Abstract
Background
Congenital Adrenal Hyperplasia (CAH) refers to a group of congenital conditions characterized by disordered cortisol synthesis. The correlation between CAH and sports performance has been less studied before and there is very limited information regarding the impacts of this congenital disease on sports performance. Probably, there are some limitations for patients who suffer from CAH in sports, but at the same time, they may enjoy some advantage due to the probable effect of endogenous hyperandrogenism on their exercise performance.
Case Presentation
The case is a 14 - year old girl with male phenotype who is a known case of congenital adrenal hyperplasia. She plays in the women's national soccer team of under 16. She has been in the first division league of indoor soccer for 4 years and was also selected in the preparation training camp of women's football team for Singapore's youth Olympic Games. Her illness and dependence on corticosteroid have caused some concerns for her participation in the international competitions of women. However, following consultations with the Therapeutic Use Exemption (TUE) Committee of games organization, she received TUE to use corticosteroid only within the games period. Despite all her problems, she is now playing in the Second Division League of indoor soccer.
Conclusions
A female adolescent with CAH may compete at the high level of outdoor and indoor soccer. However, there are many questions regarding the advantages and disadvantages of this congenital disorder and its treatment on sports related issues.
Keywords: Congenital Adrenal Hyperplasia, Doping, Gender Identity, Sports, Soccer, Therapeutic Use Exemption
INTRODUCTION
Congenital adrenal hyperplasia (CAH) refers to a group of autosomal recessive conditions characterized by disordered cortisol synthesis [1, 2]. It has a worldwide incidence of approximately one in 15,000 births [1, 3]. About 95% of CAH cases are attributable to mutations in the gene encoding the adrenal steroid 21-hydroxylase enzyme (P450c21). This enzyme catalyzes conversion of 17-hydroxyprogesterone to 11-deoxycortisol, and progesterone to deoxycorticosterone, respective precursors for cortisol and aldosterone. The blockage of cortisol synthesis leads to corticotropin stimulation of the adrenal cortex, with accumulation of cortisol precursors that are diverted to sex hormone biosynthesis [4].
Individuals with CAH generally present with signs and symptoms of androgen excess rather than symptoms reflecting glucocorticoid deficiency. During adolescence and adulthood, an ascertainment bias favors the diagnosis in females due to the nature of the hyperandrogenism symptoms. Symptoms include hirsutism, acne, alopecia, anovulation, and menstrual dysfunction [2]. In about 25% of classic CAH cases, aldosterone deficiency causes salt wasting crises, failure to thrive, hypovolemia, and shock leading to early diagnosis. A cardinal feature of classic or severe virilizing CAH in newborn females is genital ambiguity [1, 4, 5].
In patients with classic CAH, health related problems such as obesity, hyperinsulinaemia, insulin resistance and hyperleptinaemia are more often seen than in the general population. These abnormalities promote the development of a metabolic syndrome and its sequels, including endothelial dysfunction, and cardiovascular disease [6].
The diagnosis of classic 21-hydroxylase deficiency is based on a very high concentration of 17-hydroxyprogesterone (more than 242 nmol/L; normal range: less than 3 nmol/L at 3 days in full-term infant) in a randomly timed blood sample [7]. Genetic analysis can be helpful to confirm the diagnosis [8].
Glucocorticoid and mineralocorticoid replacement are the mainstays of treatment [5]. Patients on treatment, often fluctuate between periods of hyperandrogenemia and hypercortisolism. There are also reports of central precocious puberty in this population, which also contributes to increased growth velocity and premature epiphyseal closure [5].
The interaction between congenital adrenal hyperplasia and sport is less studied before and there is a very little information regarding the advantages and disadvantages of this congenital disease on sport's performance [9].
CASE PRESENTATION
Following the complaints of other players over a 14 - year old girl with male phenotype in the under 16 age- group of women's national soccer team, further evaluation about her condition was performed by the team physician.
