Abstract
In recent years, colorectal cancer (CRC) screening using computerized tomographic colonography (CTC) has attracted considerable attention. In order to better understand patient preferences for CTC versus colonoscopy, we performed a systematic review and meta-analysis of the available literature. Data sources included published studies, abstracts and book chapters, in any language, with publication dates from 1995 through February 2012, and with prospective or retrospective enrollment of diagnostic or screening patients who had undergone both procedures and explicit assessment of their preference for colonoscopy versus CTC. A predefined algorithm identified eligible studies using computer and hand searches performed by two independent investigators. We used a mixed effects model to pool preference differences (defined as the proportion of subjects who preferred CTC minus the proportion who preferred colonoscopy for each study). Twenty-three studies met inclusion criteria, totaling 5616 subjects. In 16 of these studies, patients preferred CTC over colonoscopy, while colonoscopy was preferred in three studies. Due to the high degree of heterogeneity, an overall pooled preference difference was not calculated. Stratified analysis revealed that studies published in radiology journals (preference difference 0.590 [95 % CI 0.485, 0.694]) seemed more likely than studies in gastroenterology (0.218 [–0.015–0.451]) or general medicine journals (–0.158 [–0.389-0.072]) to report preference for CTC (p < 0.001). Studies by radiology authors showed a trend towards stronger preference for CTC compared with studies by gastroenterology authors. Symptomatic patients expressed no preference, but screening patients preferred CTC. There was no difference in preferences between studies using “masked” and “unmasked” preference ascertainment methods. Three studies featuring limited bowel preparations for CTC reported marked preference for CTC. There was no evidence of publication bias, while cumulative and exclusion analysis did not show any temporal trend or dominant study. Limitations included data heterogeneity and preference ascertainment limitations. In conclusion, most included studies reported preference for CTC. On stratified analysis, screening patients preferred CTC while diagnostic patients showed no preference. Studies published in radiology journals showed significantly stronger preference for CTC compared with studies in gastroenterology or general medicine journals.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2115-4) contains supplementary material, which is available to authorized users.
KEY WORDS: colorectal cancer, adenoma, screening, colonoscopy, computerized tomographic colonography
INTRODUCTION
In recent years, colorectal cancer (CRC) screening using computerized tomographic colonography (CTC) has attracted considerable attention. With continuous technical improvements, CRC screening using CTC now exhibits accuracy rates approaching that of colonoscopy,1 and CTC every 5 years is one of the screening approaches endorsed by some current guidelines.2,3 At present, the uptake of screening colonoscopy is still relatively low,4 partly because of suboptimal patient acceptance. One of the purported advantages of CTC over colonoscopy is superior patient acceptance due to non-invasiveness. Using the available literature, we performed a systematic review and meta-analysis on patient preference for CTC versus colonoscopy.
METHODS
Study Identification
Since this is a systematic review and meta-analysis of published studies and publicly available data, it did not require institutional review board approval. In conducting this study we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.5 We searched the PubMed, EmBase and Cochrane databases from January 1995 to August 2011 using a pre-determined, systematic algorithm (Appendix A, available online), aiming to retrieve articles comparing patient preference for conventional colonoscopy versus CTC. In addition, we performed a hand search of citations in all included articles as well as editorials and reviews published in prominent gastroenterology, general medicine and radiology journals (Appendix B, available online).
The initial search strategy was designed to be highly sensitive (but with low specificity). At least one investigator manually reviewed the titles of all retrieved studies, and obviously irrelevant ones were excluded. We then reviewed the abstracts of the remaining studies. After eliminating further studies based on predefined exclusion criteria, the full texts of the remaining ones were independently reviewed by two specially trained investigators (OSL and MN).
Inclusion/Exclusion Criteria
Prospective, retrospective or cross-sectional studies that involved patients who had undergone both CTC and colonoscopy (crossover design) and compared their preferences were eligible. In prospective studies, subjects were consented before they had undergone either CTC or colonoscopy and their preferences were ascertained afterwards. In retrospective studies, investigators identified patients who had already experienced both colonoscopy and CTC in the past, and asked them to recall which procedure they preferred. Eligible studies had to be published as an abstract at a major scientific conference, a letter to the editor, or a full article in a peer-reviewed medical journal. Studies published in non-English languages were eligible. Case reports, unpublished material and studies with fewer than 20 subjects were excluded. Studies involving symptomatic patients or patients at increased risk for CRC were eligible, but were subject to subgroup analysis.
Endpoints and Data Abstraction
Two investigators (OSL and MN) independently abstracted data on patient preference, using the same abstraction form. Disagreements were resolved by face-to-face re-review of the study in question.
All eligible studies asked patients whether they preferred CTC or colonoscopy, using a direct dichotomous question administered after subjects had undergone both procedures. This question was usually of the form: “Which procedure (CTC or colonoscopy) did you prefer?” The mode of preference ascertainment for each study was categorized as either “masked” or “unmasked”. “Masked” ascertainment meant that patients gave their preference response either anonymously or outside the immediate presence of staff directly associated with either CTC or colonoscopy. For example, answering preferences questions by email or mail from home would be considered “masked” ascertainment, and answering such questions posed by a “neutral” research associate not associated with either the endoscopy or radiology units would also be considered “masked”; answering questions while the patient was still physically in the endoscopy or CTC unit would be considered “unmasked”. The primary endpoint for our meta-analysis was the “preference difference”, defined as the proportion of patients who preferred CTC minus the proportion who preferred colonoscopy; this parameter has been used in many previous meta-analyses on crossover studies involving preference ascertainment.6,7
Study Quality Assessment
Two investigators (OSL and MN) independently performed quality assessment, using a scoring system analogous to one we validated for a previous meta-analysis on observational studies.8 We assigned each study a quality score based on study design, questionnaire type, publication mode and sample size. Studies that were published as full papers, included only asymptomatic screening patients, had patient preference as the primary study outcome, assessed preference in a “masked” manner, and had ≥120 subjects, received one point for each category (for a maximum score of five). Studies that were published only as abstracts or letters, included both diagnostic and screening patients, had preference as a secondary outcome, assessed preference in an “unmasked” manner, and had <120 subjects, would receive a score of zero.
