INTRODUCTION
Despite intense research aimed at the development of effective therapeutic interventions, the means of preventing ischemic brain damage remain elusive. Among numerous therapies under current testing, approaches based on the stimulation of adenosine A1 receptors offer a substantial promise in reducing ischemia-related structural and functional damage.1–4
Multiple studies have shown that acute stimulation of adenosine A1 receptors leads to the attenuation of several cellular phenomena that constitute a part of the pathophysiological complex triggered by the arrest of the cerebral blood flow.1,2,4–7 Based upon the concept of neuroprotective effects of adenosine, several authors have shown that experimental treatment with the agonists of the adenosine A1 receptor results in a striking amelioration of postischemic morbidity and mortality.4,8 The experimental work demonstrated that adenosine receptor-based therapies against ischemic brain damage are equi- or even more potent than many other forms of treatment under evaluation. However, several major concerns emerged as well, and cardiovascular side effects, hypothermia, and unclear effects of acute vs chronic administration regimens9 were among the most often quoted side effects mitigating against clinical implementation of drugs acting at adenosine A1 receptors. Nonetheless, it needs to be emphasized that most, if not all, other experimental treatments of cerebral ischemia and stroke that are contemplated for practical use suffer from substantial side effects as well. Moreover, most of these therapies aim at but one aspect of ischemic pathophysiology. On the other hand, adenosine and its receptors constitute, together with gamma-aminobutyric acid (GABA), the principal inhibitory neuromodulator of the brain.10 Hence, the wide range of the processes that are affected either directly or indirectly by stimulation of adenosine receptors offers a wider scope of target phenomena which, if left uncontrolled, will eventually result in neuronal demise.
ENDOGENOUS ADENOSINE AND ITS RECEPTOR TYPES
Neuronal effects of adenosine are mediated via the interaction with both pre- and postsynaptic receptors. However, determination of the exact extracellular concentration of adenosine in the normal brain is complicated1,2,11,12 by its short half-life and by intrinsic fluctuations resulting from the trauma inflicted upon the investigated tissue by many of the currently used techniques.1,2,11,12 For these reasons, the range of the published levels of extracellular adenosine is quite substantial. Measured by microdialysis in awake, freely moving animals the concentration of extracellular adenosine may vary between 50 and 300 nM.1,2
Endogenously released adenosine may act at four receptor subtypes (A1, A2, A2A. A2B, and A3) whose cloning13 showed that, despite some differences in their molecular sequence, all are members of the same G protein-coupled receptor family.14 Although the detailed discussion of the distinctions among these receptor types is beyond the scope of this paper,7,15 a major difference in effector systems is that stimulation of the A1 receptor results in the depression of cyclic adenosine monophosphate (cAMP), while activation of A2 receptors results in its elevation.16 Curiously, stimulation of the A3 receptor affects two second messenger pathways, i.e., enhancement of phosphoinositide metabolism (via stimulation of phospholipase C) and/or, similar to the A1 subclass, depression of adenylate cyclase.17
NEURONAL EFFECTS OF A1 RECEPTOR STIMULATION
A1 receptors are abundant in the hippocampus, IV–VI cortical laminae, striatum, amygdala, and superior colliculus.18 Their stimulation enhances K+ 19 and voltage-dependent, non-GABAergic Cl− conductances20 and causes depression of the membrane potential. Since A1 receptor-evoked hyperpolarization of the neuronal membrane attenuates the function of presynaptic voltage-regulated calcium channels, calcium uptake5,21,22 is also inhibited. Due to A1 receptor-mediated diminution of the presynaptic Ca2+ influx the release of several neurotransmitters, e.g., glutamate, acetylcholine, dopamine, noradrenaline, and serotonin is also reduced.22 Postsynaptic control of Ca2+ influx by A1 receptors depresses their excitability.5 The functional sum of all processes that are directly attenuated by the activated A1 receptors results in the depression of neuronal excitability and firing rate,23,24 which results, in turn, in a substantially depressed metabolism of neurons.11 Obviously, when viewed in the context of ischemic pathophysiology and its prevention or amelioration, all A1 receptor-elicited effects constitute a highly desirable complex.1,2,4,7
