Abstract
Autism is a neurodevelopmental disorder in which the first diagnostic symptom is unusual reciprocal social interactions. Approximately half of the children diagnosed with an autism spectrum disorder also have intellectual impairments. General cognitive abilities may be fundamental to many aspects of social cognition. Cognitive enhancers could conceivably be of significant benefit to children and adults with autism. AMPAKINE compounds are a novel class of pharmacological agents that act as positive modulators of AMPA receptors to enhance excitatory glutamatergic neurotransmission. This class of compounds was reported to improve learning and memory in several rodent and non-human primate tasks, and to normalize respiratory abnormalities in a mouse model of Rett syndrome. Here we evaluate the actions of AMPA compounds in adult male and female BTBR mice, a well characterized mouse model of autism. Acute treatment with CX1837 and CX1739 reversed the deficit in sociability in BTBR mice on the most sensitive parameter, time spent sniffing a novel mouse as compared to time spent sniffing a novel object. The less sensitive parameter, time in the chamber containing the novel mouse versus time in the chamber containing the novel object, was not rescued by CX1837 or CX1739 treatment. Preliminary data with CX546, in which β-cyclodextrin was the vehicle, revealed behavioral effects of the acute intraperitoneal and oral administration of vehicle alone. To circumvent the artifacts introduced by the vehicle administration, we employed a novel treatment regimen using pellets of peanut butter for drug delivery. Absence of vehicle treatment effects when CX1837 and CX1739 were given in the peanut butter pellets, to multiple cohorts of BTBR and B6 control mice, confirmed that the pharmacologically-induced improvements in sociability in BTBR were not confounded by the administration procedures. The highest dose of CX1837 improved the cognitive deficit in novel object recognition in BTBR. No drug effects were detected on the high levels of repetitive self-grooming in BTBR. In open field tests, CX1837 and CX1739 did not induce hyperactivity or sedation in either strain. It is interesting to speculate that the ability of CX1837 and CX1739 to restore aspects of sociability in BTBR mice could utilize synaptic mechanisms regulating social cognition, suggesting a potential pharmacological target for interventions to treat symptoms of autism.
1. Introduction
Social cognition, a subcategory of general cognitive abilities, has been variously defined. Generally understood as the processes by which people understand themselves and other people, social cognitive abilities include learning through observation, and an understanding of the intentions and emotions of others during social interactions (Beer and Ochsner, 2006; De Jaegher et al., 2010; Frith and Frith, 2012). While social cognition abnormalities appear in many neuropsychiatric disorders such as schizophrenia (King and Lord, 2011; Nuechterlein et al., 2008; Penn et al., 1997), mood disorders (Cusi et al., 2012), and frontotemporal dementia (Gregory et al., 2002), the most iconic may be autism. Autism is a neurodevelopmental disorder in which the primary diagnostic symptom is unusual reciprocal social interactions, including dramatic deficiencies in social cognition in many cases (Chevallier et al., 2012; Constantino, 2011; Lord et al., 2000; Lord et al., 2012; Volkmar et al., 2009). Incorrect interpretations of social cues and inappropriate responses in social settings, conceptualized as impaired Theory of Mind, mentalization, or mindblindness, are hallmarks of the diagnostic symptoms of autism (Frith and Frith, 2012; Lombardo and Baron-Cohen, 2011). Eye contact is minimal while looking at the mouth rather than on the eyes is common, gaze following and joint attention are rare, imitation skills are low, and attention to inanimate objects rather than social opportunities is characteristic (Davies et al., 2011; Klin et al., 2002; McPartland et al., 2011; Pelphrey et al., 2005). Brain regions normally activated by social cues are less activated in autistic individuals, as measured by functional magnetic resonance imaging and diffusion tensor imaging. These include the frontal-parietal cortex, superior temporal sulcus, fusiform gyrus, cingulate cortex, orbitofrontal cortex, somatosensory cortex, amygdala, and their connections (Adolphs et al., 2001; Just et al., 2012; Pelphrey and Carter, 2008; Philip et al., 2012; Pina-Camacho et al., 2011; Solomon et al., 2009; Williams and Minshew, 2007). Attention is often highly focused, characterized by unusually low distractibility, inability to disengage attention, deficits in divided attention, seeing the details but not the big picture, and intense involvement with a single special interest (Casey et al., 1993; Frith, 2003; Landry and Bryson, 2004).
Approximately half of the children diagnosed with an autism spectrum disorder have intellectual disabilities, with IQ scores under 70 (Charman et al., 2011). Low functioning cases of autism often present as comorbid with Fragile X syndrome or another intellectual impairment syndrome with a known genetic cause (Fombonne, 2005; Miles et al., 2003; Muhle et al., 2004). However, intellectual impairments in autism are not diagnostic. High IQ and remarkable special abilities in categorization skills, mathematics, computer programming, music and art have been reported, particularly in cases of high functioning autism and Asperger’s syndrome (Baron-Cohen et al., 2009; Black et al., 2009; Casey et al., 1993; Frith, 2003; Kennedy and Squire, 2007; Pring et al., 1995; Williams et al., 2008).
Cognitive enhancement could benefit people with low functioning autism directly. Further, since general cognitive abilities are essential to many aspects of social cognition, higher functioning individuals could benefit indirectly through improvements in attentional abilities, ability to understand social cues, and executive functions. Compounds under consideration as cognitive enhancers include glutamaterigic AMPA receptor modulators, glycine transporter inhibitors, GABA receptor inhibitors such as alpha5 inverse agonists, stimulants such as Ritalin and modafinil, and cholinergic agonists such as nicotine and alpha4beta2 agonists (Chambers et al., 2004; Hagerman et al., 2012; Levin et al., 2011; Lynch et al., 2011; Mehta et al., 2000; Mohler et al., 2008; Robbins et al., 1997; Sarter et al., 2009; Turner et al., 2003).
AMPAKINES are particularly interesting for their mechanism of action as positive modulators of AMPA receptors, acting to increase open ion channel times to enhance excitatory glutamatergic neurotransmission and synaptic plasticity (Arai and Kessler, 2007; Lynch, 2004; Lynch et al., 2008; Mueller et al., 2011b; Mueller et al., 2011c; Mueller et al., 2011d; Suppiramaniam et al., 2001). Characterization and specificity for these molecules has been shown by potentiated glutamate evoked calcium signals in cells that express various AMPA receptor subunits, with the most potent effects at the GluR1-flop containing receptor (Mueller et al., 2011a; Street et al., 2009). These compounds improved learning and memory in several rodent and non-human primate tasks (Broberg et al., 2009; Hamlyn et al., 2009; Hampson et al., 1998a; Porrino et al., 2005; Zheng et al., 2011), reversed striatal pathology, elevated BDNF, improved rotarod motor performance in the R6/2 mouse model of Huntington’s disease (Simmons et al., 2009), normalized respiratory abnormalities in a mouse model of Rett syndrome (Ogier et al., 2007), and protected against opiate-induced respiratory depression in rats and humans (Greer and Ren, 2009; Oertel et al., 2010).
We reasoned that mouse models of autism could be employed to evaluate pharmacological agents which improve cognitive abilities in other animal models. Mouse models of autism that display both social and cognitive deficits would be the most useful for evaluating cognitive enhancers. BTBR T+tf/J (BTBR) is an inbred strain of mice that displays well-replicated social deficits, reduced ultrasonic vocalizations in social settings, and high levels of repetitive self-grooming, relevant to each of the three diagnostic categories of autism, with no abnormalities in general health or physical abilities (McFarlane et al., 2008; Scattoni et al., 2008; Scattoni et al., 2011; Silverman et al., 2010b; Silverman et al., 2010c; Wohr et al., 2011; Yang et al., 2012a; Yang et al., 2007a; Yang et al., 2007b; Yang et al., 2009). In addition, BTBR displays deficits on cognitive tasks including fear conditioning, probabilistic reversal learning and Morris water maze (Amodeo et al., 2012; MacPherson et al., 2008; Rutz and Rothblat, 2012; Yang et al., 2012a). Since BTBR is a comprehensively characterized mouse model of autism, and is a genetically homogenous, commercially available inbred strain, it provides a useful model system for assessing novel pharmacological agents in autism relevant behavioral tasks.
In the present experiments, we evaluated the actions of three AMPAKINES on social, repetitive, and cognitive behaviors in adult male and female BTBR mice. The dose regimens and time courses for CX546, CX1739, and CX1837 were based on guidance kindly contributed by Mark Varney and Stephen Johnson at Cortex Pharmaceuticals Inc., as well as previously published experimental designs for in vivo assays with this class of compounds (Carmichael et al., 2009; Lipina et al., 2007b; Ogier et al., 2007; Street et al., 2009), and the available literature on treatments in mouse models of other syndromes (Lipina et al., 2007b; Simmons et al., 2009). Using an experimental protocol previously reported for testing mGluR5 receptor compounds (Silverman et al., 2010a; Silverman et al., 2012), we employed C57BL/6J (B6) as the control strain. B6 is a standard inbred strain of mice which consistently displays normal sociability, low repetitive behaviors, and high performance on most cognitive tasks. A control for general exploratory activity in a novel open field was conducted for each drug dose. As BTBR displayed a deficit on novel object recognition, CX1837 was further evaluated in the novel object recognition cognitive task, in light of the literature on cognitive improvements with other compounds in this class (Lynch et al., 2011; Lynch et al., 2008; Simmons et al., 2009; Street et al., 2009).
Methods
2.1. Mice
C57BL/6J (B6) and BTBR T+ tf/J (BTBR) mice were the offspring of breeding pairs purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed and bred in a conventional mouse vivarium at the National Institute of Mental Health (NIMH), Bethesda, Maryland, USA, using harem breeding trios. After two weeks with a male, females were separated into individual cages (Tecniplast, USA) before parturition. Pups were kept with the dam until weaning at postnatal day 21. After weaning, juveniles were group housed in cages of three and four by sex and strain in standard plastic cages. Cages were maintained in ventilated racks in a temperature (20°C) and humidity (~55%) controlled vivarium on a 12 hour circadian cycle, lights on from 0700 to 1900 hr. Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, enrichment that included a Nestlet square, shredded brown paper and a cardboard tube were provided in each cage. Light levels were approximately 325 lux during the light phase. Background noise measured approximately 50-60 dB. All procedures were approved by the National Institute of Mental Health Animal Care and Use Committee.
