Abstract
BACKGROUND
Our understanding of the prevalence of cognitive impairment (CI) in older adults with heart failure (HF) in a nationally-representative sample is limited.
OBJECTIVES
We used a national probability sample to determine the prevalence of CI in older adults with HF.
DESIGN
Cross-sectional analysis of the 2004 wave of the nationally representative Health and Retirement Study linked to 2002–04 Medicare administrative claims
SETTING
United States, community-dwelling
PARTICIPANTS
6,189 respondents ≥ 67 years old.
MEASUREMENTS
An algorithm was developed using a combination of self- and proxy-report of a heart problem and the presence of ≥ 1 Medicare claim in administrative files using standard HF diagnostic codes. On the basis of the algorithm, 3 categories were created to characterize the likelihood of a HF diagnosis: 1) High or Moderate Probability of HF; 2) Low Probability of HF; and 3) Not a HF case. Cognitive function was assessed using a screening measure of cognitive function or by proxy rating. Age-adjusted prevalence estimates of CI were calculated for the high-moderate probability HF group, the low probability HF group, and the non-HF cases.
RESULTS
The prevalence of CI consistent with dementia in older adults with HF was 15%; while the prevalence of mild CI was 24%. The odds of dementiain those with HF was significantly increased, even after adjustment for age, education level, net worth and prior stroke (OR: 1.52; 95% CI: 1.14 – 2.02).
CONCLUSION
CI is common in older adults with HF and is independently associated with an increased risk for dementia. A cognitive assessment should be routinely incorporated into HF-focused models of care.
Keywords: Heart failure, aged, cognitive impairment, Medicare claims data
INTRODUCTION
Heart failure (HF) predominantly affects older persons in whom multiple complex chronic conditions often coexist (1–4). HF remains the leading cause of hospitalization in adults ≥ 65 years in the United States. Several initiatives exist to reduce the mortality, morbidity, and health care utilization of older adults with HF; however, these recent innovations in HF management have generally not translated into improved mortality and re-hospitalization rates(1, 5, 6). The reasons for unchanged outcomes may stem from the lack of attention given to prognostically important conditions, such as cognitive impairment (CI)(7, 8). Yet, our understanding of the prevalence of CI in older adults with HF is limited. HF patients, who have mild CI or dementia, may have an increased risk for re-hospitalization and mortality because of poor adherence to prescribed therapy due to the complexity of self-management tasks in HF management(9–11).
CI, including both dementia and mild CIis associated with cerebrovascular and cardiovascular disease and is linked to HF (6, 12, 13), but its prevalence and effects on morbidity and mortality are not well understood among those with HF. Prevalence estimates for CI among patients with HF vary widely from 5–75%, with most estimates falling between 25–46%(12–15). Prior work examining HF’s association with CI has emphasized a case-control study design using non-representative samples, including inpatients and those with a range of etiologies of HF. Furthermore, the assessment of cognition has not been standardized; investigators have employed a myriad of tools ranging from cognitive screens such as the Mini-Mental State Exam to abbreviated neuropsychological test batteries(15–17). Because of these and other limitations, it is currently difficult to confidently assess the true extent of CI among older adults with HF. Our research hypotheses are that 1) older adults with HF will have a higher prevalence of CI when compared to other heart condition subgroups; 2) HF is independently associated with a higher risk of CI.
We sought to determine the prevalence of CI among older adults with HF using a nationally representative sample, and also to determine whether HF is independently associated with CI.
METHODS
Data
We used data from the 2004 wave of the Health and Retirement Study (HRS), a biennial, longitudinal survey of a nationally representative cohort of U.S. adults aged 51 years or older.(18) The HRS provides detailed self-report information on chronic diseases and task-specific disabilities.
Sample definition
Ninety percent of HRS respondents provided consent to link their Medicare claims to their HRS survey data. We viewed claims data two years prior to the 2004 interview to capture the greatest number of HRS respondents with HF in the Medicare claims data (19, 20). In the 2004 survey wave, 9,663 respondents were aged 67 and above, ensuring that each individual had 2 full years of Medicare eligibility during our surveillance period. The sample was further reduced to 8,207 after exclusion of those HRS respondents who were not continuously enrolled in Medicare Parts A and B. We excluded 1,650 respondents who were enrolled in Medicare managed care because these plans may not have complete data. Finally, we excluded 429 subjects who were in a nursing home at the time of the analysis. Thus, our analytic sample included 6,189 respondents, representative of approximately 23.4 million community-dwelling adults ≥ 67 years old with Medicare fee-for-service in the U.S. in 2004.