A1 RECEPTOR AGONISTS AND THE EXPERIMENTAL TREATMENT OF CEREBRAL ISCHEMIA
Neurochemical and morphological correlates of protection against hypoxic/ischemic injury by adenosine A1 receptors have been described using in vitro models.25 Several recent reviews provide details of experimental in vivo therapies.1–4,8,11,12 Both focal and global ischemia have been studied, with the duration of insults varying between 5 and 30 min. Drugs were administered either prior to or shortly after cerebrocirculatory arrest, and several measures were employed in determining the outcome, e.g., mortality, neuronal loss in selectively vulnerable regions, or the extent of neurological dysfunction. The reports indicated a significant amelioration of all studied parameters,1,2,3,8 Ultimately, the effectiveness of acute adenosine A1 receptor stimulation in preventing ischemic brain damage has been stressed by several experiments in which both selective26 and nonselective27 A1 receptor antagonists have been used. Invariably, treatment with these agents resulted in a significant worsening of postischemic necrosis27 and mortality.26
Despite unequivocal, albeit experimental, demonstration that both pre- and postischemically administered adenosine A1 receptor agonists reduce postischemic neuronal loss,1–4 cardiovascular side effects (bradycardia and hypotension), which accompanied administration of even low doses of the drugs available in the recent past, mitigated against their clinical implementation. In addition, the criticism based upon the assertion that the neuroprotective effects of A1 receptor agonists were primarily based upon their hyperthermia-inducing properties provided another stumbling block.3 Subsequent experiments in which body and brain temperatures were carefully controlled1,2,4 clearly showed that hypothermia was not the principal mechanism of A1 receptor-mediated neuroprotection. Moreover, the results of these studies were also confirmed by the in vitro experiments.25 Thus, although the contributory aspect of A1 receptor agonist-induced hypothermia to neuroprotection cannot be readily dismissed, it must be viewed in the context of the overall effect of these drugs, i.e., as a direct consequence of diminished electrical activity/metabolism resulting from A1 receptor stimulation. Hence, the depression of body/brain temperature resulting from administration of A1 receptor agonists is the consequence, and not a very direct one at that, of other actions elicited by these drugs, constituting a part of the neuroprotective complex rather than a side effect.
Recently, the issue of the presence of cardiovascular complications induced by A1 agonists has been experimentally addressed. In their recent publication29 Novo Nordisk disclosed a series of potent and selective A1 agonists including NNC 21-0136, whose early postischemic administration resulted in neuroprotection, yet was remarkably free of both bradycardia and hypotension.
Virtually all early studies in which neuroprotective properties of A1 agonists have been demonstrated employed acute pre- or postischemic administration. In retrospect, such limitation of the treatment regimen is surprising, since A1 receptor therapies have been advocated not only for the treatment of acute pathologies (i.e., stroke) but also chronic, such as seizures. Studies aimed at correcting this deficiency provided a puzzling result indicating that the outcome of the treatment with A1 receptor agonists was highly dependent on the drug administration regimen.9 Thus, acute administration of the potent and selective A1 agonist N6-cyclopentyladenosine (CPA) at 1 mg/kg either 15 min prior to or after 10 min of forebrain ischemia led to increased survival and vastly improved neuronal preservation. Chronic treatment (1 mg/kg daily for 15 days) with the same drug, however, resulted in postischemic survival that was significantly worse than in the controls (60% vs 14%) and a correspondingly poor neuronal preservation in the brains of the treated animals. The results of chronic treatment with the selective A1 receptor antagonist 1,3-dipropyl-8-cyclopentylxanthihe (CPX) were exactly opposite.26 Similar data were obtained when experimental treatment of seizures with CPA was attempted.29 Although receptor downregulation/desensitization may be involved, the precise nature of this “regimen-dependent effect reversal”9 is still unclear. Interesting in terms of the understanding of basic phenomena involved, the dependence of the therapeutic outcome on the treatment regimen indicated that caution was needed when contemplating adenosine-based drugs for practical use.
