer with excitation and emission wavelength settings at 488 and 520 nm, respectively. Necrosis were assessed by detecting propidium iodide (PI) uptake of the C. albicans cells, using PI at the concentration of 20 μg/ml. Overall viability was assessed by using clonogenic assays. Cells at 1×107 per ml were diluted in series and plated in triplicate on YPD and then incubated at 30°C for 48 h. Statistical analyses were performed using ANOVA. P<0.05 was considered significant.
Caspase activity determination
Caspase activity was determined by detecting D2R staining using CaspSCREENTM Flow Cytometric Apoptosis Detection Kit (BioVision, U.S.A.) [14]. The culture samples were incubated according to the manufacturer's assay instructions and analyzed using flow cytometry with excitation at 488 nm and emission at 530 nm respectively.
Measurement of ROS
Intracellular levels of ROS were measured using DCFH-DA (Molecular Probes, U.S.A.) [6], [14]. Briefly, exponentially growing C. albicans cells were collected by centrifugation and washed three times with PBS. Subsequently, the cell samples were adjusted to 2×107 cells/ml. After incubation with 20 μg/ml of DCFH-DA for 30 min at 30°C, the cells were exposed to apoptotic stimuli and incubated at 30°C with constant shaking (200 rpm). At specified interval, cell samples were observed with a Leica TCS sp2 confocal scanning laser microscope with excitation at 485 nm and emission at 520 nm. Alternatively 1 ml of cell suspension was harvested and 100 μl of the supernatant was transferred to the wells of a flat-bottom microplate (BMG Microplate, 96well, Blank) to detect fluorescence intensities on the POLARstar Galaxy (BMG, Labtech, Offenburg, Germany) with excitation at 485 nm and emission at 520 nm. Each experiment was performed in triplicate.
Statistical analysis
All experiments were performed in triplicate and at least three independent experiments were done on separate occasions. Data are presented as mean ± SD, and the ANOVA test was employed to determine the statistical significance between experimental groups. The difference was considered significant if the P value was less than 0.05.
Acknowledgments
We thank Dr. William A. Fonzi for providing C. albicans strain CAF2-1, Dr. Leah E. Cowen for providing C. albicans strains HSP90/HSP90 (SN95) and tetO-HSP90/hsp90, and Dr. Dominique Sanglard for providing C. albicans strains DSY2091 and DSY2195.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81072678, 30825041 and 90913008) and Postdoctoral Science Foundation of China (20110491851). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hamza OJ, Matee MI, Brüggemann RJ, Moshi MJ, Simon EN, et al. (2008) Single-dose fluconazole versus standard 2-week therapy for oropharyngeal candidiasis in HIV-infected patients: a randomized, double-blind, double-dummy trial. Clin Infect Dis 47: 1270–1276. [DOI] [PubMed] [Google Scholar]
- 2. Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20: 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, et al. (2005) The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 41: 1232–1239. [DOI] [PubMed] [Google Scholar]
- 4. Mah TF, O'Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9: 34–9. [DOI] [PubMed] [Google Scholar]
- 5. Zhu J, Krom BP, Sanglard D, Intapa C, Dawson CC, et al. (2011) Farnesol-induced apoptosis in Candida albicans is mediated by Cdr1-p extrusion and depletion of intracellular glutathione. PLoS ONE 6(12): e28830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dai BD, Cao YY, Huang S, Xu YG, Gao PH, et al. (2009) Baicalein induces programmed cell death in Candida albicans . J Microbiol Biotechnol 19: 803–809. [PubMed] [Google Scholar]
- 7. Phillips AJ, Crowe JD, Ramsdale M (2006) Ras pathway signaling accelerates programmed cell death in the pathogenic fungus Candida albicans . Proc Natl Acad Sci U S A 103: 726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phillips AJ, Sudbery I, Ramsdale M (2003) Apoptosis induced by environmental stresses and amphotericin B in Candida albicans . Proc Natl Acad Sci U S A 100: 14327–14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan MA, Chock PB, Stadtman ER (2005) Knockout of caspase-like gene, YCA1, abrogates apoptosis and elevates oxidized proteins in Saccharomyces cerevisiae . Proc Natl Acad Sci U S A 102: 17326–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trompier D, Chang XB, Barattin R, Moulinet d'Hardemare A, Di Pietro A, et al. (2004) Verapamil and its derivative trigger apoptosis through glutathione extrusion by multidrug resistance protein MRP1 . Cancer Res 64(14): 4950–4956. [DOI] [PubMed] [Google Scholar]
- 11. Guaragnella N, Pereira C, Sousa MJ, Antonacci L, Passarella S, et al. (2006) YCA1 participates in the acetic acid induced yeast programmed cell death also in a manner unrelated to its caspase-like activity. FEBS Lett 580: 6880–6884. [DOI] [PubMed] [Google Scholar]
- 12. Baek YU, Kim YR, Yim HS, Kang SO (2004) Disruption of γ-glutamylcysteine synthetase results in absolute glutathione auxotrophy and apoptosis in Candida albicans . FEBS Lett 556: 47–52. [DOI] [PubMed] [Google Scholar]