2.2. Preparation of compounds
Doses of CX546, CX1837 and CX1739 were based on previous literature (Carmichael et al., 2009; Gainetdinov et al., 2001; Lipina et al., 2007a; Ogier et al., 2007; Street et al., 2009) and personal communications with Drs. Stephen Johnson and Mark Varney (Cortex, Pharmaceuticals, Inc., Irvine, CA). AMPAKINES CX1837 and CX1739 were generously contributed by Drs. Mark Varney and Stephen Johnson at Cortex Pharmaceuticals, Inc., Irvine, CA. CX546 was purchased from Sigma Aldrich, St. Louis, MO. CX546 was administered acutely by intraperitoneal (i.p.) or per os (p.o.) injection in a volume of 10 ml/kg, at doses of 25 mg/kg, 50 mg/kg, 75 mg/kg and 100 mg/kg, CX1837 was administered at doses of 0.3 mg/kg, 1.0 mg/kg and 3.0 mg/kg and CX1739 at doses of 3.0 mg/kg, 10 mg/kg and 30 mg/kg. CX1837 and CX1739 were dissolved in 25% β-cyclodextrin in water in early experiments, and CX1739 mixed into 100 mg pellets of peanut butter in later experiments. For the dietary administration, CX1837 and CX1739 were dissolved in peanut butter (Jif, Cincinnati, OH, USA) to achieve the target dosage. The mixture was heated to 37°C for approximately 15 min, spread into plastic molds (100 mg pellet molds, catalog 106A , Ted Pella, Inc., Redding, CA, USA), and stored frozen at 80°C until treatment time. Doses were calculated based on group mean weights at the start of each behavioral testing session, as described previously (Cope et al., 2005).
2.3. Administration of compounds
Mice were trained to consume pellets of peanut butter using a novel design by our laboratory, which adapted techniques learned through personal communication with Dr. Cathy Gonzales (Pfizer Global Research, Groton, CT). First, a small weigh boat filled with peanut butter was placed in the homecage overnight to avoid neophobia and allow independent exploration of the novel, palatable substance. Second, all of the mice in a single cage were provided the opportunity to consume peanut butter once a day for 5-7 days during the last hour of the light cycle, when food consumption is high. During this second stage, consumption was not monitored. In the final training phase, the home cage that housed four mice was placed adjacent to an empty cage. Three mice were placed into the empty cage and one mouse remained in his/her home cage during pellet administration. A dose was administered by placing a 100 mg peanut butter pellet on a cotton-tipped applicator and resting it on the wire insert in the top of the home cage. The experimenter held the applicator in place and/or secured it with tape. When each mouse was given its peanut butter pellet in its home cage, alone but adjacent to its cagemates with maintenance of olfactory and visual contact with cagemates, consumption of the entire peanut butter pellet was rapid, typically in less than 2 minutes. The above process was repeated for each animal until all littermates in a cage had each eaten its peanut butter pellet rapidly, daily and completely for 5-7 days. Mice were then randomly assigned to groups such that variable members of each litter were selected at random to either vehicle control or a particular compound dose, thus eliminating the confound of litter effects. The experimenter was blinded to the contents of the various pellets by a pellet tray number coding system.
All treatments were single acute doses. Compounds administered intraperitoneally or orally were given 30 min prior to behavioral testing, as previously described in the literature (Gainetdinov et al., 2001; Lipina et al., 2007b; Street et al., 2009). For the dietary administration, each subject mouse fully consumed a single dose or vehicle pellet 30 min prior to behavioral testing. Drug administration using peanut butter pellets provided a drug delivery protocol that appeared to be non-stressful, avoiding risks for behavioral effects that are sometimes associated with i.p. or p.o. injections, and biological confounds associated with various vehicles. A comparison of the effects of various vehicles given i.p. and p.o. on the three-chambered social approach task appears in the Supplementary Results, Figure S7.
2.4. Experimental Design for Behavioral Assays
We used a between-subjects design with a one week washout period, such that each mouse received a single acute dose of CX546, CX1837, CX1739 or vehicle, and was tested in a single behavioral task, one task per week. Testing was conducted in dedicated behavioral testing rooms during the standard light phase, usually between 0900 and 1600 hr. Prior to all experiments, mice were acclimatized to the behavioral testing area for at least 60 minutes. Testing began at ages 6-8 weeks. Treatment groups consisted of 10-20 mice per strain for each dose of drug or vehicle. Previous studies in our laboratory documented no sex differences on either sociability or self-grooming in BTBR or B6 (McFarlane et al., 2008; Silverman et al., 2010a; Yang et al., 2007a; Yang et al., 2007b; Yang et al., 2009). Therefore, male and female mice were used in all studies in approximately equal proportions. The behavioral task order was social approach (week 1), self-groom (week 2) and open field (week 3). A separate cohort of all male BTBR and B6 mice was utilized for the male-female social interaction task. Half of those males went on to be tested in the novel object recognition task following a one week drug washout period. The other half of the cohort tested in novel object recognition was made up of an independent group of females. Drug doses, toe tattoo patterns, and digital videotapes were coded to ensure that the raters were blind to the treatment condition. All procedures were conducted in strict compliance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committees of the National Institute of Mental Health.
2.5. Social Approach
Social approach was tested in an automated 3-chambered apparatus from a design originally developed by our group (Nadler et al., 2004), using improved methods as recently described (Silverman et al., 2010a; Silverman et al., 2012; Yang et al., 2011b; Yang et al., 2012a). Briefly, the apparatus was a rectangular, 3-chambered box made from clear polycarbonate. Retractable doorways within the two dividing walls allowed access to the side chambers. A top mounted CCTV camera (Security Cameras Direct, Luling, TX, USA) placed over the boxes recorded the session for subsequent scoring. The apparatus was cleaned with 70% ethanol and water between subjects. At least five minutes elapsed between cleaning and the start of the next test session, to allow for ethanol evaporation and clearance of ethanol vapor odors. To increase throughput, four mice were run simultaneously, in four adjacent chambers. Mice used as the novel stimulus target were 129Sv/ImJ, aged 12–20 weeks old, bred and maintained in the NIMH vivarium from breeding pairs originally purchased from JAX, and matched to the subject mice by sex and age. Time spent in each chamber and number of entries into each chamber were calculated by the automated software, based on the movements of the subject mouse in sequentially breaking and unbreaking a series of photocell beams embedded in the openings between chambers (Yang et al., 2011b). Number of entries served as a within-task control for levels of general exploratory locomotion. An observer blind to the drug treatments scored the videos with a stopwatch for cumulative time in which the subject mouse sniffed the novel target mouse and the novel object. At the end of each testing day, test chambers were thoroughly cleansed with Alconox detergent (Alconox, White Plains, NY, USA) diluted with warm water, followed by extensive rinsing with hot water and air drying.
2.6. Male-female social interaction test
The male-female reciprocal social interaction test was conducted as previously described (Bozdagi et al., 2010; Scattoni et al., 2011; Yang et al., 2012b). Each mouse was provided with a single dose or vehicle peanut butter pellet 30 min prior to the interaction session. Briefly, each freely moving male BTBR or B6 subject was paired with a freely moving unfamiliar estrus B6 female for 5 min. A digital closed-circuit television camera (Panasonic, Secaucus, NJ, USA) was positioned horizontally 30 cm from the cage. An ultrasonic microphone (Avisoft UltraSoundGate condenser microphone capsule CM15; Avisoft Bioacoustics, Berlin, Germany) was mounted 20 cm above the cage. Sampling frequency for the microphone was 250 kHz, and the resolution was 16 bits. The entire apparatus was contained in a sound-attenuating environmental chamber (ENV-018V; Med Associates, St. Albans, VT, USA) under red light illumination (10 lux). Social sniffing (sum of nose-to-nose sniffing, nose-to-anogenital sniffing, and sniffing of other body regions) and exploratory activity were scored using Noldus Observer software (Noldus Information Technology, Leesburg, VA, USA) as previously described (Scattoni et al., 2011). Ultrasonic vocalization spectrograms were displayed using Avisoft software (Bozdagi et al., 2010). Ultrasonic calls were identified manually by a highly trained investigator blinded to drug treatment.
2.7. Self-grooming assay
Mice were scored for spontaneous repetitive self-grooming behavior as previously described (Silverman et al., 2010b; Silverman et al., 2012; Yang et al., 2011a). Briefly, each mouse was placed individually into a standard mouse cage, (46 cm length × 23.5 cm wide × 20 cm high). Cages were empty, to eliminate digging in the bedding, a potentially competing behavior. The room was illuminated at ~15 lux. A trained observer uninformed of the drug treatment scored the videos. Cumulative time spent self-grooming was scored from the videos using a high-accuracy Traceable© stopwatch (Thomas Scientific, Swedesboro, NJ) with the auditory component silenced.
2.8. Open field locomotion
General exploratory locomotion in a novel open field environment was assayed as previously described (Holmes et al., 2002; Silverman et al., 2010a; Silverman et al., 2012). Individual mice were placed in a standard Accuscan open field (AccuScan Instruments, Columbus, OH, USA). Illumination in the testing room measured ~ 15 lux. Test chambers consisted of clear Plexiglas sides and floor, approximately 40 × 40 × 30.5 cm. Mice were placed in the center of the open field at the initiation of the testing session. Photocells at standard heights for recording activity were aligned 8 to a side, dividing the chamber into 64 equal squares. Total distance, horizontal activity, vertical activity, and center time were automatically collected using the Versamax activity monitor and analyzer software system. Test chambers were cleaned with 70% ethanol between test subjects. At least five minutes between cleaning and the start of the next session was allowed for ethanol evaporation and odor dissipation.
2.9. Novel object recognition
The novel object recognition test was conducted with a cohort of mice in the open field arena, using methods similar to those previously described (Yang et al., 2012b). The experiment consisted of three sessions, a 30 minute exposure to the open field arena, a 10 min familiarization session and a 5 min recognition test. On day 1, each subject was habituated to a clean empty open field arena for 30 minutes. Twenty four hours later, each mouse was provided with a single dose or vehicle peanut butter pellet 20 min prior to habituation. Each subject was returned to the open field arena for the habituation phase, for 10 minutes. The mouse was then removed from the open field and placed in a clean temporary holding cage for approximately 2 minutes. Two identical objects were placed in the arena. Each subject was returned to the open field in which it had been habituated, and allowed to freely explore for 10 minutes. After the familiarization session, subjects were returned to their holding cages, which were transferred from the testing room to a nearby holding area. The open field was cleaned with 70% ethanol and let dry. One clean familiar object and one clean novel object were placed in the arena, where the two identical objects had been located during in the familiarization phase. Thirty minutes after the end of the familiarization session, each subject was returned to its open field for a 5 min recognition test, during which time it was allowed to freely explore the familiar object and the novel object. The familiarization session and the recognition test were videotaped and subsequently scored by two highly-trained investigators, uninformed of drug treatment, whose inter-rater reliability was within 1 sec of one another or > 95%. Object investigation was defined as time spent sniffing the object when the nose was oriented toward the object and the nose-object distance was 2 cm or less. Recognition memory was defined as spending significantly more time sniffing the novel object than the familiar object. Total time spent sniffing both objects was used as a measure of general exploration. Time spent sniffing two identical objects during the familiarization phase confirmed the lack of an innate side bias. Objects utilized were plastic toys of widely divergent shapes, from the Safari coral reef and jungle bundles (Safari Ltd ®, http://www.safariltd.com). The objects sizes were approximately 4 - 7.5 cm. Objects were counterbalanced to include various combinations of the coral, cowry shell, bear, tiger and treasure chest.
