Abstract
A series of tricyclic cannabinoids incorporating a heteroaroyl group at C3 were prepared as probes to explore the binding site(s) of the CB1 and CB2 receptors. This relatively unexplored structural motif is shown to be CB2 selective with Ki values at low nanomolar concentrations when the heteroaromatic group is 3-benzothiophenyl (41) or 3-indolyl (50). When photoactivated, the lead compound 41 was shown to successfully label the CB2 receptor through covalent attachment at the active site while 50 failed to label. The benzothiophenone moiety may be a photoactivatable moiety suitable for selective labeling.
Keywords: CB2, Cannabinoid, Ligand-assisted protein structure, Photolabeling, Photoactivatable group
The known phytocannabinoids have long been known to exhibit only moderate receptor binding affinities and signaling profiles in vitro, yet they exhibit substantial potency in vivo.1,2 The best known of these classical cannabinoids, Δ9-tetrahydrocannabinol (Δ9-THC) binds with nearly equal affinity3–5 to the two known G-protein coupled cannabinoid receptors,6,7 CB18 and CB2.9 Substantial available SAR data for classical cannabinoids10 have shown that the northern β-C9 hydroxyl, C1 phenolic hydroxyl and the C3 side chain are key pharmacophores in determining receptor affinity and pharmacological potency for both CB1 and CB2. The design of novel CB1/CB2 analogues possessing higher affinities and selectivities can be based on structural information related to the interaction of cannabinergic ligands with their respective receptors. In the absence of either X-ray crystallographic or NMR data, information on the structural features of the ligand-cannabinoid receptor binding motifs can be gained through the use of carefully designed high-affinity electrophilic or photoactivatable probes. Such compounds interact with the receptor at or near the binding site and attach covalently to one or more amino acid residues. Identification of the attachment site(s) can subsequently be accomplished using targeted mutations within the receptor or by using LC/MS/MS to characterize the ligand-receptor complex. This approach that was developed in our laboratory combines the use of receptor mutants and mass spectrometry and was designated as Ligand-Assisted Protein Structure (LAPS).11 The present work describes our efforts to develop a new class of photoaffinity labels thus extending current work in our laboratory aimed at characterizing ligand-cannabinoid receptor binding motifs.
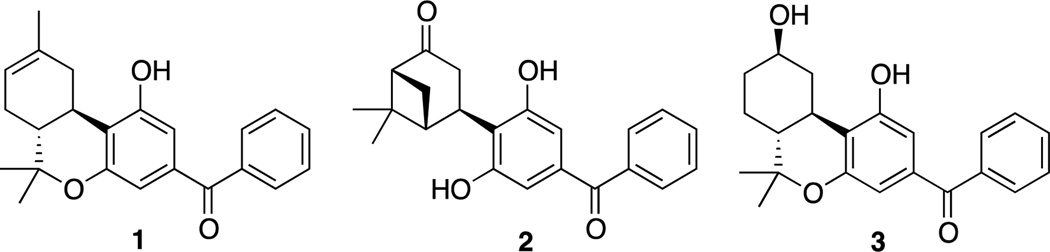
Earlier work from our laboratories had shown that 3-naphthoyl and 3-naphthylmethyl tricyclic cannabinoids have moderate affinities for the CB1 receptor.12 More recently Moore and coworkers13 showed that the tricyclic Δ8-THC analogue 1 bearing a benzoyl unit at C3 is CB2 selective while we have shown that the bicyclic analogues such as 2 are also selective for CB2 (Figure 1).14 We have now designed and synthesized a series of tricyclic cannabinoids bearing a heteroaromatic group with a carbonyl spacer at C3. Design of our novel compounds incorporates the northern β-hydroxyl pharmacophore as well as an arylphenone component, a photoactivable group capable of transforming the ligand into a GPCR covalent label.15 Earlier work from the laboratories of Martin and Dewey16a and from our laboratory16b has shown that the northern β-hydroxyl enhances affinity for both receptors while imparting the molecule with enhanced polar properties and water solubility. Our SAR approach involves the attachment of different aryl groups to the 3-keto group of the tricyclic cannabinoid moiety.
Figure 1.
Aroyl cannabinoids.