Primary Data Analysis
Using a previously validated method designed specifically for summarizing preference data in crossover trials,6,7 we calculated pooled preference differences and 95 % CI, using the Der Simonian and Laird method (weighted least squares solution) with stratifying variables as fixed effects, based on a mixed effects model.9 The degree of heterogeneity was assessed by calculating a Q statistic using the chi-squared test.
On stratified analysis, the following moderator variables were investigated: 1) Procedural indication: Some studies included only asymptomatic screening subjects, while others included both screening and symptomatic patients; 2) Bowel preparation: Standard versus “prepless” or “low-prep” regimens for CTC; 3) Publication date: Studies were stratified according to whether they were published before or after 2004 (this date was chosen because a preliminary review showed that roughly half the studies were published after 2004); 4) Sample size: Studies were stratified according to whether or not they enrolled more than 120 subjects (this number was chosen because a preliminary review showed that roughly half the studies had fewer than 120 subjects); 5) Publication format: Studies published as full papers were compared against studies published only as abstracts or letters; 6) Preference ascertainment method: Masked versus unmasked methods were compared; 7) Follow-up colonoscopy information: Studies in which subjects were told the a priori probability of needing follow-up colonoscopy after CTC were compared against studies in which subjects were not told the probability; and 8) Study quality score (scores of 0-3 versus 4-5).
Other Analysis
We also performed meta-regression, with patient preference as the dependent variable and sample size, year of publication, and quality score as independent variables.
Because our meta-analysis included only published studies and abstracts, we explored the possibility of publication bias at the study level by performing inverted funnel plot analysis, Begg and Mazumdar rank correlation (Kendall’s tau), and Egger’s regression intercept testing. Finally, using Rosenthal’s file drawer method, we estimated the number of studies with null results needed to overturn the conclusion of the meta-analysis.
CTC technique may change over time due to various factors, e.g. radiologist experience or technical improvements, therefore we performed temporal cumulative meta-analysis by publication date. We also looked for dominant studies by using exclusion analysis, i.e. excluding each study one at a time, to see if there was a marked change in the summary values.
Data analysis was performed using Comprehensive Meta-Analysis 2.0 (Biostat Inc., Englewood, New Jersey) and SPSS 18.0 (SPSS Inc., Chicago, Illinois) software.
RESULTS
Study Identification
The initial computer searches returned 204 titles (most recently performed on February 12th, 2012), of which 136 were obviously irrelevant based on the title, and were excluded. A further 27 were excluded after a review of the abstract. The remaining 41 studies underwent detailed evaluation of the entire manuscript, and another 18 were excluded. Of the excluded studies, three assessed CTC acceptance only and there was no direct comparison against colonoscopy,10–12 four focused on acceptance of various bowel preparation regimens for CTC,13–16 four reported satisfaction or acceptability without direct preference comparison between the two modalities,17–20 six compared preferences for magnetic resonance colonography (MRC) versus colonoscopy,21–26 and lastly, one study included only 11 subjects.27 Ultimately, 23 studies (comprising 5616 subjects) fulfilled all inclusion criteria and were included in this analysis (Table 1)28–50; these included three studies published only in abstract or letter form.28,29,50 All included studies were in English.
Table 1.
Outcomes and Primary Characteristics of Studies Included in the Meta-analysis. Note: The Sample Size Includes All Subjects Who Returned Preference and Acceptance Questionnaires, Including Those Who Expressed No Preference or Equal Preference for CTC and Colonoscopy, Those Who Reported “Don’t Know”, and Those Who Did Not Answer the Preference Question. Patients Who Did Not Return the Preference Questionnaire at All Were Not Included. We Used Scores from the Earliest Questionnaire Done After the Patients Had Completed Both Colonoscopy and CTC. CTC: Computerized Tomographic Colonography; GI: Gastroenterologist; CO2: Carbon Dioxide
| Study | Type | N | Prefer CTC | Prefer Colonoscopy | Procedure Indication | Main Study Purpose | Journal Specialty | Primary Author Specialty | Preference Ascertainment Timing & Methods‡ | Accuracy/Referral Data Given To Patients?§ | Quality Score | CTC Gas | “Low Prep” Regimen Used? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pineau28 | Abstract | 55 | 34 (62 %)* | 18 (33 %) | Not stated | Acceptance | GI | GI | Not stated | Equal | 1 | Not stated | Probably not |
| Forbes29 | Letter | 70 | 19 (27 %) | 16 (23 %) | Diagnostic | Acceptance | GI | GI | Not stated | Equal | 1 | Room air | Probably not |
| Akerkar30 | Paper | 295 | 107 (36 %) | 188 (64 %)* | Diagnostic & screening | Acceptance | GI | GI | Within 24 hrs, masked | No | 4 | Room air | No |
| Svensson31 | Paper | 96 | 56 (58 %)* | 12 (13 %) | Diagnostic | Acceptance | Radiology | Radiology | Within 24 hrs, masked | Equal | 3 | Room air | No |
| Thomeer32 | Paper | 124 | 88 (71 %)* | 30 (24 %) | Diagnostic & screening | Acceptance | Radiology | Radiology | 2-3 hrs after tests, not masked | No | 2 | CO2 | No |
| Gluecker33 | Paper | 494 | 357 (72 %)* | 25 (5 %) | High-risk screening | Acceptance | Radiology | Radiology | Return mail, masked | No | 5 | CO2 | No |
| Pickhardt34 | Paper | 1005 | 500 (50 %)* | 413 (41 %) | Screening | Accuracy | General | Radiology | Return mail, masked | No | 4 | Room air | No |
