Abstract
OBJECTIVE
Adopting features of the Chronic Care Model may reduce coronary heart disease risk and blood pressure in vulnerable populations. We evaluated a peer and practice team intervention on reduction in 4-year coronary heart disease risk and systolic blood pressure.
DESIGN AND SUBJECTS
A single blind, randomized, controlled trial in two adjacent urban university-affiliated primary care practices. Two hundred eighty African-American subjects aged 40 to 75 with uncontrolled hypertension.
INTERVENTION
Three monthly calls from trained peer patients with well-controlled hypertension and, on alternate months, two practice staff visits to review a personalized 4-year heart disease risk calculator and slide shows about heart disease risks. All subjects received usual physician care and brochures about healthy cooking and heart disease.
MAIN MEASURES
Change in 4-year coronary heart disease risk (primary) and change in systolic blood pressure, both assessed at 6 months.
KEY RESULTS
At baseline, the 136 intervention and 144 control subjects’ mean 4-year coronary heart disease risk did not differ (intervention = 5.8 % and control = 6.4 %, P = 0.39), and their mean systolic blood pressure was the same (140.5 mmHg, p = 0.83). Endpoint data for coronary heart disease were obtained for 69 % of intervention and 82 % of control subjects. After multiple imputation for missing endpoint data, the reduction in risk among all 280 subjects favored the intervention, but was not statistically significant (difference −0.73 %, 95 % confidence interval: -1.54 % to 0.09 %, p = 0.08). Among the 247 subjects with a systolic blood pressure endpoint (85 % of intervention and 91 % of control subjects), more intervention than control subjects achieved a >5 mmHg reduction (61 % versus 45 %, respectively, p = 0.01). After multiple imputation, the absolute reduction in systolic blood pressure was also greater for the intervention group (difference −6.47 mmHg, 95 % confidence interval: −10.69 to −2.25, P = 0.003). One patient died in each study arm.
CONCLUSIONS
Peer patient and office-based behavioral support for African-American patients with uncontrolled hypertension did not result in a significantly greater reduction in coronary heart disease risk but did significantly reduce systolic blood pressure.
KEY WORDS: coronary heart disease, hypertension, African American, peer support
INTRODUCTION
The age-adjusted death rate from heart disease is 30 % greater for African-Americans than whites.1 The higher death rate for African Americans is due, in part, to a 50 % greater prevalence of hypertension.2 Even when treated, African-Americans do not achieve blood pressure control as often as whites in similar settings.3 Failure of primary care physicians to address uncontrolled hypertension can contribute to poorer blood pressure control, but our group has found that medical intensification for uncontrolled hypertension is more likely for African-American than white patients.4 Yet, primary care physicians often neglect to address medication adherence and other unhealthy lifestyles that affect hypertension control.5,6 These facts suggest that an intervention to assist physicians in promoting healthy behaviors to reduce coronary heart disease (CHD) risk and improve hypertension control might be beneficial.
Wagner’s Chronic Care Model offers complementary approaches to physician management in order to improve chronic disease care. Four features of the Chronic Care Model are more effective in improving outcomes: registry-based information systems; team-based care; increasing providers’ expertise and skill; and educating and supporting patients.7 We report a comparative effectiveness trial of an intervention that integrates these Chronic Care Model features to reduce CHD risk and systolic blood pressure in African-American patients with sustained uncontrolled hypertension. The intervention involves counseling and behavioral support through phone calls from trained peer-patients with well-controlled hypertension and office visits with trained practice staff. We hypothesized that the 6-month intervention would result in a greater reduction in risk of CHD and, secondarily, systolic blood pressure.
