Abstract
Background
Cardiac memory refers to the observation that altered cardiac electrical activation results in repolarization changes that persist after the restoration of a normal activation pattern. Animal studies, however, have yielded disparate conclusions both regarding the spatial pattern of repolarization changes in cardiac memory and the underlying mechanisms. This study was undertaken to produce three dimensional images of the repolarization changes underlying long-term cardiac memory in humans.
Methods and Results
Nine adult subjects with structurally normal hearts and dual-chamber pacemakers were enrolled in the study. Non-invasive electrocardiographic imaging (ECGI) was used before and after one month of ventricular pacing to reconstruct epicardial activation and repolarization patterns. Eight subjects exhibited cardiac memory in response to ventricular pacing. In all subjects, ventricular pacing resulted in a prolongation of the activation recovery interval (a surrogate for action potential duration) in the region close to the site of pacemaker-induced activation from 228.4±7.6 ms during sinus rhythm to 328.3±6.2 ms during cardiac memory. As a consequence, increases are observed in both apical-basal and right-left ventricular gradients of repolarization resulting in a significant increase in the dispersion of repolarization.
Conclusions
These results demonstrate that electrical remodeling in response to ventricular pacing in human subjects results in action potential prolongation near the site of abnormal activation and a marked dispersion of repolarization. This dispersion of repolarization is potentially arrhythmogenic and, intriguingly, was less evident during continuous RV pacing, suggesting the novel possibility that continuous RV pacing at least partially suppresses pacemaker-induced cardiac memory.
Keywords: action potentials, pacemakers, remodeling, T wave memory, cardiac memory
Background
Cardiac electrical remodeling occurs in response to a variety of situations including heart failure, atrial fibrillation, and altered rate or pattern of activation.1 It is well established that periods of abnormal ventricular activation result in changes in myocardial repolarization, manifested by a persistent change in T wave axis after restoration of normal cardiac excitation.1-4 Rosenbaum and colleagues (as well as other researchers) have defined several key features of this phenomenon, including the facts that the degree and persistence of the T wave changes depended on the duration of abnormal excitation and that T wave memory exhibits accumulation.4, 5 These changes are reminiscent of memory in the central nervous system and thus the term “cardiac memory” was introduced.5
In their seminal study of the phenomenology of cardiac memory, Rosenbaum and colleagues noted that the altered T wave axis in cardiac memory recapitulated the paced QRS axis, suggesting that, after restoration of normal activation, repolarization is delayed in the region closest to the site of ectopic activation.5 This hypothesis received further support from canine studies in which epicardial pacing resulted in a prolongation of epicardial action potentials (as measured in both single isolated cells and from tissue strips).6, 7 Studies performed in intact canine hearts using unipolar electrograms to measure local activation and recovery time also demonstrated delayed epicardial repolarization near the location of the pacing site.8, 9 In contrast, using optical mapping of transmural canine wedge preparations, Jeyaraj and colleagues demonstrated action potential prolongation in the region most distant from the pacing site.10 These studies have also yielded conflicting results regarding the effect of cardiac memory on the transmural gradient of repolarization.6, 8-10 In addition to the repolarization changes that were the focus of most studies of cardiac memory, studies in the intact canine heart have suggested that delayed epicardial activation also contributes to cardiac memory.8 These disparate results, likely reflecting different experimental systems, highlight the importance of examining cardiac memory in vivo.
Several studies have suggested that altered mechanical strain during abnormal cardiac activation is the signal that results in electrical remodeling.2, 11 In a Langendorff-perfused rabbit heart model, global alterations in strain resulted in altered expression of cardiac memory and, intriguingly, local mechanical strain sufficed to induce T wave changes similar to those of cardiac memory.12 During RV apical pacing, myocardial strain and workload is increased in regions most distant from the site of pacing and reduced near the site of pacing.13 In addition, early-activated regions also displayed reduced perfusion and oxygen uptake.13, 14 A hypothesis from these observations is that heterogenous changes in strain and workload underlie the etiology of cardiac memory; however, whether the electrical changes correspond to regions of increased strain (distant from the pacing site) or reduced strain and reduced perfusion (close to the pacing site) remains a crucial and unresolved issue.
To further clarify the mechanism of cardiac memory in the human heart, we undertook the current study to clarify the spatial pattern of electrical changes underlying the body surface T wave inversions of cardiac memory. To address this question, we used the novel ECGI technology which allows reconstruction of epicardial activation and recovery patterns in patients with dual-chamber pacemakers before and after the induction of cardiac memory.
Methods
Subject enrollment
Nine adult subjects were enrolled in the study. Enrollment criteria included prior implantation of a dual chamber pacemaker, normal cardiac function, no history of valvular abnormalities or coronary artery disease, no prior percutaneous coronary interventions or surgical cardiac procedures, and minimal burdens of atrial fibrillation or ventricular pacing. All subjects provided written, informed consent and all protocols were reviewed and approved by the Human Research Protection Office at Washington University School of Medicine.
Electrocardiographic imaging (ECGI)
The ECGI methodology has been previously described.15 Briefly, body surface electrical data were acquired at 2 kHz using a 256-lead body surface mapping system (Biosemi). After electrode application, subjects underwent non-contrast thoracic gated computed tomography (CT) scans with an axial resolution of 3 mm. Scans were gated at 70% of the R-R interval. Epicardial and body surface geometry were labeled and digitized from CT images using Amira (Visage Imaging). The body-surface potentials and geometric information were combined using the ECGI algorithms in Matlab (MathWorks) to reconstruct epicardial potentials and unipolar electrograms.