| Ristvedt35 | Paper | 120 | 69 (58 %)* | 17 (14 %) | Diagnostic & screening | Acceptance | GI | Neither | 2-3 dys after both tests, not masked | No | 2 | CO2 or air | No |
| Taylor36 | Paper | 91 | 40 (44 %)* | 15 (17 %) | Diagnostic | Acceptance | Radiology | Radiology | 1 week later, masked | No | 3 | CO2 | No |
| Cotton37 | Paper | 518 | 238 (46 %) | 212 (41 %) | Diagnostic | Accuracy | General | GI | Return mail, masked | No | 3 | CO2 or air | No |
| Iannaccone38 | Paper | 162 | 99 (61 %)* | 57 (35 %) | Diagnostic & screening | Accuracy | GI | Radiology | Return mail, masked | No | 3 | Room air | Yes |
| van Gelder39 | Paper | 236 | 168 (71 %)* | 45 (19 %) | High-risk screening | Acceptance | Radiology | Radiology | Right after tests, not masked | Equal, 20 % referral for colonoscopy | 4 | CO2 | No |
| Juchems40 | Paper | 157 | 116 (74 %)* | 10 (6 %) | Diagnostic | Acceptance | Radiology | Radiology | Up to 31 mths after tests, masked | No | 4 | Room air | No |
| Bosworth41 | Paper | 581 | 145 (25 %) | 303 (52)%* | Diagnostic & screening | Accuracy† | General | Neither | Up to 3 days after tests, masked | No | 4 | Mostly room air | No |
| Florie42 | Paper | 54 | 43 (80 %)* | 7 (13 %) | High-risk screening | Accuracy | Radiology | Radiology | Right after colonoscopy, not masked | Equal, 20 % referral for colonoscopy | 2 | CO2 | Yes |
| Rajapaksa43 | Paper | 272 | 144 (53 %) | 128 (47 %) | Diagnostic | Acceptance | GI | GI | Same day, not masked | No | 3 | Room air | No |
| Roberts-Thomson44 | Paper | 195 | 119 (61 %)* | 76 (39 %) | Diagnostic | Accuracy | GI | GI | 1 week after tests, masked | No | 4 | CO2 | No |
| Jung45 | Paper | 51 | 5 (10 %) | 33 (65 %)* | Diagnostic | Acceptance | General | Neither | 24 hrs after, by phone, not masked | No | 2 | Room air | No |
| White46 | Paper | 122 | 72 (59 %)* | 29 (24 %) | Diagnostic | Accuracy | GI/Surgery | GI/Surgery | Within 2 mths, by mail, masked | No | 2 | Room air | No |
| Graser47 | Paper | 256 | 118 (46 %) | 95 (37 %) | Screening | Accuracy | GI | Radiology | Right after tests, not masked | No | 3 | Room air or CO2 | No |
| Jensch48 | Paper | 164 | 124 (76 %)* | 27 (16 %) | High-risk screening | Acceptance | Radiology | Radiology | Right after colonoscopy, not masked | Equal, 20 % referral for colonoscopy | 3 | CO2 | Yes |
| Moawad49 | Paper | 57 | 54 (95 %)* | 2 (3 %) | Screening | Acceptance | Radiology | GI | CTC after colonoscopy with variable lag, not masked | No | 3 | CO2 | No |
| Cash50 | Abstract | 441 | 340 (77 %) | 28 (6 %) | Screening | Acceptance | GI | GI/Radiology | CTC after colonoscopy with variable lag, masking not described | No | 3 | Not stated | Not stated |
*Statistically significantly superior preference
†Preference results are based on the percentage of patients who chose “most willing to have colonoscopy done again” versus the percentage of patients who chose “most willing to have CTC done again”
‡This refers to the timing of preference ascertainment, as well as the degree of anonymity or “masking”, e.g. patients were asked about their preference either anonymously or shielded from the presence of endoscopy or radiology staff
§Whether or not patients were informed about the estimated accuracy of CTC and colonoscopy for detecting polyps, and/or the referral rates for colonoscopy after CTC; “equal” means that patients were told that CTC and colonoscopy had equal sensitivity for detecting polyps
Of the included studies, 20 were prospective, with subjects committed to undergoing CTC followed by colonoscopy, regardless of the findings on CTC. The remaining three studies were retrospective and patients could have undergone either procedure first. CTC and colonoscopy were done on the same day in 18 studies. Eleven studies utilized exclusively or mostly carbon dioxide for CTC insufflation, with the remaining 12 using room air; all studies used room air for colonoscopy insufflation. Eight studies were restricted to screening patients, while 14 involved both diagnostic and screening patients (procedure indication was not described in one abstract). Finally, three studies used “low-prep” regimens for CTC.
In six studies, patients were told the estimated accuracy of CTC and colonoscopy beforehand, while in three studies, they were given the predicted referral rate for follow-up colonoscopy after CTC (Table 1). Eleven studies used “masked” preference ascertainment methods, nine used “unmasked” methods, while in three there was no description of ascertainment mode (Table 1).
Primary Preference Outcomes
Amongst the included studies, 16 (comprising 3573 subjects) showed a statistically significant preference for CTC, three (927 subjects) showed a preference for colonoscopy, and four (1116 subjects) showed no difference in preference. As expected, there was a large amount of heterogeneity in the study outcomes (Q = 125, p < 0.001), which would be expected to seriously compromise the validity of an overall pooled estimate.51 Therefore, we did not calculate an overall pooled preference difference; instead, an extensive series of stratified analyses was done to assess the effects of important moderator variables.
Stratified Analyses
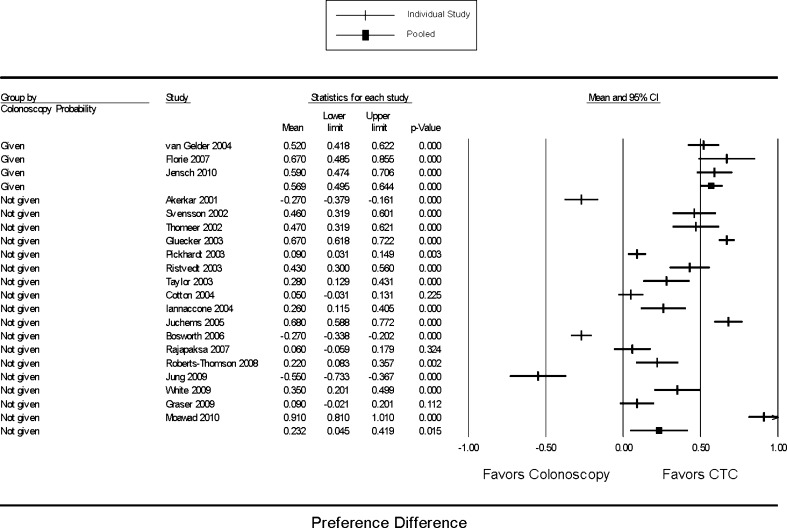
No significant differences were found when the data were stratified by main study objective (patient acceptance vs. CTC accuracy), sample size, publication date, study location, publication status, study quality score and whether or not carbon dioxide was used for CTC insufflation (data not shown). Stratified analysis comparing “masked” versus “unmasked” studies did not show any difference in outcomes (mixed effects model test p = 0.458) (Fig. 1). Most (20) of the included studies did not tell patients the a priori probability of the need for follow-up colonoscopy after CTC; the remaining three used a probability of 20 %. Stratified analysis showed a stronger preference for CTC in the latter group (p = 0.001) (Fig. 2).