2.10. Statistical analysis
For social approach, a Repeated Measures ANOVA was conducted within each drug dose group and for the vehicle group, for each strain. Since times spent in each of the three chambers added to 10 minutes, and therefore were not independent, the test condition factor compared time spent only in the right versus left chambers. Center chamber times are shown in the graphs for illustrative purposes. Time spent sniffing the novel object versus the novel mouse was similarly analyzed within each dose for each strain. For number of entries during social approach, drug effects were compared within each strain by a separate between groups drug by entries Repeated Measures ANOVA. In cases where the overall ANOVA for entries was significant, the treatment factor for each strain was further analyzed with a Dunnett’s posthoc test to compare each drug dose group to its vehicle control group. For ultrasonic vocalizations and reciprocal interactions, a one-way ANOVA for drug dose within each strain was performed. Open field dose response effects of CX1837 and CX1739 were analyzed with a Repeated Measures ANOVA using a between groups factor of drug within strain, and a within group factor of time course, for the parameters of total distance, horizontal activity, vertical activity or center time. Dose response experiments were followed by a Dunnett’s post hoc analysis, using SigmaPlot version 11.0 (Systat Inc., San Jose, CA) that compared individual means in cases where the ANOVA was significant at p < 0.05. Self-grooming was analyzed using a within strain one-way ANOVA for drug dose, using StatView statistical software (Citewise.com, Acton, MA, USA). For novel object recognition time spent sniffing, a Repeated Measures ANOVA was conducted on the raw data within each drug dose group and for the vehicle group, for each strain. For novel object recognition derived parameters of difference score and discrimination ratio, a within strain one-way ANOVA for drug dose was performed. All data were graphed using SigmaPlot version 11.0 (Systat Inc., San Jose, CA). Significance was defined as p < 0.05.
Results
3.1. CX1837 increased social sniffing in BTBR in the 3-chambered social approach task
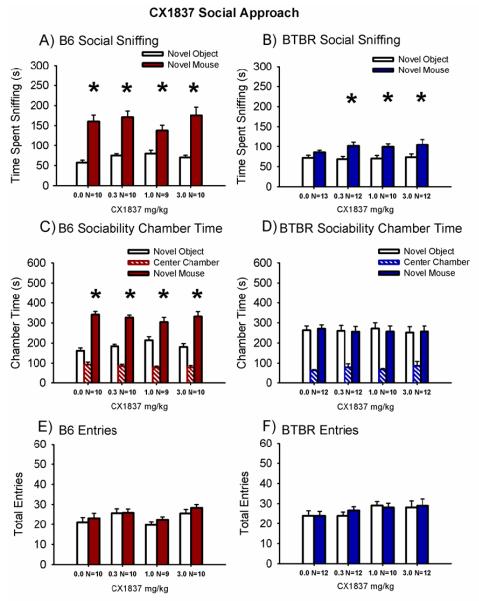
Figure 1 illustrates the sociability scores from the automated 3-chambered social approach task following a single dose of CX1837 or vehicle delivered via peanut butter pellets in B6 and BTBR mice. Sociability, defined as spending more time spent in directed sniffing to the novel mouse than in sniffing the novel object in the side chambers, was significant in B6 mice treated with peanut butter vehicle, and not significant in BTBR mice treated with the peanut butter vehicle, as expected (Panel A, B6 vehicle: F (1,9) = 35.9, p < 0.05; Panel B, BTBR vehicle: F (1,12) = 3.9, p > 0.05), and as previously reported with various other vehicles (McFarlane et al., 2008; Silverman et al., 2012; Silverman et al., 2010a; Yang et al., 2007a; Yang et al., 2007b; Yang et al., 2009). All other B6 groups treated with CX1837 exhibited scores similar to vehicle B6 and significantly spent greater time sniffing the novel mouse than sniffing the novel object (Panel A, B6 CX1837 0.3 mg/kg: F (1,9) = 34.2, p < 0.001; B6 CX1837 1.0 mg/kg: F (1,8) = 8.0, p < 0.01; B6 CX1837 3.0 mg/kg: F (1,9) = 20.4, p < 0.001). Time spent sniffing the novel mouse was greater than time spent sniffing the novel object in the BTBR treated with CX1837 (Panel B, BTBR CX1837 0.3 mg/kg: F (1,11) = 7.0, p < 0.05; BTBR CX1837 1.0 mg/kg: F (1,9) = 6.2, p < 0.05; BTBR CX1837 3.0 mg/kg: F (1,11) = 4.81, p < 0.05), at every dose, reversing BTBR’s usual low sociability sniffing scores. Time spent sniffing the novel mouse is considered a more direct and sensitive measure of sociability than chamber times (Fairless et al., 2011; Yang et al., 2011b).
Figure 1. AMPAKINE CX1837 increased social sniffing in BTBR with no deleterious effects in B6 control mice.
Social approach was assayed in a 3-chambered arena. CX1837 was administered acutely in a 100 mg peanut butter pellet, to which the subject mice were previously habituated, 30 minutes before the behavioral test session. Vehicle controls received the peanut butter pellet containing no drug. (A) The B6 control strain treated with vehicle or CX1837 exhibited characteristic sociability on the directed sniffing parameter at each dose tested. (B) BTBR exhibited its characteristic lack of sociability, i.e. did not spend more time sniffing the novel mouse versus the novel object, after treatment with vehicle. CX1837 (0.3 mg/kg, 1.0 mg/kg and 3.0 mg/kg), reversed the social sniffing deficits in BTBR. (C) B6 displayed normal sociability on the chamber time parameter, spending more time in the chamber with the novel mouse as compared to the novel object, after treatment with vehicle and at each dose of CX1837. (D) BTBR exhibited its characteristic lack of sociability on the chamber time parameter following vehicle administration and at each dose CX1837. Number of entries into the side chambers was unaffected by treatment in (E) B6 and (F) BTBR, indicating the absence of confounding increases or decreases in exploratory locomotion during the social approach task. * p < 0.05, novel mouse versus novel object. N = 9-13 per dose for each strain. Data are shown as mean ± SEM for all figures.
Sociability in B6 but not BTBR treated with the peanut butter vehicle was again observed using the time spent in the chamber parameter, comparing the time spent in the chamber with the novel mouse versus the novel object (Panel C, B6 vehicle: F (1,9) = 43.8, p < 0.001; Panel D, BTBR vehicle: F (1,11) = 0.03, p > 0.05). All B6 groups treated with CX1837 also exhibited significantly greater time spent in the chamber with the novel mouse than in the chamber with the novel object (Panel C, B6 CX1837 0.3 mg/kg: F (1,9) = 48.2, p < 0.001; B6 CX1837 1.0 mg/kg: F (1,8) = 4.9, p < 0.05; B6 CX1837 3.0 mg/kg: F (1,9) = 13.7, p < 0.05). Time in the chamber with the novel mouse was not different from time in the chamber with the novel object for BTBR treated with CX1837 (Panel D, BTBR CX1837 0.3 mg/kg: F (1,11) = 0.006, p > 0.05; BTBR CX1837 1.0 mg/kg: F (1,9) = 0.07, p > 0.05; BTBR CX1837 3.0 mg/kg: F (1,11) = 0.011, p > 0.05), similar to vehicle.
Entries into the side chambers were not affected by CX1837 in B6 (Panel E, F (3,37) = 1.8, p > 0.05) or BTBR (Panel F, F (3,47) = 1.27, p > 0.05), indicating that the drug treatment had no direct effect on exploratory locomotion during the social approach task. No innate side preference was present in B6 (F (3,37) = 2.14, p > 0.05) or BTBR (F (3,37) = 1.34, p > 0.05) during the task, as shown by similar amounts of time in the left and right side chambers during the 10 minute habituation session before the start of social testing.
3.2. CX1739 increased social sniffing in BTBR in the 3-chambered social approach task
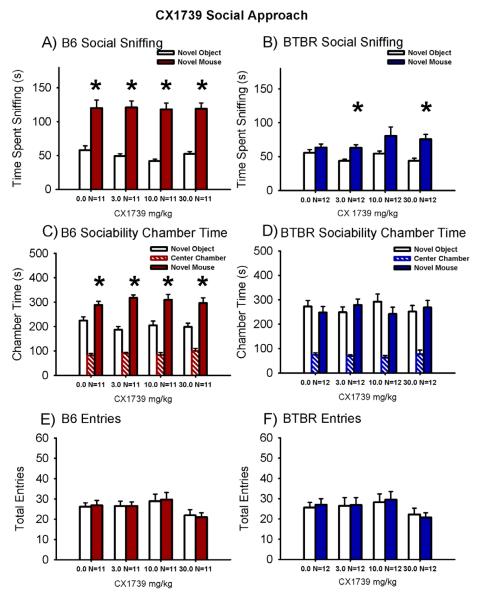
Figure 2 illustrates the sociability scores from the automated 3-chambered social approach task following a single dose of CX1739 or vehicle delivered via peanut butter pellets in B6 and BTBR mice. Sociability, defined as spending more time spent directed sniffing the novel mouse than sniffing the novel object, was significant in the peanut butter vehicle treated group of B6 mice and not significant in the peanut butter treated group of BTBR mice (Panel A, B6 vehicle: F (1,10) = 27.5, p < 0.01; Panel B, BTBR vehicle: F (1,11) = 2.12, p > 0.05), as observed in Figure 1. All B6 groups treated with CX1739 exhibited scores similar to vehicle B6 and spent significantly greater time sniffing the novel mouse than sniffing the novel object (Panel A, B6 CX1739 3.0 mg/kg: F (1,10) = 66.3, p < 0.001; B6 CX1739 10 mg/kg: F (1,10) = 81.3, p < 0.001; B6 CX1739 30 mg/kg: F (1,10) = 72.5, p < 0.001). As previously seen after CX1837, time spent sniffing the novel mouse was greater than time spent sniffing the novel object in the BTBR treated with CX1739 at two doses tested (Panel B, BTBR CX1739 3.0 mg/kg: F (1,11) = 12.3, p < 0.01 and BTBR CX1739 30 mg/kg: F (1,11) = 32.6, p < 0.001). In addition, a strong trend toward sniffing time reversal was detected in BTBR administered CX1739 at the 10 mg/kg dose (F (1,11) = 4.21, p = 0.064).