METHODS
Subject Recruitment
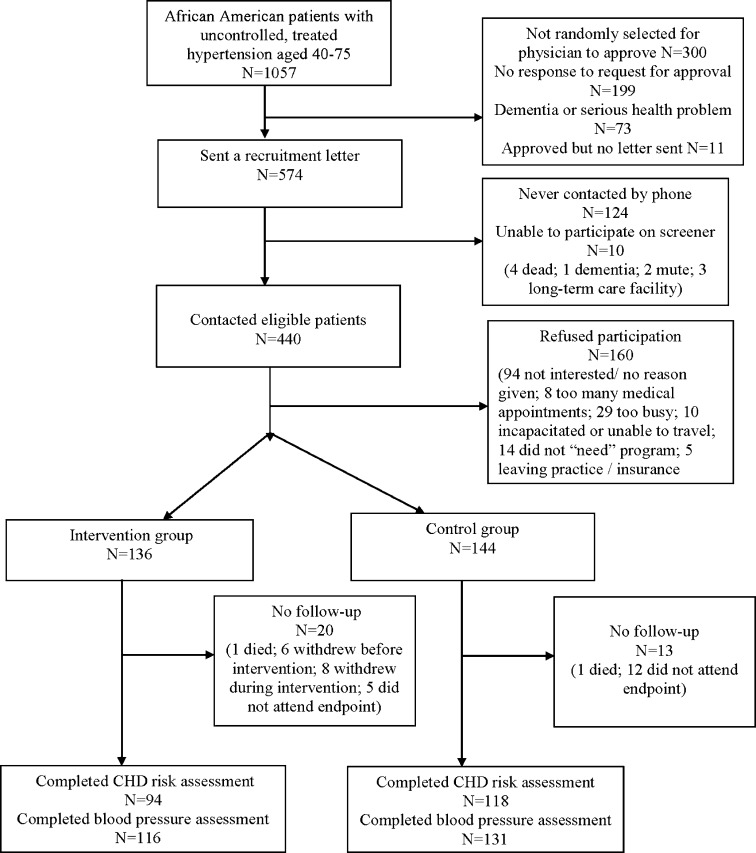
Participants were recruited during July 2007 through November 2009 from two urban academic general internal medicine practices serving a predominantly low-income minority patient population.8 From administrative data, we identified 9,135 African-American patients aged 40–75 years with 2+ practice visits in 2 consecutive years (Fig. 1). Of these, 2,092 had uncontrolled hypertension according to JNC VII targets based on the average of measurements from visits over a 2-year period9 with at least one value ≥10 mmHg above goal. Of these patients, 1,057 (51 %) met the following inclusion criteria: two or more antihypertensive drugs; lipid panel within the past 3 years; and missed or canceled <40 % of scheduled primary care visits (Fig. 1). Regarding the last criterion, persons who miss a higher proportion of visits are also non-adherent to other types of care10 and require more intensive outreach services. Of 1,057 eligible patients, we randomly selected 857 to ask their physician for approval to contact. Physicians approved 564 patients, with the most common reason for disapproval being non-response to our request. We contacted 440 of the 564 approved patients and, of these, 280 consented and were randomized at a 1:1 ratio using random computer-generated assignments. All subjects completed a 10-min baseline survey about barriers to adherence,11 smoking status, general health, Prime-MD 2 question depression screen,12 stress (one question), and knowledge about hypertension.11 All providers were blinded to the study arm.
Figure 1.
Study recruitment flow sheet.
Intervention Training Program
From lists of their African-American patients aged 50 to 75 with well-controlled hypertension, practice physicians identified those who were good communicators, adherent to medical care, and likely to be motivational. Of 20 nominees, 12 agreed and 11 completed two half-day face-to-face training sessions. Training content was developed by our six-member African-American community advisory board to address barriers and facilitators to CHD risk reduction through 12 illustrated informational slide shows (e.g., healthy food, exercise, adherence to medications, smoking cessation, stress). Our American Heart Association (AHA) advisor discussed community resources and provided brochures. Six peer coaches dropped out (4 within the first month) and were replaced by three additional coaches.
The intervention was guided by the Theory of Planned Behavior and addressed patient attitudes with evidence-based advice, social norms with role modeling, and perceived behavioral control with practical tips and links to community resources.13 Peer coaches were taught several motivational interviewing skills.14 An experienced lead peer coach demonstrated telephone etiquette and support techniques. Peer coaches practiced skills in phone calls with the study team or lead peer coach.