Figure 1.
Forest plot showing pooled summary preference difference stratified by preference ascertainment method (masked vs. unmasked). Preference difference is defined as the proportion of patients who preferred CTC minus the proportion of patients who preferred colonoscopy. Mixed effects model comparative test p = 0.458. CTC: Computerized tomographic colonography.
Figure 2.
Pooled summary preference difference, stratified by whether or not the probability of needing follow-up colonoscopy was given to subjects. Preference difference is defined as the proportion of patients who preferred CTC minus the proportion of patients who preferred colonoscopy. Mixed effects model comparative test p = 0.001. CTC: Computerized tomographic colonography.
When stratified by journal or primary author specialty, we found that studies published in radiology journals markedly favored CTC (pooled preference difference 0.590 with 95 % CI [0.485, 0.694]), but studies in gastroenterology (0.218 [-0.015, 0.451]) or general medicine journals (-0.158 [-0.389, 0.072]) showed no preference (p < 0.001) (Fig. 3). Studies by radiology primary authors showed a trend towards stronger preference for CTC (0.434 [0.269, 0.598]) than studies by gastroenterologists (0.206 [-0.075, 0.487]) or general medicine authors (0.082 [-0.528, 0.693]) (p = 0.256) (Fig. 4).
Figure 3.
Pooled summary preference difference stratified by journal specialty. “Neither” refers mostly to general medicine journals. Preference difference is defined as the proportion of patients who preferred CTC minus the proportion of patients who preferred colonoscopy. Mixed effects model comparative test p < 0.001. CTC: Computerized tomographic colonography.
Figure 4.
Pooled summary preference difference stratified by primary author specialty. “Neither” refers mostly to general medicine authors. Preference difference is defined as the proportion of patients who preferred CTC minus the proportion of patients who preferred colonoscopy. Mixed effects model comparative test p = 0.256. CTC: Computerized tomographic colonography.
Figure 5 shows the data stratified by procedure indication (diagnostic/screening vs. screening only); as expected, studies on screening patients showed a marked preference for CTC (0.530 [0.315, 0.745]), as opposed to studies that included symptomatic patients (0.158 [–0.029, 0.345]) (p = 0.011).
Figure 5.
Pooled summary preference difference stratified by procedure indication (screening vs. diagnostic/screening). Preference difference is defined as the proportion of patients who preferred CTC minus the proportion of patients who preferred colonoscopy. Mixed effects model comparative test p = 0.011. CTC: Computerized tomographic colonography.
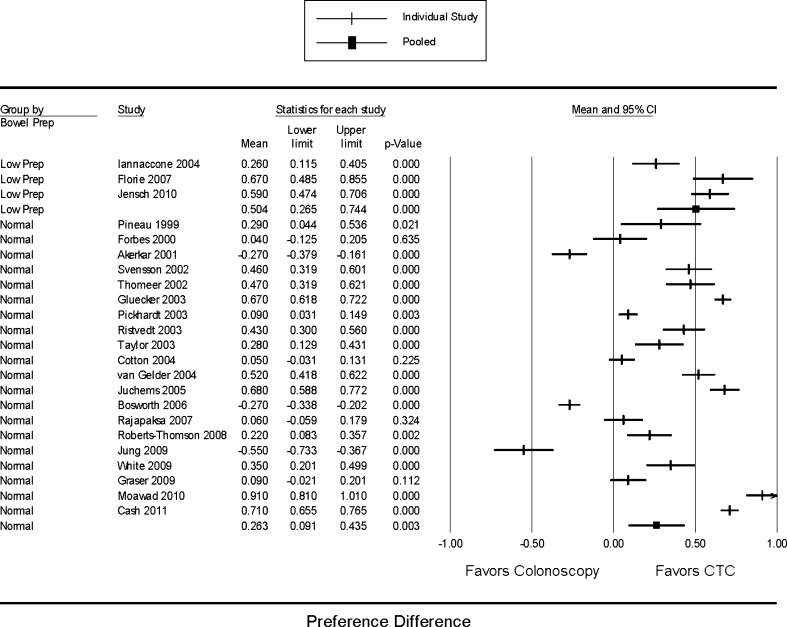
Finally, studies that used “low-prep” regimens showed a trend towards more significant results than studies that used regular preparations for CTC, although both groups demonstrated preference for CTC (p = 0.109) (Fig. 6).
Figure 6.
Pooled summary preference difference, stratified by bowel preparation type for CTC (“low-prep” vs. regular regimens). Preference difference is defined as the proportion of patients who preferred CTC minus the proportion of patients who preferred colonoscopy. Mixed effects model comparative test p = 0.109. CTC: Computerized tomographic colonography.
Other Analyses
Testing by inverted funnel plot analysis, Begg and Mazumdar rank correlation (p = 0.632), and Egger’s regression intercept (p = 0.407), showed no evidence of significant publication bias. We estimated that the number of unpublished studies with null results required to change our conclusion was 454, using Rosenthal’s file drawer method.
Meta-regression using continuous variables, such as sample size, study quality score and publication year, as input variables and preference as the output variable did not show any significant relationships (data not shown).
Temporal cumulative meta-analysis by publication date showed that the preference difference became significant in favor of CTC starting in mid-2003, but no temporal trend could be discerned (eFigure A, available online).
To test whether any study had a dominant effect on the meta-analysis, we excluded each study one by one and recalculated the summary preference difference in each case (eFigure B, available online). We did not find any dominant study.