Figure 2. AMPAKINE CX1739 increased social sniffing in BTBR with no deleterious effects in B6 control mice.
An independent cohort of B6 and BTBR mice was tested by a different investigator in the 3-chambered social approach apparatus. (A) B6 treated with CX1739 exhibited its characteristic sociability on the directed sniffing parameter at each dose tested. (B) BTBR exhibited its characteristic lack of sociability, i.e. did not spend more time sniffing the novel mouse versus the novel object, after treatment with peanut butter vehicle. CX1739 at 3.0 mg/kg and 30 mg/kg reversed the usual social sniffing deficits in BTBR on the directed sniffing parameter. A trend toward reversal was observed at 10 mg/kg (p = 0.06). (C) B6 displayed normal sociability on the chamber time parameter, spending more time in the chamber with the novel mouse as compared to the novel object, after CX1739. (D) BTBR exhibited its characteristic lack of sociability on the chamber time parameter following vehicle administration and at each dose of CX1739. No significant difference in the number of entries into the side chambers was observed in (E) B6 and (F) BTBR at any dose of CX1739, indicating the absence of confounding increases or decreases in exploratory locomotion during the social approach task. *p < 0.05, novel mouse versus novel object. N = 11-12 per dose for each strain.
Sociability in B6 but not BTBR in the peanut butter vehicle groups was observed for time spent in the chamber parameter, comparing the time spent in the chamber with the novel mouse versus the novel object (Panel C, B6 vehicle: F (1,9) = 4.10, p < 0.05; Panel D, BTBR vehicle: F (1,11) = 0.28, p > 0.05). B6 groups treated with CX1739 exhibited significantly greater time spent in the chamber with the novel mouse than in the chamber with the novel object (Panel C, B6 CX1739 3.0 mg/kg: F (1,9) = 47.6, p < 0.001; B6 CX1739 10 mg/kg: F (1,9) = 5.6, p < 0.05; B6 CX1739 30 mg/kg: F (1,8) = 7.3, p < 0.05). Time in the chamber with the novel mouse was not different from time in the chamber with the novel object for BTBR treated with CX1739 (Panel D, BTBR CX1739 3.0 mg/kg: F (1,10) = 0.41, p > 0.05; BTBR CX1739 10 mg/kg: F (1,11) = 0.75, p > 0.05; BTBR CX1739 30 mg/kg: F (1,9) = 0.11, p > 0.05).
Entries into the side chambers were not affected by CX1739 in B6 (Panel E, F (3,40) = 0.88, p > 0.05) or BTBR (Panel F, F (3, 44) = 1.60, p > 0.05), indicating that the drug treatment had no direct effect on exploratory locomotion during the social approach task. No innate side preference was present in B6 (F (3,40) = 0.52, p > 0.05) or BTBR (F (3,44) = 0.97, p > 0.05) during the task, as shown by similar amounts of time in the left and right side chambers during the 10 minute habituation session before the start of social testing.
3.3. Oral CX546 improved social sniffing in BTBR in the 3-chambered social approach task
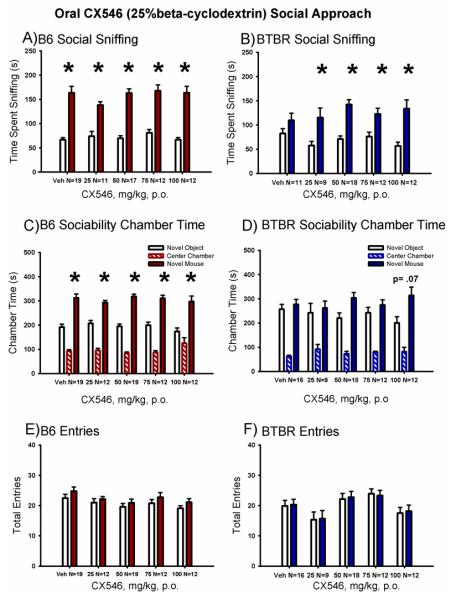
Figure 3 illustrates the sociability scores from the automated 3-chambered social approach task following a single oral dose of CX546 in 25% β-cyclodextrin in water in B6 and BTBR mice. Sociability was significant in the vehicle treated group of B6 mice but not in the p.o. vehicle injected BTBR mice (Panel A, B6 vehicle: F (1,18) = 19.52, p < 0.001; Panel B, BTBR vehicle: F (1,10) = 2.22, p > 0.05), as expected. All other B6 groups treated with CX546 exhibited scores similar to vehicle B6, by spending more time sniffing the novel mouse than sniffing the novel object (Panel A, B6 CX546 25 mg/kg: F (1,11) = 19.74, p < 0.001; B6 CX546 50 mg/kg: F (1,16) = 42.84, p < 0.001; B6 CX546 75 mg/kg: F (1,11) = 20.5, p < 0.001 and B6 CX546 100 mg/kg: F (1,11) = 15.95, p < 0.001). Time spent sniffing the novel mouse was greater than time spent sniffing the novel object in the BTBR treated with all CX546 doses tested (Panel B, BTBR CX546 25 mg/kg: F (1,8) = 7.85, p < 0.02; BTBR CX546 50 mg/kg: F (1,17) = 45.29, p < 0.001; BTBR CX546 75 mg/kg: F (1,11) = 11.76, p < 0.01 and BTBR CX546 100 mg/kg: F (1,11) = 16.79, p < 0.001).
Figure 3. Oral administration of CX546 increased social sniffing in BTBR with no deleterious effects in B6 control mice.
(A) B6 control strain treated with CX546 exhibited characteristic sociability on the directed sniffing parameter at each dose tested. (B) BTBR exhibited its characteristic lack of sociability, i.e. did not spend more time sniffing the novel mouse versus the novel object. Vehicle was 25% β-cyclodextrin in water, administered orally. CX546 (25 mg/kg, 50 mg/kg, 75 mg/kg and 100 mg/kg), reversed the low social sniffing in BTBR on the directed sniffing parameter. A trend toward restored sociability was seen in BTBR treated with the cyclodextrin vehicle (p = 0.064). In other cohorts of BTBR employed in preliminary CX546 experiments, sociability was significant on the sniff time parameter in BTBR, prompting the search for vehicles that do not affect sociability in mice. (C) B6 displayed normal sociability on the chamber time parameter, spending more time in the chamber with the novel mouse as compared to the novel object, after treatment with vehicle and at each dose of CX546 tested. (D) BTBR exhibited its characteristic lack of sociability on the chamber time parameter following vehicle administration and at each dose of CX546. A trend toward restored sociability (p = 0.07) on the chamber time parameter, time spent in the chamber with the novel mouse versus time spent in the chamber with the novel object, was observed at the highest dose of CX546 in BTBR (100 mg/kg). No significant difference in the number of entries into the side chambers was observed in (E) B6 and (F) BTBR following treatment with CX546, indicating the absence of confounding increases or decreases in exploratory locomotion during the social approach task. *p < 0.05, novel mouse versus novel object. N = 9-16 per dose for each strain.
Sociability in vehicle (25% β-cyclodextrin, p.o.) treated B6 but not BTBR was again observed using the time spent in the chamber parameter, comparing the time spent in the chamber with the novel mouse versus the novel object (Panel C, B6 vehicle: F (1,16) = 64.12, p < 0.001; Panel D, BTBR vehicle: F (1,16) = 0.24, p > 0.05). All B6 groups treated with CX546, p.o. also exhibited significantly greater time spent in the chamber with the novel mouse than in the chamber with the novel object (Panel C, B6 CX546 25 mg/kg: F (1,11) = 55.6, p < 0.001; B6 CX546 50 mg/kg: F (1,16) = 92.34, p < 0.05; B6 CX546 75 mg/kg: F (1,11) = 65.20, p < 0.001; B6 CX546 100 mg/kg: F (1,11) = 32.53, p < 0.001). Time in the chamber with the novel mouse was not different from time in the chamber with the novel object for BTBR treated with CX546, p.o. (Panel D, BTBR CX546 25 mg/kg: F (1,8) = 0.09, p > 0.05; BTBR CX546 50 mg/kg: F (1,17) = 3.70, p = 0.07; BTBR CX546 75 mg/kg: F (1,11) = 0.53, p > 0.05; BTBR CX546 100 mg/kg: F (1,11) = 4.02, p > 0.05).
Entries into the side chambers were not affected by CX546, p.o. in B6 (Panel E, F (4,69) = 1.71, p > 0.05) or BTBR (Panel F, F (4,62) = 0.76, p > 0.05), indicating that the drug treatment had no direct effect on exploratory locomotion during the social approach task. No innate side preference was present in B6 (F (4,69) = 1.15, p > 0.05) or BTBR (F (4,62) = 2.25, p > 0.05) during the task, as shown by similar amounts of time in the left and right side chambers during the 10 minute habituation session before the start of social testing.
3.4. Effects of CX1837 on repetitive self-grooming and open field locomotion in B6 and BTBR
Figure 4A,B illustrates self-grooming scores in B6 and BTBR mice treated with vehicle (25% β-cyclodextrin in water) or CX1837 (0.3 mg/kg, 1.0 mg/kg and 3.0 mg/kg), delivered by injection per os. The cyclodextrin vehicle was employed in the grooming experiments because it produced no effects alone in this assay. Bioavailability is likely higher with the p.o. injection route than with a dietary route. If the compound given p.o. produced sedating effects on motor activity that could potentially confound the self-grooming results, these should easily have been detected in the open field assay with the same doses given in peanut butter pellets. CX1837 had no significant effect on the normal self-grooming scores in B6 (Panel A, F (3,27) = 3.6, p > 0.05), or the high levels of repetitive self-grooming in BTBR (Panel B, F (3,33) = 0.71, p > 0.05), as compared to vehicle treatment. Figure 4C,D illustrates CX1837 dose-response curves for distance travelled in the Accuscan open field which measured exploratory locomotion in B6 and BTBR, beginning 30 minutes after CX1837 (0.3 mg/kg, 1.0 mg/kg and 3.0 mg/kg) delivered orally via peanut butter pellets. Across the 30 minute session, the time course for total distance traversed by both B6 and BTBR declined as expected, representing habituation to the novel open field (Panel C, B6: F (5,38) = 20.9, p < 0.0001; Panel D, BTBR: F (5,43) = 113.2, p < 0.0001). Total distance scores were similar across doses of CX1837 and vehicle in B6 (Panel C, B6: F (3,38) = 1.27, p > 0.05) and BTBR (Panel D, BTBR: F (3,43) = 1.54, p > 0.05), indicating the absence of confounding hyperactivation or sedation following CX1837 administration. Additional CX1837 open field results appear in Supplementary Figure S3.