Analysis
The raw dataset consisted of reconstructed unipolar epicardial electrograms for 512 nodes on the epicardial surface. Local activation time (AT) for each node was defined as the time of minimal dV/dt during the local QRS complex; local recovery time (RT) for each node was determined using both the traditional Wyatt method (the time of maximal dV/dt during the local T wave).16 The local activation-recovery interval (ARI) was calculated for each node as the difference between local AT and local RT; the ARI was corrected for heart rate using Bazett's formula (corrected ARI = ARI/(RR0.5)). The QRST integral was calculated for each node by summing the recorded potentials from the onset of the QRS complex to the end of the T wave and multiplying by the sampling interval. For each subject, four consecutive beats were reconstructed and analyzed; the local AT, RT, and ARI at each node during each beat were then averaged. The epicardial surface was divided into 18 regions (basal, mid, and apical segments for anterior, lateral, and inferior regions of both the right ventricle (RV) and left ventricle (LV)). Regions were assigned without visualization of either activation or recovery patterns. For each subject, all nodes within each region were averaged together.
Statistics
Within each cardiac region, each parameter (activation time, recovery time, ARI, and QRST integral) was analyzed across pacing conditions using a repeated measures one-way ANOVA, followed by post-hoc t-tests with a Bonferroni correction. Comparisons between baseline (normal sinus rhythm) and the onset of RV pacing, between the onset of RV pacing and after 1 month of RV pacing, and between baseline and after cessation of RV pacing (i.e. cardiac memory) were pre-specified for the purpose of post-hoc analyses. No correction was made for repeated analyses in different cardiac regions. Apical-basal gradients were calculated in each subject between anterior apical LV and anterior basal LV; RV-LV gradients were calculated between mid-lateral RV and mid-lateral LV. Statistical analyses were carried out using Prism 5 (GraphPad).
Results
Subjects and study design
The clinical characteristics of the participants are summarized in Table 1. All subjects had dual chamber cardiac pacemakers implanted for either sick sinus syndrome (n=7), a history of transient AV block (n=1), or neurocardiogenic syncope with a prominent cardioinhibitory component (n=1) and no history of coronary, valvular, or other structural cardiac disease. One subject had a previous radiofrequency ablation for atrial flutter and one had a previous radiofrequency ablation for atrial fibrillation; none had a history of ventricular arrhythmias or ventricular ablations. At baseline, the subjects required minimal ventricular pacing (3.2±1.3%). However, one subject (Subject 8) exhibited an unexpected 20% ventricular pacing at the first study-related visit and was excluded from further analysis. Interestingly, we noted that this subject displayed, at the first visit, the same repolarization changes recorded in the other participants after 1 month of ventricular pacing (discussed below), suggesting that substantially less than 100% ventricular pacing suffices to induce cardiac memory.
Table 1.
Summary of clinical characteristics of study participants.
| Subject | Age | Gender | Cardiac diagnoses | Cardiac medications |
|---|---|---|---|---|
| 1 | 44 | Male | Sick sinus syndrome | Propanolol |
| 2 | 56 | Female | Neurocardiogenic/cardioinhibitory syncope | Metoprolol Lipitor |
| Enalapril | ||||
| Aspirin | ||||
| 3 | 80 | Female | Sick sinus syndrome | metoprolol |
| Triamterene/HCTZ | ||||
| Lovastatin | ||||
| 4 | 72 | Male | Sick sinus syndrome s/p atrial flutter ablation | None |
| Aspirin | ||||
| 5 | 50 | Female | Sick sinus syndrome | Metoprolol XL Ezetimibe/simvastatin |
| 6 | 77 | Female | Sick sinus syndrome | Sotalol |
| 7 | 51 | Female | h/o Mobitz 2, Type 1 AV block | Atorvastatin |
| 8 | 52 | Male | Sinus bradycardia | None |
| 9 | 63 | Male | Sick sinus syndrome, s/p atrial fibrillation ablation | Sotalol |
s/p=status post, AV=atrioventricular, HCTZ=hydrochlorothiazide, h/o=history of
Subjects underwent baseline ECGI during supraventricular activation (either normal sinus rhythm (NSR) or atrial pacing) after which the pacemaker was reprogrammed with a short AV delay, determined by maximizing the width of the body-surface QRS. Pacemaker parameters for each subject are summarized in Table 2; on average, the paced AV delay was 128±8 ms and the sensed AV delay was 101±7 ms. These settings achieved nearly 100% ventricular pacing during the study (98.8±0.7%). Subjects immediately underwent repeat ECGI after pacemaker reprogramming. These settings were maintained for approximately 1 month (30±3 days) after which the subjects underwent a third ECGI (during continued ventricular pacing). The pacemakers were then restored to the original, clinical settings and a final ECGI was performed during supraventricular activation (either NSR or atrial pacing); it is under this final condition that cardiac memory is most evident.
Table 2.
Pacemaker parameters for study participants.
| Subject | Baseline VP% | Baseline AP% | Paced AV delay (ms) | Sensed AV delay (ms) | VP% during study | AP% during study |
|---|---|---|---|---|---|---|
| 1 | 3.8 | 10.1 | 110 | 80 | 100 | 13.7 |
| 2 | 12 | 86 | 110 | 80 | 100 | 87 |
| 3 | 1 | 96 | 150 | 120 | 94.5 | 97 |
| 4 | 1.9 | 99 | 160 | 130 | 98 | 97.9 |
| 5 | 0.3 | 36.4 | 120 | 100 | 99.9 | 27.6 |
| 6 | 1.4 | 95.8 | 120 | 100 | 99.2 | 96 |
| 7 | 1.5 | 1.1 | 150 | 120 | 99.1 | 4.2 |
| 8 | 20 | 31 | 120 | 80 | 97 | 53 |
| 9 | 3.8 | 1.4 | 100 | 80 | 99.8 | 1.6 |
VP=ventricular paced, AP=atrial paced, AV=atrioventricular.
The entire study therefore comprises four sequential ECGI conditions: 1) baseline (either NSR or atrial pacing – henceforth referred to as “NSR” for simplicity), 2) onset of ventricular pacing, 3) ventricular pacing after one month of pacing, and 4) after restoration of either NSR or atrial pacing (henceforth referred to as “cardiac memory” for simplicity, although evidence of cardiac memory remodeling is evident during pacing, as discussed below). To eliminate any possible effects of activation pattern on the intrinsic repolarization indices (ARI and QRST integral), we confined the repolarization analysis to comparisons during the same activation pattern.