DISCUSSION
Although surveys generally show a high level of interest in CRC screening,52 patient acceptance of colonoscopy is limited by several factors, including embarrassment, bowel preparation and procedural discomfort.53,54 Even amongst screening-eligible, insured Americans with a primary care provider, the prevalence of screening colonoscopy was still relatively low (22 %) as of 2003.55 However, in recent years, CRC screening uptake has increased from 25 % to 55 %, mostly attributed to the growth of screening colonoscopy,56 such that now the prevalence of lifetime colonoscopy or sigmoidoscopy is about 56 % of the population aged ≥50.57
Choice of modality for evaluation of the colon can be affected by procedure-related factors such as accuracy, discomfort, invasiveness, embarrassment or inconvenience,52,58 as well as patient-related factors such as ethnicity or education level.43 Some advantages of colonoscopy mentioned by patients include being able to watch the procedure on screen, getting immediate feedback on results, having the opportunity to “sleep through” the procedure, and being able to undergo biopsy or polypectomy during the same session.31,35,45 On the other hand, reported advantages of CTC include non-invasiveness, short duration, relative painlessness and novel technology.31,32,35,40,45 With increasing passage of time after the procedures, comfort, embarrassment and inconvenience considerations wane and outcome considerations become more prominent.39
One of the purported advantages of CTC over colonoscopy is improved patient acceptance, but the data are extremely heterogeneous. A previous meta-analysis, published only in abstract form, included 11 studies, of which 9 reported that patients preferred CTC over colonoscopy (51 % of subjects preferred CTC versus 27 % who preferred colonoscopy).59 The available studies are very heterogenous in design, in terms of CTC technique, procedure indication, study location, bowel preparation regimens, preference ascertainment methods and other variables. In such situations, stratified analysis can be helpful in determining the impact of various factors on outcomes.
Because acceptance and preference are “soft” measures, they are prone to bias. This is reflected by the differences seen when studies are stratified by journal type or lead author specialty. Studies published in radiology journals demonstrated a much more marked preference for CTC compared with studies in gastroenterology journals. Regarding methodological differences between the two groups, we note that radiology studies were more likely to include screening cohorts (6 out of 11 studies) compared with gastroenterology or general medicine studies (2 of 11); furthermore, all three studies using “low prep” regimens for CTC were by radiology authors. These factors may partially explain why radiology studies seem to favor CTC more; any remaining differences can be attributed to “subconscious investigator bias”, an unquantifiable element. It has been demonstrated that wording, context and ascertainment methods can significantly impact responses.60–62 In particular, studies employing “unmasked” ascertainment methods are prone to so-called “social desirability response bias” (patients giving better responses than they actually believe because they feel it is more acceptable) and “ingratiating response bias” (patients giving better responses than they feel because they wish to ingratiate themselves with their providers). If accuracy data or referral rates for colonoscopy are not given or are distorted, this can further bias responses. However, it should be noted that all the included studies involved patients filling out the preference questionnaires after they had already undergone both procedures and knew the CTC results; in such cases, the a priori probability of needing follow-up colonoscopy may not be as influential. All these potential pitfalls underscore the importance of describing the methodology in detail when reporting patient preference studies.
Our other findings on stratified analysis are consistent with what would be expected. For example, screening patients were more likely to favor CTC, while diagnostic patients reported no difference in preference. Patients who are symptomatic or already have a known colonic condition (such as inflammatory bowel disease) are understandably less favorably disposed towards imaging tests incapable of biopsy or polypectomy.39,43 Nevertheless, CTC remains an important option for symptomatic patients unable or unwilling to undergo colonoscopy.
Finally, as expected, studies that used “low-prep” regimens seemed to favor CTC more than studies using regular preparations. Previous studies have consistently shown that bowel preparation is the worst part of the colonoscopy experience.53 However, it must be kept in mind that in clinical practice, “low-prep” regimens would necessitate a second, fuller bowel preparation for colonoscopy if pathology (e.g. polyp) is discovered; this may impact the preference of some patients.
Because of concerns about radiation exposure, MRC has been investigated. We did not include MRC in this systematic review because it is very different from CTC in terms of its developmental lifecycle, with MRC being more of an emerging technology. Compared with CTC, MRC entails a longer procedure time in a more confined environment, and insufflation of the colon is done with water rather than air. In general, most available studies have not demonstrated any preference for MRC over colonoscopy.21–26
Although the proportion of subjects who prefer colonoscopy is inversely related to the proportion who prefer CTC, the “preference difference” is well established as the endpoint in meta-analyses summarizing studies comparing preferences in a single cohort of subjects with a cross-over design, which is the type of study that is the focus of our meta-analysis. Pooling proportions is a feasible alternative approach, but is less commonly used for meta-analyses of this type, and is also affected by the same inverse relationship between those who prefer colonoscopy and those who prefer CTC.
Currently, the “STrengthening the Reporting of OBservational studies in Epidemiology” (STROBE) initiative and Newcastle-Ottawa scale are two available measures for assessing the quality of observational studies.63,64 STROBE consists of a checklist of features to ensure quality; however, there is no easy way to score this, making it difficult to use for stratified analysis. As for the Newcastle-Ottawa scale, its content validity and inter-rater reliability have been established, but its criterion validity and intra-rater reliability are still being validated. Furthermore, it seems geared towards case-control or prospective cohort studies, different from those in our meta-analysis. For these reasons, we elected to use the quality scale we had developed and partially validated in our previous meta-analysis for observational studies on distal polyps.8
Our review focuses on the preferences of patients experienced in both procedures. However, pre-procedure perception in those who have never undergone CTC or colonoscopy is perhaps even more important in predicting the behavior of patients. Only a few studies have assessed pre-procedure perception, all of which showed that subjects preferred CTC prior to being informed about the detailed technical and performance features of both procedures.17,28,35,58,65–67 However, once they were educated about CTC and colonoscopy (including sedation practices, accuracy data, complication rates, bowel preparation requirements and probability of referral for colonoscopy after CTC), there was a marked improvement in the perception of colonoscopy,28,35,66 in some cases leading to an outright reversal in preference.65 A similar pattern was seen when primary care providers were surveyed.66 In general, both providers and patients were concerned more about “outcome features”, such as accuracy, colonoscopy referral rates or complications, than about “process features”, such as pain, embarrassment or inconvenience.58,65,67
In conclusion, amongst patients who had undergone both procedures, CTC is preferred over colonoscopy in most of the studies in this review. The use of CTC in situations where colonoscopy cannot be completed, such as tortuous colons or obstructive cancers, is already well established, and studies show that large-scale screening CTC is feasible.68 Inadequate insurance coverage is currently a major barrier to the adoption of this technology.69 However, even if CTC becomes widely reimbursable, whether it can improve overall compliance with CRC screening is uncertain. In some studies, offering multiple screening methods did not result in higher screening rates,70,71 and adding the CTC option to standard modalities (fecal occult blood testing, sigmoidoscopy and colonoscopy) would increase screening uptake by only 2 %.58 However, a recent abstract reported that a substantial proportion of patients who had undergone CTC screening would not have undergone screening at all if the CTC option had not been available.72 In addition to insurance coverage, close logistical cooperation between the radiology and gastroenterology departments (to facilitate same-day colonoscopy after a positive CTC) would be necessary in order for diagnostic or screening CTC to become the standard of care. Further research should focus on CTC acceptance amongst patients who have yet to undergo either procedure, either in the form of a trial in which patients are randomized to CTC or colonoscopy and then their acceptance is assessed, or a prospective cohort study in which patients are asked to choose between CTC or colonoscopy.