Figure 4. CX1837 did not alter levels of self-grooming in B6 or BTBR and did not induce hyperactivity or sedation in a novel open field.
Cumulative time spent engaged in self-grooming behavior over a 10 minute session was scored by investigators blind to drug treatment. (A) B6 mice displayed their normally low levels of self-grooming after oral administration of CX1837. (B) BTBR displayed their usual high levels of repetitive self-grooming after CX1837 administration. For the grooming experiments, CX1837 was administered in 25% β-cyclodextrin in water, in accordance with lack of vehicle issues on self-grooming, discussed in the text. N = 7-10 per dose for each strain. Total distance traversed during a 30 minute test session in an Accuscan open field in BTBR and B6 was unaffected, following peanut butter delivery of vehicle (0.0 mg/kg) or CX1837 treatment at doses of 0.3 mg/kg, 1.0 mg/kg and 3.0 mg/kg in (C) B6 and (D) BTBR. Vehicle for the open field experiments was peanut butter pellets. Data for the open field graphs are shown in 5 minute time bins as mean ± SEM. *p < 0.05 as compared to vehicle. N=9-13 per strain per dose. See Supplementary Material S3 for additional open field parameters.
3.5. Effects of CX1739 on repetitive self-grooming and open field locomotion in B6 and BTBR
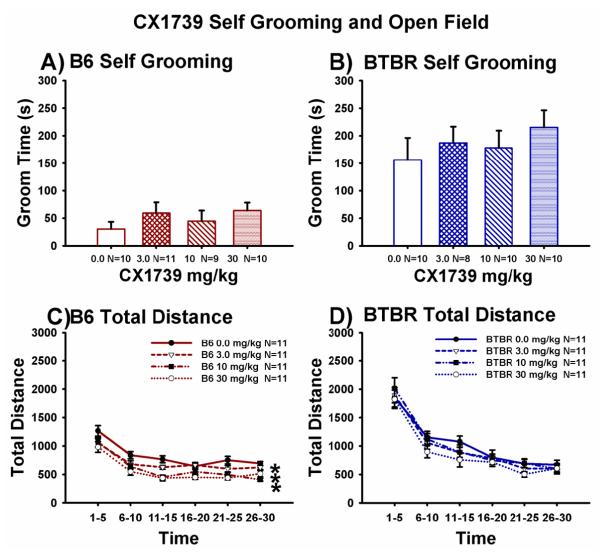
Figure 5A,B illustrates self-grooming scores in B6 and BTBR mice treated with vehicle (25% β-cyclodextrin in water) or CX1739 (3.0 mg/kg, 10 mg/kg and 30 mg/kg), delivered by injection per os. CX1739 had no significant effect on the normal self-grooming scores in B6 (Panel A, F (3,36) = 0.81, p > 0.05), or the high levels of repetitive self-grooming in BTBR (Panel B, F (3,34) = 0.55, p > 0.05), as compared to vehicle treatment. Figure 5C,D also illustrates CX1739 dose-response curves for distance travelled in the Accuscan open field which measured exploratory locomotion in B6 and BTBR, beginning 30 minutes after CX1739 (3.0 mg/kg, 10 mg/kg and 30 mg/kg) delivered orally via peanut butter pellets. Across the 30 minute session, the time course for total distance traversed by both B6 and BTBR declined as expected, representing habituation to the novel open field (Panel C, B6: F (5,60) = 135.3, p < 0.0001; Panel D, BTBR: F (5,77) = 169.84, p < 0.0001). Total distance scores were similar across doses of CX1739 and vehicle in BTBR (Panel D, F (3,77) = 0.68, p > 0.05), indicating the absence of confounding hyperactivity or sedation follow CX1739 administration in BTBR. However, total distance scores were slightly lower in the CX1739 treated B6 (Panel C, F (3,60) = 6.67, p < 0.01, Dunnett’s p < 0.05 for doses of 3.0 mg/kg, 10 mg/kg and 30 mg/kg as compared to vehicle). Additional CX1739 open field results appear in Supplementary Figure S4.
Figure 5. CX1739 did not alter levels of self-grooming in B6 or BTBR and did not induce hyperactivity or sedation in an open field.
(A) Normally low levels of self-grooming in B6 mice were unchanged after treatment with CX1739. (B) BTBR displayed their innately high levels of repetitive self-grooming after vehicle and CX1739. For the grooming experiments, CX1739 was administered in 25% β-cyclodextrin in water. N = 7-10 per dose for each strain. (C) Total distance traveled in a 30 minute test session in an Accuscan open field was lower after CX1739 (3.0 mg/kg, 10 mg/kg and 30 mg/kg) in B6. *p < 0.05 as compared to peanut butter vehicle. (D) Total distance traveled in a 30 minute test session in an Accuscan open field was not significantly different after CX1739 or peanut butter vehicle treatment in BTBR (D). N=11 per strain per dose. See Supplementary Material S4 for additional open field parameters.
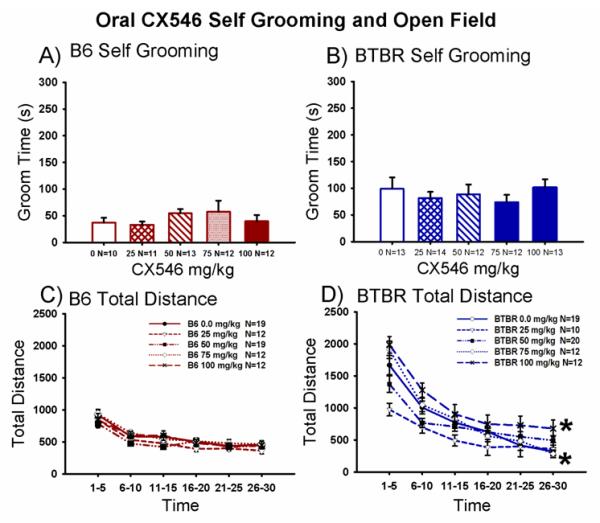
3.6. Effects of oral administration of CX546 on repetitive self-grooming and open field locomotion in B6 and BTBR
Figure 6A,B illustrates self-grooming scores in B6 and BTBR mice treated with vehicle (25% β-cyclodextrin in water) or CX546 (25 mg/kg, 50 mg/kg, 75 mg/kg and 100 mg/kg), delivered per os. CX546 p.o. had no significant effect on the normal self-grooming scores in B6 (Panel A, F (4,69) = 1.18, p > 0.05), or the high levels of repetitive self-grooming in BTBR (Panel B, F (4,64) = 1.69, p > 0.05), as compared to p.o. vehicle (25% β-cyclodextrin in water) administration. Figure 6C,D also illustrates orally delivered CX546 dose-response curves for distance traveled in the Accuscan open field which measured exploratory locomotion in B6 and BTBR, beginning 30 minutes after CX546 (25 mg/kg, 50 mg/kg, 75 mg/kg and 100 mg/kg). Across the 30 minute session, the time course for distance traversed by both B6 and BTBR declined as expected, representing habituation to the novel open field (Panel C, B6: F (5,69) = 100.2, p < 0.0001; Panel D, BTBR: F (5, 68) = 141.12, p < 0.0001). Total distance traversed did not differ in B6 across groups treated with CX546, at any dose (Panel C, B6: F (4,69) = 0.87, p > 0.05). Total distance traversed was elevated in BTBR at the highest dose, 100 mg/kg, and reduced in BTBR at the lowest dose of CX546, 25 mg/kg, (Panel D, F (4,68) = 3.78, p < 0.01, Dunnett’s p < 0.05 for doses 25 mg/kg and 100 mg/kg compared to vehicle). Additional orally delivered CX546 open field results appear in Supplementary Figure S5.
Figure 6. CX546, delivered orally, did not alter levels of self-grooming in B6 or BTBR but induced mild hyperactivity and sedation in an open field.
(A) Normally low levels of self-grooming in B6 mice were unchanged after treatment with CX546. (B) BTBR displayed their innately high levels of repetitive self-grooming after vehicle and CX546. CX546 was administered in 25% β-cyclodextrin in water, per os. N = 10-13 per dose for each strain. Total distance traveled in a 30 minute test session in an Accuscan open field in B6, following administration of vehicle (25% β-cyclodextrin in water, p.o.) or CX546 displayed no significant differences (C). Total distance scores in BTBR were elevated at 100 mg/kg and lowered at 25 mg/kg compared to BTBR treated with vehicle (D). *p < 0.05, versus vehicle. N=10-20 per strain per dose.
3.7. Novel object recognition in BTBR following CX1837
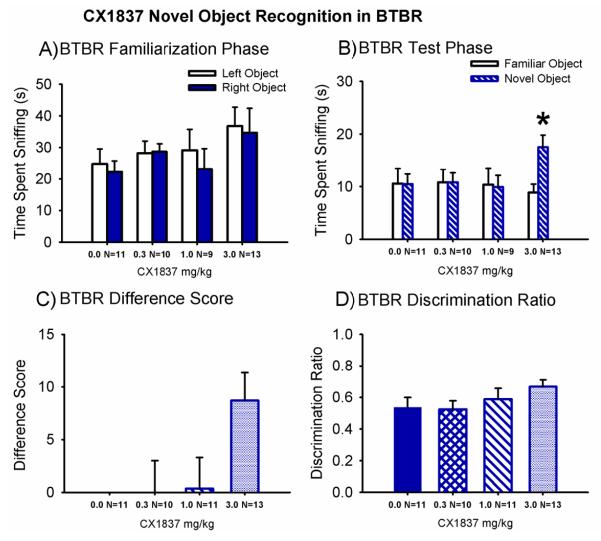
Figure 7 illustrates scores for BTBR on the novel object recognition task. Cognitive deficits in BTBR on other tasks have been published, and novel object recognition deficits in other cohorts of BTBR were observed in unpublished data from our laboratory (Amodeo et al., 2012; MacPherson et al., 2008; Yang et al., 2012a). During the 10 minute familiarization session, no innate preference for the object position was exhibited in BTBR treated with peanut butter (100 mg/kg) vehicle (Panel A, F (1,10) = 0.28, p > 0.05) or at any dose of CX1837 (Panel A, BTBR CX1837 0.3 mg/kg: F (1,9) = 0.04, p > 0.05; BTBR CX1837 1.0 mg/kg: F (1,9) = 0.51, p > 0.05; BTBR CX1837 3.0 mg/kg: F (1,10) = 0.07, p < 0.05), as shown by similar amounts of time sniffing the left and right objects. During the test session, BTBR treated with vehicle did not display novel object recognition, while BTBR treated with CX1837 3.0 mg/kg showed a significant preference for the novel object, 3.0 mg/kg (Panel B, vehicle F (1,10) = 0.28, p > 0.05; 3.0 mg/kg: F (1,12) = 10.7, p < 0.01). No significant effect of CX1837 was observed when using the commonly transformed parameters of difference score (Panel C, F (3,39) = 2.15, p = 0.10) and discrimination ratio (Panel D, F (3,39) = 1.32, p > 0.05). However, trends toward significance were observed in both derived parameters.