Chemistry
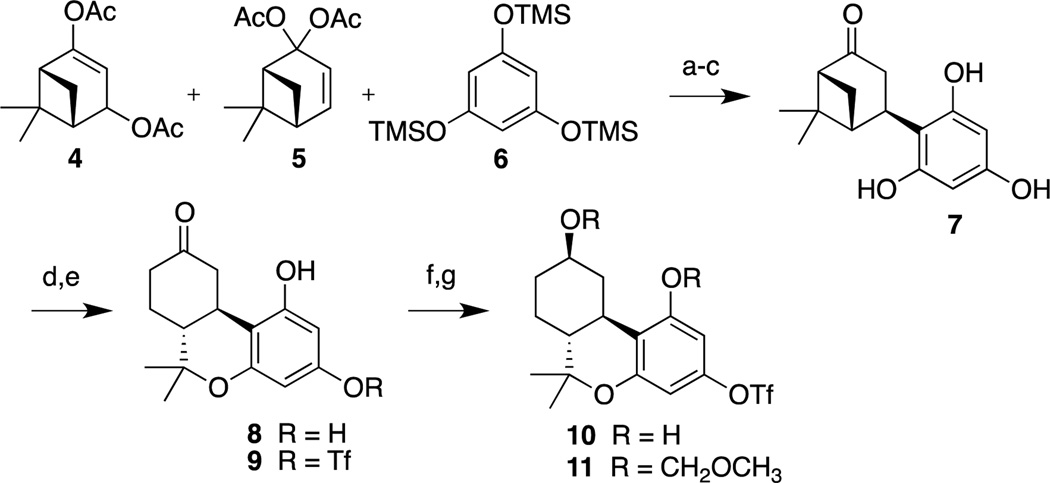
Utilizing a strategy that has been developed in our group,17,18 we prepared bicyclic intermediate 7 via the acid catalyzed condensation between persilylated phloroglucinol 6 and a mixture of diacetates 4 and 5 (Scheme 1) following a general approach that was applied to the synthesis of nabilone by the Eli Lilly group.19 It should be noted that persilylating phloroglucinol was essential to improve solubility in the reaction medium so as to ensure a high yield of 7. Ketone 7 was subsequently treated with TMSOTf to promote the rearrangement-cyclization to yield tricyclic compound 8. Selective conversion of the C3 phenolic hydroxyl group to the corresponding triflate led to 9 in 57% overall yield from 7. Reduction of ketone 9 with NaBH4 led to a 95/5 mixture of C9 diastereoisomers in 97% yield. Simultaneous protection of the phenolic and aliphatic hydroxy groups in 10 as methoxymethyl ether groups (MOM) led to 11 in 93% yield.
Scheme 1.
Reagents and conditions: (a) pTsOH, CHCl3/acetone (4/1), 0 °C, 1 h; rt, 1 h; (b) CH2Cl2, cat. DMAP, pyr, Ac2O, 0 °C to rt, 12 h; (c) KOH, MeOH, 0 °C, 1.5 h; 68% from 4, 5; (d) TMSOTf, MeNO2, 0 °C, 2.5 h; (e) PhNTf2, Et3N, CH2Cl2, 0 °C to rt; 57% from 7; (f) NaBH4, MeOH, rt, 1 h; β/α ca. 95/5, 97%; (g) MeOCH2Cl, iPr2NEt, CH2Cl2, 0 °C to rt, 2.5 h; 93%.
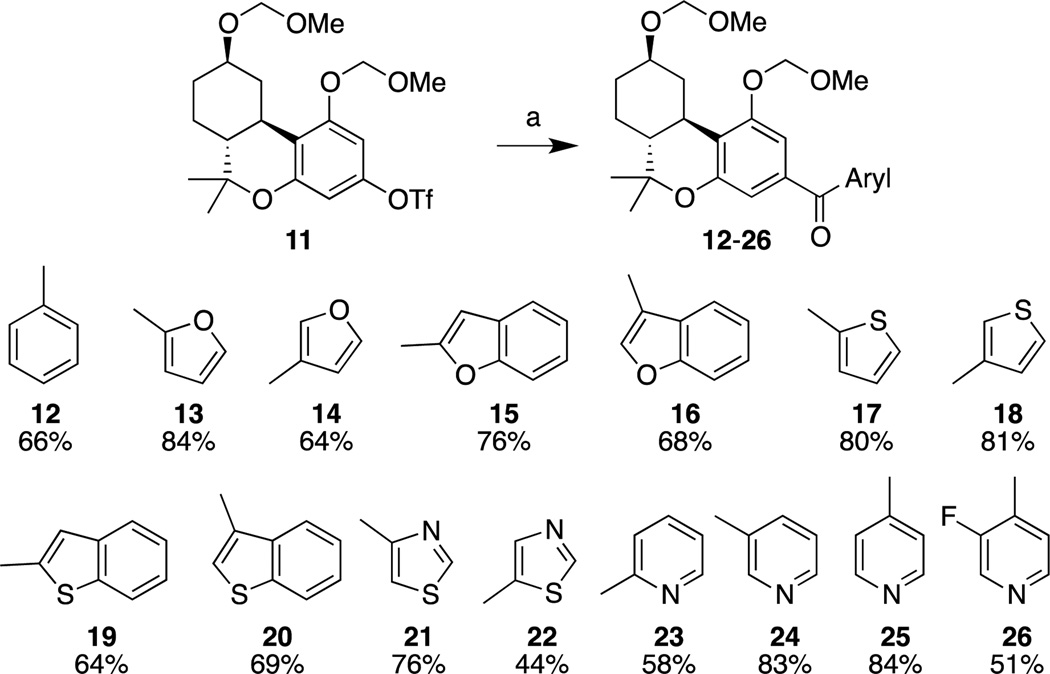
As in earlier work,18 we wanted to prepare all compounds from 11, a common advanced intermediate, utilizing a cross coupling procedure. The carbonylative Stille coupling was an attractive option for the installation of the heteroaroyl unit due to the large variety of commercially available heteroaryl stannanes and their relative ease of preparation from their corresponding aryl bromide or iodide. Treatment of triflate 11 with a slight excess of heteroaryl stannane, LiCl, 4 Å molecular sieves (MS), 2,6-di-tert-butyl-4-methylphenol (BHT) and catalytic 1,1'-bis(diphenylphosphino)ferrocene palladium(II) dichloride dichloromethane complex (PdCl2(dppf)·DCM) under an atmosphere of CO at 110 °C in N,N-dimethylformamide (DMF) for 24 h yielded heteroaroyl cannabinoids 12 – 26 in moderate to excellent yields (Scheme 2). Under these reaction conditions none of the direct coupling of triflate with stannane was observed.