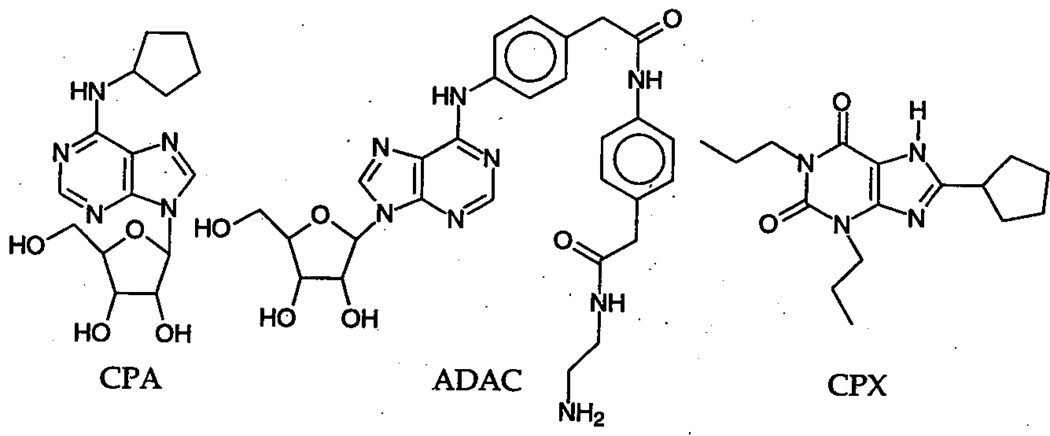
It is unknown whether the therapeutic properties of novel A1 agonists recently disclosed by Novo Nordisk28 will be sustained also in the chronic administration. While synthesis of the latter drugs is based on the “classical” medicinal chemistry of adenosine receptor-active agents, the functionalized congener concept, i.e., addition of a long side chain to the phenyl substituent of N6-phenyladenosine introduced a series of highly selective and potent A1 receptor agonists and antagonists.30 One of these drugs, adenosine amine congener (ADAC, Fig. 1) has been studied extensively as a potential candidate for the treatment of ischemic brain damage.31–32 The therapeutic dose of ADAC is much lower (micrograms) than that of the hitherto studied agents: either pre- or postischemic administration of ADAC at 75–100 µg/kg substantially reduces postischemic mortality, and leads to a high degree of neuroprotection. Furthermore, preischemic treatment with the drug given at 100 µg/kg results in a virtually complete elimination of postischemic spatial memory and learning deficits (Figs. 2 and 3). However, in contrast to most other A1 agonists, ADAC does not induce cardiovascular side effects (Table 1).
FIGURE 1.
Chemical structure of the A1 agonists CPA and ADAC and the A1 antagonist CPX.
FIGURE 2.
Effect of ADAC administration (single dose of 100 µg/kg) at 6 hr and 12 hr postischemia on neuronal survival following 5 min ischemia in gerbils. ap < 0.05. Bars: SEM.
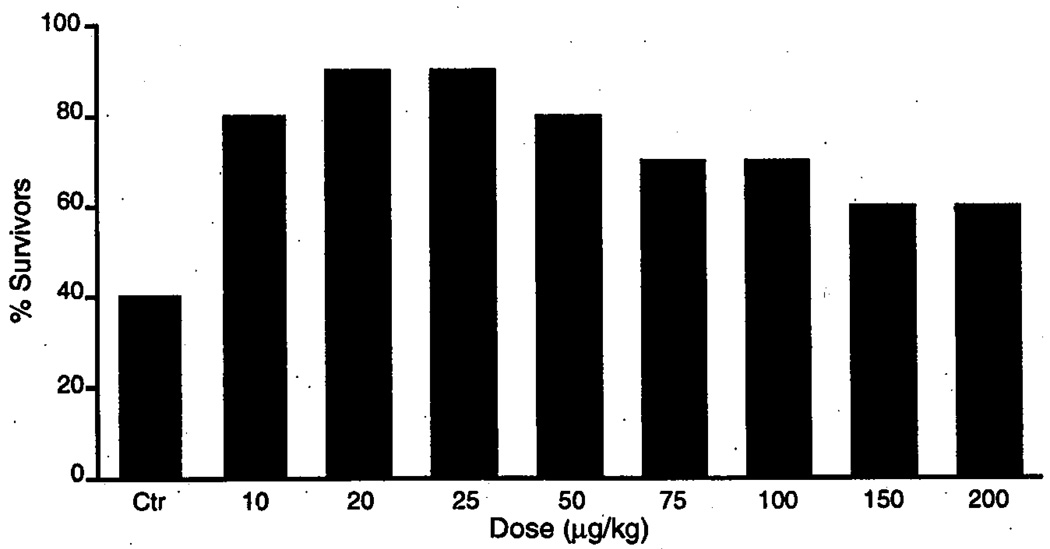
FIGURE 3.
10 day survival of gerbils following 10 min ischemia after chronic administration of ADAC (60 days) at different doses.
TABLE 1.