Electronic Supplementary Material
(DOC 46 kb)
Acknowledgments
The authors would like to thank Jane Babione for her assistance and input.
There was no internal or external funding or grant support provided for this study.
Dr. Otto Lin gave an oral presentation of a preliminary version of this study at Digestive Disease Week in June of 2010 in New Orleans.
Conflict of Interest
None of the authors have any financial conflicts of interest to report, with the following exceptions: 1) A research grant from Cumberland Pharmaceuticals to Dr. Otto Lin, for a pilot study assessing the efficacy and safety of crystalline lactulose as a colonoscopy bowel preparation agent; 2) Consultancies for Epigenomics Inc. and Salix Pharmaceuticals for Dr. Jason Dominitz.
Grant Support or Funding
None
REFERENCES
- 1.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 4.Schenck AP, Peacock SC, Klabunde CN, Lapin P, Coan JF, Brown ML. Trends in colorectal cancer test use in the medicare population, 1998-2005. Am J Prev Med. 2009;37(1):1–7. doi: 10.1016/j.amepre.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 6.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. 1. Chichester: Wiley; 2000. p. 317. [Google Scholar]
- 7.Gotzsche PC. Patients' preference in indomethacin trials: an overview. Lancet. 1989;1(8629):88–91. doi: 10.1016/S0140-6736(89)91439-6. [DOI] [PubMed] [Google Scholar]
- 8.Lin OS, Gerson LB, Soon MS, Schembre DB, Kozarek RA. Risk of proximal colon neoplasia with distal hyperplastic polyps: a meta-analysis. Arch Intern Med. 2005;165(4):382–390. doi: 10.1001/archinte.165.4.382. [DOI] [PubMed] [Google Scholar]
- 9.Hedges LV, Pigott TD. The power of statistical tests for moderators in meta-analysis. Psychol Methods. 2004;9(4):426–445. doi: 10.1037/1082-989X.9.4.426. [DOI] [PubMed] [Google Scholar]
- 10.Jensch S, Vries AH, Pot D, et al. Image quality and patient acceptance of four regimens with different amounts of mild laxatives for CT colonography. AJR Am J Roentgenol. 2008;191(1):158–167. doi: 10.2214/AJR.07.3128. [DOI] [PubMed] [Google Scholar]
- 11.Wagner C, Knight K, Halligan S, et al. Patient experiences of colonoscopy, barium enema and CT colonography: a qualitative study. Br J Radiol. 2009;82(973):13–19. doi: 10.1259/bjr/61732956. [DOI] [PubMed] [Google Scholar]
- 12.Edwards JT, Mendelson RM, Fritschi L, et al. Colorectal neoplasia screening with CT colonography in average-risk asymptomatic subjects: community-based study. Radiology. 2004;230(2):459–464. doi: 10.1148/radiol.2302021422. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SA, Slater A, Burling DN, et al. CT colonography: optimisation, diagnostic performance and patient acceptability of reduced-laxative regimens using barium-based faecal tagging. Eur Radiol. 2008;18(1):32–42. doi: 10.1007/s00330-007-0631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zalis ME, Perumpillichira JJ, Magee C, Kohlberg G, Hahn PF. Tagging-based, electronically cleansed CT colonography: evaluation of patient comfort and image readability. Radiology. 2006;239(1):149–159. doi: 10.1148/radiol.2383041308. [DOI] [PubMed] [Google Scholar]
- 15.Lefere PA, Gryspeerdt SS, Dewyspelaere J, Baekelandt M, Holsbeeck BG. Dietary fecal tagging as a cleansing method before CT colonography: initial results polyp detection and patient acceptance. Radiology. 2002;224(2):393–403. doi: 10.1148/radiol.2241011222. [DOI] [PubMed] [Google Scholar]
- 16.Liedenbaum MH, Vries AH, Gouw CI, et al. CT colonography with minimal bowel preparation: evaluation of tagging quality, patient acceptance and diagnostic accuracy in two iodine-based preparation schemes. Eur Radiol. 2009;20(2):367–376. doi: 10.1007/s00330-009-1570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46(9 Suppl 1):S10–S16. doi: 10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 18.Taylor SA, Bomanji JB, Manpanzure L, et al. Nonlaxative PET/CT colonography: feasibility, acceptability, and pilot performance in patients at higher risk of colonic neoplasia. J Nucl Med. 2010;51(6):854–861. doi: 10.2967/jnumed.109.072728. [DOI] [PubMed] [Google Scholar]
- 19.Nagata K, Okawa T, Honma A, Endo S, Kudo SE, Yoshida H. Full-laxative versus minimum-laxative fecal-tagging CT colonography using 64-detector row CT: prospective blinded comparison of diagnostic performance, tagging quality, and patient acceptance. Acad Radiol. 2009;16(7):780–789. doi: 10.1016/j.acra.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 20.Farrell RJ, Morrin MM, Silas A, Raptopoulos V, McGee JB. Virtual colonoscopy in patients undergoing elective colonoscopy: diagnostic accuracy and patient tolerance. Gastroenterology. 2000;118(4):A258. doi: 10.1016/S0016-5085(00)83109-1. [DOI] [Google Scholar]
- 21.Leung WK, Lam WW, Wu JC, et al. Magnetic resonance colonography in the detection of colonic neoplasm in high-risk and average-risk individuals. Am J Gastroenterol. 2004;99(1):102–108. doi: 10.1046/j.1572-0241.2003.04008.x. [DOI] [PubMed] [Google Scholar]
- 22.Goehde SC, Descher E, Boekstegers A, et al. Dark lumen MR colonography based on fecal tagging for detection of colorectal masses: accuracy and patient acceptance. Abdom Imaging. 2005;30(5):576–583. doi: 10.1007/s00261-004-0290-4. [DOI] [PubMed] [Google Scholar]
- 23.Florie J, Birnie E, Gelder RE, et al. MR colonography with limited bowel preparation: patient acceptance compared with that of full-preparation colonoscopy. Radiology. 2007;245(1):150–159. doi: 10.1148/radiol.2451061244. [DOI] [PubMed] [Google Scholar]