Scheme 2.
Reagents and conditions: (a) DMF, CO, LiCl, BHT, 4Å MS, 110 °C, ArSnBu3, PdCl2(dppf)·CH2Cl2, 24 h.
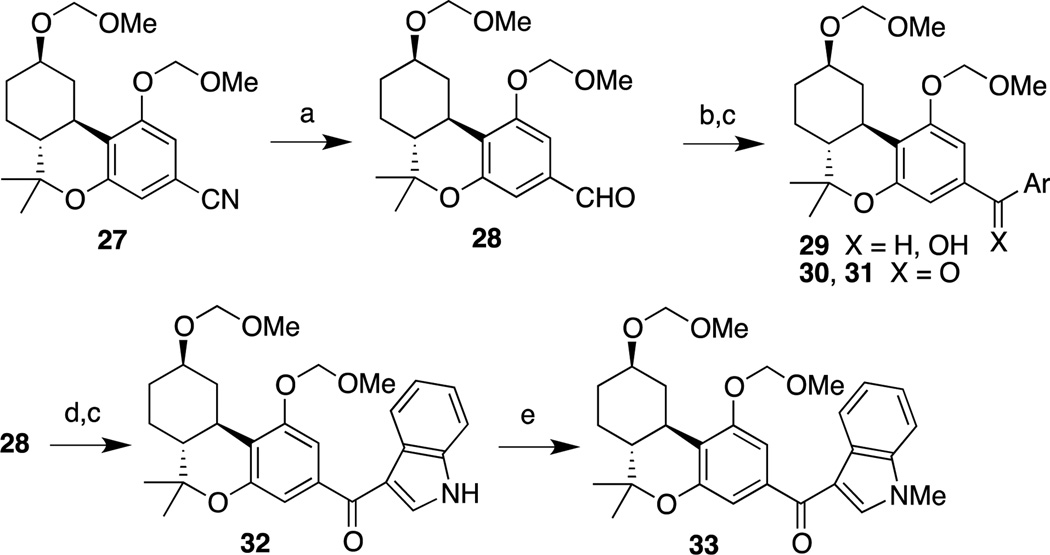
In cases in which the stannane was either not commercially available or was unreactive, a slightly modified synthetic procedure was used in order to prepare the heteroaroyl cannabinoids. We have shown in earlier work that triflate 11 can be converted to nitrile 27 (Scheme 3) in 96% yield.18 Reduction of 27 to aldehyde 28 with diisobutylaluminum hydride (DIBAL) took place in 96% yield. Nucleophilic addition of the aryllithium reagents derived from 3-bromofluorobenzene and 3-bromobenzotrifluoride to 28 followed by oxidation of the respective products with active manganese dioxide led to phenones 30 and 31 in 74% and 81% yield, respectively. Direct addition of various aryllithium or arylmagnesium compounds to nitrile 27 failed to produce the desired phenones, necessitating the two-step procedure. Exposure of 28 to indole in methanolic KOH followed by oxidation of the resulting alcohol led to 32 in 52% yield for the two steps. N-Methylation of 32 with NaH and CH3I in DMF furnished 33 in 97% yield.
Scheme 3.
Reagents and conditions: (a) CH2Cl2, DIBAL, −78 °C; 96%; (b) ArBr, n-BuLi, THF, −78 °C; (c) MnO2, CH2Cl2; 30, Ar = 3-fluorophenyl, 74%; 31, Ar = 3-(trifluoromethyl)phenyl, 81%; 32, 52% (2 steps); (d) KOH, MeOH, indole; (e) DMF, NaH, MeI; 97%.
