Abstract
PKMζ is an autonomously active, atypical protein kinase C (aPKC) isoform that is both necessary and sufficient for maintaining long-term potentiation (LTP) and long-term memory. The myristoylated ζ-pseudosubstrate peptide, ZIP, potently inhibits PKMζ biochemically in vitro, within cultured cells, and within neurons in hippocampal slices, and reverses LTP maintenance and erases long-term memory storage. A recent study (Wu-Zhang, et al., 2012), however, suggested ZIP was not effective on a PKMζ fusion protein overexpressed in cultured cells. Chelerythrine, a redox-sensitive PKC inhibitor that inhibits PKMζ and disrupts LTP maintenance and memory storage, was also reported by Wu-Zhang, et al. (2012) not to inhibit the expressed PKMζ fusion protein. However, the efficacy of inhibitors on endogenous enzymes in cells may not be adequately assessed in expression systems in which exogenous enzymes are present at much higher levels. Thus, we show, biochemically, that when PKMζ reaches a level beyond that necessary for substrate phosphorylation such that much of the enzyme is excess or ‘spare’ kinase, ZIP and chelerythrine do not effectively block substrate phosphorylation. We also show that the cellular overexpression techniques used by Wu-Zhang, et al. (2012) induce a ~30-40 fold increase in kinase levels. Using a mathematical model we show that at such level of overexpression, standard concentrations of inhibitor should have no noticeable effect. Furthermore, we demonstrate the standard concentrations of ZIP, but not scrambled ZIP, inhibit the ability of PKMζ to potentiate AMPAR responses at postsynaptic sites, the physiological function of the kinase. Wu-Zhang, et al. (2012) had also claimed that staurosporine, a general kinase inhibitor that does not effectively inhibit PKMζ biochemically in vitro, nonetheless indirectly blocked the PKMζ fusion protein overexpressed in cultured cells by inhibiting phosphoinositide-dependent protein kinase-1 (PDK1). However, here we show that staurosporine does not affect PDK1 phosphorylation of the endogenous PKMζ in hippocampal slices. Thus, the biochemical in vitro effects of PKMζ inhibitors correspond with their intracellular effects, and ZIP and chelerythrine, together with scrambled ZIP and staurosporine as controls, are effective tools to examine the function of PKMζ in neurons.
Keywords: PKMzeta, PKM zeta, aPKC, ZIP, chelerythrine, staurosporine
1. Introduction
PKMζ is both necessary and sufficient for maintaining LTP and long-term memory storage (Sacktor, 2011). Increasing PKMζ activity potentiates synaptic transmission (Ling et al., 2006; Ling et al., 2002; Serrano et al., 2005) and enhances both new and previously established long-term memory (Drier et al., 2002; Shema et al., 2011). Conversely, decreasing PKMζ activity by either pharmacological or dominant negative inhibitors blocks LTP maintenance (Ling et al., 2002; Serrano et al., 2005) and reverses established long-term memory (Drier et al., 2002; Pastalkova et al., 2006; Serrano et al., 2008; Shema et al., 2011).
One widely used inhibitor of PKMζ is the zeta inhibitory peptide (ZIP). ZIP consists of the 13 amino-acid pseudosubstrate sequence from the regulatory domain of the full-length PKCζ, which has been conjugated on its N-terminal to a myristoyl group that allows peptide penetration into cells (Laudanna et al., 1998). In the full-length PKCζ, the pseudosubstrate of the ζ regulatory domain interacts with and inhibits the catalytic domain, maintaining the isoform in a basally autoinhibited state. Second messengers activate PKCζ by binding to the regulatory domain and inducing a conformational change that releases the autoinhibition of the pseudosubstrate from the catalytic domain. PKMζ, in contrast, is an atypical PKC (aPKC) isoform that consists of the independent ζ catalytic domain without a PKCζ regulatory domain, and, lacking the autoinhibition of the regulatory domain’s pseudosubstrate, is constitutively and therefore persistently active. ZIP inhibits PKMζ by providing the autoinhibition of the missing PKCζ regulatory domain and thus reverses LTP maintenance and long-term memory.
Applied extracellularly to neurons, ZIP blocks the action of PKMζ perfused into CA1 pyramidal cells in hippocampal slices (Serrano et al., 2005), PKMζ transfected into primary cultured hippocampal neurons (Shao et al., 2011), and PKCζ introduced into sensory neurons (Zhang et al., 2012). The IC50 of bath applications of ZIP that inhibit the ability of intracellular PKMζ to augment postsynaptic AMPAR responses in CA1 pyramidal cells is ~1 μM, nearly identical to the IC50 for the reversal of the established late-phase of LTP maintenance (Serrano et al., 2005). Applied intracranially, ZIP disrupts long-term memories stored in a variety of neural circuits, including spatial and trace memories in the hippocampus, aversive memories in the basolateral amygdala, appetitive memories in the nucleus accumbens, habit memories in the dorsal lateral striatum, and elementary associations and skilled sensorimotor memories in neocortex (see Supplementary information in (Sacktor, 2011) and (Li et al., 2011; Pauli et al., 2012)). When administered in vivo, ZIP also disrupts long-term sensitization of reflex responses in the model system Aplysia californica (Cai et al., 2011). The mechanism by which ZIP reverses late-LTP and erases long-term memory is through inhibiting the specific AMPAR trafficking mechanism by which PKMζ potentiates synaptic transmission, confirming that ZIP acts by inhibiting PKMζ in brain slices and in vivo (Migues et al., 2010). Because both full-length aPKC isoforms, PKCζ and PKCτ/λ, contain the identical pseudosubstrate sequence, ZIP is also the standard reagent to inhibit the function of full-length aPKC within cells (Laudanna et al., 1998) and to identify intracellular aPKC substrates (Suzuki et al., 2004).
Inconsistent with this literature, a recent study showed that ZIP did not inhibit a PKMζ fusion protein that was transfected into cultured cells (Wu-Zhang et al., 2012). In addition, chelerythrine, a second inhibitor of the PKC catalytic domain (Herbert et al., 1990) that inhibits PKMζ biochemically in vitro and within neurons and disrupts LTP and long-term memory (Cai et al., 2011; Li et al., 2011; Ling et al., 2006; Ling et al., 2002; Serrano et al., 2005), also did not inhibit the overexpressed kinase in cultured cells.