Body surface and epicardial T wave changes
As has been reported previously, NSR after a period of RV pacing was associated with the development of body surface T wave inversions which mirror the QRS axis of RV pacing (Figure 1).1, 3, 5 In this study, seven out of eight participants developed body surface T wave inversions indicative of cardiac memory. Interestingly, the RV lead in the one participant without T wave inversions was implanted in a high septal location whereas the other seven participants had an RV apical lead placement; whether this contributed to the lack of cardiac memory requires further study. The remaining analysis focuses on the epicardial electrical changes associated with cardiac memory in the seven participants with body surface T wave inversions.
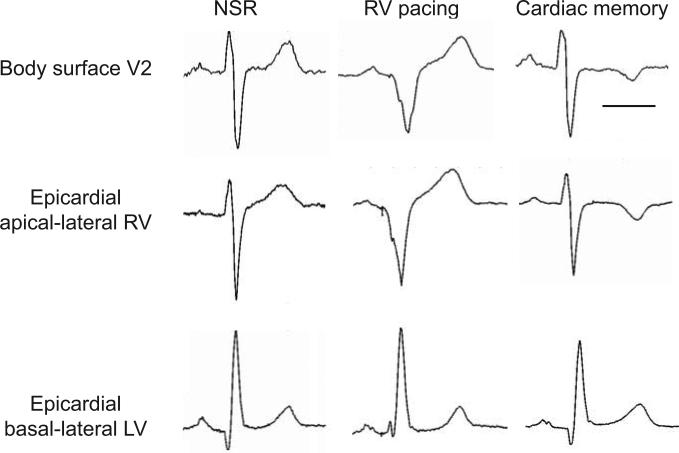
Figure 1.
Epicardial electrograms exhibit localized regional T wave inversions after RV pacing. Representative electrocardiograms demonstrate body surface T wave inversions in lead V2, which mirror the QRS complex present during RV pacing. Similarly, the epicardial electrogram from the apical-lateral RV exhibits T wave inversion after RV pacing whereas the epicardial electrogram from the basal lateral LV does not exhibit changes after RV pacing. Scale bar = 250 ms.
Inspection of epicardial electrograms revealed that epicardial T waves exhibited behavior similar to body surface T waves, i.e. becoming inverted in regions with a negative epicardial QRS complex during pacing. Specifically, epicardial T wave inversions were evident during cardiac memory in the apical-lateral RV but not in the basal-lateral LV (Figure 1).
Activation Patterns
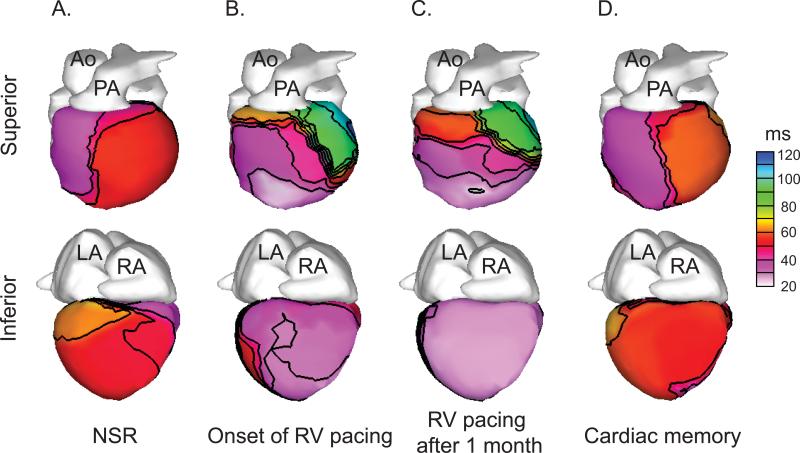
In all patients, the epicardial activation pattern during NSR was characterized by a broad activation breakthrough on the RV free wall followed by rapid activation of the ventricular surface with the latest activation occurring at the LV base, as was described previously for normal subjects17 (Figure 2A); on average, this RV breakthrough occurred 16.5±2.2 ms after the onset of the body surface QRS (all timepoints are in reference to the onset of the body surface QRS). During RV pacing, a smaller focus of early activation typically appeared over the RV apex (Figure 2B-C); the earliest epicardial activation during RV pacing occurred at 10.7±2.1 ms (p=0.31 compared to earliest AT during NSR). In contrast to the relatively subtle changes in earliest activation time, the time of latest epicardial activation was markedly different during RV pacing. RV pacing delayed the time of latest epicardial activation (located in the basal-lateral LV) from 66.6±2.9 ms to 123.7±6.7 ms (p<0.001).
Figure 2.
Epicardial activation times do not exhibit persistent changes after a period of RV pacing. Representative epicardial activation time isochrones are shown. A. Epicardial activation during normal sinus rhythm exhibits a broad area of early activation in the RV. B-C. Epicardial activation during RV pacing exhibits a site of early activation over the apical RV followed by delayed activation of the basal LV. D. Epicardial activation after restoration of NSR is similar to the baseline activation pattern (in A). Ao = aorta; PA = pulmonary artery; LA = left atrium; RA = right atrium; NSR = normal sinus rhythm. Time values (in ms) are in reference to the onset of the body-surface QRS.
During cardiac memory, both the times of earliest (21.9±3.9 ms) and latest (70.4±2.4 ms) epicardial activation did not differ from baseline earliest (16.5±2.2 ms) and latest (66.6±2.9 ms) epicardial activation (p=0.39 for earliest AT and p=1.86 for latest AT). The mean activation time of apical-lateral RV was unchanged after ventricular pacing (41.5±1.8 ms vs. 40.6±2.2 ms, p=2.49) as was the activation time of the basal-lateral LV (52.6±2.9 ms vs. 55.1±4.1 ms, p=2.14). In addition, visual inspection of the pattern of epicardial activation did not suggest a change in the overall pattern of epicardial activation after the induction of cardiac memory (Figure 2D). Taken together, these results indicate that cardiac memory does not result in persistent changes in the epicardial activation pattern.