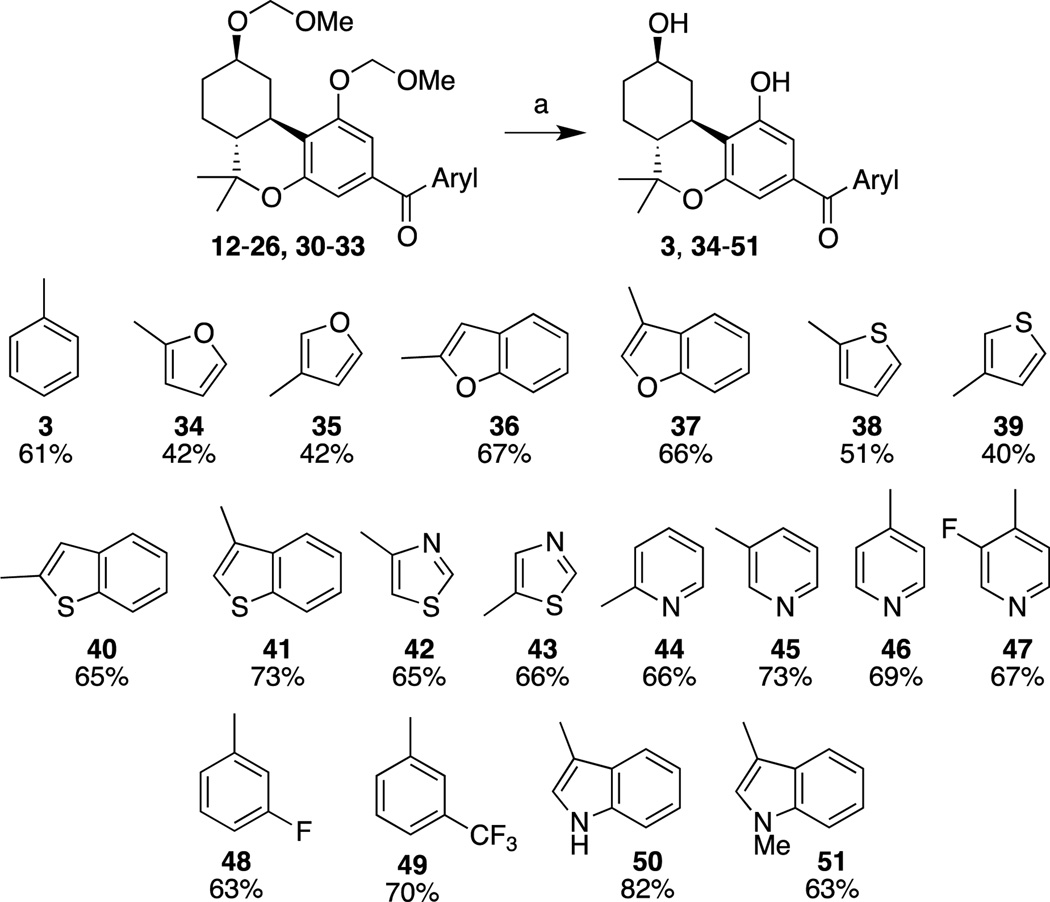
Removal of the methoxymethyl ether protecting groups from 12–26 and 30–33 with TMSBr led to 3 and 34 – 51 in moderate to good yields (Scheme 4). The low yield for deprotection of the furyl and thiophenyl compounds can be attributed to the high nucleophilicity of the electron rich aromatic ring. Reaction with the methoxymethyl bromide that is generated during deprotection may be responsible for the appearance of byproducts. Since poor yields were also observed in these cases in the presence of poly(4-vinylpyridine), it is unlikely that the poor yields of deprotected products can be attributed to the presence of strong acid. Other common conditions to remove methoxymethyl ethers such as methanolic HCl or ZnBr2/n-BuSH, which served us well in the past, also failed to improve the yields.
Scheme 4.
Reagents and conditions: (a) TMSBr, CH2Cl2, −40 °C, 1.5 h; 0 °C, 1 h.
Structure-Activity Relationships
Earlier work from our laboratory12 as well as from the Moore group13 explored the role of aroyl groups as substitutions at the C-3 position in the classical cannabinoid in lieu of the traditionally used alkyl side chain. It was shown13 that introduction of a 3-benzoyl substituent in a Δ8-THC tricyclic structure results in a compound (1) with high affinity for CB2. We have now extended the limited available SAR in this class of cannabinoids with a series of analogues carrying the cannabinoid receptor-favorable 9β-OH group10, 15 as well as different heteroaryl groups at the 3-position. These structural modifications were aimed at identifying novel ligands and photoaffinity probes for the CB2 cannabinoid receptor with improved overall profiles. Our work has led to the discovery of a novel effective covalent probe for this receptor. The SAR of all novel arylphenone analogues was evaluated by measuring their respective affinities for the rat CB1 (rCB1), mouse CB2 (mCB2) and human CB2 (hCB2) receptors (Table 1). All synthesized novel analogues exhibited reduced affinities for both CB1 and CB2 receptors when compared with the earlier synthesized benzophenone analogue 1.13
Table 1.