In all their attempts to inhibit the overexpressed enzyme, however, Wu-Zhang et al. (2012) used doses of inhibitors previously shown to be effective on the intracellular actions of PKMζ that had been postsynaptically perfused into neurons (Ling et al., 2006; Ling et al., 2002; Serrano et al., 2005; Zhang et al., 2012). Therefore, an assumption was made that these doses were adequate, regardless of the amount of enzyme in the transfected cell. However, systems in which enzymes are expressed to levels much higher than endogenous levels cannot be used to accurately determine the concentrations of inhibitors required to inactivate endogenous enzymes in cells. For example, if a kinase is in 10-fold excess of the maximal concentration required to phosphorylate substrate, inhibiting 90% of the kinase will have no effect on phosphorylation.
In this study, we first demonstrate that ZIP is a competitive inhibitor of substrate binding to PKMζ, and, as expected, high enzyme concentrations reduce and even eliminate the efficacy of both ZIP and chelerythrine when inhibitor concentrations are not appropriately adjusted. Second, we show that the cellular overexpression techniques used by Wu-Zhang et al. (2012) increase PKMζ protein levels 30- to 40-fold above normal levels in transfected cells. Third, using a mathematical model of a kinase-inhibitor system, we show that at these levels of kinase overexpression standard concentrations of inhibitor are not expected to have a noticeable effect. Fourth, we demonstrate the efficacy of standard concentrations of ZIP, but not scrambled ZIP, on the physiological action of PKMζ at postsynaptic sites — the potentiation of postsynaptic AMPAR responses. Fifth, we show that, contrary to a claim by Wu-Zhang et al. (2012) about the PKMζ fusion protein overexpressed in cultured cells, the inhibitor staurosporine does not decrease activation loop phosphorylation of endogenous PKMζ in neurons. Thus, the PKMζ inhibitors ZIP and chelerythrine, together with scrambled ZIP and staurosporine as controls, are effective tools to examine the function of PKMζ in neurons.
2. Materials and methods
2.1 Reagents
The myristoylated ζ-pseudosubstrate peptide (myr-SIYRRGARRWRKL-OH) and its corresponding scrambled control peptide (myr-RLYRKRIWRSAGR-OH; both from AnaSpec) (Laudanna et al., 1998) were dissolved in an aqueous stock concentration of 10 mM, stored at −20°C, and diluted in the reaction mixture, or in physiological saline for hippocampal slice experiments, immediately before use at the designated concentrations. PKCε substrate was from AnaSpec. Chelerythrine and staurosporine (stored in DMSO, which was diluted to 0.001% in physiological saline) were from Enzo Life Sciences. Phorbol 12,13-dibutyrate (stored in DMSO, which was diluted to 0.01% in physiological saline) and other reagents unless specified otherwise were from Sigma. Peptide and protein concentrations were determined by assay using bicinchoninic acid (Pierce).
2.2 PKMζ phosphorylation assay
PKMζ was recombinantly expressed and purified as previously described (Ling et al., 2002). The reaction mixture (50 μl final volume) contained: 50 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 10 μM dithiothreitol (DTT), 25 μM PKCε substrate, and PKMζ (concentrations as noted in the figures), except for 1 mM DTT as noted in Fig. 1E and for the Dixon plot, Fig. 1A. For the Dixon plot, myelin basic protein (0.75 and 1.5 μM) was substituted for PKCε substrate. The reaction, begun with the addition of 50 μM ATP (final concentration, ~1-3 μCi [γ-32P]/assay), was for 30 min at 30°C, which up to 10 nM PKMζ/assay is in the linear range for time and enzyme concentration (Fig. 1B and data not shown). The reaction was stopped by addition of 25 μl of 100 mM cold ATP and 100 mM EDTA, and 40 μl of the assay was spotted onto phosphocellulose paper and counted by liquid scintillation. PKMζ activity was measured as the difference between counts incorporated in the presence and absence of enzyme.
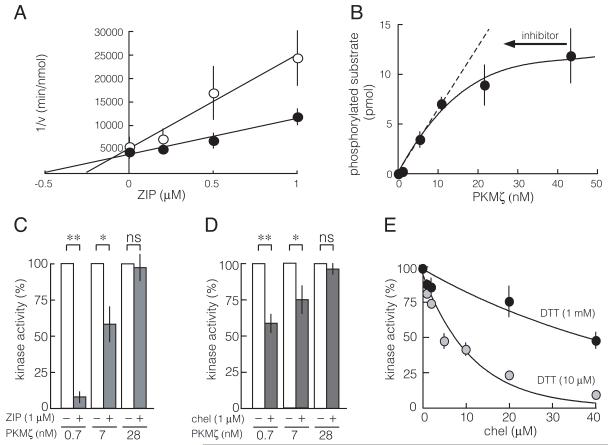
Fig. 1.
Increasing PKMζ concentration decreases the efficacy of the competitive inhibitors ZIP and chelerythrine. A. Dixon plot shows ZIP is a competitive inhibitor of protein substrate binding to PKMζ. Myelin basic protein (0.75 μM, open circles; 1.5 μM, filled circles) is the protein substrate; PKMζ concentration is 4 nM; n = 4. B. At high concentrations of PKMζ, further increasing enzyme concentration has little effect on substrate phosphorylation. Inhibitors act by reducing the effective concentration of enzyme (arrow); thus, at high enzyme concentrations, inhibitors will have less effect on phosphorylation. N = 5. C. 1 μM ZIP is not effective as enzyme levels increase; **, p < 0.01; *, p < 0.05; ns, not significant; n = 4 for all groups. D. Chelerythrine (chel, 1 μM) also becomes less effective as enzyme increases; **, p < 0.01; *, p < 0.05; ns, not significant; n = 4 for all groups. E. Chelerythrine is a redox-sensitive inhibitor of PKMζ. Chelerythrine is effective with DTT at 10 μM, but much less effective at 1 mM. PKMζ concentration is 5 nM; n = 4. For all figures, if a bar is not shown, the size of the standard error is less than the size of the symbol for that data point.