Definition of heart failure and other heart problems by self- or proxy-report
Self-or proxy-report heart problem including HF was determined based on survey responses to questions in the HRS 2004 core interview wave. All respondents and proxies were asked: “Has a doctor ever told you that you had a heart attack, coronary heart disease, angina, congestive heart failure (CHF), or other heart problems?” The response to this question was used to sort respondents into two mutually exclusive heart problem categories: 1) no HF or other heart problem; 2) heart problem(including HF).
Claims-based diagnosis of heart failure
HF was defined with Medicare claims by making use of the International Classification of Diseases-Ninth Revision-Clinical Modification (ICD-9-CM) diagnosis codes for primary and secondary diagnoses. Using Medicare Part A and Part B claim files, we determined the claims-based diagnosis of HF for the 2-year period prior to each respondent’s HRS interview date (19). We employed ICD-9-CM coding algorithms to identify HF as a diagnosis in inpatient, outpatient and carrier files using codes 398.91, 402.01–402.91, 404.01–404.93, and 428.0–428.90 in a two-year period(19).
Heart Failure case-finding approach
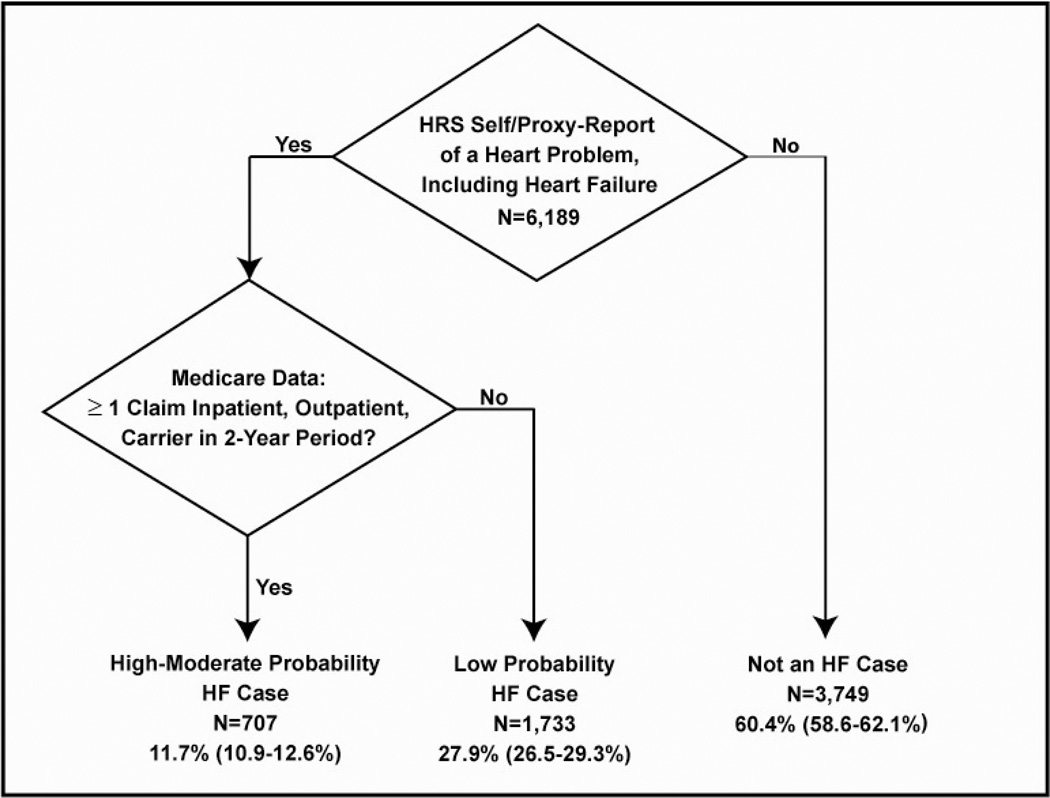
Our prior work identified the test-operating characteristics of self- or proxy-report of HF by comparing to HF diagnosis codes in linked Medicare claims as a diagnostic gold standard (sensitivity: 25%; specificity: 99%)(21). Older adults in the HRS were more likely to report HF if they had poorer health status. Under-reporting of HF was much more common this study, in particular within minority populations and in those with fewer Medicare claims, perhaps suggesting less severe disease. Therefore, we utilized the presence of a heart problem to better capture respondents who may be unaware of HF as a diagnosis. A newly-developed algorithm was derived from this work (see Figure 1). We utilized a combination of self- and proxy-report of a heart problem and the presence of ≥ 1 Medicare claim in inpatient, outpatient, and carrier files using standard HF diagnostic codes to increase our yield of HF cases. On the basis of the algorithm, we created 3 categories to characterize the likelihood of having a HF diagnosis: 1) High/Moderate Probability of HF; 2) Low Probability of HF; and 3) Not a HF case.