- 24.Achiam MP, Logager V, Chabanova E, Thomsen HS, Rosenberg J. Patient acceptance of MR colonography with improved fecal tagging versus conventional colonoscopy. Eur J Radiol. 2010;73(1):143–7. [DOI] [PubMed]
- 25.Langhorst J, Kuhle CA, Ajaj W, et al. MR colonography without bowel purgation for the assessment of inflammatory bowel diseases: diagnostic accuracy and patient acceptance. Inflamm Bowel Dis. 2007;13(8):1001–1008. doi: 10.1002/ibd.20140. [DOI] [PubMed] [Google Scholar]
- 26.Kinner S, Kuehle CA, Langhorst J, et al. MR colonography vs. optical colonoscopy: comparison of patients' acceptance in a screening population. Eur Radiol. 2007;17(9):2286–2293. doi: 10.1007/s00330-007-0643-9. [DOI] [PubMed] [Google Scholar]
- 27.Bielen D, Thomeer M, Vanbeckevoort D, et al. Dry preparation for virtual CT colonography with fecal tagging using water-soluble contrast medium: initial results. Eur Radiol. 2003;13(3):453–458. doi: 10.1007/s00330-002-1755-x. [DOI] [PubMed] [Google Scholar]
- 28.Pineau BC, Sevick MA, Mikulaninec C, Vining DJ. Evaluation of patient preference: virtual colonoscopy versus Endoscopic. Gastroenterology. 1999;116(4):A486. [Google Scholar]
- 29.Forbes GM, Mendelson RM. Patient acceptance of virtual colonoscopy. Endoscopy. 2000;32(3):274–275. [PubMed] [Google Scholar]
- 30.Akerkar GA, Yee J, Hung R, McQuaid K. Patient experience and preferences toward colon cancer screening: a comparison of virtual colonoscopy and conventional colonoscopy. Gastrointest Endosc. 2001;54(3):310–315. doi: 10.1067/mge.2001.117595. [DOI] [PubMed] [Google Scholar]
- 31.Svensson MH, Svensson E, Lasson A, Hellstrom M. Patient acceptance of CT colonography and conventional colonoscopy: prospective comparative study in patients with or suspected of having colorectal disease. Radiology. 2002;222(2):337–345. doi: 10.1148/radiol.2222010669. [DOI] [PubMed] [Google Scholar]
- 32.Thomeer M, Bielen D, Vanbeckevoort D, et al. Patient acceptance for CT colonography: what is the real issue? Eur Radiol. 2002;12(6):1410–1415. doi: 10.1007/s003300101082. [DOI] [PubMed] [Google Scholar]
- 33.Gluecker TM, Johnson CD, Harmsen WS, et al. Colorectal cancer screening with CT colonography, colonoscopy, and double-contrast barium enema examination: prospective assessment of patient perceptions and preferences. Radiology. 2003;227(2):378–384. doi: 10.1148/radiol.2272020293. [DOI] [PubMed] [Google Scholar]
- 34.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349(23):2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 35.Ristvedt SL, McFarland EG, Weinstock LB, Thyssen EP. Patient preferences for CT colonography, conventional colonoscopy, and bowel preparation. Am J Gastroenterol. 2003;98(3):578–585. doi: 10.1111/j.1572-0241.2003.07302.x. [DOI] [PubMed] [Google Scholar]
- 36.Taylor SA, Halligan S, Saunders BP, Bassett P, Vance M, Bartram CI. Acceptance by patients of multidetector CT colonography compared with barium enema examinations, flexible sigmoidoscopy, and colonoscopy. AJR Am J Roentgenol. 2003;181(4):913–921. doi: 10.2214/ajr.181.4.1810913. [DOI] [PubMed] [Google Scholar]
- 37.Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004;291(14):1713–1719. doi: 10.1001/jama.291.14.1713. [DOI] [PubMed] [Google Scholar]
- 38.Iannaccone R, Laghi A, Catalano C, et al. Computed tomographic colonography without cathartic preparation for the detection of colorectal polyps. Gastroenterology. 2004;127(5):1300–1311. doi: 10.1053/j.gastro.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Gelder RE, Birnie E, Florie J, et al. CT colonography and colonoscopy: assessment of patient preference in a 5-week follow-up study. Radiology. 2004;233(2):328–337. doi: 10.1148/radiol.2331031208. [DOI] [PubMed] [Google Scholar]
- 40.Juchems MS, Ehmann J, Brambs HJ, Aschoff AJ. A retrospective evaluation of patient acceptance of computed tomography colonography ("virtual colonoscopy") in comparison with conventional colonoscopy in an average risk screening population. Acta Radiol. 2005;46(7):664–670. doi: 10.1080/02841850500216277. [DOI] [PubMed] [Google Scholar]
- 41.Bosworth HB, Rockey DC, Paulson EK, et al. Prospective comparison of patient experience with colon imaging tests. Am J Med. 2006;119(9):791–799. doi: 10.1016/j.amjmed.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Florie J, Gelder RE, Schutter MP, et al. Feasibility study of computed tomography colonography using limited bowel preparation at normal and low-dose levels study. Eur Radiol. 2007;17(12):3112–3122. doi: 10.1007/s00330-007-0668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajapaksa RC, Macari M, Bini EJ. Racial/ethnic differences in patient experiences with and preferences for computed tomography colonography and optical colonoscopy. Clin Gastroenterol Hepatol. 2007;5(11):1306–1312. doi: 10.1016/j.cgh.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Roberts-Thomson IC, Tucker GR, Hewett PJ, et al. Single-center study comparing computed tomography colonography with conventional colonoscopy. World J Gastroenterol. 2008;14(3):469–473. doi: 10.3748/wjg.14.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung HS, Park DK, Kim MJ, et al. A comparison of patient acceptance and preferences between CT colonography and conventional colonoscopy in colorectal cancer screening. Korean J Intern Med. 2009;24(1):43–47. doi: 10.3904/kjim.2009.24.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White TJ, Avery GR, Kennan N, Syed AM, Hartley JE, Monson JR. Virtual colonoscopy vs conventional colonoscopy in patients at high risk of colorectal cancer–a prospective trial of 150 patients. Colorectal Dis. 2009;11(2):138–145. doi: 10.1111/j.1463-1318.2008.01554.x. [DOI] [PubMed] [Google Scholar]