Binding Affinities (Ki) for CB1 and CB2 cannabinoid receptors
| Compound | Ki (nM)a | ||
|---|---|---|---|
| rCB1 | mCB2 | hCB2 | |
| 3 | 968 | 247 | 587 |
| 34 | >1000 | >1000 | - |
| 35 | >1000 | >1000 | - |
| 36 | 1356 | 163.3 | 209.4 |
| 37 | 2880 | 169.2 | 118.2 |
| 38 | >1000 | >1000 | - |
| 39 | 1037 | 525 | 1551 |
| 40 | 156.6 | 152.1 | 113.1 |
| 41 | 1254 | 34.2 | 124.8 |
| 42 | >1000 | >1000 | >1000 |
| 43 | >1000 | >1000 | - |
| 44 | >1000 | >1000 | >1000 |
| 45 | >1000 | >1000 | >1000 |
| 46 | >1000 | >1000 | >1000 |
| 47 | >1000 | >1000 | - |
| 48 | 460 | 370 | - |
| 49 | 61.7 | 45.8 | 37.3 |
| 50 | 1045 | 60.4 | 158.6 |
| 51 | 3270 | 406 | 3006 |
Binding affinities for CB1 and CB2 were determined using rat brain (CB1) or membranes from HEK293 cells expressing mouse or human CB2 and [3H]CP-55,940 as the radioligand following previously described procedures.20 Ki values for these compounds were obtained from one experiment (8 point) run in triplicate when experiments using the two point data (in triplicate) showed Ki values below 1000 nM.
All novel 9β-OH analogues were shown to have reduced binding affinities for both receptors when compared to Δ8-THC analogue 1. The reason for this observation is unclear. Arguably, the additional interaction of analogue 3 caused by the 9β-OH group may be orienting the planar benzophenone side chain differently in the receptor hydrophobic pocket to cause an overall unfavorable interaction. Our SAR data show that all analogues containing 1’-five-membered heteroaromatic ring (34, 35, 38, 39, 42, and 43) exhibited significantly diminished affinities for both CB1 and CB2 receptors. This is presumably due to lack of sufficient (hydrophobic) interaction of this ring with the hydrophobic pocket of the receptor which, in general, is a determining factor for the affinity, potency and selectivity of classical cannabinoids.10 To further probe the interaction of this 3-benzophenone group, we incorporated a nitrogen atom into various positions of the aromatic ring (44 – 47). All of these compounds exhibited further reduction in activity at both CB receptors. Incorporation of a meta fluorine substituent in the aromatic ring (48) improved binding at CB1 with a slight loss in binding affinity at mCB2. Replacement of the fluorine group with a lipophilic trifluoromethyl group (49) gave encouraging results. Compound 49 exhibited significantly improved affinity for both CB receptors (Ki = 61.7 nM at rCB1, 45.8 nM at mCB2 and 37.3 nM at hCB2).
Our most promising results with regard to CB2 affinities and selectivities were observed with analogues carrying fused bicyclic 3-heteroaroyl substitutions. The best were those carrying 3-benzofuran (37; Ki mCB2 169.2 nM rCB1/mCB2=17-fold), 3-benzothiopheno (41; Ki mCB2 34.2 nM rCB1/mCB2=37-fold) and 3-indole (50; Ki mCB2 60.4 nM rCB1/mCB2=17-fold) substituents while those with 2-benzothiopheno (40) or 3-(N-methylindole; 51) had somewhat reduced affinities or CB2 selectivities. The binding data on the analogues included in this study point to steric factors playing a key role in determining the effectiveness of the 3-aroyl pharmacophore’s ability to interact with each of the two receptors.