2.3 Hippocampal slice preparation, stimulation, and recording
Hippocampi of male, 3- to 4-week-old Sprague Dawley rats were removed after decapitation under isofluorane-induced anesthesia, according to State University of New York Downstate Medical Center Animal Use and Care Committee standards. All efforts were made to minimize animal suffering and to reduce the number of animals used. For whole-cell recordings, hippocampal slices (400 μm) were prepared using a Vibratome tissue sectioner. The slices were transferred to an incubation chamber at 32°C in oxygenated (95% O2-5% CO2) physiological saline containing the following (in mM): 124 NaCl, 5 KCl, 26 NaHCO3, 1.6 MgCl2, 4 CaCl2, and 10 glucose, for a minimum of 1.5 h before recording. After incubation, single slices were transferred to a 1.5 ml recording chamber placed on the stage of an upright microscope (Zeiss Axioskop 2; Carl Zeiss) and perfused with warm (31-33°C) saline at ~5 ml/min. Visualized CA1 pyramidal cells were held at −75 mV, the empirically determined reversal potential of the GABAA receptor (Ling et al., 2002). Synaptic events were evoked every 15 s by extracellular stimulation with bipolar electrodes placed in stratum radiatum. The recording pipettes had tip resistance of 2-4 MΩ and contained (in mM) 130 Cs-MeSO4, 10 NaCl, 2 EGTA, 10 HEPES, 1 CaCl2, 2 Na-ATP, and 0.5 Na-GTP, with or without PKMζ (10 nM, 1.0 pmol/min/μl phosphotransferase activity (Ling et al., 2002)). The pH value of the pipette solution was adjusted to 7.3 with CsOH. Cs was used to block potassium currents, including GABAB responses. Evoked AMPAR-mediated EPSCs were recorded under voltage-clamp mode with a Warner Instruments PC-501A amplifier and filtered at 2 kHz (−3 dB, four-pole Bessel). Brief voltage steps (−5 mV, 5 or 10 ms) from holding potential were applied during the course of recording to monitor cell access resistance, input resistance, and capacitance. Only cells with an initial input resistance of >100 MΩ and an initial access resistance of <20 MΩ with insignificant change (<20%) during the course of recording were accepted for study. Signals were digitized with Digidata 1322A and acquired and analyzed with pClamp software (Molecular Devices) running on a Pentium microcomputer. The peak amplitude of EPSCs was further analyzed with Excel (Microsoft). The mean ± SEM of 1 min bins of the responses were plotted in the figures.
Field EPSPs (fEPSPs) were recorded using glass microelectrodes with a resistance of 3-8 MΩ, filled with the recording saline, and positioned 200 μm from the stimulating electrodes in stratum radiatum. Current intensity of test stimuli (20-40 μA, 0.1 ms duration) was set to produce one-third maximal fEPSPs (1-2 mV). The frequency of test stimulation was every 15 s and the mean ± SEM of 1 min bins of responses plotted in the figures. The slope of the fEPSP was measured between 10 and 50% of the initial phase of the fEPSP response.
2.4 PKMζ Sindbis virus and expression plasmids
Sindbis virus vectors were used to overexpress N-terminally tagged, human influenza hemagglutinin (HA)-PKMζ in primary cultures of hippocampal neurons, as previously described (Shao et al., 2011). Control hippocampal neurons were transfected with Sindbis virus expressing eGFP (Shao et al., 2011). Membranous and cytosolic fractions were prepared as previously described (Sacktor et al., 1993), and 10 μg total protein per lane analyzed by immunoblot.
PKMζ overexpression in 293T cells was as described in Wu-Zhang et al. (2012), except that enhanced green fluorescent protein (eGFP) was fused on the N-terminal of PKMζ, rather than red fluorescent protein (RFP) to the C-terminal (Wu-Zhang et al., 2012). The C-terminal of PKMζ is a PDZ-binding domain and blocking the C-terminal with fusion protein as in Wu-Zhang et al. (2012) prevents normal binding interactions between PKMζ and PDZ-containing proteins (Kelly et al., 2007). Control 293T cells were transfected with eGFP alone.
2.5 Immunoblots
Immunoblots with ζ-specific catalytic domain antiserum (Hernandez et al., 2003), 1:2000) for total PKMζ and PKCζ, and activation loop phosphorylation of PKMζ (phospho-PKCζ/λ [Thr410/403] antibody #9378, Cell Signaling, 1:1000), actin (1:5000), and GAPDH (1:20,000, Santa Cruz) were performed as previously described (Kelly et al., 2007; Li et al., 2010). Ser(P) PKC substrate antiserum (Cell Signaling) was used at 1:500.
For staurosporine experiments, hippocampal slices were prepared as described above and incubated in the physiological saline for a minimum of 1.5 hr, and then incubated in the saline with or without 100 nM staurosporine for 4 hr. Two slices from each group were collected and homogenized in 100 μl of homogenization buffer, as previously described (Sacktor et al., 1993). Homogenate was then centrifuged at 13,000 × g for 3 min. Supernatant was collected and prepared for immunoblots, and the densitometry of the bands performed as described (Sacktor et al., 1993). The identity of the T410 band on immunoblot was confirmed as PKMζ by immunoprecipitation with C-terminal ζ antiserum, as described in Kelly et al., 2007 (data not shown).
2.6 Statistics
Values are presented as mean ± SEM. Within-group differences were determined by paired t test (Fig. 1, 2, 4B, 5A, 5B, PDBu). Between-group comparisons were determined by one-way ANOVA (Fig. 4A), unpaired t test (Fig. 5B, PDBu with or without staurosporine), or one-way ANOVA, repeated measures, followed by Tukey’s post hoc tests (Fig. 5B, staurosporine + PDBu).
Fig. 2.