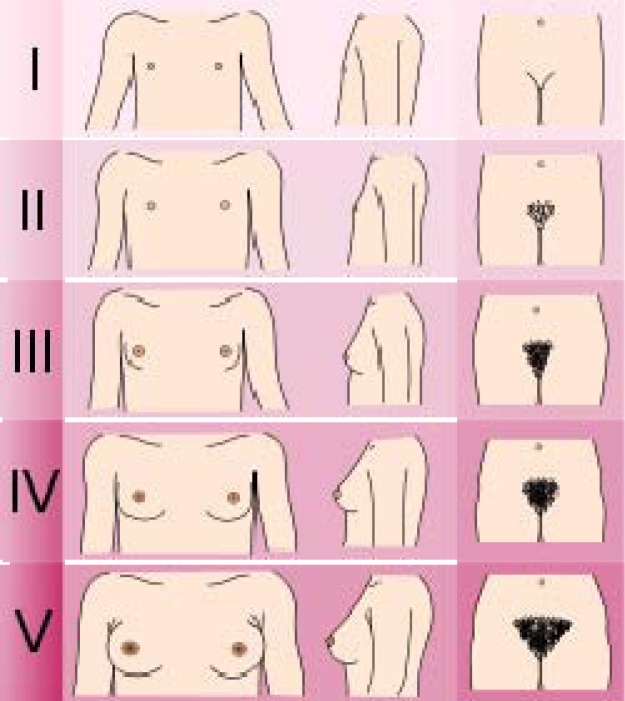
In her general appearance, she had hirsutism, increased muscle bulk, dark skin, voice deepening and short stature. In her physical examination her specifications were as below: height: 145 cm, weight: 46 Kg, body mass index (BMI): 21.8, Tanner staging: 1 (Fig. 1), lack of menstrual period, clitoral enlargement, good strength and excessive fatigue after exercise.
Fig. 1.
Illustration of the Tanner scale for females
In her past medical history, following her family's concern about her ambiguous genitalia, she underwent a number of specific laboratory tests and chromosomal karyotype and based on hormonal assessment the final diagnosis was Congenital Adrenal Hyperplasia (C.A.H) due to 21 hydroxylase deficiency. Afterwards, she had undergone surgery (vaginoplasty) when she was 3 years old and also she had been admitted in hospital 2 times because of electrolytes imbalance.
In her drug history, she had been using 30 mg hydrocortisone daily in three divided doses (15.8 mg/m2·d) plus 100 mg fludrocortisone daily in 2 divided doses, but recently her drug doses had been changed to 7/5 mg prednisolone in the morning and 5 mg at night.
In her sports career, she used to be in First Division League of indoor soccer for 4 years and she was also selected in preparation training camp of women football team for Singapore's youth Olympic Games.
She plays as a striker. She is a speedy player with a high level of technique. Despite her impressive role in the team, she usually cannot play more than one halftime because of excessive fatigue and vomiting due to electrolytes imbalance.
As her karyotype was XX, based on the IOC (International Olympic Committee) rules, there was no limitation for her participation in international competitions of women. However, following consultations with the TUE (Therapeutic Use Exemption) Committee of games organization, she received TUE to use corticosteroid only within the games period.
Now, mainly because of some problems in her medical conditions including prolonged dysfunction due to her ankle sprain, she is playing in the Second Division League of indoor soccer.
Because of her irregular check-ups, there isn't much laboratory tests available. Her most recent one, requested by the team physician is shown in the Table 1.
Table 1.
The last hormonal profile of the case
| Hormone/ Prohormone | Result(unit) | Normal range |
|---|---|---|
| TSH | 2.8 µIU/ml | 0.3 – 6 µIU /ml |
| FSH | 11 µIU / ml | 1.8 – 10.9 µIU / ml |
| LH | 4 µIU / ml | 0.4 – 11.5 µIU / ml |
| Prolactin | 13 ng/ml | ./1 – 28/5 ng/ml |
| Testosterone | 2 ng/ml | 1-1.2 ng/ml |
| 17 OH progestrone | 3.6 ng/ml | 0.2 – 1.3 ng/ml |
| DHEA sulfate | 3.9 µgr/dl | 0.4 – 4.6 µgr/dl |
DISCUSSION
Existing scientific evidence is too sparse to determine whether a performance advantage actually exists for CAH patients and how influential it might be. The specific genetic and/or intrinsic hormonal milieus that contribute to athletic performance are not clearly understood. Androgens are thought to play an important role in exercise-induced target tissue response [9]. For example, the available scientific literature describes that exogenous administration of these drugs by athletes can increase strength and body weight. Strength gains of about 5-20% of the initial strength and increments of 2-5 kg body weight, that may be attributed to an increase of the lean body mass, have been observed. Also, androgens may affect erythropoiesis and blood hemoglobin concentration response [10]. So, in adult athletes, the use of exogenous anabolic steroids can occasionally improve athletic performance, decrease fatigue, increase muscle mass, and increase aggressiveness. However, the benefits of these substances in adolescents are not clear. Moreover, the role of endogenous androgen secretion for competitive performance success is far less studied [9]. The aspects related to the effect of endogenous hyperandrogenism on exercise performance, as seen in congenital adrenal hyperplasia are not clear. Probably, there are some limitations for patients with congenital adrenal hyperplasia in sports, but at the same time, they may enjoy some advantages due to the probable effect of endogenous hyperandrogenism on their exercise performance. In women, excess production of endogenous testosterone due to inborn disorders of sexual development (DSD) may convey a competitive advantage. As understanding of DSD has expanded in recent years, women with DSD are increasingly able to continue athletic competition [11]. There are no published studies in the scientific literature that have specifically shown or suggested that any of the DSDs give an unfair advantage to the individual [12]. Accordingly, the International Amateur Athletics Federation (IAAF) groups the DSD conditions into two broad categories, (i) those that accord no advantage over other females and (ii) those that may accord some advantage but are nevertheless acceptable. CAH is an example of the latter group [13].
