Abstract
Background
Matrigels, solubilized basement membrane preparations, are often used to support tumor development in animal models. However, tumors formed by a mixture of tumor cells and Matrigel may vary significantly. The purpose of this study was to compare tumor development and growth of LNCaP human prostate cancer cells mixed with Matrigel or in gelatin sponges.
Methods
LNCaP cells were mixed with Matrigel or absorbed into VETSPON, a gelatin sponge, and inoculated into the subcutis of nude mice. Tumor incidence and growth rate were determined. Gene expression and cell growth and survival in tumor lesions were evaluated by immunohistochemistry (IHC), immunoblotting, and RT-PCR.
Results
All mice (12/12) inoculated with LNCaP cells in VETSPON produced tumors, compared to 70% (19/27) of mice injected with the cells with Matrigel. Tumor volume also varied less with VETSPON implants. No significant differences were observed in gene expression, cell growth, apoptosis, and microvessel density in tumors established from the two types of implants. However, in samples collected on days 1 and 4, more cells in Matrigel implants than those in VETSPON implants were stained positive for cleaved-caspase 3 and -PARP1. Expression of VEGF-A, HIF-1α, and Bcl-2 was elevated in the early VETSPON implants.
Conclusion
These data indicate that VETSPON promotes tumor cell survival at the early stage of implantation and suggest that the gelatin sponge is superior to Matrigel in supporting development and progression of human prostate cancer in nude mice. This model should be useful for preclinical studies in nude mice using LNCaP cells.
Keywords: Prostate cancer, Matrigel, hemostatic gelatin sponge, VETSPON, animal model
Introduction
Prostate cancer is the sixth most common cancer in the world and the second leading cause of cancer death in American men (1). As detection techniques improve, more patients are diagnosed with localized disease and can be cured by either surgery or radiation therapy. Metastasis in many patients, however, still occurs prior to the initial diagnosis. Hormonal therapies, using castration and an antiandrogen alone or the combination of castration and an antiandrogen (complete androgen blockade, CAB), are the mainstay treatment for advanced diseases. However, these treatments are only palliative: delaying tumor progression to castration-refractory prostate cancer (CRPC) by an average of less than 18 months (2,3). Further improvement of therapeutic efficacy requires better understanding of cancer biology and development of novel therapeutic agents, in which a reliable model of human cancer xenograft in nude mice is invaluable.
LNCaP human prostate adenocarcinoma cells, isolated from a metastatic supraclavicular lymph node (4,5), are androgen-sensitive and express prostatic specific antigen (PSA). The xenograft model of LNCaP tumors in nude mice recapitulate many properties of human prostate cancer and, hence, is commonly used in numerous preclinical studies. Matrigel is a solubilized basement membrane preparation extracted from Engelbreth-Holm-Swarm (EHS) mouse sarcoma containing many growth factors (6,7), Matrigel is often inoculated with LNCaP cells to facilitate tumor development (8-10). However, the incidence and volume of LNCaP tumors varies significantly in this model, which present difficulties for effectively evaluating effects of novel therapeutics on development of LNCaP tumors (10,11).
It was reported that Gelfoam, derived from hemostatic gelatin sponges, liquefies within a week or less and is completely absorbed in four to six weeks, without inducing excessive scar formation (12-14). Moreover, gelatin sponge does not present any antigenicity under physiological conditions. Gelatin sponges, such as Gelfoam and VETSPON, have been widely used in veterinary surgeries as a wound dressing, adhesive and absorbent pad (15). Gelatin sponge was also used as a scaffold to support osteoblasts and to promote bone regeneration in defective areas (16). Furthermore, gelatin sponges were used previously to study human bone fibroblasts and growth factors on development of LNCaP tumors in mice (17-19). The purpose of this study was to compare tumorigenicity and growth rate of LNCaP tumors inoculated with Matrigel and gelatin sponge in nude mice. Our data show that gelatin sponge is a superior matrix for supporting establishment of LNCaP tumors in comparison with Matrigel and should be useful tool for translational researches involving LNCaP cells.
Materials and methods
Reagents
RPMI 1640, Ca2+, Mg2+-free Hanks' balanced salt solution (HBSS), and fetal bovine serum (FBS) were purchased from M. A. Bioproducts (Walkersville, MD). Gelatin sponge (VETSPON) was purchased from Ferrosan Inc (Duncanville, TX). Growth factor reduced-Matrigel was purchased from BD Biosciences (Bedford, MA). The TRIzol total cellular RNA isolation kit was purchased from Invitrogen (San Diego, CA). System of cDNA synthesis was purchased from Promega (Madison, WI): M-MLV Reverse Transcriptase (Cat: M170B), dNTP mix (Cat: U151B), random primers (Cat:C118A), RNAse Inhibitor (Cat:N251A). Fast SYBR Green Master Mix (Cat:4385612, Applied Biosystems) was used for RT PCR. Antibodies used in the studies were purchased from Cell Signaling Technology (Danvers, MA): the 70 kilodalton heat shock proteins(HSP70) (Cat: #4873), Proliferating cell nuclear antigen (PCNA) (Cat: 2586), Cleaved caspase-3 (cat: 9661), Cleaved PARP (Cat: 9541); Epitomics Inc (Burlingame, CA): Androgen receptor(AR) (Cat: # 3184) for immunoblotting; Dako Cytomation (Glostrup, Denmark): PSA (Cat: A0562), Santa Cruz Biotechnology (Santa Cruz, CA): Prostate Stem Cell Antigen (PSCA) (Cat: sc-28820); Millipore (Temecula, CA):AR (Cat: 06-680) for IHC. The second antibodies for immunoblotting were purchased from GE Health Care UK Limited (Little Chalfont Buckinghamshire HP7 9NA, England): ECL™ horseradish peroxidase-conjugated donkey anti-rabbit IgG antibody (NA934V) and ECL™ horseradish peroxidase-conjugated sheep anti-mouse IgG antibody (NA931V). The secondary antibodies for IHC were purchased from Vector Laboratories, Inc. (Burlingame, CA): biotinylated Goat anti-rabbit IgG (Cat: BA-1000) and anti-mouse IgG (Cat: BA-9200).