- 47.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58(2):241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 48.Jensch S, Bipat S, Peringa J, et al. CT colonography with limited bowel preparation: prospective assessment of patient experience and preference in comparison to optical colonoscopy with cathartic bowel preparation. Eur Radiol. 2010;20(1):146–156. doi: 10.1007/s00330-009-1517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moawad FJ, Maydonovitch CL, Cullen PA, Barlow DS, Jenson DW, Cash BD. CT colonography may improve colorectal cancer screening compliance. AJR Am J Roentgenol. 2010;195(5):1118–1123. doi: 10.2214/AJR.10.4921. [DOI] [PubMed] [Google Scholar]
- 50.Cash BD, Riddle MS, Baumel MJ, et al. Patient opinions of screening CT colonography: Results of a multicenter survey of more than 1400 patients. Gastroenterology. 2011;140(5):S409. [Google Scholar]
- 51.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 52.Wardle J, Sutton S, Williamson S, et al. Psychosocial influences on older adults' interest in participating in bowel cancer screening. Prev Med. 2000;31(4):323–334. doi: 10.1006/pmed.2000.0725. [DOI] [PubMed] [Google Scholar]
- 53.Harewood GC, Wiersema MJ, Melton LJ., 3rd A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002;97(12):3186–3194. doi: 10.1111/j.1572-0241.2002.07129.x. [DOI] [PubMed] [Google Scholar]
- 54.Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38(5):536–550. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 55.Lafata JE, Williams LK, Ben-Menachem T, Moon C, Divine G. Colorectal carcinoma screening procedure use among primary care patients. Cancer. 2005;104(7):1356–1361. doi: 10.1002/cncr.21333. [DOI] [PubMed] [Google Scholar]
- 56.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 57.Stock C, Haug U, Brenner H. Population-based prevalence estimates of history of colonoscopy or sigmoidoscopy: review and analysis of recent trends. Gastrointest Endosc. 2010;71(2):366–381. doi: 10.1016/j.gie.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 58.Marshall DA, Johnson FR, Phillips KA, Marshall JK, Thabane L, Kulin NA. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health. 2007;10(5):415–430. doi: 10.1111/j.1524-4733.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- 59.Padron MC, Jimenez B, Urma D, Mitty RD. Patients' preferences and perceptions on virtual and conventional colonoscopy: a systematic review. Am J Gastroenterol. 2006;101(9):S548–S549. [Google Scholar]
- 60.Burroughs TE, Waterman BM, Gilin D, Adams D, McCollegan J, Cira J. Do on-site patient satisfaction surveys bias results? Jt Comm J Qual Patient Saf. 2005;31(3):158–166. doi: 10.1016/s1553-7250(05)31021-x. [DOI] [PubMed] [Google Scholar]
- 61.Gribble RK, Haupt C. Quantitative and qualitative differences between handout and mailed patient satisfaction surveys. Med Care. 2005;43(3):276–281. doi: 10.1097/00005650-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Hodlewsky RT, Decker FH. The problem of bias when nursing facility staff administer customer satisfaction surveys. Jt Comm J Qual Improv. 2002;28(10):546–554. doi: 10.1016/s1070-3241(02)28058-x. [DOI] [PubMed] [Google Scholar]
- 63.Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 64.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 65.Wagner C, Halligan S, Atkin WS, Lilford RJ, Morton D, Wardle J. Choosing between CT colonography and colonoscopy in the diagnostic context: a qualitative study of influences on patient preferences. Health Expect. 2009;12(1):18–26. doi: 10.1111/j.1369-7625.2008.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angtuaco TL, Banaad-Omiotek GD, Howden CW. Differing attitudes toward virtual and conventional colonoscopy for colorectal cancer screening: surveys among primary care physicians and potential patients. Am J Gastroenterol. 2001;96(3):887–893. doi: 10.1111/j.1572-0241.2001.03639.x. [DOI] [PubMed] [Google Scholar]
- 67.Marshall DA, Johnson FR, Kulin NA, et al. How do physician assessments of patient preferences for colorectal cancer screening tests differ from actual preferences? A comparison in Canada and the United States using a stated-choice survey. Health Econ. 2009;18(12):1420–1439. doi: 10.1002/hec.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357(14):1403–1412. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 69.Ho W, Broughton DE, Donelan K, Gazelle GS, Hur C. Analysis of barriers to and patients' preferences for CT colonography for colorectal cancer screening in a nonadherent urban population. AJR Am J Roentgenol. 2010;195(2):393–397. doi: 10.2214/AJR.09.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott RG, Edwards JT, Fritschi L, Foster NM, Mendelson RM, Forbes GM. Community-based screening by colonoscopy or computed tomographic colonography in asymptomatic average-risk subjects. Am J Gastroenterol. 2004;99(6):1145–1151. doi: 10.1111/j.1572-0241.2004.30253.x. [DOI] [PubMed] [Google Scholar]
- 71.Griffith JM, Lewis CL, Brenner AR, Pignone MP. The effect of offering different numbers of colorectal cancer screening test options in a decision aid: a pilot randomized trial. BMC Med Inform Decis Mak. 2008;8:4. doi: 10.1186/1472-6947-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones LE, Cash BD, Stamps K. Computed tomographic colonography increases colorectal cancer screening compliance. Gastroenterology. 2011;140(5):S565–S566. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 46 kb)