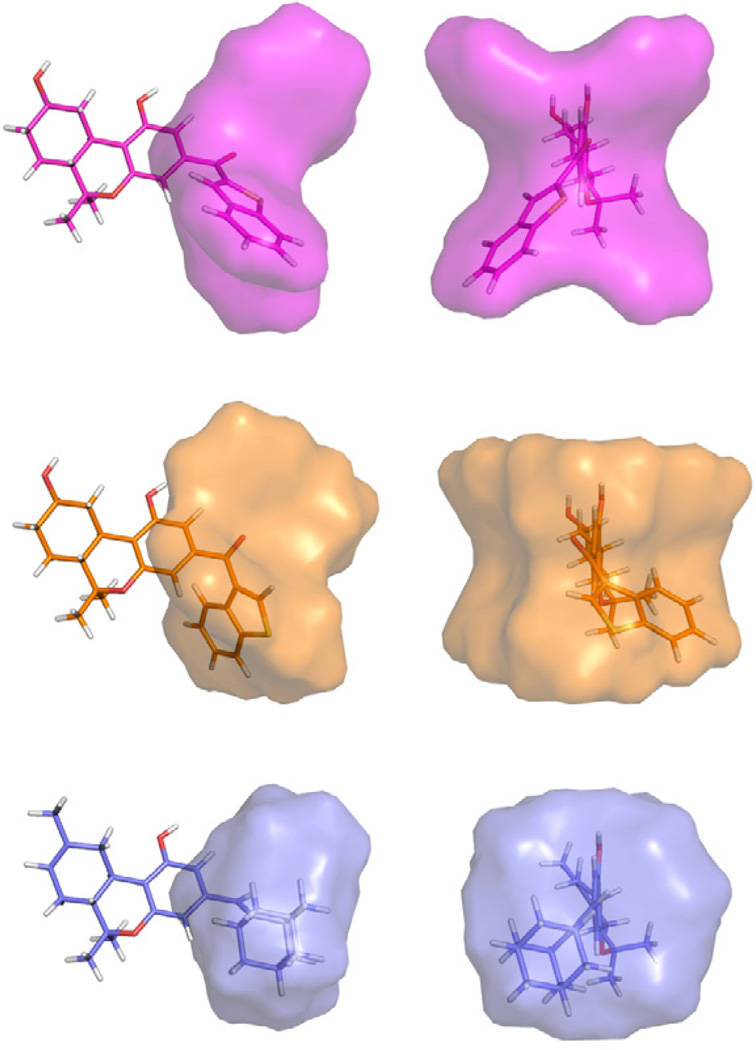
An interesting observation in our SAR is the significant difference in mCB2 affinities between the lead 3-benzothiophene analogue 41 and its 2-regioisomer 40. To better interpret these interesting results we have explored the rotational space available by these two substituents. We also included in our comparison results for the earlier published 3-vinyladamantyl analogue AM755 that also exhibited CB2 selectivity.22 A comparison of the computational data (Figure 2) points out the steric similarities between the respective pharmacophores for the two analogues with favorable CB2 affinities and selectivities (41, AM755) and the distinct differences when their conformational spaces are compared with the analogue (40) with lower affinity and selectivity for mCB2. The limited binding data included here did not allow us to carry out a full exploration of the arylphenone pharmacophore for the CB2 receptor. This will be attempted in future work when a larger database becomes available. However, our computational exercise underscores the steric factors associated with mCB2 binding and provide a basis for the design of higher affinity analogues.
Figure 2.
Accessible conformers within 6 kcal mol−1 of the global energy minimum for 40 (magenta), 41 (orange), and AM755 (blue). Analogues are shown superimposed at their aromatic rings. The global minimum energy conformer for each compound is shown in stick representation.21
Photolabeling of mCB2
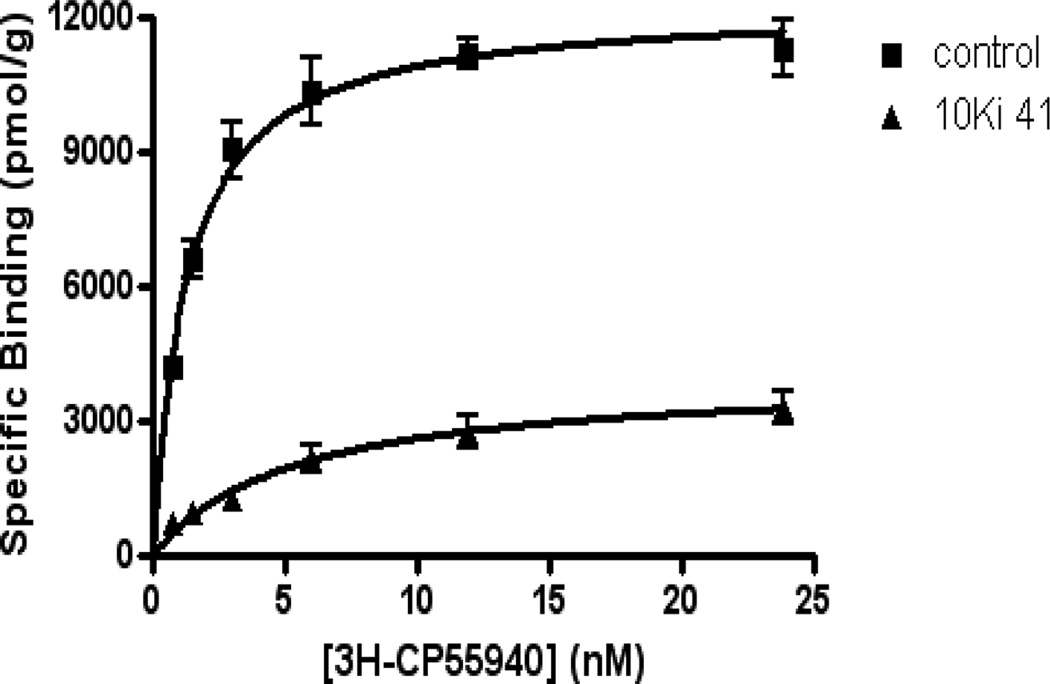
To explore the value of this class of cannabinoid analogues as photolabeling reagents for the CB receptors we tested some of our compounds for their abilities to interact covalently with the mCB2 receptor. The experiment was carried out using membrane preparations obtained from a HEK293 cell line expressing mCB2. We used methodology developed in our laboratory with cannabinergic ligands carrying different photoactivatable groups23 while employing conditions reported earlier for labeling the NK-1 receptor with ligands incorporating a benzophenone moiety.24 Of the heteroaryl benzophenones tested, the two benzothiophenones (40 and 41) exhibited the highest ability to photolabel the mCB2 receptor (77% and 67% respectively; Fig. 3). The meta-trifluoro analogue 49 also labeled, however, less effectively.25 Conversely, the 3-indolyl analogue (50) failed to label mCB2. These very successful results confirmed the value of the 3-arylphenone moieties as useful photolabels for the CB2 receptors.