Mice
Athymic male nude mice were purchased from Harlan Laboratories (Indianapolis, IN). The mice were used when they were 8-10 weeks of age and maintained in a facility approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the U. S. Department of Agriculture, U. S. Department of Health and Human Services, and the National Institute of Health. The animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) and executed according to IACUC guidelines.
LNCaP cells and culture
LNCaP (5,20) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained in RPMI 1640 supplemented with 10% FBS at 37 °C in 5% CO2. The cells were passaged 3 times in the subcutis of nude mice before being used in the study. Cells in exponential phase of growth were harvested by a 1-3 min treatment with a 0.25% trypsin-0.02% EDTA solution, suspended in RPMI 1640 supplemented 10% FBS, washed in cold HBSS twice (5 min centrifugation, 800 × g), resuspended in cold HBSS (2×107 cells/ml), Cell viability was ascertained by trypan blue exclusion assay and only cells with viability exceeding 95% were used.
Tumor cell inoculation
Mice were given pre-emptive analgesics, anesthetized by using Isoflurane and placed in the prone position. For inoculation of LNCaP cells in Matrigel, the Matrigel was first thawed on ice. All equipment (syringes and 21 gauge needles) was chilled on ice prior to be used in tumor cell inoculation. LNCaP cells (2×107/ml) in 0.1 ml cold HBSS were mixed with an equal volume Matrigel and injected subcutaneously into the right flank of each mouse. For inoculation of LNCaP cells with VETSPON, VETSPON was cut to 0.5 cm3 and placed in 48-well cell culture plates on ice. LNCaP cells (2×106) in 0.1 ml cold HBSS were seeded into VETSPON, gently mixed by using a pipette tip until the cells were absorbed completely. A small incision was created longitudinally on the dorsal lateral chest wall. The cell-soaked VETSPONs were placed under the skin. The wound was closed with a metal clip (Autoclip, Clay Adama, Parsippany, NJ) which were removed in 2 weeks after the surgery.
Tumor measurement and sampling
From day 8 after tumor cell implantation, tumor sizes were measured by using a caliper twice a week. Tumor volume (mm3) was calculated according the formula: (width2 × Length)/2. The experiment was terminated on day 40 after tumor cell inoculation and tumor volume and incidence were recorded. Blood of tumor-bearing mice was collected for serum PSA measurement prior to the mice being sacrificed. Tumor tissues were sampled for analysis as detailed below. In addition, on days 1 and 4 after the inoculation, several mice were anaesthetized, and Matrigel and VETSPON implants recovered and sampled for in vitro analyses.
Immunoblotting analysis of tumor samples
Tumor samples were snap-frozen in liquid nitrogen and homogenized in PBS-1% Triton X-100 containing a proteinase inhibitor cocktail (Sigma Chemicals). After a centrifugation at 12,000 rpm for 20 min, the supernatants were collected and subjected to immunoblotting analysis as described in our previous study (21). After incubation with the secondary antibodies, the immunoreactive signals were revealed using SuperSignal West Dura Extended Duration Substrate (34076, Thermo) and visualized in a KODAK Image Station IS4000MM Digital Imaging System (Eastman Kodak Co., Rochester, NY). Gene expression levels and were quantitated in the linear range of exposure and normalized to human HSP70.