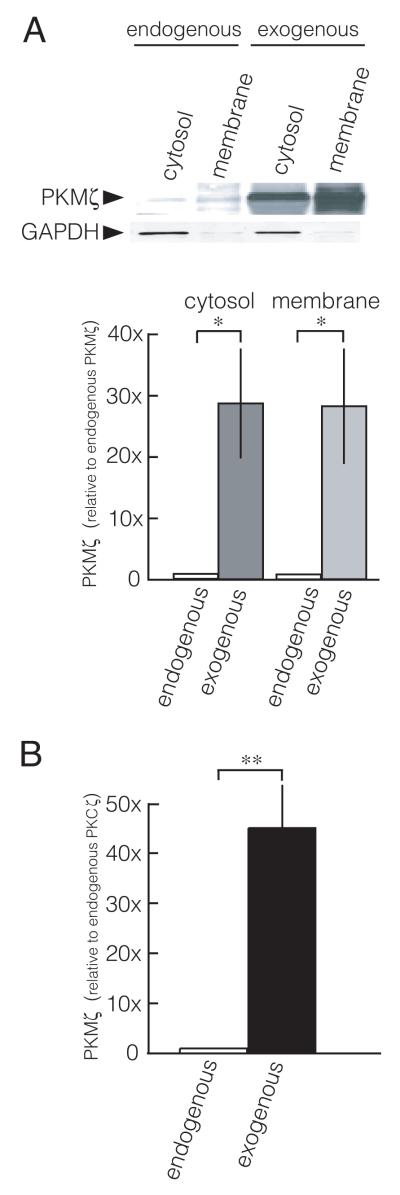
Cellular overexpression increases PKMζ above endogenous levels. A. Overexpression of PKMζ in primary cultures of hippocampal neurons increases PKMζ over endogenous PKMζ levels. Hippocampal neurons express endogenous PKMζ, but only trace amount of PKCζ (data not shown). Top, representative immunoblot; bottom, group data, mean ± SEM; *, p < 0.05, n = 4. B. Overexpression of PKMζ in 293T cells increases PKMζ ~40-fold over endogenous PKCζ levels (**, p < 0.01, n = 3). The 293T cells express endogenous PKCζ, but not PKMζ (data not shown).
Fig. 4.
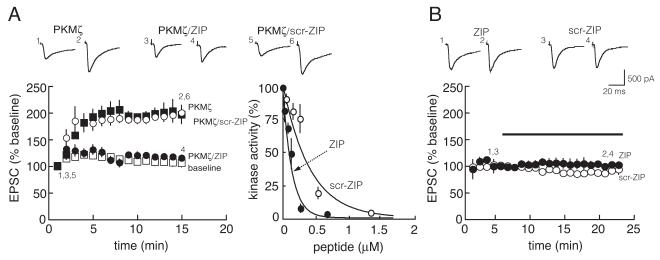
ZIP (5 μM), but not an equivalent concentration of scrambled ZIP, inhibits intracellular PKMζ-mediated potentiation of postsynaptic AMPAR responses in CA1 pyramidal cells of hippocampal slices. A. Left, PKMζ potentiates postsynaptic AMPARs (filled squares; p < 0.0001, 1 min after cell breakthrough compared to 15 min). ZIP blocks the effect of PKMζ (filled circles; p = 0.8, 1 min after cell breakthrough compared to 15 min). Scrambled ZIP (5 μM) has no effect on PKMζ-mediated potentiation of postsynaptic AMPAR responses (open circles; p = 1.0, compared to PKMζ alone at 15 min). Baseline, open squares. Traces above are from the time points denoted in time course below; n = 4 for all groups. Right, scrambled ZIP is less effective on inhibiting PKMζ biochemically in vitro than ZIP. B. Neither ZIP (filled circles; p = 0.5, comparing 1 min before application to 15 min after) nor scrambled ZIP (open circles; p = 0.6) has an effect on baseline postsynaptic AMPAR responses. Bar denotes duration of drug application; n = 4 for all groups.
Fig. 5.
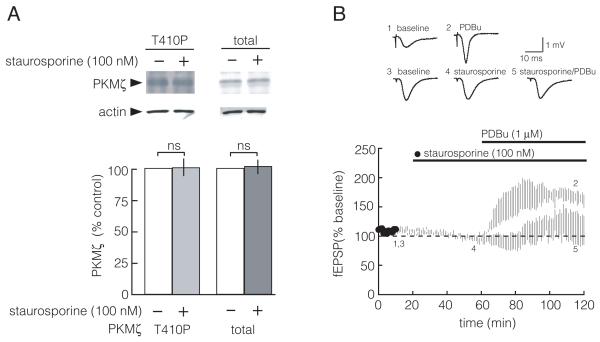
Staurosporine does not affect PDK1 phosphorylation of PKMζ in hippocampal slices. A. Staurosporine (100 nM) applied for 4 hr to the bath of hippocampal slices does not alter the phosphorylation state of the activation loop of PKMζ. Top, representative immunoblots; bottom, group data (between control and staurosporine, PKMζ T410P, p = 0.9; total PKMζ, p = 0.8; n = 5 for each group). B. Staurosporine (100 nM) inhibits phorbol ester-mediated potentiation of synaptic transmission. Unpaired t test, p < 0.05, between PDBu alone (open circles) and PDBu + staurosporine (filled circles) for fEPSPs at 120 min of recording; and paired t test, p < 0.05, comparing before and after PDBu alone, at 10 and 120 min, respectively. Staurosporine has no effect on baseline (one-way ANOVA, repeated measures, followed by Tukey’s post hoc tests, p = 0.8, between 10 and 60 min), and prevents potentiation by PDBu (p = 0.9, between 10 and 120 min; p = 0.8, between 60 and 120 min). Traces above are from the time points denoted in time course below. Bars denote duration of drug application; n = 4.
2.7 Kinetic model
We implemented a mass action model of a system with a kinase, a phosphatase, and a competitive kinase inhibitor. To this system protein synthesis and degradation could also be added, but the simulation results shown in this paper do not include synthesis and turnover based on the assumption that they operate on a slower time scale; however, additional simulations not shown here with synthesis and degradation are nearly identical to those without.
The product formation (phosphorylation phase) is described by the two following standard model enzymatic reaction equations (Segel, 1980):
Where E is the enzyme, in this case PKMζ, S is the substrate to be phosphorylated by PKMζ, and P is the product, the phosphorylated substrate. The variable C1 is the complex of bound substrate and enzyme. The competitive binding is modeled here by the following standard equation:
Where I is the inhibitor (in this case ZIP) and C2 is the bound complex. Here we assume for simplicity that C2 cannot bind to the substrate. The reaction that turns product into substrate (dephosphorylation) is again modeled by standard enzymatic kinetics:
Here Ph is the phosphatase concentration, and C3 the concentration of product bound to phosphatase. The total concentration of substrate: ST=S+P+C1+C3, is conserved, and so are the total concentration of enzymes ET=E+C1 and PhT=Ph+C3, and of inhibitor IT=I+C2.