Recovery Pattern
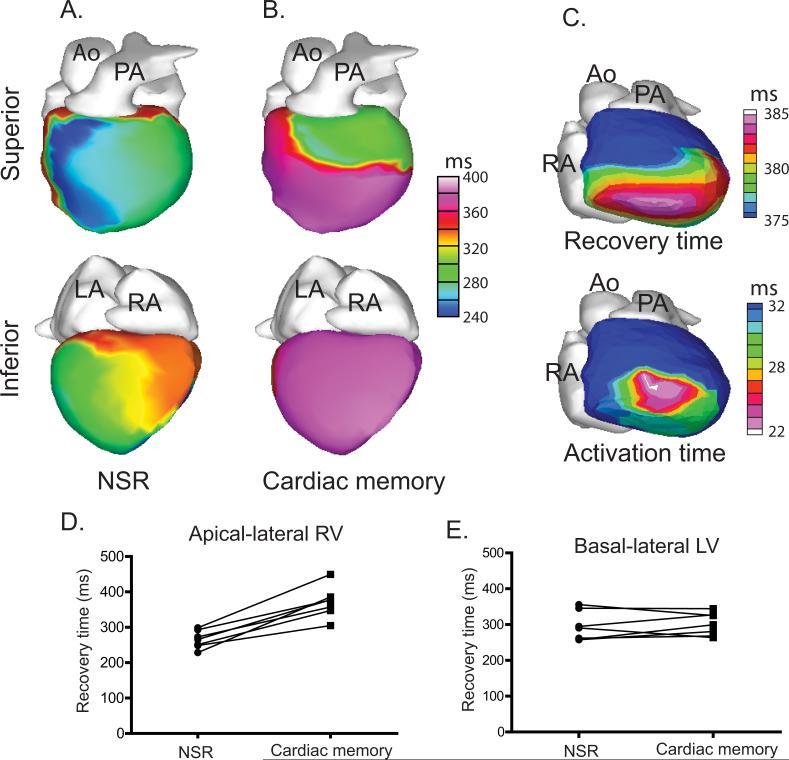
After cessation of RV pacing, there was a slight delay in the earliest recovery time (from 200.5±8.7 ms during NSR to 226.1±11.7 ms during cardiac memory, p=0.045) and no significant change in the latest recovery time (383.3±30ms during NSR vs. 412.5±17.3 ms during cardiac memory, p=0.30). There were, however, marked local changes in recovery pattern, specifically a region of delayed recovery over the right ventricle (Figure 3A-B). Although the exact extent of this delayed region varied from subject to subject, it was invariably centered over the site of earliest pacemaker-induced activation (Figure 3C). In the apical-lateral RV, the mean±SEM recovery time was delayed from 265.4±9.5 ms during NSR to 371.2±16.6 ms after restoration of NSR (p<0.001). In contrast, no significant changes in RT occurred in the basal-lateral LV as a result of RV pacing (294.9±15.5 ms during NSR vs. 301.2±12.0 ms after RV pacing, p=1.93). Importantly, every subject with evidence of body surface T wave memory exhibited a delayed apical-lateral RV recovery time after RV pacing but no significant change in basal-lateral LV recovery time (Figure 3D-E).
Figure 3.
Epicardial recovery times exhibit marked regional delays after RV pacing. A-B. Representative epicardial recovery time isochrones are shown during NSR (A) and after the period of pacing (B) demonstrating a significant delay in recovery time over the RV, the inferior LV and the apical-lateral LV. C. Within the region of delayed recovery time, the region of the greatest delay (upper panel – same data as panel B but displayed on a different scale) corresponds approximately to the area of earliest pacemaker-induced activation (lower panel – same data as Figure 2B but displayed on a different scale) D-E. All subjects exhibited a recovery time delay in the apical-lateral RV but exhibited no consistent effect in the basal-lateral LV. Time values (in ms) are in reference to the onset of the body-surface QRS.
Activation-recovery intervals
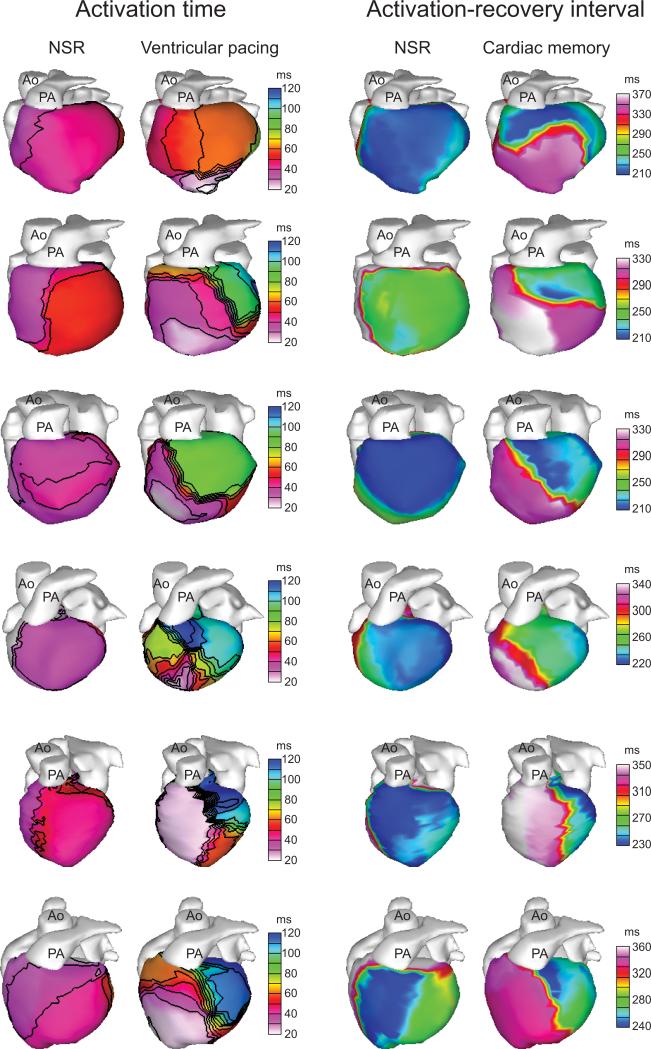
The difference between local AT and local RT is termed the activation-recovery interval (ARI) and serves as a surrogate for local APD.16, 18, 19 Several previous studies have confirmed that ARI correlates closely with APD under a variety of conditions.16, 18, 19 A significant prolongation of the local ARI occurred in the apical-lateral RV as a consequence of RV pacing (228.4±7.6 ms during NSR vs. 328.3±6.2 ms during cardiac memory, p<0.001). In contrast, no significant ARI change occurred in the basal-lateral LV (248.2±11.7 ms during NSR vs. 245.2±7.5 ms during cardiac memory, p=2.08). While the precise size and location of the region of ARI prolongation varied slightly between subjects, there was a clear relationship between the region of early, pacemaker-induced activation and the region which exhibited ARI prolongation after the cessation of pacing (Figure 4).