Total RNA isolation and analysis
Total cellular RNA was extracted from tumor tissues using a TRIzol RNA extraction kit (Invitrogen) and analyzed by real-time reverse transcriptional PCR (RT-PCR) as detailed in our previous studies (22,23). Briefly, 2 μg of total RNA was reverse transcribed at 42°C in a 25 μl reaction buffer containing 200 units of M-MLV Reverse Transcriptase, 500 ng of random primers, and 500 nM dNTP mix. The obtained cDNA was amplified in a 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA) with Fast SYBR Green Master Mix following the manufacturer's instructions. The cycle threshold values were used to calculate the normalized expression of specific genes against β-actin using the Q-Gene software (24). The sequences of the primer pairs are listed below: β-actin, 5′-CCAACTGGGACGACATGGAGAAA-3′/5′-ACCGGAGTCCATCA CGATGCCA-3′; AR, 5′GGTGAGCAGAGTGCCCTATC-3′/5′-GGCAGTCTCCAAACGCA TGTC-3′; PSA, 5′-GACCACCTGCTACGCCTCA-3′/5′-AACTTGCGCACACACGTCATT-3′; PCNA, 5′-AAGGTGTTGGAGGCACTCAAA-3′/5′-CAAAGAGACGTGGGACGAGTC-3′; PSCA, 5′-GTTGGCCTCCTGACCGTCAT-3′/5′-GGCGTTGCACAAGTCGGTGTC-3′; Vascular endothelial growth factor A (VEGF-A), 5′-GAATCATCACGAAGTGGTGAA-3′/5′-TGGTGATGTTGGACTCCTCA-3′; B-cell lymphoma 2(Bcl-2), 5′-ATGTGTGTGGAG AGCGTCAACC-3′/5′-TGAGCAGAGTCTTCAGAGACAGCC-3′; DNA fragmentation factor 40 (DFF40), 5′-AAGGCTTGGAGTCCCGATTTC-3′/5′-CCTGAGCCTCCGCACCC-3′; DNA fragmentation factor 45(DFF45), 5′-TTCTGGCCATTGATAAGTCC-3′/5′-TGCC CATTTCTCATTACTAGC-3′; Hypoxia-inducible factor 1, alpha subunit (HIF1α, 5′-ACTTC TGGATGCCGGTGGT-3′/5′-GTCGCCGTCATCTGTTAGCAC-3′.
Serum PSA measurement
The blood of tumor bearing mice was allowed to clot for 2 hours at room temperature. After a centrifugation at 3000 rpm for 30 min to remove the clotted material, serum was collected and analyzed by a PSA ELISA kit (Cat: CM-401, United Biotech Inc.) following the protocol provided by the manufacturer.
Histology and immunohistochemistry (IHC) analyses
The histology and IHC staining was performed as described previously (25,26). Briefly, tumor tissues were rinsed with PBS, fixed in formalin, embedded in paraffin, and sectioned. Hematoxylin-Eosin (HE) staining was performed by a technician in our histology core facility. Tissue sections (4 μm) were dewaxed and rehydrated prior to antigen retrieval using Target Retrieval Solution, followed by a treatment with 3% hydrogen peroxide (H2O2) in methanol (v/v). The treated slides were incubated in a blocking solution and then reacted for 18 hr at 4°C in a humidified chamber with first antibodies. The sections were rinsed and incubated with peroxidase-conjugated secondary antibodies. A positive reaction was visualized by incubating the slides with stable DAB and counterstaining with Mayer's hematoxylin. The slides were dried and mounted with Universal mount, and images were digitized using an Olympus CCD camera (Olympus, Tokyo, Japan) and a personal computer equipped with Optimas Image Analysis Software (Optimas Corp., Bothell, WA). The positive cells in the ten representative microscopic fields were counted and the percentage of positive cells was calculated.
Statistical analysis
Continuous variables were compared by analysis of variance (ANOVA) and dichotomous variables were analyzed by Fisher's exact test. All the p values were for a two-sided test and p ≤ 0.05 was considered as statistically significant.
Results
Comparison of tumorigenicity and growth of LNCaP cells inoculated with Matrigel and VETSPON
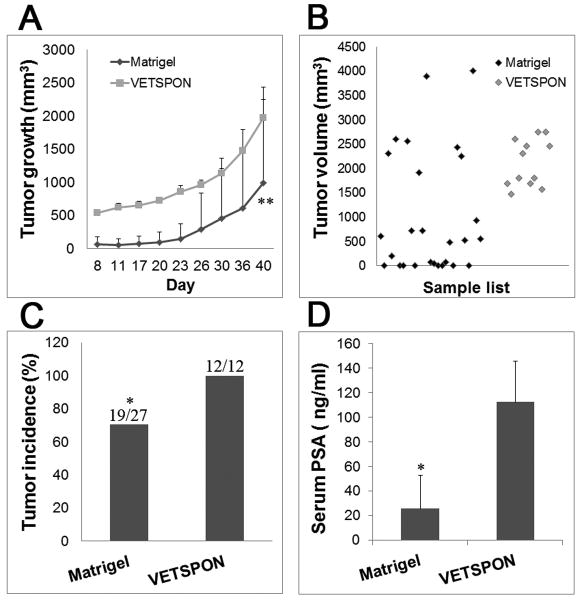
Several experiments were combined to include 27 mice injected with LNCaP cells mixed with Matrigel and 12 mice implanted with LNCaP cells absorbed into VETSPON. As shown in Figure 1A, larger masses were observed in the subcutis of mice implanted with LNCaP cells in VETSPON than those produced by the cells in Matrigel at the beginning of the experiments, due to the slower absorption of VETSPON. Tumors formed by LNCaP cells in both Matrigel and VETSPON grew rapidly at the fourth week after inoculation. On day 40, when VETSPON was almost completely absorbed (data not shown), LNCaP cells inoculated in VETSPON produced larger tumors (p<0.01): the tumor volumes of LNCaP cells in Matrigel and in VETSPON were 996 ± 1251 mm3 and 1972 ± 465 mm3, respectively. The volumes of tumors formed by LNCaP cells in Matrigel and VETSPON ranged from 0 to 4000 mm3 and 1470 to 2746 mm3, respectively (Figure 1B, p<0.05). The tumor incidence of LNCaP cells inoculated with Matrigel and with VETSPON was also significantly different. Whereas all mice (12/12) inoculated with LNCaP cells with VETSPON produced detectable tumors, only 70% of mice (19/27) injected with the cells in Matrigel induced tumor formation (Figure 1C, p<0.05). Consistently, PSA levels in the serum of mice inoculated with LNCaP cells in VETSPON were significantly higher than those in mice injected with the cells in Matrigel (112 ± 33 ng/ml vs. 25 ±27 ng/ml, p < 0.05) (Figure 1D). Taken together, these data demonstrate that the variations of both incidence and volume from tumors formed by LNCaP cells inoculated with VETSPON were significantly lower than those of tumors formed by cells inoculated with Matrigel.