Figure 7. CX1837 increased novel object recognition in BTBR.
Novel object recognition was assayed in a Plexiglas arena, after vehicle (100 mg peanut butter pellet) or CX1837 treatment. The testing sequence consisted of a 60 minute habituation session in the empty arena, a 10 minute familiarization session in which two identical objects were placed on the floor of the arena, and a 5 minute test session in which one previous object and one new object was placed on the floor of the arena. No innate object preference or side bias was observed during the familiarization session (A), in BTBR mice treated with vehicle (100 mg peanut butter pellet) or CX1837. (B) BTBR failed to exhibit novel object recognition after treatment with vehicle and CX1837 (0.3 mg/kg and 1.0 mg/kg), consistent with previously reported cognitive deficits in BTBR mice. CX1837, 3.0 mg/kg, rescued the deficit. (C) A commonly used derived parameter, object recall difference, demonstrated scores ~ 0 for the vehicle and CX1837 (0.3 mg/kg and 1.0 mg/kg) treatment groups. A trend (p = 0.09) for a significant novel object recall measured by difference score was observed in BTBR treated at the 3.0 mg/kg dose of CX1837. (D) Another commonly used derived parameter, the object recall discrimination ratio, showed absence of novel object recognition, ratios ~ 0.5, for vehicle and CX1837 (0.3 mg/kg and 1.0 mg/kg) treatment groups. A trend (p = 0.09) for a significant novel object recall by discrimination ratio was observed for BTBR treated with the 3.0 mg/kg dose. *p < 0.05, novel object versus familiar object. N = 9-13 per dose for each strain.
3.8. Additional results
from assays of reciprocal social interactions, social approach, open field parameters and self-grooming, with CX1837, CX1739, CX546, and various vehicles are presented in Supplementary Figures S1-S9.
4. Discussion
Abnormalities in social cognition have been reported for neurodevelopmental and neuropsychiatric disorders including autism and schizophrenia (Couture et al., 2010; Sasson et al., 2011; Schreibman, 1988; Solomon et al., 2009; Sugranyes et al., 2011). Therapeutics that improve general cognitive abilities have the potential to improve specific cognitive skills that are required to understand social cues and formulate appropriate responses. Evaluating cognitive enhancers in animal models that display social deficits could reveal pharmacological interventions that ameliorate social dysfunctions in human syndromes.
BTBR is an inbred strain of mice which displays well replicated social deficits, reduced ultrasonic vocalizations in social settings, and high levels of repetitive self-grooming, thus incorporating analogies to the first, second, and third diagnostic symptoms of autism (Blanchard et al., 2011; Bolivar et al., 2007; Defensor et al., 2011; Gould et al., 2011; McFarlane et al., 2008; Pearson et al., 2011; Pobbe et al., 2010; Pobbe et al., 2011; Scattoni et al., 2008; Scattoni et al., 2011; Wohr et al., 2011; Yang et al., 2009; Yang et al., 2007a; Yang et al., 2007b; Yang et al., 2012a). We and others are employing BTBR and the control strain B6 to test interventions that may rescue autism-relevant phenotypes in BTBR, as a preclinical translational approach to discovering treatments for autism spectrum disorders (Gould et al., 2012; Silverman et al., 2010a; Silverman et al., 2012;). Although BTBR is an inbred strain rather than a targeted gene mutation with construct validity to a mutation identified in autism, the inbred strain approach may be more analogous to the large majority of cases of autism in which no genetic mutation has been identified, and useful for discovering therapeutic interventions that generalize across larger numbers of cases. Learning and memory deficits have been reported for BTBR on fear conditioning, water maze reversal and probabilistic reversal learning (Amodeo et al., 2012; MacPherson et al., 2008; Moy et al., 2007; Rutz and Rothblat, 2012; Yang et al., 2012a). We reasoned that the BTBR mouse model of autism would be an appropriate research tool to test cognitive enhancers for improvement of both social and cognitive deficits. As a first test of this hypothesis, we focused on AMPAKINES, a class of AMPA receptor modulators that has previously been shown to improve learning and memory in various tasks in several species (Baudry et al., 2012; Broberg et al., 2009; Hamlyn et al., 2009; Hampson et al., 1998a, b; Porrino et al., 2005; Simmons et al., 2009; Zheng et al., 2011). The commercially available CX546 and two new compounds, CX1837 and CX1739, were selected for these initial experiments.
In the 3-chambered test for social approach, we detected an improvement in social interactions in BTBR mice treated with CX1837 on time spent sniffing a novel mouse as compared to time spent sniffing a novel object. Time in the chamber containing the novel mouse versus time in the chamber containing the novel object, was not affected by CX1837 treatment, indicating that the social improvement was not complete. Similarly, CX1739 improved sociability on the sniffing parameter, although not at all doses, and again not on the chamber time parameter. Neither compound affected the normal sociability in the control B6 strain. Concordance of results fromCX1837 and CX1739, administered to two independent groups of BTBR and B6 mice, supports the reliability of the improvement in one parameter of the 3-chambered social approach task. Sniff time is a more sensitive measure of sociability than chamber time in this assay (Fairless et al., 2011; Yang et al., 2011b). This is likely because time in the chamber with the novel mouse or novel object includes time spent exploring the chamber environment, including the floor areas and walls that are distant from the novel mouse. Sniff time is a more pure measure of social approach tendencies, wherein the subject mouse is in close proximity or in physical contact with the novel mouse or novel object. The subject mouse extends its nose toward the novel mouse or object, and engages in directed, interactive sniffing. Sniffing is a primary approach behavior through which mice initiate social interactions. Drug-induced increases in sniffing the novel mouse versus the novel object in this task, therefore, represent a more socially meaningful indicator than time spent in the chamber with the novel mouse versus novel object.
Doses of CX1837 that improved sociability in BTBR were used to test reciprocal social interactions in BTBR and B6. CX1837 did not rescue any of the parameters of interactions between pairs of freely moving male BTBR and estrus female B6, including following, crawling over/pushing past, nose-to-nose sniffing, nose-to-anogenital sniffing, whole body sniffing, or ultrasonic vocalization emissions. CX1837 similarly did not improve or impair the normally high reciprocal social interactions in B6 control mice. It is essential to recognize that the partial rescues observed at this time are the result of acute single dose administration. It is possible that following a continued treatment period, effects on various parameters of reciprocal social interactions may be detected. Future studies will be necessary to analyze this class of compounds on social behaviors following longer subchronic and chronic treatment regimens. BTBR mice display generally normal olfactory habituation/dishabituation to both non-social and social odors (Yang et al., 2012). It will be interesting to investigate whether CX1837 and CX1739 have any direct effect on general olfactory abilities and discrimination, given the robust olfactory cues of the social stimuli in the social approach paradigm. CX1837, CX1739 and CX546 did not reduce the high levels of repetitive self-grooming in BTBR, and had no effect on the low levels of self-grooming in B6 controls, in contrast to another report in which CX546 reduced repetitive behavior (Iijima et al., 2010). These findings argue against a global ameliorative action of CX1837 on multiple components of behavioral phenotypes relevant to the three diagnostic symptoms of autism. Rather, this profile of improvement on sniff time but not general proximity nor freely moving interactions indicates a partial rescue of some of the social deficits in BTBR. Dissecting the mechanisms through which these compounds improve some aspects of social interaction, but not others, will be necessary to understand the potential therapeutic usefulness of AMPA receptor modulators.
Because social behaviors in mice depend on intact exploratory activity and motor abilities, we conducted internal and external control experiments to evaluate the effects of the treatments on exploratory locomotion. During the 3-chambered social approach task, movements between the three compartments are detected by the photocells in the border panels and automatically recorded as number of entries. Chamber entries after CX1837 and CX1739 were not significantly different than chamber entries after vehicle treatment, either during the 10 minute sociability test session or during the preceding 10 minute habituation session. General exploratory locomotion was independently tested in a standard 30 minute open field test session. Small increases in locomotion at the highest doses were detected in BTBR of some cohorts on one parameter. A small decrease in locomotion at the highest dose of CX1739 was detected in B6 in some cohorts on one parameter. The variability of changes in locomotor parameters across these cohorts and compound doses indicates a minor effect, likely of minimal biological meaning. However, it is possible that AMPAKINE treatments contributed an activating effect in BTBR on general activity and/or exploratory tendencies. A profile of increased exploration concomitant with increased social sniffing was seen in BTBR mice treated with an mGluR5 negative allosteric modulator (Silverman et al., 2012). Increased interest in exploration of the environment could lead to increased interest in exploring other individuals. Mechanistic investigations of this class of compounds at specific brain regions mediating exploration, social interest, and reward will be important to pursue.
One confound throughout our experiments was the behavioral effects of the vehicles employed to administer the compounds. The standard vehicle used for CX546, CX1837, and CX1739 is 2-hydroxypropyl-β-cyclodextrin (β-cyclodextrin) in water administered intraperitoneally or orally (Gainetdinov et al., 2001; Hess et al., 2003; Lipina et al., 2007a; Ogier et al., 2007). We found that this vehicle alone produced an improvement in social sniffing in some cohorts of BTBR. Other vehicles were then attempted. Methylcellulose 0.5% in saline did not affect the low sociability in BTBR or the high sociability in B6, but the compounds assessed in the present study did not completely suspend in the 0.5% methylcellulose solution, and thus accurate dosing was not possible. DMSO 20% in saline was particularly problematic, decreasing sociability in B6 and increasing sociability in BTBR. The mechanism(s) by which these vehicles influence mouse behaviors are not known, but could involve the stress of the injection procedure on social tendencies, the viscosity of the solution, or direct gastrointestinal or subcutaneous sensory input. The 15-30 minute interval between the injection and the start of the social task is usually sufficient for mice to recover from injection stressors. Longer intervals were not evaluated because of the short half-life of the compounds tested. Similar vehicle effects were problematic in our pilot experiments with the commercially available CX546. Thus, the apparent beneficial actions of CX1837 were not separable from vehicle effects when the compound was injected intraperitoneally or orally.