In the simulations, we used the following constants. ST=1, k1=0.025,k-1=0.5, k2=1, kI=1, k-I=0.35, kp1=0.1, kp-1=0.1, kp2=0.5. Basal concentration of enzyme is 1, and for Fig. 3A, It=1. Kinetic coefficients for this system are not experimentally known; instead some were arbitrarily set, whereas others were adjusted to obtain results consistent qualitatively with experimental data of experiments herein. Coefficients and results are given in arbitrary units [AU] because we do not know the real coefficients, and to stress the qualitative and general applicability of these results. In Fig. 3A, ET was varied as shown, and in Fig. 3B, ET was varied and for each ET we found IT such that we had 50% inhibition of product at baseline. Results were obtained through integration of this mono-stable until steady state.
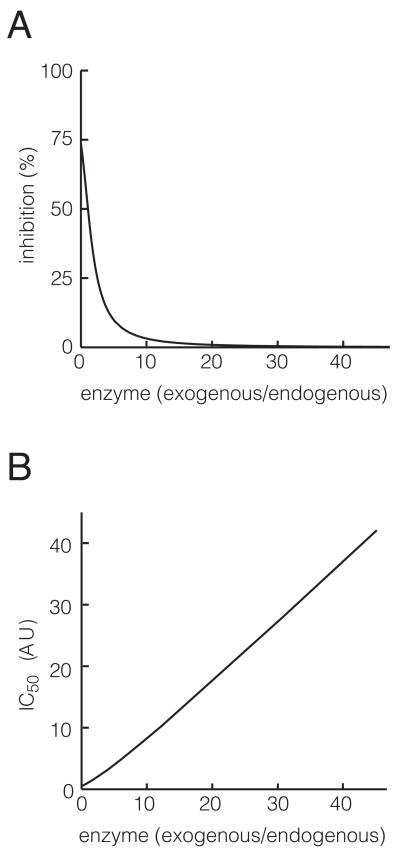
Fig 3.
Modeling of intracellular PKMζ-ZIP interactions. A. The percent inhibition of product at steady state resulting from a fixed level of competitive inhibitor (ZIP), as the amount of total enzyme changes. The level of competitive inhibitor is set to 1 in arbitrary units [AU]. B. The amount of inhibitor required for inhibiting 50% of product concentration at baseline (IC50), as a function of the total enzyme concentration.
3. Results
3.1 The ability of ZIP and chelerythrine to inhibit phosphorylation decreases with increasing PKMζ concentrations
ZIP is a competitive inhibitor of PKMζ with a Ki of 76 nM (Fig. 1A). In most of their experiments testing the efficacy of ZIP on PKMζ-RFP fusion protein in transfected cells, Wu-Zhang et al. (2012) used 1 μM, a concentration near the half-maximal concentration for inhibiting PKMζ’s effects within CA1 pyramidal cells (Serrano et al., 2005). We therefore examined the consequence of increasing PKMζ concentration on the ability of 1 μM ZIP to inhibit PKMζ phosphorylation. As PKMζ concentrations increase, ZIP’s efficacy decreases (Fig. 1B, C). One μM ZIP fails to block phosphorylation at high concentrations of PKMζ (Fig. 1C), when further increasing enzyme concentration has little effect on substrate phosphorylation (Fig. 1B).
Chelerythrine is a competitive inhibitor of the catalytic domain of PKC, highly effective on PKM forms (Fig. 1D) (Ling et al., 2002), that is sensitive to redox state, with high levels of the reducing agent dithiothreitol (DTT) reducing inhibition (Fig. 1E). Wu-Zhang et al. (2012) used 1 μM chelerythrine, a dose effective on PKMζ introduced into neurons (Ling et al., 2006; Ling et al., 2002), in their attempts to inhibit overexpressed PKMζ-RFP. Like ZIP, chelerythine effectively inhibits PKMζ, but not if the kinase is in excess (Fig. 1D).
3.2 Cellular overexpression increases PKMζ ~30- to 40-fold above endogenous levels
These results suggest that ZIP and chelerythrine are ineffective at high concentrations of PKMζ. Wu-Zhang et al. (2012) did not report for their experiments the level of expression of exogenous PKMζ-RFP relative to endogenous PKMζ or PKCζ. We therefore examined PKMζ protein levels expressed using the techniques of Wu-Zhang et al. (2012). In cultured hippocampal neurons, the Sindbis virus overexpression technique employed by Wu-Zhang et al. (2012) increases exogenous PKMζ ~30-fold above endogenous levels, in both cytosolic and membranous compartments (Fig. 2A). Similarly, in 293T cells used by Wu-Zhang et al. (2012), which express PKCζ but not PKMζ, the liposome-mediated transfection methods employed increase PKMζ ~40-fold over endogenous PKCζ levels (Fig. 2B). Because ~50% of the hippocampal neurons and 293T cells were transfected (data not shown), and the immunoblots examined the protein extracted from all of the cells whether transfected or not, these measurements substantially underestimate the amount of overexpressed protein within the transfected cells.
In the 293T cells overexpressed with PKMζ, we reproduced the results of Wu-Zhang et al. (2012), finding that ZIP at 1 μM for 1 hr does not inhibit the phosphorylation of the ~180 kDa band recognized by a Ser(P) PKC substrate antiserum. Extending the concentration to 100 μM, beyond the concentration that they used, was also ineffective (data not shown).
3.3 Modeling the inhibition of PKMζ by ZIP at normal and overexpressed levels of kinase
In order to evaluate the consequences of such high levels of cellular expression of PKMζ on the efficacy of competitive inhibitors, we modeled how the effect of increasing total PKMζ changes the ability of a fixed concentration of competitive inhibitor (ZIP or chelerythrine) to inhibit the steady state level of product. Our in vitro biochemical experiments (Fig. 1) are a system with no phosphatases or protein turnover. In such a system the steady state levels for any amount of inhibitor or enzyme are such that all substrate is converted into product; all that changes with the different conditions is the rate of product formation. Cells, however, contain phosphatases, as well as the means of protein production and turnover, and steady state levels of kinase products depend on the concentration of inhibitors. We therefore modeled the effect of the competitive inhibitor in a cell (see Methods), in order to understand how inhibition depends on the level of total enzyme. This model depends on various kinetic coefficients and concentrations of chemicals that are not experimentally known; however, the qualitative results generalize over a wide range of parameters.