Figure 1. Incidences and growth rates of LNCaP cells inoculated with VETSPON and Matrigel.
A. Growth curves of LNCaP tumors formed by a mixture of cells with Matrigel or absorbed in VETSPON. B. The volume of each tumor formed by LNCaP cells injected with Matrigel or VETSPON. C. Tumor incidence in each group. D. PSA levels (ng/ml) in serum of tumor-bearing mice determined by using the ELISA. * p < 0.05; **p < 0.001.
Comparison of expression of genes pertinent to prostate cancer and cell survival in established tumors
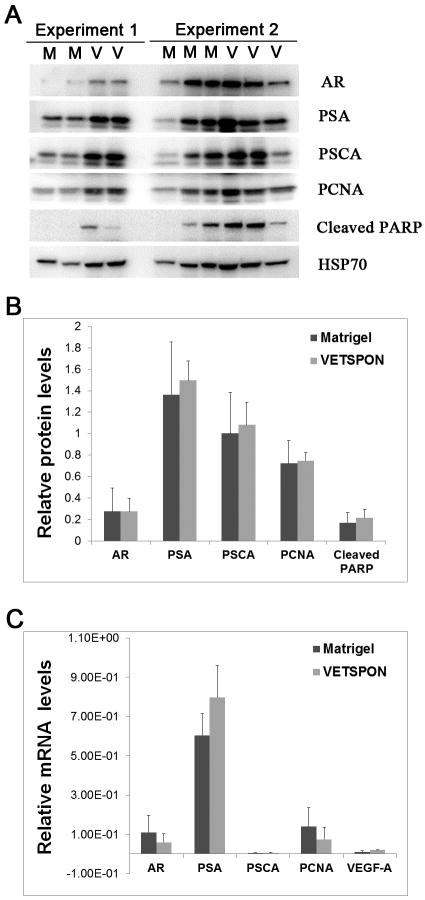
Immunoblotting was performed to evaluate protein expression of genes pertinent to prostate cancer (AR, PSA, and PCSA), cell proliferation (PCNA), and apoptosis (cleaved PARP) in the tumor lesions. Five tumors from 2 independent experiments were analyzed with human heat shock protein 70 (HSP70) as loading control (Figure 2A). Densitometry analysis of expression levels of the protein showed that there was no significant difference in protein expression of all genes analyzed (Figure 2B). Similarly, real time RT-PCR analysis showed no difference in mRNA expression levels of AR, PSA, PCNA, PSCA and VEGF-A between tumors formed by LNCaP cells in VETSPON and those in Matrigel (Figure 2C).
Figure 2. Expression of prostate-specific, cell replication-, and cell survival-related genes in established tumors.
A. Protein levels were analyzed by immunoblotting using human HSP70 as a loading control. B. Quantitative analysis of the immunoblotting bands by densitometry. C: Quantitative RT-PCR analysis of mRNA levels of AR, PSA, PCNA, PSCA and VEGF-A in the established tumors normalized with human β-actin.
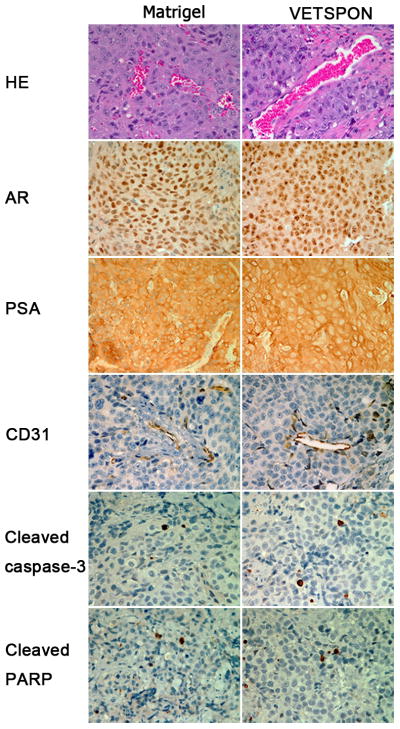
Microvessel densities were also similar in tumors formed by LNCaP cells in VETSPON and in Matrigel (Figure. 3, HE and CD31 staining). However, some blood vessels appeared inflated in the tumor formed using VETSPON (Figure 3). Consistent with the observations in the immunoblotting analysis, IHC staining did not detect any significant difference in AR, PSA, cleaved-caspase-3 and cleaved-PARP protein levels between the tumor formed by the cells in VETSPON and those in Matrigel (Figure 3). These data indicated that there was no discernible difference in the expression of genes pertinent to prostate cancer, cell growth, and apoptosis between tumors formed by LNCaP cells in VETSPON and in Matrigel.