Three African-American staff members (medical assistant, licensed practical nurse, and chronic disease health educator) were trained in practice-based counseling with the 12 slideshows used to train peer coaches and trained to demonstrate the impact of reducing personal CHD risks using an interactive computer 4-year risk15 calculator modeled after a 10-year CHD risk program16 (available on request).
Intervention Program
Intervention and control subjects received AHA brochures designed for African-Americans about healthy recipes and reducing CHD risk. Intervention subjects were phoned by peer coaches every other month for 6 months (3 calls) and received office-based counseling in 2 15–30-min visits. All subjects received $50 in gift certificates ($20 at enrollment and $30 at endpoint visit) and coverage for transportation.
Peer coaches received $20 per completed phone call and concurrently followed a mean of eight patients. Peer coaches shared concerns to address at office counseling visits in confidential voicemails to the research coordinator who recorded them in medical record messages to staff counselors. The lead peer coach supervised the peer coaches as described previously.17 All protocol and consent procedures were reviewed and approved by the University of Pennsylvania Institutional Review Board.
Study Outcome Measures
The primary outcome was change in 4-year CHD risk 6 months after baseline using a measure developed by D’Agostino and colleagues from Framingham data that assess the risk of a primary or secondary CHD event.15 In contrast to the 10-year Framingham risk model, this measure is applicable for all subjects. Our secondary study outcome was a 5 mmHg or greater reduction in systolic blood pressure 6 months after baseline. As a related outcome, we examined absolute changes in systolic and diastolic blood pressure. The 6-month endpoint blood pressure was performed by blinded office medical assistants following the same office procedures as at baseline with an appropriate-sized, calibrated automatic cuff (repeated if elevated). The endpoint lipid panel was drawn by blinded technicians and performed at the health system laboratory. At least six attempts were made to complete the endpoint assessment.
Statistical Analyses
Since no other study has used reduction in 4-year coronary heart disease risk as an outcome, we estimated our sample size based on the REACH OUT trial that used the 10-year Framingham risk measure. Baseline 10-year risk was 17 % with a calculated standard deviation of 0.07 % for the intervention group (n = 524).16 For our 4-year risk outcome, we assumed a 7 % baseline risk with standard deviation of 0.035 %. To be able to detect a 20 % relative risk reduction, a sample size of 105 subjects per group achieves 80 % power (two-sided Mann-Whitney test) with α = 0.05. With a 20 % loss to follow-up, we required 140 (>105/0.8) subjects per group.
We examined change in the predicted 4-year CHD for intervention and control groups using a complete case analysis. We also performed a sensitivity analysis by imputing missing values needed to calculate the 4-year CHD (e.g., blood pressure, total cholesterol, triglycerides).18 We used a multiple imputation with chained equations (MICE) approach to create ten imputed data sets19 and combined these results following Rubin's rules.20 We fitted multiple linear regression models with change in 4-year CHD risk as the dependent variable and intervention group as the independent variable, and adjusted for confounders (i.e., age, gender, CHD or CHD equivalent, diabetes, and tobacco use). Similar procedures were followed for analyses of absolute change in systolic and diastolic blood pressures. For ≥5 mmHg reduction in systolic blood pressure among those with an endpoint, we used the chi-square test, and, in a sensitivity analysis, we assumed that all subjects with a missing value had no change in blood pressure.
RESULTS
We recruited 280 African-American subjects (49 % of 574 subjects approved by their physicians and 64 % of 440 contacted subjects; Fig. 1). The baseline characteristics of the 136 intervention and 144 control subjects did not differ except for HDL cholesterol (Table 1). Subjects had a mean age of 62, and nearly two-thirds were women. Most subjects had compromised health (good, fair, or poor). Thirty (21 %) subjects had a prior history of CHD or CHD equivalent; 62 (43 %) subjects reported depressive symptoms, and 65 (45 %) reported feeling more stressed than others. The average baseline 4-year risk of a CHD event was 6.1 % (5.8 % for intervention vs 6.4 % for control, p = 0.39), while the mean systolic and diastolic blood pressures were 140.5 and 81 mmHg, respectively, for both study groups.
Table 1.