We first examined how the steady state levels of product depend on the relative enzyme concentration, at a fixed level of a competitive inhibitor (Fig. 3A). Qualitatively similar to velocity measurements of in vitro models of enzyme kinetics (Segal, 1975), we find that as enzyme concentration increases, the effect of the inhibitor drops rapidly. Here inhibitor level is set so that it produces a 50% reduction in steady state product for an endogenous level of product. If the enzyme levels are increased 30- to 40-fold compared to endogenous, the same level of inhibitor will produce almost no inhibition.
The model can also be used to investigate what level of inhibitor is required for inhibiting 50% of product at steady state (IC50) at every enzyme level (Fig. 3B). The analysis indicates that when the level of enzyme expressed is 30- to 40-fold more than the endogenous enzyme level, the IC50 will also increase by roughly 30- to 40-fold.
3.4 ZIP, but not scrambled ZIP, inhibits the physiological effect of intracellular PKMζ in neurons
To demonstrate that ZIP inhibits a physiologically relevant function of PKMζ, we introduced PKMζ (10 nM in the whole-cell recording pipette) into CA1 pyramidal cells and examined the effect of bath applications of the inhibitor on PKMζ-mediated augmentation of postsynaptic AMPAR responses. Previous results have shown that 5 μM ZIP is close to the lowest dose that completely blocks the enhancement of AMPAR-mediated EPSCs by PKMζ (Serrano et al., 2005). Reproducing this result, 5 μM ZIP completely inhibited PKMζ-mediated AMPAR potentiation (Fig. 4A, left). The same concentration of ZIP had no effect on baseline AMPAR-mediated EPSCs (Fig. 4B), indicating the drug had no effect on other cellular processes regulating basal excitatory synaptic transmission.
Scrambled ZIP has been used as a control for ZIP, because it has the identical amino acids as ZIP but in an altered sequence that decreases its efficacy 3- to 4-fold (Fig. 4A, right). Thus as expected, the equivalent concentration of scrambled ZIP had no effect on PKMζ-mediated AMPAR potentiation (Fig. 4A, left). Like ZIP, scrambled ZIP also had no effect on baseline AMPAR-mediated EPSCs (Fig. 4B).
3.5 Staurosporine does not inhibit PDK1 phosphorylation of endogenous PKMζ
Staurosporine, an effective inhibitor of conventional/novel PKCs and other protein kinases in the low nanomolar range, is an ineffective inhibitor of PKMζ up to 100 nM, and has been used as a control for ZIP (Li et al., 2011; Ling et al., 2002; Pastalkova et al., 2006). Wu-Zhang et al. (2012), however, claimed that the drug could inhibit the cellularly overexpressed PKMζ fusion protein indirectly through inhibition of PDK1, which phosphorylates the activation loop of PKMζ. However, inhibiting PDK1 is not expected to have an effect on endogenous PKMζ, because the PDK1 phosphorylation of the activation loop of PKMζ occurs as a co-translational step that is completed within minutes of PKMζ synthesis (Kelly et al., 2007). Thus, staurosporine is unlikely to alter the phosphorylation of the activation loop of PKMζ after its synthesis in neurons (Kelly et al., 2007).
We directly tested whether staurosporine alters the phosphorylation of the activation loop of endogenous PKMζ in hippocampal slices. We bath applied 100 nM staurosporine for 4 hours to hippocampal slices, which is the concentration and duration used to demonstrate that staurosporine does not reverse LTP maintenance (Ling et al., 2002). We found staurosporine has no effect of the state of T410 phosphorylation in the activation loop of endogenous PKMζ (using the notation for the amino acid sequence of PKCζ, Fig. 5A). This dose of staurosporine was effective in the hippocampal slices because it prevented LTP induction (Ling et al., 2002), and it blocks synaptic potentiation produced by applications of phorbol ester, an activator of the conventional and novel PKC isoforms (Fig. 5B).
4. Discussion
Here we show that ZIP is an effective inhibitor of PKMζ both assayed biochemically and applied to neurons. We find, biochemically in vitro, that when PKMζ reaches a level such that further increasing enzyme concentration has little effect on substrate phosphorylation, ZIP, and a second competitive PKMζ inhibitor chelerythrine, do not effectively reduce substrate phosphorylation. We also show the viral and liposome-mediated overexpression techniques used by Wu-Zhang et al. (2012) cause a ~30-40 fold increase in kinase levels in transfected cells. Using a mathematical model we further show that at such level of overexpression, standard concentrations of inhibitor should have no noticeable effect. These standard concentrations, however, completely inhibit the ability of PKMζ to enhance postsynaptic AMPAR responses in CA1 pyramidal cells.
In the interpretations of their experiments, Wu-Zhang et al. (2012) had assumed that ZIP would inhibit PKMζ at the doses previously shown to inhibit the kinase in neurons (Ling et al., 2006; Ling et al., 2002; Serrano et al., 2005; Zhang et al., 2012), regardless of the amount of enzyme expressed in the transfected cells. However, if PKMζ concentrations are high relative to substrate, much of the transfected enzyme will be ‘spare’ kinase, analogous to spare receptors, and inhibitors will be less effective or ineffective on blocking PKMζ’s action (Figs. 1, 3). Thus, the viral and liposome-mediated transfection methods used by Wu-Zhang et al. (2012), which produce very high levels of PKMζ in cultured cells (Fig. 2), are problematic for accurately determining the inhibitor concentrations that inactivate endogenous levels of PKMζ.
In addition to the high levels of kinase expression in their transfected cells, Wu-Zhang et al. (2012) had also allowed the PKMζ-RFP expression to proceed for 1-2 days prior to the addition of inhibitors. This experimental design increases the likelihood that the cellular substrates were converted to phosphorylated products. In this state, very little kinase is required to phosphorylate the depleted substrate, and thus the amount of spare kinase is even higher. In turn, an even higher concentration of inhibitor would be required to reduce phosphorylation.