Figure 3. Histology and IHC analysis of established tumors.
Paraffin-embedded sections of LNCaP tumors were stained by HE or IHC with antibodies specific to human AR, PSA, mouse CD31, Cleaved caspase-3 and Cleaved PARP.
Comparison of expression of cell survival and tumor angiogenesis-related genes in the early implants
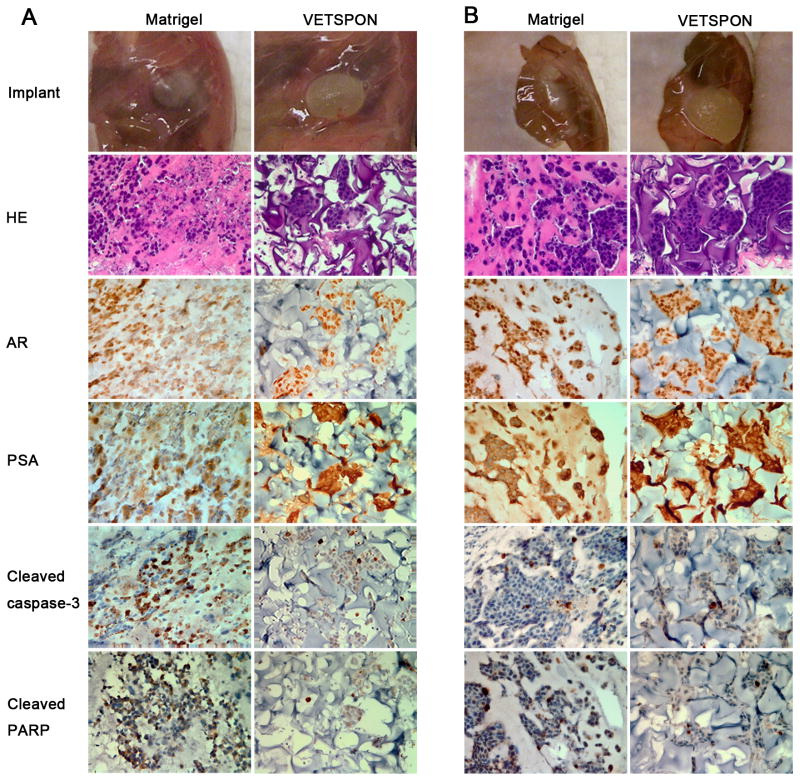
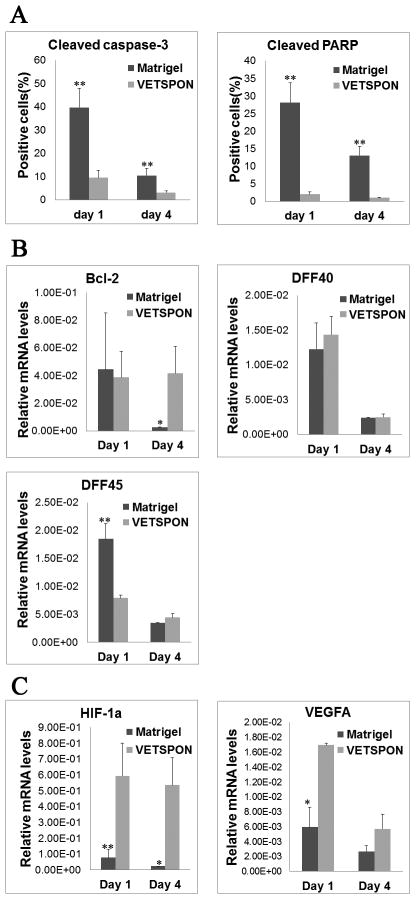
To elucidate mechanisms underlying the differential effects of VETSPON and Matrigel on tumorigenicity of LNCaP cells, the implants were harvested on day 1 and 4 for in vitro analyses. Macroscopically, the implants derived from LNCaP cells in VETSPON were larger than those from the cells in Matrigel (Figure 4A and B): the implants of LNCaP cells in VETSPON were filled with body fluid. HE staining showed that LNCaP cells formed clumps in Matrigel. In contrast, LNCaP cells in VETSPON distributed more evenly scattered in VETSPON (Figure 4A and 4B). IHC staining showed that AR and PSA protein expression levels were similar in LNCaP cells implanted with Matrigel and VETSPON on both day 1 and day 4. However, in comparison with those in VETSPON, significantly more cells in the Matrigel implants were stained positive by antibodies against the cleaved caspase-3 and cleaved PARP, two apoptotic markers (Figure 4). Approximately, 30-40% of cells implanted in Matrigel stained positive for cleaved caspase 3 and cleaved PARP on day 1, whereas only 10-20% of cells implanted in VETSPON were positive for both markers (Figure 5A). Similarly, the percentages of cells stained positively by the apoptotic markers in the VETSPON implants were also significantly lower than those in the Matrigel implants harvested on day 4 (Figure 5A). Therefore, more cells in the Matrigel implants underwent apoptotic death during the first and fourth days.