To circumvent the artifacts introduced by the vehicles administered intraperitoneally and orally, we sought other approaches. Mice readily eat peanut butter. A method for administering compounds in the diet by mixing in peanut butter pellets has been reported (Cope et al., 2005). Following this protocol, we mixed CX1837 and CX 1739 in peanut butter and fashioned 100 mg pellets containing the indicated doses of each compound. One pellet per day was administered to each subject mouse on a moistened cotton swab. After habituation to the peanut butter in the home cage, and subsequent daily consumption of non-drug peanut butter pellets on the swab in mouse home cages, subject mice quickly consumed their daily peanut butter pellet, usually within 2-3 minutes. When peanut butter pellets containing the compounds were then administered, subject mice similarly quickly consumed their daily dose. Using the non-stressful peanut butter pellet route of administration for CX1837 and CX1739, we detected no effect of the peanut butter vehicle on any of the behaviors tested. Effects of CX1837 and CX1739 were therefore not confounded by vehicle effects in the social, cognitive, and locomotor tasks conducted. With this treatment regimen, both CX1837 and CX1739 restored sociability in BTBR mice on time spent engaged in social sniffing as compared to non-social object sniffing. This dietary route of administration appears to be advantageous for pharmacological treatment of mice on behavioral tasks that are impacted by oral and intraperitoneal injections. The peanut butter pellet approach has the advantages of low cost and simplicity, as compared to the expense of commercial preparations of specialized food pellets containing the compound. One disadvantage of the peanut butter approach is the lengthy training period until the mice rapidly consume the pellets, requiring laborious and time-intensive preparation of an adequate pellet supply. A further issue that will require additional experiments is the bioavailability and pharmacokinetics of compounds mixed in peanut butter and consumed orally.
AMPAKINES have been proposed as cognitive enhancers, based on both their ability to lower the induction threshold and increase the magnitude of electrophysiological long term potentiation, enhance the trophic effects of excitatory transmission, and behavioral assays of cognitive measures in rodents and non-human primates (Baudry et al., 2012; Broberg et al., 2009; Hamlyn et al., 2009; Lauterborn et al., 2003; Lauterborn et al., 2009; Lynch, 2002; Lynch and Gall, 2006; Lynch et al., 2011; Lynch et al., 2008; Simmons et al., 2009; Street et al., 2009; Zheng et al., 2011). To investigate the cognitive effects of doses of CX1837 employed in our social assays, we administered CX1837 in peanut butter pellets 20 minutes before the habituation session of the novel object recognition task. B6 control mice displayed robust novel object recognition, while BTBR mice failed to display significant novel object recognition. CX1837 restored significant novel object recognition in BTBR at the highest dose tested, when analyzed as the time engaged in exploring the novel versus the familiar object, although significance was not reached when the data were analyzed on standard derivative measures of difference scores and discrimination ratio. These preliminary data indicate that CX1837 has a positive effect on rescuing the cognitive deficit on the novel object task in BTBR mice, consistent with previous literature demonstrating positive actions of other AMPA compounds on other cognitive tasks in other animal models.
In summary, we hypothesize that AMPA receptor modulation may represent a treatment strategy for ameliorating specific components of low sociability in autism spectrum disorders. It is interesting to speculate that the ability of CX1837 and CX1739 to restore sociability in BTBR mice on the sniffing parameter in the social approach task represents an improvement in social cognition. Mice appear to detect other mice and respond by approaching and exploring a novel individual through nose-to-nose and nose-to-anogenital sniffing. In the 3-chambered task, it seems possible that our pharmacological interventions enabled BTBR to better able to detect, recognize, and/or be attracted to the subject mouse inside the wire cage. However, BTBR did not engage in more interactions with freely moving novel mice. We have previously noted that BTBR avoid direct interactions with conspecifics, while remaining interested in social odors on inanimate objects (Yang et al., 2012a). The tendency of this inbred strain to avoid social contact when approached by other mice may be harder to reverse. It may be easier to rescue the reduced tendencies of BTBR to approach another mouse that is contained in a wire cage, and therefore cannot initiate chasing or aggressive interactions. Further assays of other social behaviors will be needed to evaluate the promise of an AMPA modulation strategy on social behaviors in mouse models of autism spectrum disorders. Our early data demonstrate that modulation of glutamatergic neurotransmission by AMPA receptor modulators improved some components of social deficits in one mouse model, suggesting a pharmacological intervention strategy that could convey benefits for the improvement of components of social cognition deficits in human syndromes.
Supplementary Material
Research Highlights of the manuscript “AMPAKINE enhancement of social interaction in the BTBR mouse model of autism” by Jill L Silverman, Chicora F Oliver, Michael N Karras, Philip T Gastrell and Jacqueline N Crawley.
CX1837, CX1739 and oral CX546 reversed the sensitive parameter of sociability in the BTBR mouse model of autism
CX1837, CX1739 and CX546 had no effects on normal or repetitive self-grooming in B6 and BTBR
CX1837, CX1739 and CX546 had no confounding effects on locomotion in B6 and BTBR
Acknowledgments
We are sincerely grateful to Drs. Mark Varney and Stephen Johnson for their generous donation of CX1837 and CX1739 for our experiments, and for their essential advice on doses, time points, and vehicles throughout the design and implementation of these studies. We thank Cathy Gonzalez, Pfizer Global Research, Groton, CT for guidance in the methods for peanut butter pellet formulation and administration. Our gratitude is extended to Drs. Jennifer Brielmaier, Dr. Mu Yang, Ms. Julia Senerth, and Danielle Abrams, in our Laboratory of Behavioral Neuroscience, who contributed valuable assistance in developing the protocol for novel object recognition. Supported by the National Institute of Mental Health Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos Trans R Soc Lond B Biol Sci. 2009;364:1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Kramar E, Xu X, Zadran H, Moreno S, Lynch G, Gall C, Bi X. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol Dis. 2012 Aug;47(2):210–5. doi: 10.1016/j.nbd.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS, Ochsner KN. Social cognition: a multi level analysis. Brain Res. 2006;1079:98–105. doi: 10.1016/j.brainres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Black DO, Wallace GL, Sokoloff JL, Kenworthy L. Brief report: IQ split predicts social symptoms and communication abilities in high-functioning children with autism spectrum disorders. J Autism Dev Disord. 2009;39:1613–1619. doi: 10.1007/s10803-009-0795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Defensor EB, Meyza KZ, Pobbe RL, Pearson BL, Bolivar VJ, Blanchard RJ. BTBR T+tf/J mice: Autism-relevant behaviors and reduced fractone-associated heparan sulfate. Neurosci Biobehav Rev. 2011 Jan;36(1):285–96. doi: 10.1016/j.neubiorev.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg BV, Glenthoj BY, Dias R, Larsen DB, Olsen CK. Reversal of cognitive deficits by an ampakine (CX516) and sertindole in two animal models of schizophrenia--sub-chronic and early postnatal PCP treatment in attentional set-shifting. Psychopharmacology (Berl) 2009;206:631–640. doi: 10.1007/s00213-009-1540-5. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Nair S, Lynch G, Clarkson AN. AMPAKine-Mediated Post-Stroke Functional Improvements. Society for Neuroscience; Chicago, IL: 2009. pp. 639–632. [Google Scholar]
- Casey BJ, Gordon CT, Mannheim GB, Rumsey JM. Dysfunctional attention in autistic savants. J Clin Exp Neuropsychol. 1993;15:933–946. doi: 10.1080/01688639308402609. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, Ferris P, Hobbs SC, O’Connor D, Marshall G, Rycroft W, Macleod AM. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA alpha5 receptors with cognition enhancing properties. J Med Chem. 2004;47:5829–5832. doi: 10.1021/jm040863t. [DOI] [PubMed] [Google Scholar]
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP) Psychol Med. 2011;41:619–627. doi: 10.1017/S0033291710000991. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. The quantitative nature of autistic social impairment. Pediatr Res. 2011;69:55R–62R. doi: 10.1203/PDR.0b013e318212ec6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope MB, Nagy TR, Fernandez JR, Geary N, Casey DE, Allison DB. Antipsychotic drug-induced weight gain: development of an animal model. Int J Obes (Lond) 2005;29:607–614. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychol Med. 2010;40:569–579. doi: 10.1017/S003329170999078X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusi AM, Nazarov A, Holshausen K, Macqueen GM, McKinnon MC. Systematic review of the neural basis of social cognition in patients with mood disorders. J Psychiatry Neurosci. 2012;37:154–169. doi: 10.1503/jpn.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MS, Dapretto M, Sigman M, Sepeta L, Bookheimer SY. Neural bases of gaze and emotion processing in children with autism spectrum disorders. Brain Behav. 2011;1:1–11. doi: 10.1002/brb3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jaegher H, Di Paolo E, Gallagher S. Can social interaction constitute social cognition? Trends Cogn Sci. 2010;14:441–447. doi: 10.1016/j.tics.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless AH, Shah RY, Guthrie AJ, Li H, Brodkin ES. Deconstructing sociability, an autism-relevant phenotype, in mouse models. Anat Rec (Hoboken) 2011;294:1713–1725. doi: 10.1002/ar.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66(Suppl 10):3–8. [PubMed] [Google Scholar]
- Frith CD, Frith U. Mechanisms of social cognition. Annu Rev Psychol. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the Enigma Blackwell Publishing. 2003.