Baseline Characteristics of African-American Patients with Uncontrolled Hypertension
| Characteristics* | Total study population | Intervention group | Control group |
|---|---|---|---|
| (N = 280) | (N = 136) | (N = 144) | |
| Age, years (standard deviation) | 61.9 (8.83) | 61.2 (9.29) | 62.6 (8.34) |
| Gender N (%) | |||
| Female | 183 (65) | 95 (70) | 88 (61) |
| Male | 97 (35) | 41 (30) | 56 (39) |
| General health N (%) | |||
| Excellent or very good | 93 (33) | 46 (34) | 47 (33) |
| Good | 128 (46) | 64 (47) | 64 (44) |
| Fair or poor | 58 (21) | 26 (19) | 32 (22) |
| Missing | 1 (<1) | 0 (0) | 1 (1) |
| Clinical conditions N (%) | |||
| Diabetes mellitus | 151 (54) | 76 (56) | 75 (52) |
| Coronary artery disease or equivalent | 50 (18) | 20 (15) | 30 (21) |
| Depressive symptoms | 117 (42) | 55 (40) | 62 (43) |
| More stressed than others | 120 (43) | 55 (40) | 65 (45) |
| Current smoker | 52 (19) | 27 (20) | 25 (17) |
| Baseline blood pressure, mmHg (standard deviation) | |||
| Systolic | 140.5 (9.08) | 140.5 (9.34) | 140.5 (8.86) |
| Diastolic | 81.2 (7.26) | 81.4 (7.84) | 81 (6.69) |
| Baseline lipid levels, mg/dl (standard deviation) | |||
| LDL cholesterol | 114.8 (29.35) | 116.2 (29.46) | 113.4 (29.29) |
| HDL cholesterol | 55.7 (15.37) | 57.5 (15.4) | 54 (15.21) |
| Triglycerides | 143.8 (85.07) | 138.1 (74.6) | 149.1 (93.84) |
| Total cholesterol | 198.2 (33.73) | 200.5 (33.63) | 196.1 (33.79) |
| 4-Year CHD risk % (standard deviation) | 6.1 (7) | 5.8 (7) | 6.4 (6) |
*Entries are mean (SD) and count (proportion) for continuous and categorical outcomes, respectively
In regard to baseline self-care behaviors (Table 2), approximately 25 % of subjects admitted to recently missing doses, while half ran out of medication, and one-third decided to skip their medication on occasion. Nearly half of the subjects admitted missing physician appointments, and 28 % had trouble following their physician’s advice.
Table 2.
Self-reported Anti-hypertensive Medication Adherence Measures and Barriers to Adherence
| Characteristics | Total study population | Intervention group | Control group |
|---|---|---|---|
| (N = 280) | (N = 136) | (N = 144) | |
| Last time missed antihypertensive medication N (%) | |||
| Past week | 72 (26) | 37 (27) | 35 (24) |
| 1 to 2 weeks ago | 26 (9) | 12 (9) | 14 (10) |
| 2 to 4 weeks ago | 10 (4) | 1 (1) | 9 (6) |
| 1 to 3 months ago | 27 (10) | 16 (12) | 11 (8) |
| >3 months ago | 141 (50) | 66 (49) | 75 (52) |
| Missing | 4 (1) | 4 (3) | 0 (0) |
| Run out of medications N (%) | |||
| Often/sometimes/rarely | 135 (48) | 62 (46) | 73 (51) |
| Never | 142 (51) | 71 (52) | 71 (49) |
| Missing | 3 (1) | 3 (2) | 0 (0) |
| Decide not to take meds N (%) | |||
| Often/sometimes/rarely | 95 (34) | 47 (35) | 48 (33) |
| Never | 181 (65) | 87 (64) | 94 (65) |
| Missing | 4 (1) | 2 (2) | 2 (1) |
| Miss doctor appointments N (%) | |||
| Often/sometimes/rarely | 130 (46) | 68 (50) | 62 (43) |
| Never | 149 (53) | 68 (50) | 81 (56) |
| Missing | 1 (<1) | 0 (0 %) | 1 (1) |
| Trouble following doctor’s advice N (%) | |||
| Often/sometimes/rarely | 78 (28) | 44 (32) | 34 (24) |
| Never | 196 (70 %) | 89 (65) | 107 (74) |
| Missing | 6 (2) | 3 (2) | 3 (2) |
| Forget to fill prescription N (%) | |||
| Often/sometimes/rarely | 87 (31) | 46 (34) | 41 (29) |
| Never | 189 (68) | 88 (65) | 101 (70) |
| Missing | 4 (1) | 2 (2) | 2 (1) |
After 6 months, 94 (69 %) intervention subjects and 118 (82 %) control subjects had 4-year CHD risk assessed (p = 0.012). Lipid panels were not obtained for 53 (19 %) study subjects (35 intervention and 18 control). In a complete case analysis (n = 222), change in 4-year CHD risk was −0.5 % and 0.3 % for intervention and control groups, respectively, with a difference of 0.82 % in favor of the intervention (p = 0.02). With multiple imputation for missing values (Table 3), the change in CHD was 0.7 % (95 % CI −1.54 % to 0.09 %) greater for the intervention than the control group (p = 0.08).