Figure 4.
The region of ARI prolongation in all subjects corresponds to the region of early pacemaker-induced activation. Each row represents an individual subject in the study. For each subject, the left two columns show isochrones of epicardial activation during NSR and RV pacing to demonstrate the location of early pacemaker-induced activation. The right two columns show epicardial ARI maps during NSR and cardiac memory, demonstrating a similar pattern of ARI prolongation during cardiac memory to the early activation during RV pacing.
Gradients of repolarization underlying T wave inversions
As discussed above, altered repolarization gradients in any axis could theoretically affect the body surface T wave. Both the apical-basal and RV-LV gradients in recovery time became significantly greater after induction of cardiac memory (apical-basal gradient: -3.7±6.7 ms during NSR vs. 36.0±16.7 ms during cardiac memory, p=0.017; RV-LV gradient: -18.9±10.7 ms during NSR vs. 61.4±12.8 ms during cardiac memory, p<0.001).
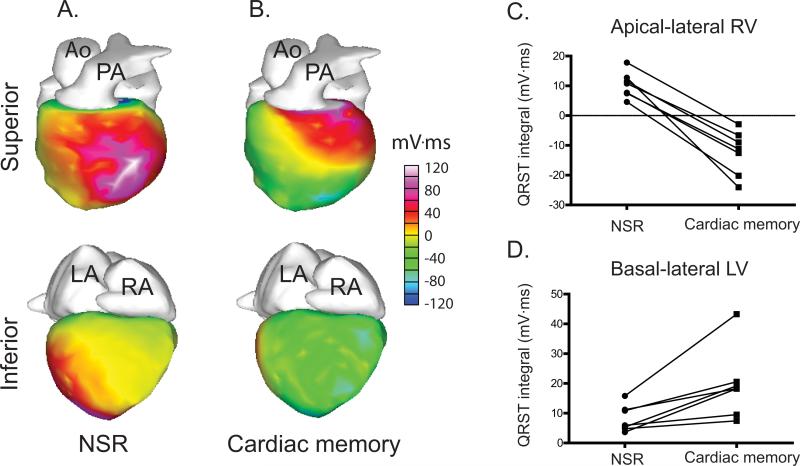
To further define repolarization dispersion, we used an additional independent index of repolarization, the QRST integral. The QRST integral reflects only the gradient of repolarization by cancelling the gradient of activation20, 21 and has been shown to reflect changes in local action potential duration.22 A negative QRST integral implies that the local APD is longer than more distant (either transmural or lateral) regions whereas a positive QRST integral indicates that local APD is shorter than in more distant regions. After RV pacing, the apical-lateral RV QRST integral became significantly more negative (10.4±1.6 mV·ms during NSR vs. -12.4±2.8 mV·ms during cardiac memory, p<0.001) whereas the basal-lateral LV became significantly more positive (8.2±1.7 mV·ms during NSR vs. 19.5±4.4 mV·ms during cardiac memory, p=0.001) (Figure 5). These changes are consistent with action potential prolongation in the apical lateral RV relative to other regions.
Figure 5.
QRST integral changes as a result of RV pacing. A-B. Representative QRST integral maps are shown during NSR and cardiac memory. C. All subjects exhibit an evolution from positive to negative QRST integral in the apical-lateral RV after RV pacing. D. All subjects exhibit an increase in QRST integral in the basal-lateral LV after RV pacing.
Repolarization changes present during RV pacing
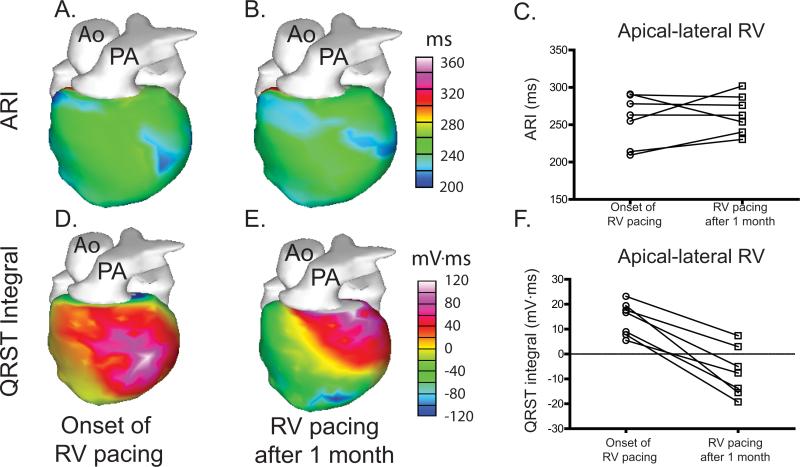
To explore whether epicardial indices of repolarization exhibit changes during continued pacing, we compared measurements made at the onset of RV pacing with measurements made during continued RV pacing after one month of continuous pacing. Surprisingly, no changes in ARI in the apical-lateral RV evolved over the course of one month of RV pacing (257.3±12.8 ms at the onset of pacing vs. 264.6±9.7 ms at the end of pacing, p=1.34) (Figure 6A-C). Whether this reflects a limited sensitivity of ARI to repolarization changes or a suppression of ARI prolongation due to continued pacing remains unclear (see Discussion).