Cardiovascular Effects of ADAC Administration at a Dose of 100 µg/kg to Nonischemic Gerbils
| Time | Cardiac Rate (Beats/Min ± SEM) |
Blood Pressure (mm Hg ± SEM) |
|---|---|---|
| Preinjection | 384 ± 10 | 81 ± 2 |
| 5 min post | 385 ± 6 | 83 ± 1 |
| 15 min post | 382 ± 7 | 81 ± 1 |
| 30 min post | 383 ± 8 | 82 ± 2 |
| 60 min post | 383 ± 6 | 80 ± 2 |
ADAC has been shown to reduce glutamate release from gerbil cortical slices in a dose dependent manner.33 However, involvement of other mechanisms is quite likely in view of the fact that postischemic treatment with ADAC protects against neuronal damage and memory loss even when instituted as late as 12 hr postischemia,32 i.e., the time when ischemia-elevated glutamate release is fully normalized. Immunocytochemical studies emphasize the extensive character of ADAC-mediated neuroprotection as evidenced by a significant reduction in the degeneration of microtubule associated protein 2 (MAP-2).
The finding that acute treatment with ADAC effectively prevents brain damage even when instituted as late as 12 hr after the insult (Fig. 2) conflicts with the belief that the “time window” for A1 receptor therapy is rather brief.8 It does, however, underline the emerging notion that the interplay between the effects elicited by stimulation of A1 receptors located on different cell types (i.e., neurons, glia) may be exceedingly complex and far from fully elucidated.4,34
ADAC proved equally effective in chronic administration. Figure 3 shows the effect of different doses of the drug given once daily for 60 days. As can be seen, prolonged treatment with ADAC at doses of 10–100 µg/kg resulted in a significant increase of postischemic survival and neuronal preservation (Fig. 3). Most importantly, however, the results of chronic treatment with ADAC are in stark contrast to those obtained with CPA (see above), i.e., long-term administration of ADAC is not associated with the “regimen-dependent effect reversal.”
In summary, the currently available data indicate that ADAC, and possibly other compounds based upon the functionalized congener concept,30 may belong among the leading candidates for practical treatment of neurological diseases. The newly emerging compounds are free of cardiovascular side effects and appear to be equally effective in acute as well as chronic administration. The latter aspect is particularly important in view of the fact that adenosine A1-based therapies have been suggested not only for stroke but also for other disorders of the central nervous system (e.g., seizures) in which long-term treatment is mandatory. Unquestionably, several aspects of adenosine receptor involvement in neurodegenerative processes that accompany ischemia and other neuron-destroying diseases need further studies. Nonetheless, it also appears that, despite the initial setbacks, the notion of clinical applications of A1 receptor-acting drugs is now more real than ever.
REFERENCES
- 1.Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Cerebrovasc. Brain Metab. Rev. 1992;4:346–369. [PubMed] [Google Scholar]
- 2.Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Trends Pharmacol. Sci. 1992;13(12):439–445. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- 3.Miller LP, Hsu C. J. Neurotrauma. 1992;9(Suppl. 2):S563–S577. [PubMed] [Google Scholar]
- 4.von Lubitz DKJE, Carter MF, Beenhakker M, Lin RC-S, Jacobson KA. Adenosine: a prototherapeutic concept in neurodegeneration. In: Trembly B, Slikker W Jr, editors. Neuroprotective Agents: Clinical and Experimental Aspects. Vol. 765. Ann. N. Y. Acad. Sci.; 1995. pp. 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubert P, Kreutzberg GW. Neuroprotective mechanisms of endogenous adenosine action and pharmacological implications. In: Krieglstein J, Oberpichler H, editors. Pharmacology of Cerebral Ischemia. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 1990. p. 417. [Google Scholar]
- 6.von Lubitz DKJE, Marangos PJ. In: Emerging Strategies in Neuroprotection. Marangos PJ, Lal H, editors. Basel: Birkhäuser; 1992. pp. 151–186. [Google Scholar]