Figure 3.
Compound 41 inhibits the specific binding of [3H]CP-55,940 to mCB2 receptor. HEK293 cell membranes expressing wild type mouse cannabinoid receptor 2 (mCB2) were suspended in TME buffer (25 mM Tris-Base, 5 mM MgCl2, 1 mM EDTA, pH 7.4) with 0.1% BSA, containing 0.34 µM 41 (i.e. 10-fold Ki of 41 for WT mCB2). A membrane devoid of 41 was used as a parallel control. Incubations of both samples were performed in silanized glass tubes for 30 min in a 37°C water bath. Subsequently, the samples were irradiated for 1 h using Black-Ray long wavelength ultraviolet lamp at 365 nm in ice-cold silanized Petri dishes.24 The membranes were washed once with 1% BSA TME buffer to remove unbound ligands, and once with TME buffer (no BSA) to remove BSA. Saturation binding assays were carried out after photo-labeling using [3H]CP-55940 as a radioligand.20 The membrane sample with 41 (34.2 nM; 10 × Ki) exhibited a 67% reduction in its Bmax when compared to control.
Conclusions
In this SAR study we explored the value of cannabinoid analogues carrying 3-arylphenone moieties in lieu of the 3-alkyl chains in the phytocannabinoid structures as potential photoaffinity ligands for the mCB2 receptor. The lead compound 41 provided evidence that the 3-benzothiophene analogue had the highest affinity and selectivity for mCB2. Our results underscore the important steric requirements of the arylphenone pharmacophoric moiety for the CB2 receptor and provide the basis for the design of later generation analogues with improved affinity profiles. Importantly, we demonstrated that 41 is capable of photolabeling the mCB2 receptor in excellent yields. This compound will be used in future studies to obtain information on the binding motifs of the arylphenone cannabinoid analogues for the CB2 receptor. Additionally, our results suggest that the 3-benzothiopheno group is an excellent moiety to be incorporated in cannabinergic photolabeling probes.
Supplementary Material
Acknowledgments
Acknowledgment is made to The National Institute on Drug Abuse (DA07215, 2P01 DA09158) for generous support of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data associated with this article is attached.