Figure 6.
Repolarization changes during continuous RV pacing. A-B. Representative ARI maps are shown for a subject at the onset of RV pacing and during continued RV pacing after 1 month of pacing. C. No consistent change in ARI during continued RV pacing is observed among the subjects. D-E. Representative QRST maps are shown for the same subject at the onset of RV pacing and during continued RV pacing after 1 month of pacing. F. All subjects exhibit a decrease in QRST integral of the apical-lateral RV during continuous RV pacing.
In contrast, the QRST integral exhibited a significant change from a positive to a negative value during the course of RV pacing from 14.2±2.5 mV·ms at the onset of RV pacing to - 7.2±3.7 mV·ms at the end of RV pacing (p<0.001) (Figure 6D-F), indicative of a local prolongation of APD relative to more distant regions. Given the lack of changes in epicardial ARI, this observation suggests that a repolarization gradient has developed either across the ventricular wall or involving the septum (neither of which can be directly observed using ECGI).
Discussion
This study used ECGI to determine the spatial pattern of activation and repolarization in the human heart during RV pacing-induced cardiac memory. Our results provide novel insights into the pattern of pacing-induced electrical changes in vivo in the human heart. These results indicate that the body surface T wave inversions of cardiac memory result from delayed repolarization of regions close to the site of ectopic pacing, reflecting action potential prolongation in this region and a resultant increase in both apical-basal and RV-LV gradients of repolarization. Interestingly, these changes are only partially evident during continuous RV pacing as indicated by a QRST integral change without an associated change in the local ARI, whereas changes in both metrics of repolarization are evident in NSR following induction of cardiac memory.
Spatial gradients of repolarization
In principle, the body surface T wave axis can be altered due to changes in apical-lateral, RV-LV, or transmural gradients of repolarization and different experimental approaches have previously led to disparate conclusions regarding the altered repolarization gradient in cardiac memory.6, 8, 10 In the current study, we observed several spatial gradients of repolarization after induction of cardiac memory including both RV-LV and apical-basal gradients of repolarization. In addition, the presence of a more negative QRST integral during continued RV pacing (indicative of a repolarization gradient) in the absence of changes in the epicardial repolarization time indirectly suggests a transmural gradient of repolarization. It thus appears that multiple spatial gradients underlie the T wave inversions of cardiac memory.
Spatial pattern of action potential prolongation
The subjects in this study exhibited consistent patterns of repolarization underlying body surface T wave inversions; all subjects had similar delayed repolarization due to ARI prolongation in the region of earliest pacemaker activation despite different underlying pathological processes. This finding supports the original inference of Rosenbaum5 that repolarization changes would be prominent in the region of earliest paced activation and contrasts with the findings of Jeyaraj and colleagues.3 With regard to the contrasting results of Jeyaraj and colleagues, we note that the canine study was performed using LV epicardial pacing rather than RV endocardial pacing; determining whether these disparate findings reflect the effects of different pacing sites or species-specific differences will require further study.
Repolarization changes during RV pacing
Although it seems intuitive that the molecular and cellular remodeling of cardiac memory are present during RV pacing (rather than appearing quite suddenly after the cessation of pacing), relatively little research has focused on identifying the manifestations of cardiac memory during RV pacing due to the fact that the secondary T wave changes during pacing obscure the changes of cardiac memory. One study reports that the amplitude of the vectorcardiographic T wave is reducing during pacing, although the axis change is not evident until restoration of NSR.23 Additionally, Rosenbaum noted that the body surface QRST integral became more negative during continued RV pacing.5 The current findings shed further light on the repolarization changes present during pacing. In the cardiac regions destined to exhibit a prolonged ARI after cessation of pacing, the QRST integral becomes more negative during pacing and remains stable after cessation of pacing, indicating that at least some components of the repolarization gradient have evolved during pacing although the body surface T wave manifestations remain partially masked.
In contrast, the epicardial ARI in this study exhibits the unpredicted behavior of remaining unchanged during continuous pacing and then becoming prolonged immediately upon cessation of pacing. Although the ARI is a well-validated albeit indirect measure of APD under a variety of conditions in animal models,16, 19 it remains possible that during RV endocardial pacing in human subjects, it fails to accurately reflect local APD. Alternatively, it is possible that the epicardial ARI prolongation is suppressed during continued RV pacing. Intriguingly, Libbus et al. reported a similar observation on their study of pacing-induced changes in a canine transmural wedge preparation; epicardial APD shortened slightly during epicardial pacing but then became markedly prolonged after restoration of endocardial activation.24 While not identical to the current study, this finding lends support to the hypothesis that continued pacing can suppress APD changes which then become manifest after cessation of pacing. These observations contrast with a previous ECGI study of cardiac memory in which ARI prolongation was evident both prior to and after WPW pathway ablation.25 The reason for this discrepancy remains unclear but may relate to the fact that in WPW, the majority of the myocardium is activated through the normal conduction system whereas in RV pacing most activation occurs through slower direct myocyte connections.