- Gainetdinov RR, Mohn AR, Bohn LM, Caron MG. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci U S A. 2001;98:11047–11054. doi: 10.1073/pnas.191353298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Seillier A, Weiss G, Giuffrida A, Burke TF, Hensler JG, Rock C, Tristan A, McMahon LR, Salazar A, O’Connor JC, Satsangi N, Satsangi RK, Gu TT, Treat K, Smolik C, Schultz ST. Acetaminophen differentially enhances social behavior and cortical cannabinoid levels in inbred mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012 Aug 7;38(2):260–9. doi: 10.1016/j.pnpbp.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Ren J. Ampakine therapy to counter fentanyl-induced respiratory depression. Respir Physiol Neurobiol. 2009;168:153–157. doi: 10.1016/j.resp.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, Hodges JR. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. 2002;125:752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Fragile X syndrome and targeted treatment trials. Results Probl Cell Differ. 2012;54:297–335. doi: 10.1007/978-3-642-21649-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn E, Brand L, Shahid M, Harvey BH. The ampakine, Org 26576, bolsters early spatial reference learning and retrieval in the Morris water maze: a subchronic, dose-ranging study in rats. Behav Pharmacol. 2009;20:662–667. doi: 10.1097/FBP.0b013e328331ba1b. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Rogers G, Lynch G, Deadwyler SA. Facilitative effects of the ampakine CX516 on short-term memory in rats: correlations with hippocampal neuronal activity. J Neurosci. 1998a;18:2748–2763. doi: 10.1523/JNEUROSCI.18-07-02748.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Rogers G, Lynch G, Deadwyler SA. Facilitative effects of the ampakine CX516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance. J Neurosci. 1998b;18:2740–2747. doi: 10.1523/JNEUROSCI.18-07-02740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess US, Whalen SP, Sandoval LM, Lynch G, Gall CM. Ampakines reduce methamphetamine-driven rotation and activate neocortex in a regionally selective fashion. Neuroscience. 2003;121:509–521. doi: 10.1016/s0306-4522(03)00423-8. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Crawley JN. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J Mol Neurosci. 2002;18:151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- Iijima M, Kurosu S, Chaki S. Effects of agents targeting glutamatergic systems on marble-burying behavior. Neurosci Lett. 2010;471:63–65. doi: 10.1016/j.neulet.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36:1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Squire LR. An analysis of calendar performance in two autistic calendar savants. Learn Mem. 2007;14:533–538. doi: 10.1101/lm.653607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BH, Lord C. Is schizophrenia on the autism spectrum? Brain Res. 2011;1380:34–41. doi: 10.1016/j.brainres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. J Child Psychol Psychiatry. 2004;45:1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Pineda E, Chen LY, Ramirez EA, Lynch G, Gall CM. Ampakines cause sustained increases in brain-derived neurotrophic factor signaling at excitatory synapses without changes in AMPA receptor subunit expression. Neuroscience. 2009;159:283–295. doi: 10.1016/j.neuroscience.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bushnell PJ, Rezvani AH. Attention-modulating effects of cognitive enhancers. Pharmacol Biochem Behav. 2011;99:146–154. doi: 10.1016/j.pbb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina R, Palecek T, Reguli S, Kovarova M. Death in consequence of late failure of endoscopic third ventriculostomy. Childs Nerv Syst. 2007a;23:815–819. doi: 10.1007/s00381-007-0316-7. [DOI] [PubMed] [Google Scholar]
- Lipina T, Weiss K, Roder J. The ampakine CX546 restores the prepulse inhibition and latent inhibition deficits in mGluR5-deficient mice. Neuropsychopharmacology. 2007b;32:745–756. doi: 10.1038/sj.npp.1301191. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Baron-Cohen S. The role of the self in mindblindness in autism. Conscious Cogn. 2011;20:130–140. doi: 10.1016/j.concog.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Petkova E, Hus V, Gan W, Lu F, Martin DM, Ousley O, Guy L, Bernier R, Gerdts J, Algermissen M, Whitaker A, Sutcliffe JS, Warren Z, Klin A, Saulnier C, Hanson E, Hundley R, Piggot J, Fombonne E, Steiman M, Miles J, Kanne SM, Goin-Kochel RP, Peters SU, Cook EH, Guter S, Tjernagel J, Green-Snyder LA, Bishop S, Esler A, Gotham K, Luyster R, Miller F, Olson J, Richler J, Risi S. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69:306–313. doi: 10.1001/archgenpsychiatry.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. Memory enhancement: the search for mechanism-based drugs. Nat Neurosci. 2002;5(Suppl):1035–1038. doi: 10.1038/nn935. [DOI] [PubMed] [Google Scholar]
- Lynch G. AMPA receptor modulators as cognitive enhancers. Curr Opin Pharmacol. 2004;4:4–11. doi: 10.1016/j.coph.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Lynch G, Gall CM. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29:554–562. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lynch G, Palmer LC, Gall CM. The likelihood of cognitive enhancement. Pharmacol Biochem Behav. 2011;99:116–129. doi: 10.1016/j.pbb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain Res. 2008;1210:179–188. doi: 10.1016/j.brainres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McPartland JC, Webb SJ, Keehn B, Dawson G. Patterns of visual attention to faces and objects in autism spectrum disorder. J Autism Dev Disord. 2011;41:148–157. doi: 10.1007/s10803-010-1033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JH, Takahashi TN, Haber A, Hadden L. Autism families with a high incidence of alcoholism. J Autism Dev Disord. 2003;33:403–415. doi: 10.1023/a:1025010828304. [DOI] [PubMed] [Google Scholar]
- Mohler H, Rudolph U, Boison D, Singer P, Feldon J, Yee BK. Regulation of cognition and symptoms of psychosis: focus on GABA(A) receptors and glycine transporter 1. Pharmacol Biochem Behav. 2008;90:58–64. doi: 10.1016/j.pbb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R, Li YX, Hampson A, Zhong S, Harris C, Marrs C, Rachwal S, Ulas J, Nielsson L, Rogers G. Benzoxazinones as potent positive allosteric AMPA receptor modulators: part I. Bioorg Med Chem Lett. 2011a;21:3923–3926. doi: 10.1016/j.bmcl.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Mueller R, Rachwal S, Lee S, Zhong S, Li YX, Haroldsen P, Herbst T, Tanimura S, Varney M, Johnson S, Rogers G, Street LJ. Benzotriazinone and benzopyrimidinone derivatives as potent positive allosteric AMPA receptor modulators. Bioorg Med Chem Lett. 2011b;21:6170–6175. doi: 10.1016/j.bmcl.2011.07.098. [DOI] [PubMed] [Google Scholar]
- Mueller R, Rachwal S, Tedder ME, Li YX, Zhong S, Hampson A, Ulas J, Varney M, Nielsson L, Rogers G. Substituted benzoxazinones as potent positive allosteric AMPA receptor modulators: part II. Bioorg Med Chem Lett. 2011c;21:3927–3930. doi: 10.1016/j.bmcl.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Mueller R, Rachwal S, Varney MA, Johnson SA, Alisala K, Zhong S, Li YX, Haroldsen P, Herbst T, Street LJ. Benzobistriazinones and related heterocyclic ring systems as potent, orally bioavailable positive allosteric AMPA receptor modulators. Bioorg Med Chem Lett. 2011d;21:7455–7459. doi: 10.1016/j.bmcl.2011.09.132. [DOI] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Felden L, Tran PV, Bradshaw MH, Angst MS, Schmidt H, Johnson S, Greer JJ, Geisslinger G, Varney MA, Lotsch J. Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clin Pharmacol Ther. 2010;87:204–211. doi: 10.1038/clpt.2009.194. [DOI] [PubMed] [Google Scholar]
- Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RL, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121:114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- Philip RC, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci Biobehav Rev. 2012;36:901–942. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Pina-Camacho L, Villero S, Fraguas D, Boada L, Janssen J, Navas-Sanchez FJ, Mayoral M, Llorente C, Arango C, Parellada M. Autism spectrum disorder: Does neuroimaging support the DSM-5 proposal for a symptom dyad? A systematic review of functional magnetic resonance imaging and diffusion tensor imaging studies. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1360-4. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2011;216:446–451. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring L, Hermelin B, Heavey L. Savants, segments, art and autism. J Child Psychol Psychiatry. 1995;36:1065–1076. doi: 10.1111/j.1469-7610.1995.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, McAlonan G, Muir JL, Everitt BJ. Cognitive enhancers in theory and practice: studies of the cholinergic hypothesis of cognitive deficits in Alzheimer’s disease. Behav Brain Res. 1997;83:15–23. doi: 10.1016/s0166-4328(97)86040-8. [DOI] [PubMed] [Google Scholar]
- Rutz HLH, Rothblat LA. Intact and impaired executive abilities in the BTBR T+ tf/J mouse model of autism. Behavioural Brain Research. 2012 Jun 5; doi: 10.1016/j.bbr.2012.05.048. [Epub] [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78:658–667. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Pinkham AE, Carpenter KL, Belger A. The benefit of directly comparing autism and schizophrenia for revealing mechanisms of social cognitive impairment. J Neurodev Disord. 2011;3:87–100. doi: 10.1007/s11689-010-9068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibman L. Diagnostic features of autism. J Child Neurol. 1988;3(Suppl):S57–64. doi: 10.1177/0883073888003001s11. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med. 2012;4:131ra151. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010a;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010b;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010c;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM, Lynch G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington’s disease knockin mice. Proc Natl Acad Sci U S A. 2009;106:4906–4911. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street LJ, Zhong S, Mueller R, Rachwal S, Johnson S, Haroldson PE, Herbst T, Julas J, Li Y, Yu W, Hampson A, Tinsley M, G. R, Varney MA. Society for Neuroscience. Chicago, IL: 2009. Characterization of CX1837: a positive AMPA receptor modulator (AMPAKINE®) with pro-cognitive activity; pp. 529–522. [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS One. 2011;6:e25322. doi: 10.1371/journal.pone.0025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppiramaniam V, Bahr BA, Sinnarajah S, Owens K, Rogers G, Yilma S, Vodyanoy V. Member of the Ampakine class of memory enhancers prolongs the single channel open time of reconstituted AMPA receptors. Synapse. 2001;40:154–158. doi: 10.1002/syn.1037. [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl) 2003;165:260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, State M, Klin A. Autism and autism spectrum disorders: diagnostic issues for the coming decade. J Child Psychol Psychiatry. 2009;50:108–115. doi: 10.1111/j.1469-7610.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Kojkowski N, Minshew NJ. Do individuals with high functioning autism have the IQ profile associated with nonverbal learning disability? Res Autism Spectr Disord. 2008;2:353–361. doi: 10.1016/j.rasd.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Minshew NJ. Understanding autism and related disorders: what has imaging taught us? Neuroimaging Clin N Am. 2007;17:495–509. ix. doi: 10.1016/j.nic.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, Silverman JL, Crawley JN. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012a doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wohr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent shank3 null mutant mice. J Neurosci. 2012b;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011a;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant Mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011b doi: 10.1002/0471142301.ns0826s56. Chapter 8, Unit 8 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Balabhadrapatruni S, Masumura C, Darlington CL, Smith PF. Effects of the putative cognitive-enhancing ampakine, CX717, on attention and object recognition memory. Curr Alzheimer Res. 2011;8:876–882. doi: 10.2174/156720511798192709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.