Wu-Zhang et al. (2012) also assumed that the effects of inhibitors on the substrates they examined apply to the substrates that mediate the physiological function of PKMζ. In a cell, however, the ability to observe a reduction in phosphorylation by a kinase inhibitor depends on the efficacy of endogenous phosphatases, and the specificity of phosphatases may be different for the physiologically relevant products of PKMζ phosphorylation that mediate synaptic enhancement than for those examined by Wu-Zhang et al. (2012). Furthermore, because ZIP and chelerythrine are competitive inhibitors of substrate binding (Fig. 1A and Herbert et al. 1990), their effective concentrations will depend on substrate affinity and abundance, with higher concentrations required to inhibit substrates that are of high affinity or high abundance. The kinase substrates examined by Wu-Zhang et al. (2012) were not characterized as to affinity or abundance.
Indeed, the only bona fide physiological substrate of aPKC examined by Wu-Zhang et al. (2012) was MARK2/Par1b. It is important to note, however, that ZIP applied to cells was used to demonstrate that MARK2/Par1b is an intracellular substrate of endogenous aPKC (Suzuki et al., 2004). In Wu-Zhang et al. (2012), the experiments examining MARK2/Par1b were the only ones to employ a higher dose of ZIP — 10 μM, instead of the 1 μM that was used in all their other experiments. In a dose-response curve, however, Suzuki et al. (2004) had found that ZIP inhibited endogenous aPKC phosphorylation of MARK2/Par1b with an IC50 of ~20 μM.
How much ZIP would be required to inhibit PKMζ phosphorylation of MARK2/Par1b under the conditions of overexpression used by Wu-Zhang et al. (2012)? Our model of ZIP inhibition of PKMζ in cells indicates that at high concentrations of enzyme, the increase in IC50 of a competitive inhibitor is proportional to the increase in the level of expressed kinase relative to that of the endogenous kinase. Therefore, a 30- to 40-fold increase of PKMζ (Fig. 2) would require a dose of ~700 μM ZIP to achieve half-maximal inhibition of the phosphorylation of MARK2/Par1b.
Because the Km’s of the endogenous substrates of PKMζ are likely to differ, and therefore effective concentrations of competitive inhibitors will vary, it is crucial to test the inhibitors of PKMζ on the physiologically relevant function of the kinase. ZIP inhibits the ability of intracellularly perfused PKMζ to increase postsynaptic AMPAR responses within CA1 pyramidal cells (Serrano et al., 2005, and Fig. 4A, left), and, at higher doses, the action of expressed PKMζ on PSD-95 aggregation in cultured hippocampal neurons (Shao et al., 2011). In hippocampal slices, a dose-response curve shows that 5 μM ZIP is close to the lowest dose that completely blocks the ability of PKMζ to enhance AMPAR responses at postsynaptic sites and, in separate experiments, to reverse established LTP maintenance (Serrano et al., 2005). At equivalent doses, scrambled ZIP is ineffective on both postsynaptically perfused PKMζ (Fig. 4A, left) and LTP maintenance (Serrano et al., 2005). Because scrambled ZIP will also inhibit PKMζ at higher doses (Fig. 4A, right), it is important to use the control agent only when applying appropriate, low effective doses of ZIP. ZIP and scrambled ZIP do not affect baseline synaptic transmission (Fig. 4B), indicating that neither the myristoyl group nor the peptide moeity has an adverse effect on basal excitatory synaptic function.
Wu-Zhang et al. (2012) had also claimed that chelerythrine is not an inhibitor of PKC either biochemically in vitro or in cells. Chelerythrine, however, is well-documented by biochemical in vitro studies to be an inhibitor of the catalytic domain of PKC (Herbert et al., 1990; Thompson and Fields, 1996), particularly effective on PKM forms (Ling et al., 2002) (Fig. 1E). Because chelerythrine is a redox-sensitive, competitive inhibitor of PKC (Fig. 1E) (Herbert et al., 1990), biochemical in vitro studies failing to observe effective inhibition may not have controlled for protein substrate concentration or redox state (Davies et al., 2000; Lee et al., 1998). The efficacy of chelerythrine on PKMζ’s physiological actions in neurons is also well-established (Ling et al., 2006; Ling et al., 2002).
Finally, Wu-Zhang et al. (2012) had reported that staurosporine, a general kinase inhibitor that is ineffective on PKMζ biochemically in vitro, nonetheless inhibited cellularly overexpressed PKMζ-RFP by acting on PDK1. Staurosporine inhibits many protein kinases at low nanomolar concentrations, including conventional and novel PKCs, Ca2+/calmodulin-dependent protein kinase II (CaMKII), cAMP-dependent protein kinase (PKA), and PDK1, but not PKMζ (Komander et al., 2003; Ling et al., 2002). During the induction phase of LTP, PKMζ is synthesized and then binds to and is phosphorylated by PDK1 on its activation loop (Kelly et al., 2007). This activation loop phosphorylation of PKMζ is completed within minutes, however, and thus endogenous PKMζ in the hippocampus is maximally phosphorylated on its activation loop (Kelly et al., 2007). Therefore, ongoing PDK1 activity is not thought to be required for activation loop phosphorylation of endogenous PKMζ in neurons, and staurosporine would not be expected to inhibit activation loop phosphorylation of PKMζ after its synthesis. Indeed, 100 nM staurosporine has no effect on the state of PDK1 phosphorylation of endogenous PKMζ in hippocampal slices (Fig. 5A). The mechanism for the stability of the PKMζ activation loop phosphorylation by PDK1 and its resistance to phosphatases is not known, but may be related to the stable complex formed between PDK1 and the catalytic domain of PKCζ and PKMζ (Balendran et al., 2000; Kelly et al., 2007). Although the concentration of staurosporine used in our experiments does not inhibit PKMζ (Ling et al., 2002), it inhibits conventional and novel PKCs when applied to hippocampal slices, as shown by inhibition of phorbol ester-mediated potentiation of synaptic transmission (Fig. 5B). This concentration was also shown to block LTP induction, but not LTP maintenance (Ling et al., 2002).