Figure 4. Histology and IHC analysis of implants of LNCaP cells inoculated with Matrigel or VETSPON.
Implants of LNCaP cells in Matrigel or VETSPON were harvested on day 1 (A) or day 4 (B) for in vitro analyses. The implants were shown on the top panel. Paraffin-embedded sections were stained by HE and IHC with antibodies against AR, PSA, cleaved caspase-3 and cleaved PARP.
Figure 5. Analyses of genes expressed in early stage implants of LNCaP cells inoculated with Matrigel or VETSPON.
A. Quantitative analysis of cells positively stained by cleaved caspase-3 and Cleaved PARP in the implant sampled on day 1 and 4. B. Quantitative RT-PCR analysis of Bcl-2, DFF40 and DFF45 mRNA levels in the implants harvested on day 1 and day 4. The relative expression levels were normalized by human β-actin. C. Quantitative RT-PCR analysis of HIF-1α and VEGF-A mRNA levels in the implants harvested on day 1 and day 4. The relative expression levels were normalized by human β-actin. *p< 0.05; **p < 0.01.
In samples collected on day 4, expression of Bcl-2 mRNA, an anti-apoptotic gene, was significantly higher in cells implanted in VETSPON than those in Matrigel (Figure 5B). Expression levels of DFF40 mRNA, an endonuclease that cleaves DNA in apoptotic cells (27) were similar in cells implanted with Matrigel and VETSPON on both day 1 and day 4 (Figure 5B). However, in samples collected on day 1 after the inoculation, the level of DFF45, the DFF40 chaperone responsible for proper folding of DFF40 during its synthesis (28,29), was significantly higher in cells implanted in Matrigel than that in cells implanted with VETSPON (Figure 5B).
Finally, expression of HIF-1α and VEGF-A, two genes critical for cell survival and tumor angiogenesis, was determined in the early implants. As shown in Figure 5C, expression of HIF-1α mRNA was significantly elevated in the LNCaP cell-VETSPON implants harvested on both day 1 and 4 in comparison with that in LNCaP cell-Matrigel implants (p < 0.05). Similarly, expression of VEGF-A mRNA, which can be regulated by HIF-1, was also elevated in the LNCaP cell-VETSPON implants (p < 0.05).
Discussion
A reliable animal model is critical for cancer research. The xenograft model of LNCaP human prostate cancer cells recapitulates many properties of human disease and has been used in numerous studies over the last decades (5,8-10). Currently, subcutaneous tumors of LNCaP cells were mostly established by inoculation of a mixture of LNCaP cells and Matrigel (8-10). This model, however, does not exhibit predictable tumorigenicity and growth rate, often leading to significant variations in tumor incidence and volumes, which may reduce its efficiency in evaluating therapeutic agents. In order reduce the variation, therapy or treatment often begins when the tumor has grown successfully or has reached certain volume and requires more nude mice (10,11). We compared formation of tumors in nude mice by LNCaP cells inoculated with Matrigel and VETSPON gelatin sponges. Our data clearly show that LNCaP cells inoculated with VETSPON produce tumors in all mice and that tumor volume variation is significantly reduced, thus overcoming the drawbacks of the model in which LNCaP cells are injected as mixture with Matrigel.
A detailed analyses of prostate-specific genes, such as AR, PSA, and PSCA, the cell growth gene PCNA, apoptosis-related genes, including cleaved PARP and cleaved caspase 3, and angiogenesis-related gene (VEGF-A) and micovessel (CD31), showed no discernible differences between established tumors formed LNCaP cells inoculated with Matrigel and VETSPON. To elucidate underlying mechanisms responsible for the significant differences in the incidence and growth rate between the two different models, we analyzed tumor cells implants on day 1 and day 4 after inoculation. LNCaP cells formed cell clumps in the Matrigel implants. The volume of a Matrigel significantly reduced during the first 4 days, which was consistent with a previous report that there was a rapid loss of liquid from a Matrigel in the early stage (30). In contrast, VETSPON implant was filled with body fluid and LNCaP cells distributed more evenly in the sponge matrix. This physical property of VETSPON (31) may provide structural support for the distribution of the LNCaP cell and make the LNCaP cells scatter in VETSPON. The body fluid in the gelatin sponge may also provide additional nutrients supporting the survival of LNCaP cells at the early stage. Indeed, in comparison with those in the implants of VETSPON, more cells in the Matrigel implants stained positive by cleaved caspase-3 and cleaved PARP, whereas Bcl-2 expression level was reduced, indicating that more cells in the Matrigel went apoptosis in the early stage soon after the inoculation.