References and notes
- 1.Pertwee RG. Br. J. Pharmacol. 2006;147:S163. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Marzo V, Petrocellis LD. Ann. Rev. Med. 2006;57:553. doi: 10.1146/annurev.med.57.011205.135648. [DOI] [PubMed] [Google Scholar]
- 3.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1932. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compton DR, Prescott WR, Jr, Martin BR, Siegel C, Gordon PM, Razdan RK. J. Med. Chem. 1991;34:3310. doi: 10.1021/jm00115a023. [DOI] [PubMed] [Google Scholar]
- 5.Martin BR, Jefferson R, Winckler R, Wiley JL, Huffman JW, Crocker PJ, Saha B, Razdan RK. J. Pharmacol. Exp. Ther. 1999;290:1065. [PubMed] [Google Scholar]
- 6.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. Pharmacol. Rev. 2002;54:161. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 7.Lambert DM, Fowler CJ. J. Med. Chem. 2005;48:5059. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Nature. 1990;346:561. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 9.Munro S, Thomas KL, Abu-Shaar M. Nature. 1993;365:61. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 10.Thakur GA, Nikas SP, Li C, Makriyannis A. In: Handbook of Experimental Pharmacology. Pertwee RG, editor. Vol. 168. New York: Springer-Verlag; 2005. pp. 209–246. (Cannabinoids) [DOI] [PubMed] [Google Scholar]
- 11.Pei Y, Mercier RW, Anday JK, Thakur GA, Zvonok AM, Hurst D, Reggio PH, Janero DR, Makriyannis A. Chem. & Biol. 2008;15:1207. doi: 10.1016/j.chembiol.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papahatjis DP, Kourouli T, Makriyannis A. J. Heterocycl. Chem. 1996;33:559. [Google Scholar]
- 13.Krishnamurthy M, Ferreira AK, Moore BM., II Bioorg. Med. Chem. Lett. 2003;13:3487. doi: 10.1016/s0960-894x(03)00729-7. [DOI] [PubMed] [Google Scholar]
- 14.Makriyannis A, Nikas SP, Khanolkar AD, Thakur GA, Lu D. Novel bicyclic cannabinoids. 2007/0135388 A1. US. 2007
- 15.(a) Dorman G, Prestwich GD. Biochemistry. 1994;33:5661. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]; (b) Galardy RE, Craig LC, Printz MP. Nat. New Biol. 1973;242:127. doi: 10.1038/newbio242127a0. [DOI] [PubMed] [Google Scholar]
- 16.(a) Wilson RS, May EL, Martin BR, Dewey WL. J. Med. Chem. 1976;19:1165–1167. doi: 10.1021/jm00231a017. [DOI] [PubMed] [Google Scholar]; (b) Drake DJ, Jensen RS, Busch-Petersen J, Kawakami JK, Fernandez-Garcia MC, Fan P, Makriyannis A, Tius MA. J. Med. Chem. 1998;41:3596. doi: 10.1021/jm960677q. [DOI] [PubMed] [Google Scholar]
- 17.Le Goanvic D, Tius MA. J. Org. Chem. 2006;71:7800. doi: 10.1021/jo061352c. [DOI] [PubMed] [Google Scholar]
- 18.Dixon DD, Sethumadhavan D, Benneche T, Banaag AR, Tius MA, Thakur GA, Bowman A, Wood J, Makriyannis A. J. Med. Chem. 2010;53:5656. doi: 10.1021/jm100390h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archer RA, Blanchard WB, Day WA, Johnson DW, Lavagnino ER, Ryan CW, Baldwin JE. J. Org. Chem. 1977;42:2277. doi: 10.1021/jo00433a020. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y, Prusoff WH. Biochem. Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 21.A conformational search of the aryl substituent was performed using optimized potentials for liquid simulations (OPLS) force field, the cannabinoid tricyclic moiety was held fixed. Conformers with greater than 0.5 Å root-mean square deviation (rmsd) within 6 kcal mol−1 of the global minimum were retained. All calculations were performed in Macromodel. See: Jorgensen WL, Tirado-Rives J. J. Am. Chem. Soc. 1988;110:1657. doi: 10.1021/ja00214a001. Kaminski GA, Friesner RA, Tirado-Rives J, Jorgensen WL. J. Phys. Chem. B. 2001;105:6474. MacroModel, version 9.7. New York, NY: Schrödinger, LLC; 2009.
- 22.Lu D, Meng Z, Thakur GA, Fan P, Steed J, Tartal CL, Hurst DP, Reggio P, Deschamps JR, Parrish DA, George C, Jarbe TUC, Lamb RJ, Makriyannis A. J. Med. Chem. 2005;48:4576. doi: 10.1021/jm058175c. [DOI] [PubMed] [Google Scholar]
- 23.(a) Burstein SH, Audette CA, Charalambous A, Doyle SA, Guo Y, Hunter SA, Makriyannis A. Biochem. Biophys. Res. Commun. 1991;176:492. doi: 10.1016/0006-291x(91)90951-3. [DOI] [PubMed] [Google Scholar]; (b) Li C, Xu W, Vadivel SK, Fan P, Makriyannis A. J. Med. Chem. 2005;48:6423. doi: 10.1021/jm050272i. [DOI] [PubMed] [Google Scholar]
- 24.(a) Bremer AA, Leeman SE, Boyd ND. FEBS Lett. 2000;486:43. doi: 10.1016/s0014-5793(00)02228-6. [DOI] [PubMed] [Google Scholar]; (b) Bremer AA, Leeman SE, Boyd ND. J. Biol. Chem. 2001;276:22857. doi: 10.1074/jbc.M100824200. [DOI] [PubMed] [Google Scholar]
- 25.Analogue 49 was used to label hCB2. The extent of labeling was 30%.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.