Mechanism of cardiac memory
The molecular and cellular mechanism(s) underlying cardiac memory remain incompletely understood.11 In their original description of cardiac memory, Rosenbaum and colleagues speculated that altered electrotonic interactions due to the altered pattern of excitation gave rise to cardiac memory.5 This hypothesis gained experimental support from (short-term) experiments on canine wedge preparations.24 Interestingly, long-term cardiac memory in canine hearts has been shown to be associated with the downregulation of connexin 43 and a redistribution of gap junctions in epicardial cells, potentially altering local electrotonic interactions.26 It has also been suggested that the well-documented alterations in mechanical strain resulting from abnormal activation13 lead to cardiac memory via angiotensin II signaling.11 However, the most marked region of altered strain is located distant from the site of pacing whereas most (but not all)10 studies indicate that the most marked electrical remodeling occurs close to the site of pacing. Conversely, regions close to the site of pacing exhibit reduced strain, perfusion, and oxygen uptake;13, 14 whether these conditions contribute to local electrical remodeling remains unknown. The results reported here indicate that electrical remodeling is maximal close to the site of pacing and thus, indirectly, favor the hypotheses that local electrotonic interactions and/or reduced mechanical strain underlie cardiac memory.
Clinical implications
T wave inversions occur in a variety of disease processes, including neurologic injury, hypertrophic cardiomyopathy, and cardiac ischemia and infarction, in addition to cardiac memory; their prognostic significance depends on the causative pathology and on the uniformity of the underlying repolarization changes.27, 28 It has been suggested that the abrupt repolarization changes which occur upon restoration of NSR after induction of cardiac memory may constitute an arrhythmogenic substrate, implying that frequent switching between pacing and NSR may be more unstable than maintaining pacing.29 The results of this study offer a potential mechanistic insight into these observations. Although some repolarization changes occur during continuous RV pacing (as evidenced by the altered QRST integral), epicardial ARI prolongation is suppressed by continued RV pacing. While it remains possible that this reflects merely a limitation of the ARI measure during endocardial pacing, we note the work of Libbus and colleagues which also demonstrates that APD changes can be suppressed by an altered activation pattern.24 Taken together, these results suggest the intriguing possibility that continuous pacing may be less arrhythmogenic than intermittent pacing. Further work is necessary to define whether there are clinically adverse outcomes as a consequence of intermittent RV pacing and, if so, to fully define the responsible mechanisms.
Recent studies have drawn attention to the potentially deleterious effects of long-term right ventricular (RV) pacing on cardiac mechanical function. In addition, RV pacing is known to result in electrical changes (as manifest by an altered T-wave axis), a phenomenon described as cardiac memory, although a detailed understanding of the spatial pattern of repolarization changes resulting from RV pacing has remained lacking. Using noninvasive ECGI, this study provides novel insights into the pattern of electrical remodeling induced by RV pacing. Two new insights emerged from this study. First, the region close to the site of pacing exhibits a local action potential prolongation resulting in a potentially arrhythmogenic dispersion of repolarization. Second, this dispersion of repolarization is only partially evident during continuous RV pacing, raising the intriguing possibility that the potentially arrhythmogenic substrate induced by RV pacing is only fully present after cessation of pacing. The clinical implications of these findings require further study, but the results offer mechanistic insights into the potential clinical sequelae of RV pacing.
Acknowledgments
The authors thank Dr. Pamela Woodard and Mr. Tim Street for expert assistance with CT scans and Mr. Eric Novak for assistance with statistical analysis.
Funding Sources: This study was supported by NIH R01-HL-033343-26 and R01-HL-049054-18 grants (to Dr. Rudy) and Washington University School of Medicine (Department of Internal Medicine) Mentors in Medicine grant (to Dr. Marrus). Dr. Marrus was also supported by NIH T32 HL 007275.
Footnotes
Conflict of Interest Disclosures: Dr. Yoram Rudy co-chairs the advisory board of and holds equity in CardioInsight Technologies. CardioInsight Technologies does not support any research conducted by Dr. Rudy including that presented here. Dr. Yoram Rudy is the Fred Saigh Distinguished Professor at Washington University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patberg KW, Shvilkin A, Plotnikov AN, Chandra P, Josephson ME, Rosen MR. Cardiac memory: Mechanisms and clinical implications. Heart Rhythm. 2005;2:1376–1382. doi: 10.1016/j.hrthm.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Marrus SB, Nerbonne JM. Mechanisms linking short- and long-term electrical remodeling in the heart...Is it a stretch? Channels (Austin) 2008;2:117–124. doi: 10.4161/chan.2.2.6104. [DOI] [PubMed] [Google Scholar]
- 3.Wecke L, Gadler F, Linde C, Lundahl G, Rosen MR, Bergfeldt L. Temporal characteristics of cardiac memory in humans: Vectorcardiographic quantification in a model of cardiac pacing. Heart Rhythm. 2005;2:28–34. doi: 10.1016/j.hrthm.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee K, Harris A, Davies G, Leatham A. Electrocardiographic changes subsequent to artificial ventricular depolarization. British heart journal. 1969;31:770–779. doi: 10.1136/hrt.31.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenbaum MB, Blanco HH, Elizari MV, Lazzari JO, Davidenko JM. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol. 1982;50:213–222. doi: 10.1016/0002-9149(82)90169-2. [DOI] [PubMed] [Google Scholar]