The reason Wu-Zhang et al. (2012) observed staurosporine to reduce activation loop phosphorylation of overexpressed PKMζ-RFP in cultured cells is not clear. It is possible that the red fluorescent protein fused to the C-terminal of the PKMζ changes the conformation of the kinase and alters the phosphate turnover on the PDK1 phosphorylation site of the activation loop. The C-terminal of PKMζ is a PDZ-binding domain and blocking the endogenous C-terminal with a fusion protein will prevent normal interactions between PKMζ and PDZ-containing proteins (Kelly et al., 2007). This possibility is supported by the observation that staurosporine at 100 nM for 4 hr has no effect on PDK1 phosphorylation of N-terminally tagged, eGFP-PKMζ overexpressed in 293T cells (A. Tcherepanov and T. C. Sacktor, unpublished data). Moreover, Wu-Zhang et al. (2012) did not report the efficacy of ZIP, chelerythrine, or staurosporine on the biochemical activity of the fusion protein in vitro, so it is not known to what extent the altered pharmacology they reported is due to having studied a fusion protein rather than actual PKMζ. The artificial overexpression of large amounts of PKMζ-RFP in the cultured cells appears to have transformed the PDK1 phosphorylation of PKMζ from the brief, co-translational step found in neurons into a rate-limiting step that can be inhibited by staurosporine. Regardless of the reason for the modified regulation of overexpressed PKMζ-RFP in cultured cells, staurosporine does not affect activation loop phosphorylation of endogenous PKMζ in neurons, and the results of Wu-Zhang et al. (2012) are not relevant to the physiological function of PKMζ.
In summary, we have demonstrated that the biochemical effects of PKMζ inhibitors correspond with their cellular effects, and thus ZIP and chelerythrine, together with scrambled ZIP and staurosporine as controls, are effective tools to examine the function of PKMζ in neurons. With the appropriate use of these reagents, together with other methodologies such as genetic manipulations, neuroscientists can characterize the mechanisms by which PKMζ enhances synaptic transmission and maintains long-term memory storage.
Highlights.
ZIP is a competitive PKMζ inhibitor that blocks PKMζ-mediated synaptic potentiation
Chelerythrine is a redox-sensitive PKC inhibitor, effective on PKMζ
High levels of PKMζ render standard doses of ZIP and chelerythrine ineffective
Overexpression methodologies increase PKMζ 30- to 40-fold over endogenous levels
Staurosporine does not inhibit PDK1 phosphorylation of endogenous PKMζ in neurons
Acknowledgements
Supported by R37MH057068 (TCS), R01MH53576 (TCS), and R01DA034970-01 (HS and TCS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balendran A, Biondi RM, Cheung PC, Casamayor A, Deak M, Alessi DR. A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Czeta (PKCzeta) and PKC-related kinase 2 by PDK1. J Biol Chem. 2000;275:20806–20813. doi: 10.1074/jbc.M000421200. [DOI] [PubMed] [Google Scholar]
- Cai D, Pearce K, Chen S, Glanzman DL. Protein kinase M maintains long-term sensitization and long-term facilitation in aplysia. J Neurosci. 2011;31:6421–6431. doi: 10.1523/JNEUROSCI.4744-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drier EA, Tello MK, Cowan M, Wu P, Blace N, Sacktor TC, Yin JC. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat Neurosci. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Comm. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC. Protein kinase Mζ synthesis from a brain mRNA encoding an independent protein kinase Cζ catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Kelly MT, Crary JF, Sacktor TC. Regulation of protein kinase Mζ synthesis by multiple kinases in long-term potentiation. J Neurosci. 2007;27:3439–3444. doi: 10.1523/JNEUROSCI.5612-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D, Kular GS, Bain J, Elliott M, Alessi DR, Van Aalten DM. Structural basis for UCN-01 (7-hydroxystaurosporine) specificity and PDK1 (3-phosphoinositide-dependent protein kinase-1) inhibition. Biochem J. 2003;375:255–262. doi: 10.1042/BJ20031119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998;273:30306–30315. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- Lee SK, Qing WG, Mar W, Luyengi L, Mehta RG, Kawanishi K, Fong HH, Beecher CW, Kinghorn AD, Pezzuto JM. Angoline and chelerythrine, benzophenanthridine alkaloids that do not inhibit protein kinase C. J Biol Chem. 1998;273:19829–19833. doi: 10.1074/jbc.273.31.19829. [DOI] [PubMed] [Google Scholar]
- Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330:1400–1404. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- Li YQ, Xue YX, He YY, Li FQ, Xue LF, Xu CM, Sacktor TC, Shaham Y, Lu L. Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci. 2011;31:5436–5446. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Sacktor TC. Protein kinase Mζ enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMζ maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Pauli WM, Clark AD, Guenther HJ, O’Reilly RC, Rudy JW. Inhibiting PKMzeta reveals dorsal lateral and dorsal medial striatum store the different memories needed to support adaptive behavior. Learn Mem. 2012;19:307–314. doi: 10.1101/lm.025148.111. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMζ maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the ζ isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci U S A. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. John Wiley and Sons; New York: 1975. [Google Scholar]
- Segel LA. Mathematical Models in Molecular and Cellular Biology. Cambridge University Press; Cambridge, UK: 1980. [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, et al. PKMζ maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao CY, Sondhi R, van de Nes PS, Sacktor TC. PKMζ is necessary and sufficient for synaptic clustering of PSD-95. Hippocampus. 2011 doi: 10.1002/hipo.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y. Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science. 2011;331:1207–1210. doi: 10.1126/science.1200215. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14:1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Thompson LJ, Fields AP. betaII protein kinase C is required for the G2/M phase transition of cell cycle. J Biol Chem. 1996;271:15045–15053. doi: 10.1074/jbc.271.25.15045. [DOI] [PubMed] [Google Scholar]
- Wu-Zhang AX, Schramm CL, Nabavi S, Malinow R, Newton AC. Cellular Pharmacology of Protein Kinase Mzeta (PKMzeta) Contrasts with Its in Vitro Profile: Implications for PKMzeta as a mediator of memory. J Biol Chem. 2012;287:12879–12885. doi: 10.1074/jbc.M112.357244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Kays J, Hodgdon KE, Sacktor TC, Nicol GD. Nerve growth factor enhances the excitability of rat sensory neurons through activation of the atypical protein kinase C isoform, PKMzeta. J Neurophysiol. 2012;107:315–335. doi: 10.1152/jn.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]