Table 3.
Change in Predicted 4-Year Cardiovascular Heart Disease (CHD) Risk, Systolic Blood Pressure, and Diastolic Blood Pressure for Intervention versus Control Groups
| Outcome | Baseline | 6 Months | CFB* | Intervention effect† | |||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | Mean (SD) | CCA‡ | MICE§ | |
| 4-year CHD risk | −0.82 % | −0.73 | |||||
| Intervention | 136 | 5.81 % (0.07) | 94 | 5.17 % (0.06) | −0.51 % (0.02) | (−1.48, −0.15 %) | (−1.54, 0.09 %) |
| Control | 144 | 6.44 % (0.06) | 118 | 7.11 % (0.07) | 0.31 % (0.03) | p = 0.016 | p = 0.08 |
| Systolic blood pressure (mmHg) | −6.38 | −6.47 | |||||
| Intervention | 136 | 140.47 (9.34) | 116 | 131.84 (14.69) | −7.15 (15.61) | (−10.52, −2.25) | (−10.69, −2.25) |
| Control | 144 | 140.54 (8.86) | 131 | 139.94 (18.13) | −0.77 (17.22) | p = 0.003 | p = 0.003 |
| Diastolic blood pressure (mmHg) | −1.99 | −2.42 | |||||
| Intervention | 136 | 81.37 (7.84) | 116 | 76.41 (9.40) | −4.61 (8.93) | (−4.23, 0.24) | (−4.72, −0.11) |
| Control | 144 | 80.98 (6.69) | 131 | 78.57 (10.41) | −2.62 (8.89) | p = 0.081 | p = 0.040 |
*CFB: change from baseline
†Difference in CFB between intervention and control groups (intervention minus control). 95 % confidence intervals (CIs) and p values were also provided
‡CCA: 222 complete cases analysis, simple linear regression without adjusting for any covariates
§Missing data imputed by using the multiple imputation by chained equations (MICE) approach18 with ten multiply imputed data sets and the results were combined using Rubin's rules19
At the 6-month endpoint, 247 subjects had a blood pressure determination [116 (85 %) intervention subjects and 131 (91 %) control subjects, p = 0.141]. A 5 mmHg or greater reduction was achieved by 71 (61 %) of intervention and 53 (45 %) of controls (p = 0.01). In a sensitivity analysis assuming no change in systolic blood pressure for those with a missing endpoint, 71 (52 %) of 136 intervention and 59 (41 %) of 144 control subjects achieved a 5-mm decrement in SBP (p = 0.06). In regard to absolute reductions, systolic pressure was reduced by 7.2 mmHg versus 0.8 mmHg for the intervention and control groups, respectively, resulting in a 6.4-mmHg greater reduction for the intervention (p = 0.003). For diastolic blood pressure, the mean change from baseline was 2.0 mmHg greater for intervention versus control groups (p = 0.08). With multiple imputations for missing values, the absolute reductions in systolic and diastolic pressures were significantly greater for the intervention group than control group. Adjustment for potential confounders in multiple linear regression models did not add independent predictive value to the analysis of change in CHD risk or blood pressure.