Figure 1.
Ascertainment of HF cases in HRS-Medicare data via a newly-developed algorithm, weighted % (95% Confidence Interval)
Wtd.= weighted
Sd=standard deviation
CI=cognitive impairment
95% CI=95% confidence interval
Prob.=probability
Survey-based assessment of cognitive performance
For self-respondents, the presence of dementia was assessed with a modified version of the Telephone Interview for Cognitive Status (TICS-m), a validated cognitive screening instrument patterned on the Mini-Mental State Examination(22). The TICS-m used in the HRS is a 27-point scale and has high sensitivity and specificity for cognitive impairment (CI) and dementia in community samples of older adults (22–24). The test items include: 1) immediate and delayed 10-word recall test to measure memory; 2) serial seven subtraction test to measure working memory; and 3) counting backward test to measure speed of mental processing (25, 26). Cut-scores to identify subjects with CI were based on findings from the Aging, Demographics and Memory Study(27). Based on prior work validating these cutpoints, cognitive function was categorized as normal (≥ 12 points) or CI (<12 points) with the CI category divided in to mild (7–11) and moderate/severe impairment (0–6) consistent with dementia (27). Cognitive categories for respondents with proxy informants were based on both the proxy and interviewer assessments of cognitive function. Proxies assessed memory and whether the respondent was cognitively impaired. Cognitive function scores were created based on the proxy interview ratings and the number of IADL limitations, resulting in a scale ranging from 0 to 11, with scores 3–5 indicating, mild cognitive impairment (CI) and scores 6–11 indicating CI consistent with dementia.
Independent variables
The socio-demographic variables included in the analysis as independent variables were: age, race, gender, household net worth, and years of education. Self-reported chronic conditions included were stroke and diabetes. All measures were obtained from the HRS core interview in 2004.
Data analysis
All analyses were conducted using STATA/SE 11. To adjust for the complex sample design of the HRS, the differential probability of selection, and the probability of a respondent’s consent to accessing their Medicare data, all analyses were appropriately weighted. Analyses were conducted to calculate population-based characteristics and the prevalence of CI based on sample data. Socio-demographic characteristics, TICS-m, and Proxy rating of cognitive status were compared across the 3 heart condition categories (not HF, low probability, and moderate/high probability HF groups) using adjusted Wald and Pearson chi-square test statistics, as appropriate. We compared prevalence estimates of cognitive function with categorical cognitive variables (normal, mild CI, and moderate/severe CI) by adjusted Wald chi-square statistical tests. Taylor series Linearization was used to estimate design-based standard errors for population estimates (incorporating the stratification and clustering of the HRS sample along with the weights) and compute 95% confidence intervals for population parameters.
We used multivariate logistic regression to identify covariates independently associated with the likelihood of moderate/severe cognitive impairment (CI), by heart disease category. Mild CI was combined with the normal cognition subgroup for these analyses. The final logistic models included the sociodemographic and health status factors that are associated with CI. Model 1 is an univariate analysis. Model 2 is age-adjusted only. Model 3 includes age, race, educational level, net worth, and self-reported prior stroke.
RESULTS
Sample Characteristics
Among the 6,189 respondents in the analytic sample, our case-finding algorithm identified 707 subjects (11.7% of the population) with a moderate/high probability of HF. This corresponds to approximately 2,700,000 Medicare fee-for-service benificiaries ≥ 67 years with HF in the U.S.A. in 2004. The mean age was 76.5 years old, 42% were male, 8% were Black, and 4% were Hispanic (Table 1). The mean number of years of education was 12.1. Approximately, 5,800 (92%) respondents were self-respondents; 436 (8%) were represented by a proxy-informant.
Table 1.