- 6.Shvilkin A, Danilo P, Jr., Wang J, Burkhoff D, Anyukhovsky EP, Sosunov EA, Hara M, Rosen MR. Evolution and resolution of long-term cardiac memory. Circulation. 1998;97:1810–1817. doi: 10.1161/01.cir.97.18.1810. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, McKinnon D, Dixon JE, Gao J, Wymore R, Cohen IS, Danilo P, Jr., Shvilkin A, Anyukhovsky EP, Sosunov EA, Hara M, Rosen MR. Transient outward current, Ito,1, is altered in cardiac memory. Circulation. 1999;99:1898–1905. doi: 10.1161/01.cir.99.14.1898. [DOI] [PubMed] [Google Scholar]
- 8.Coronel R, Opthof T, Plotnikov AN, Wilms-Schopman FJ, Shlapakova IN, Danilo P, Jr., Sosunov EA, Anyukhovsky EP, Janse MJ, Rosen MR. Long-term cardiac memory in canine heart is associated with the evolution of a transmural repolarization gradient. Cardiovasc Res. 2007;74:416–425. doi: 10.1016/j.cardiores.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Opthof T, Coronel R, Wilms-Schopman FJ, Plotnikov AN, Shlapakova IN, Danilo P, Jr., Rosen MR, Janse MJ. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm. 2007;4:341–348. doi: 10.1016/j.hrthm.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Jeyaraj D, Wilson LD, Zhong J, Flask C, Saffitz JE, Deschenes I, Yu X, Rosenbaum DS. Mechanoelectrical feedback as novel mechanism of cardiac electrical remodeling. Circulation. 2007;115:3145–3155. doi: 10.1161/CIRCULATIONAHA.107.688317. [DOI] [PubMed] [Google Scholar]
- 11.Ozgen N, Rosen MR. Cardiac memory: A work in progress. Heart Rhythm. 2009;6:564–570. doi: 10.1016/j.hrthm.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Sosunov EA, Anyukhovsky EP, Rosen MR. Altered ventricular stretch contributes to initiation of cardiac memory. Heart Rhythm. 2008;5:106–113. doi: 10.1016/j.hrthm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: Experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33:1735–1742. doi: 10.1016/s0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu L, Imai K, Okabe A, Mashima S, Takahashi N, Kato K. A possible mechanism for pacemaker-induced T-wave changes. Eur Heart J. 1992;13:1173–1179. doi: 10.1093/oxfordjournals.eurheartj.a060333. [DOI] [PubMed] [Google Scholar]
- 15.Cuculich PS, Zhang J, Wang Y, Desouza KA, Vijayakumar R, Woodard PK, Rudy Y. The electrophysiological cardiac ventricular substrate in patients after myocardial infarction: Noninvasive characterization with electrocardiographic imaging. J Am Coll Cardiol. 2011;58:1893–1902. doi: 10.1016/j.jacc.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haws CW, Lux RL. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation. 1990;81:281–288. doi: 10.1161/01.cir.81.1.281. [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan C, Jia P, Ghanem R, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proc Natl Acad Sci U S A. 2006;103:6309–6314. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millar CK, Kralios FA, Lux RL. Correlation between refractory periods and activation-recovery intervals from electrograms: Effects of rate and adrenergic interventions. Circulation. 1985;72:1372–1379. doi: 10.1161/01.cir.72.6.1372. [DOI] [PubMed] [Google Scholar]
- 19.Coronel R, de Bakker JM, Wilms-Schopman FJ, Opthof T, Linnenbank AC, Belterman CN, Janse MJ. Monophasic action potentials and activation recovery intervals as measures of ventricular action potential duration: Experimental evidence to resolve some controversies. Heart Rhythm. 2006;3:1043–1050. doi: 10.1016/j.hrthm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Plonsey R. A contemporary view of the ventricular gradient of wilson. J Electrocardiol. 1979;12:337–341. doi: 10.1016/s0022-0736(79)80001-1. [DOI] [PubMed] [Google Scholar]
- 21.Geselowitz DB. The ventricular gradient revisited: Relation to the area under the action potential. IEEE Trans Biomed Eng. 1983;30:76–77. doi: 10.1109/tbme.1983.325172. [DOI] [PubMed] [Google Scholar]
- 22.Ghanem RN, Burnes JE, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, ii: Noninvasive reconstruction of epicardial measures. Circulation. 2001;104:1306–1312. doi: 10.1161/hc3601.094277. [DOI] [PubMed] [Google Scholar]
- 23.Shvilkin A, Bojovic B, Vajdic B, Gussak I, Zimetbaum P, Josephson ME. Vectorcardiographic determinants of cardiac memory during normal ventricular activation and continuous ventricular pacing. Heart Rhythm. 2009;6:943–948. doi: 10.1016/j.hrthm.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Libbus I, Wan X, Rosenbaum DS. Electrotonic load triggers remodeling of repolarizing current ito in ventricle. Am J Physiol Heart Circ Physiol. 2004;286:H1901–1909. doi: 10.1152/ajpheart.00581.2003. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Rhee EK, Avari JN, Woodard PK, Rudy Y. Cardiac memory in patients with Wolff-Parkinson-White syndrome: Noninvasive imaging of activation and repolarization before and after catheter ablation. Circulation. 2008;118:907–915. doi: 10.1161/CIRCULATIONAHA.108.781658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel PM, Plotnikov A, Kanagaratnam P, Shvilkin A, Sheehan CT, Xiong W, Danilo P, Jr., Rosen MR, Peters NS. Altering ventricular activation remodels gap junction distribution in canine heart. J Cardiovasc Electrophysiol. 2001;12:570–577. doi: 10.1046/j.1540-8167.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 27.Hanna EB, Glancy DL. ST-segment depression and T-wave inversion: Classification, differential diagnosis, and caveats. Cleveland Clinic journal of medicine. 2011;78:404–414. doi: 10.3949/ccjm.78a.10077. [DOI] [PubMed] [Google Scholar]
- 28.Killeen MJ, Sabir IN, Grace AA, Huang CL. Dispersions of repolarization and ventricular arrhythmogenesis: Lessons from animal models. Progress in biophysics and molecular biology. 2008;98:219–229. doi: 10.1016/j.pbiomolbio.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Wecke L, Rubulis A, Lundahl G, Rosen MR, Bergfeldt L. Right ventricular pacing-induced electrophysiological remodeling in the human heart and its relationship to cardiac memory. Heart Rhythm. 2007;4:1477–1486. doi: 10.1016/j.hrthm.2007.08.001. [DOI] [PubMed] [Google Scholar]