In regard to adverse events, one person died in each arm of the study. The intervention subject died at home with severely uncontrolled diabetes (hemoglobin A1c = 15 %), hypertension, and hyperlipidemia. The death certificate was unavailable, but the chart noted that the subject was unable to afford medications. The death certificate for the deceased control subject noted chronic obstructive pulmonary disease (COPD) as the cause of death. No other adverse events occurred during the study.
DISCUSSION
This is the first randomized trial of a theory-based, peer and primary care staff-based behavioral support designed to reduce both CHD risk and systolic blood pressure in African-American patients with sustained poorly controlled hypertension. Our complete case analysis revealed a 0.8 % greater reduction in 4-year CHD risk for the intervention group versus control group (p = 0.02), but, in an intent-to-treat analysis with multiple imputation, the reduction of 0.7 % was no longer statistically significant (p = 0.08). These primary endpoint results should be viewed with caution because of differential loss to follow-up due primarily to fewer intervention than control subjects (69 vs 82 %) obtaining an endpoint lipid panel. We speculate that intervention subjects might have been more reluctant to have their lipid levels tested because the primary focus of the intervention to reduce CHD risk factors was on improving blood pressure control, with limited attention to lowering cholesterol.
Indeed, 85 % of control subjects and 91 % of intervention subjects had a blood pressure endpoint determination. A 5 mmHg reduction in systolic blood pressure was achieved by 61 % of the intervention group and 45 % of the control group (p = 0.01). If we conservatively estimate change in blood pressure among the 13 % of study subjects missing this endpoint, this change is not significant (p = 0.06). However, the absolute reduction in systolic blood pressure was more than 6 mmHg greater for the intervention group with and without imputation for missing values (p < 0.01). A novel trial of peer storytelling DVDs for African-Americans also achieved a 6.4 mmHg reduction in systolic blood pressure after 6 to 9 months in the subgroup with baseline uncontrolled hypertension.21 These effects on systolic blood pressure are similar to the pooled reduction of 6.8 mmHg from eight trials of angiotensin converting enzyme inhibitors in African-Americans.22
Our African-American study subjects were clinically complex; over half had diabetes, nearly 20 % had a prior CHD event, and over 40 % had depressive symptoms. We employed several aspects of the Chronic Care Model in our intervention: use of a patient registry, team-based care, increasing provider expertise, and patient education.7 Our intervention has the distinctive feature of using peer coaches with well-controlled hypertension from the same practice. A trial of mutual support by pairs of peers, both of whom had poor diabetes control, achieved significant reductions in hemoglobin A1c levels.23 However, an Irish trial of peer education to reduce CHD risk reported no benefit, but lacked a theoretical basis and required subjects to attend nine office-based didactic group sessions led by peers.24 Our intervention was structured by the Theory of Planned Behavior and emphasized interactive role modeling, phone outreach, and practical, culturally appropriate tips.25
In addition to peer support, African-American practice staff provided counseling with entertaining, culturally appropriate, educational slideshows and an interactive computer-based CHD tool. It is possible that physicians need to reinforce messages in order achieve a significant reduction in CHD risk as reported in other studies.16 For this study, we used the 4-year CHD risk model developed by D’Agostino and colleagues from Framingham data.15 It has the advantage of being applicable to all patients because it calculates risk of either a primary or a secondary CHD event. Possibly because of the longer time horizon, offering information about 10-year risk did not significantly change CHD risk factors in a primary care setting.26
This trial has several strengths. First, our primary endpoint of reducing CHD risk set a high standard for a peer-office staff intervention. Second, our peer coaches were recruited from the same practices as patients as in our colon cancer prevention intervention. This approach may make it easier to identify potential coaches. Second, we used an electronic medical record registry to identify at-risk patients as recommended by the Chronic Care Model. Fourth, our team-based intervention involved training African-American office staff who had not previously been engaged in supporting at-risk patients. Fifth, D'Agostino's 4-year CHD risk model can be applied to all primary care patients. Sixth, our intervention addresses the needs of a clinically complex group of African-American patients with many challenges in adhering to medications.