Bcl-2 is a multifunctional protein. In addition to its antiapoptotic activity, bcl-2 was reported to promote HIF-1-mediated VEGF expression in tumors under hypoxia conditions (32,33) and induce HIF-1 activation in hypoxic tumor cells in culture (34). Enhanced levels of HIF-1α, the oxygen-regulated subunit of HIF-1, are often associated with increased tumor angiogenesis, metastasis, therapeutic resistance and poor prognosis (34). HIF-1α binds to the promoter region of VEGF and apparently thereby increases VEGF expression (35). The higher HIF-1α and VEGF-A mRNA expression levels in VETSPON implants suggest that the gelatin sponge and/or the body fluid in the sponge at the early stage may promote tumor cell survival and angiogenesis, by which VETSPON promotes tumor development and progression.
In summary, our data clearly show that VETSPON has some advantages over Matrigel in this LNCaP human prostate cancer xenograft model: (a) It is easier to store at room temperature compared with Matrigel at -20 degree; (b) It is easier to control the quality of VETSPON versus Matrigel which should be thawed low temperature and need chilled equipment; (c) LNCaP cells inoculated with VETSPON produce tumors in all mice, whereas the cells injected with Matrigel only have a tumor incidence of approximately 70%; and (d) the volume of tumors formed by cells implanted with VETSPON is significantly lower than that of tumors inoculated with Matrigel. Taken together, our data indicate that hemostatic gelatin sponge, such as VETSPON, appears to be a superior matrix supporting LNCaP tumor development, which should be useful for evaluating therapeutic effects of various agents as well as other biological modifiers.
Acknowledgments
The authors would like to thank Ms. Kelsey L. Dillehay (Department of Internal Medicine, University of Cincinnati College of Medicine) for critical reading of this manuscript.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
This work was supported in parts by grants from University of Cincinnati Cancer Center (ZD), Grants NIH-NCI 1 R01 CA131137-01A1 (ZD), and a Scholarship (2010704039) from China Scholarship Council (LC)
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED. Challenges in the management of prostate cancer. Br J Urol. 1992;70(Suppl 1):33–38. doi: 10.1111/j.1464-410x.1992.tb15865.x. [DOI] [PubMed] [Google Scholar]
- 3.Gulley J, Figg WD, Dahut WL. Treatment options for androgen-independent prostate cancer. Clin Adv Hematol Oncol. 2003;1(1):49–57. [PubMed] [Google Scholar]
- 4.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 5.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43(4):1809–1818. [PubMed] [Google Scholar]
- 6.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochem. 1982;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 8.Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinol. 2004;145(8):3961–3970. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 9.Eder IE, Hoffmann J, Rogatsch H, Schafer G, Zopf D, Bartsch G, Klocker H. Inhibition of LNCaP prostate tumor growth in vivo by an antisense oligonucleotide directed against the human androgen receptor. Cancer Gene Ther. 2002;9(2):117–125. doi: 10.1038/sj.cgt.7700416. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Cui XX, Gao Z, Zhao Y, Lin Y, Shih WJ, Huang MT, Liu Y, Rabson A, Reddy B, Yang CS, Conney AH. Atorvastatin and celecoxib in combination inhibits the progression of androgen-dependent LNCaP xenograft prostate tumors to androgen independence. Cancer Prev Res (Phila) 2010;3(1):114–124. doi: 10.1158/1940-6207.CAPR-09-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P, Benedict R, Urzua F, Fischbach C, Mooney D, Polverini P. Combination treatment significantly enhances the efficacy of antitumor therapy by preferentially targeting angiogenesis. Lab Invest. 2005;85(6):756–767. doi: 10.1038/labinvest.3700272. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins HP, Senz EH, et al. Present status of gelatin sponge for the control of hemorrhage; with experimental data on its use for wounds of the great vessels and the heart. J Am Med Assoc. 1946;132(11):614–619. doi: 10.1001/jama.1946.02870460004002. [DOI] [PubMed] [Google Scholar]
- 13.Barnes AC. The use of gelatin foam sponges in obstetrics and gynecology. Am J Obstet Gynecol. 1947;54(1):105–107. doi: 10.1016/s0002-9378(16)39476-5. [DOI] [PubMed] [Google Scholar]
- 14.Rarig HR. Successful use of gelatin foam sponge in surgical restoration of fertility. Am J Obstet Gynecol. 1963;86:136. doi: 10.1016/0002-9378(63)90086-3. [DOI] [PubMed] [Google Scholar]
- 15.Charlesworth TM, Agthe P, Moores A, Anderson DM. The use of haemostatic gelatin sponges in veterinary surgery. J Small Anim Pract. 2012;53(1):51–56. doi: 10.1111/j.1748-5827.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 16.Rohanizadeh R, Swain MV, Mason RS. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med. 2008;19(3):1173–1182. doi: 10.1007/s10856-007-3154-y. [DOI] [PubMed] [Google Scholar]