- 7.von Lubitz DKJE. Adenosine and acute treatment of ischemic brain disorders—put out more flags! In: Jacobson KA, Jarvis MF, editors. Purinergic Approaches in Experimental Therapeutics. New York: Wiley; 1997. In press. [Google Scholar]
- 8.Rudolphi KA, Schubert P. Purinergic interventions in traumatic and ischemic injury. In: Peterson PL, Phillis JW, editors. Novel Therapies for CNS Injuries, Rationales and Results. Boca Raton: CRC Press; 1996. p. 327. [Google Scholar]
- 9.Jacobson KA, von Lubitz DKJE, Daly JW, Fredholm BB. Trends Pharmacol. Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kostopoulos GK, Phillis JW. Exp. Neurol. 1977;55:719–724. doi: 10.1016/0014-4886(77)90296-5. [DOI] [PubMed] [Google Scholar]
- 11.von Lubitz DKJE, Marangos PJ. J. Mol. Neurosci. 1990;2(1):53–59. doi: 10.1007/BF02896926. [DOI] [PubMed] [Google Scholar]
- 12.Phillis JW. Adenosine and Adenine Nucleotides as Regulators of Cellular Functions. Boca Raton: CRC Press; 1991. [Google Scholar]
- 13.Ji XD, von Lubitz DKJE, Olah M, Stiles GL, Jacobson KA. Drag Dev. Res. 1994;33:51–59. doi: 10.1002/ddr.430330109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Galen PJM, Stiles GL, Michaels G, Jacobson KA. Med. Res. Rev. 1992;12:423–471. doi: 10.1002/med.2610120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson KA, Suzuki F. Drag Dev. Res. 1997 In press. [Google Scholar]
- 16.van Calker D, Müller M, Hamprecht G. J. Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramkumar V, Stiles GL, Beaven MA, Ali H. J. Biol. Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- 18.Jarvis MF, Williams M. Eur. J. Pharmacol. 1989;168:243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- 19.Trussel LO, Jackson MB. Proc. Natl. Acad. Sci. USA. 1985;82:4857–4860. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mager R, Ferroni S, Schubert P. Brain Res. 1990;532:58–62. doi: 10.1016/0006-8993(90)91741-x. [DOI] [PubMed] [Google Scholar]
- 21.Schubert P, Heinemann U, Kolb R. Brain Res. 1986;376:382–386. doi: 10.1016/0006-8993(86)90204-0. [DOI] [PubMed] [Google Scholar]
- 22.Fredholm BB, Dunwiddie TV. Trends Pharmacol. Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- 23.Dunwiddie TV. Epilepsia. 1980;21:541–548. doi: 10.1111/j.1528-1157.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- 24.Dunwiddie TV. Intl. Rev. Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg MP, Monyer H, Weiss JH, Choi DW. Neurosci. Lett. 1988;89:323–327. doi: 10.1016/0304-3940(88)90547-2. [DOI] [PubMed] [Google Scholar]
- 26.von Lubitz DKJE, Lin RC-S, Melman N, Ji X-D, Carter MF, Jacobson KA. Eur. J. Pharmacol. 1994;256:161–167. doi: 10.1016/0014-2999(94)90241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudolphi KA, Keil M, Hinze HJ. J. Cereb. Blood Flow Metab. 1987;7:74–81. doi: 10.1038/jcbfm.1987.11. [DOI] [PubMed] [Google Scholar]
- 28.Knutsen LJS, Lau J, Petersen H, Thomsen C, Weis JW, Shalmi M, Judge ME, Hansen AJ, Sheardown MJ. J. Med. Chem. 1999;42:3463–3477. doi: 10.1021/jm960682u. [DOI] [PubMed] [Google Scholar]
- 29.von Lubitz DKJE, Paul IA, Ji X-D, Carter MF, Jacobson KA. Eur. J. Pharmacol. 1994;253:95–99. doi: 10.1016/0014-2999(94)90762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson KA, Kirk KL, Padgett WL, Daly JW. J. Med. Chem. 1985;28:1341–1346. doi: 10.1021/jm00147a039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Lubitz DKJE, Beenhakker M, Lin RC-S, Carter MF, Paul IA, Bischofberger N, Jacobson KA. Eur. J. Pharmacol. 1996;302(1–3):43–48. doi: 10.1016/0014-2999(96)00101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Lubitz DKJE, Lin RC-S, Beenhakker M, Boyd M, Bischofberger N, Jacobson KA. Eur. J. Pharmacol. 1996 doi: 10.1016/s0014-2999(96)00667-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd M, Meshulam Y, Jacobson KA, von Lubitz DKJE. In Vitro, FASEB Abstracts. Washington, DC: 1996. Apr 14–17, [Google Scholar]
- 34.Schubert P, Ogata T. J. Neurochem. 1996;66(S2):S4. [Google Scholar]