This study also has a number of limitations. First, as noted previously, we had a differential follow-up in regard to our CHD endpoint. Failure to obtain a lipid determination at endpoint was the main reason for this differential. The intervention subjects received substantial support to improve poor blood pressure control, but many had no lipid abnormality and might have questioned the relevance of rechecking this value. Second, almost half of the trained peer coaches dropped out. In a qualitative study with ten peer coaches, motivation to continue in this role involved a mix of personal gain, gratitude to the physician, and a spirit of volunteerism.20 These motivators can inform future efforts to recruit peer coaches. Third, it is unclear whether both peer and staff support is required to reduce blood pressure. Fourth, this is a 6-month trial, and, in other studies, benefits observed at 6 months did not persist at 18 months.27 Fifth, the intervention is primarily generalizable to academic primary care practices that care for poor, minority populations.8 Sixth, contamination across study arms is possible but unlikely because patients’ physicians were not part of the intervention, and notes in the electronic medical record were directed to the intervention staff. Seventh, the research team coordinated and scheduled the intervention components, and, if adopted in clinical practice, these services would have to be performed by office staff. Lastly, one subject died in each arm of the study, but these events—uncontrolled diabetes and COPD exacerbation—were unlikely to result from the trial.
In summary, a complementary peer- and practice-based intervention resulted in a non-significant reduction of 4-year CHD risk, but a clinically significant drop in systolic blood pressure in African-Americans with poorly controlled hypertension. Effective interventions to reduce national disparities in morbidity and mortality have a high priority according to a recent US federal report.28 This trial should prompt similar studies of features of the Chronic Care Model to reduce the substantial risk of heart disease in African-Americans while evaluating sustainability and cost effectiveness.
Acknowledgments
We would like to thank our funding support from the Robert Wood Johnson Foundation and the staff of the Finding Answers, Disparities Research for Change Program. We also received an unrestricted grant from Pfizer, Inc., for the conduct of this study.
We would also like to thank the American Heart Association and especially Laura Dawson, Director, Cultural Health Initiatives, Great Rivers Affiliate, American Heart Association for her support throughout the project. We thank our peer coaches and community advisors, especially Mrs. Claire Walters, our lead peer coach, for her incredible talent and dedication to this project. We would like to also thank Josh Benner, PhD, for his assistance in the development of this project.
Conflict of Interest
The project received an unrestricted supplementary grant from Pfizer, Inc., to the University of Pennsylvania. The authors declare that they have no other conflicts.
Footnotes
Trial Registration: (ClinicalTrials.gov number NCT00948714 Support for Cardiovascular Health in African American Primary Care Patients). Funded by the Robert Wood Johnson Foundation Informing Disparities program and Pfizer, Inc.
References
- 1.Centers for Disease Control and Prevention. National Vital Statistic Report. Vol. 57, Num 14 Table B. http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_19.pdf, accessed Jan 5, 2012.
- 2.Centers for Disease Control and Prevention. Health United States, 2008. Table 71. http://www.cdc.gov/nchs/data/hus/hus08.pdf, accessed Jan 5, 2012.