- 13. Cao YY, Huang S, Dai BD, Zhu ZY, Lu H, et al. (2009) Candida albicans cells lacking CaMCA1-encoded metacaspase show resistance to oxidative stress induced death and change in energy metabolism. Fungal Genet Biol 46: 183–189. [DOI] [PubMed] [Google Scholar]
- 14. Lu H, Zhu Z, Dong L, Jia X, Sun X, et al. (2011) Lack of trehalose accelerates H2O2-induced Candida albicans apoptosis through regulating Ca2+ signaling pathway and caspase activity. PLoS ONE 6(1): e15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, et al. (2002) Calcineurin is essential for survival during membrane stress in Candida albicans . EMBO J 21: 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinbach WJ, Reedy JL, Cramer RA Jr, Perfect JR, Heitman J, et al. (2007) Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 5: 418–430. [DOI] [PubMed] [Google Scholar]
- 17. Rusnak F, Mertz P (2000) Calcineurin: Form and function. Physiol Rev 80: 1483–1521. [DOI] [PubMed] [Google Scholar]
- 18. Wang L, Chang JH, Paik SY, Tang Y, Eisner W, et al. (2011) Calcineurin (CN) activation promotes apoptosis of glomerular podocytes both in vitro and in vivo. Mol Endocrinol 25: 1376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun X, Wu Y, Chen B, Zhang Z, Zhou W, et al. (2011) Regulator of calcineurin 1 (RCAN1) facilitates neuronal apoptosis through caspase-3 activation. J Biol Chem 286: 9049–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J (2003) Calcineurin A of Candida albicans: Involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol 48: 959–976. [DOI] [PubMed] [Google Scholar]
- 21. Santos M, de Larrinoa IF (2005) Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr Genet 48: 88–100. [DOI] [PubMed] [Google Scholar]
- 22. Karababa M, Valentino E, Pardini G, Coste AT, Bille J (2006) CRZ1, a target of the calcineurin pathway in Candida albicans . Mol Microbiol 59: 1429–1451. [DOI] [PubMed] [Google Scholar]
- 23. Stathopoulos AM, Cyert MS (1997) Calcineurin acts through the CRZ1/TCN1- encoded transcription factor to regulate gene expression in yeast. Gene Dev 11: 3432–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cowen LE, Singh SD, Köhler JR, Collins C, Zaas AK, et al. (2009) Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A 106: 2818–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, et al. (2006) Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 440(7087): 1013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cowen LE, Lindquist S (2005) Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309: 2185–2189. [DOI] [PubMed] [Google Scholar]
- 27. Robbins N, Uppuluri P, Nett J, Rajendran R, Ramage G, et al. (2011) Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog 7: e1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. LaFayette SL, Collins C, Zaas AK, Schell WA, Betancourt-Quiroz M, et al. (2010) PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog 6: e1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, et al. (2009) Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol 19: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, et al. (2009) Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via Calcineurin. PLoS Pathog 5: e1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galea-Lauri J, Richardson AJ, Latchman DS, Katz DR (1996) Increased heat shock protein 90 (hsp90) expression leads to increased apoptosis in the monoblastoid cell line U937 following induction with TNF-alpha and cycloheximide: a possible role in immunopathology. J Immunol 157: 4109–4118. [PubMed] [Google Scholar]
- 32. Lee MW, Park SC, Chae HS, Bach JH, Lee HJ, et al. (2001) The protective role of HSP90 against 3-hydroxykynurenine-induced neuronal apoptosis. Biochem Biophys Res Commun 284(2): 261–7. [DOI] [PubMed] [Google Scholar]
- 33. Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, et al. (2000) Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J 19(16): 4310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rezzani R, Rodella L, Dessy C, Daneau G, Bianchi R, et al. (2003) Changes in Hsp90 expression determine the effects of cyclosporine A on the NO pathway in rat myocardium. FEBS Lett 552(2–3): 125–9. [DOI] [PubMed] [Google Scholar]
- 35. Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, et al. (1996) Human ICE/CED-3 protease nomenclature. Cell 87(2): 171. [DOI] [PubMed] [Google Scholar]
- 36. Salvesen GS, Dixit VM (1997) Caspases: intracellular signaling by proteolysis. Cell 91(4): 443–6. [DOI] [PubMed] [Google Scholar]
- 37. Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, et al. (2012) Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148(5): 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, et al. (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91(4): 479–89. [DOI] [PubMed] [Google Scholar]
- 39. Kirsch DG, Santiago PM, di Tomaso E, Sullivan JM, Hou WS, et al. (2010) p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 327(5965): 593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, et al. (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325(5938): 332–6. [DOI] [PubMed] [Google Scholar]
- 41. Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, et al. (2005) Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120(5): 715–27. [DOI] [PubMed] [Google Scholar]