However, the above-mentioned CAH case can be viewed from six different perspectives by sports medicine clinicians:
1. An interesting and extraordinary point is that a female adolescent with a severe (and sometimes fatal) congenital abnormality may improve her sport career to the level of national and international competitions in high demanding sports such as indoor or outdoor soccer, despite lack of appropriate medical supervision and further follow ups.
2. An important concern is whether this type of congenital disorder may bring significant advantages or disadvantages over the teammates. For example, it seems that excess of endogenous androgens may enhance some fitness variables such as strength, anaerobic power, agility and speed. On the other hand, some variables may be impaired. However, there is no evidence to support these hypotheses and further studies are needed to objectively elucidate the changes. On the contrary, the administered drugs (glucocorticoids and mineralocorticoids) may be hazardous because of their effects on bone loss, muscle weakness and fatigue, weight gain, fluid retention, mood swings, delayed healing, tachycardia, high blood pressure and shortened career (due to frequent injuries and healing delay).
Some features in the history of our case such as quickness, good agility (as a striker), early fatigue, lack of cardiopulmonary endurance, short stature and longstanding and debilitating injuries may demonstrate some possible links between CAH and performance. So, it is not clear that finally CAH itself or its treatment have positive or negative impact on sport performance.
3. According to the mentioned effects of disease and its treatment, the important question is how to consult these patients to select sport disciplines consistent with their advantages. For instance, whether it is reasonable to recommend the patients to participate and compete in power demanding and short duration sports such as combat sports, which of course require further studies.
4. Another notable concern is about therapeutic use exemption. An athlete, like any other person, may have illnesses or conditions that require the use of particular medications as treatment. However, substances an athlete is required to take as a treatment may fall under the prohibited list, such as the long term use of systemic corticosteroids in our case. In this situation, a Therapeutic Use Exemption (TUE) may, under strict conditions, provide an athlete with the authorization to take the needed medicine, all the while competing in sport, with no resulting doping offence.
However, four criteria should be fulfilled to grant a TUE according to the standards of world anti-doping agency (WADA). These criteria are: A) The athlete would experience a significant impairment to health if the prohibited substance or method were to be withheld in the course of treating an acute or chronic medical condition. B) The therapeutic use of the prohibited substance or method would produce no additional enhancement of performance other than that which might be anticipated by a return to a state of normal health following the treatment of a legitimate medical condition. C) There is no reasonable therapeutic alternative to the use of the otherwise prohibited substance or method, and D) The necessity for the use of the otherwise Prohibited Substance or Prohibited Method cannot be a consequence, on the whole or in part, of prior non-therapeutic use of any substance from the Prohibited List [14].
A TUE can only be granted if all four criteria are fulfilled [14]. Although the first, third and fourth criteria are fulfilled in our case, the second criteria is under question. Although the aim of treatment is to suppress the inappropriate production of adrenal sex steroids, this treatment seldom returns the level of testosterone and its related prohormones to the normal state, as it is shown in paraclinical data of our case (testosterone and DHEA sulfate much more than normal range).