- 3.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Jinagouda S, Shea S. Racial-ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17(10):963–70. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Umscheid CA, Gross R, Weiner MG, Hollenbeak CS, Tang SS, Turner BJ. Racial disparities in hypertension control, but not treatment intensification. Am J Hypertens. 2010;23(1):54–61. doi: 10.1038/ajh.2009.201. [DOI] [PubMed] [Google Scholar]
- 5.Bosworth HB, Powers B, Grubber JM, Thorpe CT, Olsen MK, Orr M, et al. Racial differences in blood pressure control: potential explanatory factors. J Gen Intern Med. 2008;23(5):692–8. doi: 10.1007/s11606-008-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bokhour BG, Berlowitz DR, Long JA, Kressin NR. How do providers assess antihypertensive medication adherence in medical encounters? J Gen Intern Med. 2006;21:577–83. doi: 10.1111/j.1525-1497.2006.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the chronic care model in the new millennium. Health Aff (Millwood) 2009;28(1):75–85. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moy E, Valente E, Jr, Levin RJ, Griner PF. Academic medical centers and the care of underserved populations. Acad Med. 1996;71(12):1370–7. doi: 10.1097/00001888-199612000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 10.Turner BJ, Weiner M, Yang C, TenHave T. Predicting adherence to colonoscopy or flexible sigmoidoscopy on the basis of physician appointment-keeping behavior. Ann Intern Med. 2004;140:528–32. doi: 10.7326/0003-4819-140-7-200404060-00013. [DOI] [PubMed] [Google Scholar]
- 11.Turner BJ, Hollenbeak C, Weiner MG, Have T, Roberts C. Barriers to adherence and hypertension control in a racially diverse representative sample of elderly primary care patients. Pharmacoepidemiol Drug Safe. 2009;18:672–81. doi: 10.1002/pds.1766. [DOI] [PubMed] [Google Scholar]
- 12.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282(18):1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 13.Azjen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50:179–211. doi: 10.1016/0749-5978(91)90020-T. [DOI] [Google Scholar]
- 14.Miller WR, Rollnick S. Motivational Interviewing: preparing People for Change. 2. New York: Guilford Press; 2002. [Google Scholar]
- 15.D'Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PW, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. 2000;139(2 Pt 1):272–81. Erratum in: Am Heart J. 2002;143(1):21. [DOI] [PubMed]
- 16.Benner JS, Erhardt L, Flammer M, Moller RA, Rajicic N, Changela K, et al. A novel programme to evaluate and communicate 10-year risk of CHD reduces predicted risk and improves patients' modifiable risk factor profile. Int J Clin Pract. 2008;62(10):1484–98. doi: 10.1111/j.1742-1241.2008.01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaffer ML, Chinchilli VM. Including multiple imputation in a sensitivity analysis for clinical trials with treatment failures. Contemp Clin Trials. 2007;28(2):130–7. doi: 10.1016/j.cct.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Raghunathan TW, Lepkowksi JM, Hoewyk J, Solenbeger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 19.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 1987. [Google Scholar]
- 20.Barg FK, Weiner MG, Joseph S, Pandit K, Turner BJ. Qualitative analysis of peer coaches' experiences with counseling African Americans about reducing heart disease risk. J Gen Intern Med. 2012;27(2):167–72. doi: 10.1007/s11606-011-1883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houston TK, Allison JJ, Sussman M, Horn W, Holt CL, Trobaugh J, et al. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med. 2011;154(2):77–84. doi: 10.7326/0003-4819-154-2-201101180-00004. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Kraja AT, Oberman A, Lewis CE, Ellison RC, Arnett DK, et al. A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens. 2005;18:935–42. doi: 10.1016/j.amjhyper.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507–15. doi: 10.7326/0003-4819-153-8-201010190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM, Paul G, Kelly A, Whitford DL, O'Shea E, O'Dowd T. Peer support for patients with type 2 diabetes: cluster randomised controlled trial. BMJ. 2011;342. doi:10.1136/bmj.d715. [DOI] [PMC free article] [PubMed]
- 25.Kressin NR, Orner MB, Manze M, Glickman ME, Berlowitz D. Understanding contributors to racial disparities in blood pressure control. Circ Cardiovasc Qual Outcomes. 2010;3(2):173–80. doi: 10.1161/CIRCOUTCOMES.109.860841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koelewijn-van Loon MS, Weijden T, Ronda G, Steenkiste B, Winkens B, Elwyn G, et al. Improving lifestyle and risk perception through patient involvement in nurse-led cardiovascular risk management: a cluster-randomized controlled trial in primary care. Prev Med. 2010;50(1–2):35–44. doi: 10.1016/j.ypmed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Svetkey LP, Pollak KI, Yancy WS, Jr, Dolor RJ, Batch BC, Samsa G, et al. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension. 2009;54(6):1226–33. doi: 10.1161/HYPERTENSIONAHA.109.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention CDC health disparities and inequality report–United States 2011. MMWR. 2011;60(Suppl):1–20. [PubMed] [Google Scholar]