- 17.Chung LW, Li W, Gleave ME, Hsieh JT, Wu HC, Sikes RA, Zhau HE, Bandyk MG, Logothetis CJ, Rubin JS, et al. Human prostate cancer model: roles of growth factors and extracellular matrices. J Cell Biochem Suppl. 1992;16H:99–105. doi: 10.1002/jcb.240501222. [DOI] [PubMed] [Google Scholar]
- 18.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991;51(14):3753–3761. [PubMed] [Google Scholar]
- 19.Gleave ME, Hsieh JT, von Eschenbach AC, Chung LW. Prostate and bone fibroblasts induce human prostate cancer growth in vivo: implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. J Urol. 1992;147(4):1151–1159. doi: 10.1016/s0022-5347(17)37506-7. [DOI] [PubMed] [Google Scholar]
- 20.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57(3):205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 21.Dong Z, Liu Y, Lu S, Wang A, Lee K, Wang LH, Revelo M, Lu S. Vav3 oncogene is overexpressed and regulates cell growth and androgen receptor activity in human prostate cancer. Mol Endocrinol. 2006;20(10):2315–2325. doi: 10.1210/me.2006-0048. [DOI] [PubMed] [Google Scholar]
- 22.Olson MV, Lee J, Zhang F, Wang A, Dong Z. Inducible nitric oxide synthase activity is essential for inhibition of prostatic tumor growth by interferon-beta gene therapy. Cancer Gene Ther. 2006;13(7):676–685. doi: 10.1038/sj.cgt.7700941. [DOI] [PubMed] [Google Scholar]
- 23.Lu S, Dong Z. Characterization of TGF-beta-regulated interleukin-8 expression in human prostate cancer cells. Prostate. 2006;66(9):996–1004. doi: 10.1002/pros.20424. [DOI] [PubMed] [Google Scholar]
- 24.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32(6):1372–1374. 1376, 1378–1379. [PubMed] [Google Scholar]
- 25.Zhang F, Lee J, Lu S, Pettaway CA, Dong Z. Blockade of transforming growth factor-beta signaling suppresses progression of androgen-independent human prostate cancer in nude mice. Clin Cancer Res. 2005;11(12):4512–4520. doi: 10.1158/1078-0432.CCR-04-2571. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Lu W, Dong Z. Tumor-infiltrating Macrophages Are Involved in Suppressing Growth and Metastasis of Human Prostate Cancer Cells by INF-beta Gene Therapy in Nude Mice. Clin Cancer Res. 2002;8(9):2942–2951. [PubMed] [Google Scholar]
- 27.Ninios YP, Sekeri-Pataryas KE, Sourlingas TG. Histone H1 subtype preferences of DFF40 and possible nuclear localization of DFF40/45 in normal and trichostatin A-treated NB4 leukemic cells. Apoptosis. 2010;15(2):128–138. doi: 10.1007/s10495-009-0418-7. [DOI] [PubMed] [Google Scholar]
- 28.Neimanis S, Albig W, Doenecke D, Kahle J. Sequence elements in both subunits of the DNA fragmentation factor are essential for its nuclear transport. J Biol Chem. 2007;282(49):35821–35830. doi: 10.1074/jbc.M703110200. [DOI] [PubMed] [Google Scholar]
- 29.Widlak P, Lanuszewska J, Cary RB, Garrard WT. Subunit structures and stoichiometries of human DNA fragmentation factor proteins before and after induction of apoptosis. J Biol Chem. 2003;278(29):26915–26922. doi: 10.1074/jbc.M303807200. [DOI] [PubMed] [Google Scholar]
- 30.Wilson MJ, Sinha AA. Human prostate tumor angiogenesis in nude mice: metalloprotease and plasminogen activator activities during tumor growth and neovascularization of subcutaneously injected matrigel impregnated with human prostate tumor cells. Anat Rec. 1997;249(1):63–73. doi: 10.1002/(SICI)1097-0185(199709)249:1<63::AID-AR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Yang SH, Hsu CK, Wang KC, Hou SM, Lin FH. Tricalcium phosphate and glutaraldehyde crosslinked gelatin incorporating bone morphogenetic protein--a viable scaffold for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(1):468–475. doi: 10.1002/jbm.b.30200. [DOI] [PubMed] [Google Scholar]
- 32.Iervolino A, Trisciuoglio D, Ribatti D, Candiloro A, Biroccio A, Zupi G, Del Bufalo D. Bcl-2 overexpression in human melanoma cells increases angiogenesis through VEGF mRNA stabilization and HIF-1-mediated transcriptional activity. FASEB J. 2002;16(11):1453–1455. doi: 10.1096/fj.02-0122fje. [DOI] [PubMed] [Google Scholar]
- 33.Biroccio A, Candiloro A, Mottolese M, Sapora O, Albini A, Zupi G, Del Bufalo D. Bcl-2 overexpression and hypoxia synergistically act to modulate vascular endothelial growth factor expression and in vivo angiogenesis in a breast carcinoma line. FASEB J. 2000;14(5):652–660. doi: 10.1096/fasebj.14.5.652. [DOI] [PubMed] [Google Scholar]
- 34.Trisciuoglio D, Gabellini C, Desideri M, Ziparo E, Zupi G, Del Bufalo D. Bcl-2 regulates HIF-1alpha protein stabilization in hypoxic melanoma cells via the molecular chaperone HSP90. PLoS One. 2010;5(7):e11772. doi: 10.1371/journal.pone.0011772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24(19):3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]