5. Another concern is about the higher frequency of acute and overuse injuries of these athletes due to continuos use of corticosteroids [15], which may shorten the athlete's career. Furthermore, some advantages for athletes during childhood and adolescence such as faster skeletal growth may disappear during adulthood and the athlete will lose these advantages with time [16]. So, it is a controversy whether it is ethical to impose significant stress and sometimes lifelong injuries of professional sport on these athletes, because of their transient advantages over their teammates.
6. A further important worry backs to the higher likelihood of dehydration and salt wasting in these cases [17] which may endanger the health and performance of the athlete, especially during streneous workouts in hot and humid weather. So, it will be prudent to emphasize proper hydration before, during and after the exercise bout and also consider specific modifications in contents of sports drinks, if applicable.
CONCLUSION
A female adolescent with CAH may compete at the highest level of outdoor and indoor soccer. However, there are many questions regarding the advantages and disadvantages of this congenital disorder and its treatment on sport performance, doping issues, early retirement of sport career, and ethical considerations. It seems necessary to conduct high quality studies to find appropriate answers for these questions.
REFERENCES
- 1.Merke DP, Bornstein SR. 2005 Congenital adrenal hyperplasia. Lancet. 2005;365:2125–36. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 2.Witchel SF, Azziz R. Congenital adrenal hyperplasia. J Pediatr Adolesc Gynecol. 2011;24:116–26. doi: 10.1016/j.jpag.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, et al. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–90. doi: 10.1542/peds.101.4.583. [DOI] [PubMed] [Google Scholar]
- 4.Muthusamy K, Elamin MB, Smushkin G, et al. Adult Height in Patients with Congenital Adrenal Hyperplasia: A Systematic Review and Metaanalysis. J Clin Endocrinol Metab. 2010;95:4161–72. doi: 10.1210/jc.2009-2616. [DOI] [PubMed] [Google Scholar]
- 5.White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocrine Rev. 2000;21:245–91. doi: 10.1210/edrv.21.3.0398. [DOI] [PubMed] [Google Scholar]
- 6.Ambroziak U, Bednarcz T, Ginalska-Malinowska M, et al. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency — management in adults. Endokrynol Pol. 2010;61:142–55. [PubMed] [Google Scholar]
- 7.New MI, Lorenzen F, Lerner AJ, et al. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J Clin Endocrinol Metab. 1983;57:320–26. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- 8.Nordenstrom A, Thilen A, Hagenfeldt L, et al. Genotyping is a valuable diagnostic complement to neonatal screening for congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1999;84:1505–9. doi: 10.1210/jcem.84.5.5651. [DOI] [PubMed] [Google Scholar]
- 9.Eliakim A, Nemet D. Endogenous hyperandrogenism and exercise capacity lessons from the exercise-congenital adrenal hyperplasia model. J Pediatr Endocrinol Metab. 2010;23:1213–9. doi: 10.1515/jpem.2010.194. [DOI] [PubMed] [Google Scholar]
- 10.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34(8):513–54. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 11.Wood RI, Stanton SJ. Testosterone and sport: Current perspectives. Horm Behav. 2012;61:147–55. doi: 10.1016/j.yhbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson BD, Genel M, Robinowitz CB. Gender verification of female Olympic athletes. Med Sci Sports Exerc. 2002 Oct;34(10):1539–42. doi: 10.1097/00005768-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 13.IAAF/IAF. Reports on Approved Methods of Femininity Verification; Monte Carlo; 1990. Nov, [Google Scholar]
- 14.World Anti-Doping Agency (WADA) International standard – Therapeutic use exemptions. Available at: http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-IS-TUE/2011/WADA_ISTUE_2011_revJanuary-2012_EN.pdf. Access date: Feb, 2012.
- 15.Bannwarth B. Drug-Induced Musculoskeletal Disorders. Drug Safety. 2007;30:27–46. doi: 10.2165/00002018-200730010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Magiakou MA. Growth in disorders of adrenal hyperfunction. Pediatr Endocrinol Rev. 2004;1(Suppl 3):484–9. [PubMed] [Google Scholar]
- 17.Kochli A, Tenenbaum-Rakover Y, Leshem M. Increased salt appetite in patients with congenital adrenal hyperplasia 21-hydroxylase deficiency. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1673–81. doi: 10.1152/ajpregu.00713.2004. [DOI] [PubMed] [Google Scholar]