Characteristics of Older Adults ≥ 67 years with HRS Data Linked to Medicare Claims (n=6,189)
| Characteristics N (wtd. % or mean) weighted n |
Not a HF case 3,749 (60.5%) 14,000,000 |
Low Probability HF 1,733 (27.8%) 6,500,000 |
Moderate/High Probability HF 707 (11.7%) 2,700,000 |
Total 6,189 23,365,184 |
p-value |
|---|---|---|---|---|---|
| Age mean (sd) | 75.6 (6.51) | 77.4 (6.75) | 78.6 (6.86) | 76.5 (6.71) | < .001 |
| Race/ethnicity n (wtd. %) | |||||
| Hispanic | 229(4) | 75 (3) | 37 (3) | 341 (4) | .37 |
| Black | 435 (8) | 211(7) | 85 (9) | 731 (8) | |
| White/Other | 3,085 (88) | 1,447 (89) | 585 (88) | 5,117 (88) | |
| Gender n (wtd. %) | <.001 | ||||
| Male | 1,414 (38) | 828 (48) | 334 (47) | 2,576 (42) | |
| Female | 2,335 (62) | 905 (52) | 373 (53) | 3,613 (58) | |
| Years of education | <.001 | ||||
| Mean (sd) | 12.27 (3.21) | 11.92 (3.32) | 11.45 (3.32) | 12.07 (3.26) | |
| Net worth (quartile) N (wtd. %) | <.001 | ||||
| ≤ $61,000 | 931 (23) | 503 (27) | 263 (36) | 1,697 (26) | |
| 61,001–203,400 | 917 (24) | 476 (27) | 198 (28) | 1,591 (25) | |
| 203,401–512,000 | 966 (27) | 405 (24) | 140 (19) | 1,511 (25) | |
| ≥ 512,000 | 935 (26) | 349 (22) | 106 (17) | 1,390 (24) | |
| Stroke n (wtd. %) | 246 (6) | 232 (14) | 151 (21) | 629 (10) | <.001 |
| Diabetes n (wtd. %) | 593 (15) | 418 (24) | 234 (.32) | 1,245 (20) | < .001 |
|
Characteristics N (row, col %) Weighted n |
Not a HF case 3,749 (60.5%) 14,000,000 |
Low Probability HF 1,733 (27.8%) 6,500,000 |
Moderate/High Probability HF 707 (11.7%) 2,700,000 |
Total 6,189 23,365,184 |
p- value |
| Self –respondents | 3,526 (61, 93) | 1,607 (28, 91) | 620 (11, 85) | 5,753 (92) | <.001 |
| 13,000,000 | 5,900,000 | 2,300,000 | 21,000,000 | ||
| Proxy respondents | 223 (51, 7) | 126 (29, 9) | 87 (21, 15) | 436 (8) | |
| 1,000,000 | 600,000 | 400,000 | 1,900,00 | ||
| 27-point TICS-m score, Overall mean (sd) | 14.5 (4.34) | 13.7 (4.57) | 12.7 (4.26) | 14.1 (4.44) | <.001 |
| 11-point Proxy assessment of memory Overall mean (sd) | 5.09 (3.38) | 5.04 (3.32) | 5.69 (3.20) | 5.20 (3.33) | .41 |
| *Cognitive categoryn,(weighted % ; 95% CI) | <.001 | ||||
| Normal | 2,698 (71; 70–73) | 1,126 (68; 66–70) | 411 (61;57–65) | 4,235 (69; 68–71) | |
| Mild CI | 778 (21; 19–22) | 419 (22; 22–24) | 184 (24;21–28) | 1,381 (21; 20–23) | |
| Moderate/Severe CI | 271 (8;7–9) | 188 (10; 9–11) | 112 (15; 12–18) | 571 (9; 8–10) | |
Adjusted for age
The subgroup with moderate/high probability of HF was older, with a mean age of 78.6 years compared to the low probability and no HF subgroups (p < .001). The moderate-high probability HF subgroup had a lower education level.
The majority of respondents in all heart condition categories were self-respondents; however, 8% of respondents utilized a proxy-informant. Twenty-percent of proxy-informants had a high likelihood of HF compared with 29% in the low-probability HF group and 51% in the no HF subgroup. The mean TICS-m score for those who could self-respond was 14.1±4.44. The high/moderate probability HF subgroup had the lowest mean TICS-m score 12.67 ±4.26 (low probability HF 13.68 vs. no HF 14.50; p < .001). There was no statistically significant difference in proxy rating score between the heart condition subgroups.
Prevalence of cognitive impairment (CI), by heart condition category
Theage-adjusted cognitive performance of respondents with HRS-Medicare data, by heart condition category is described in Table 1. The age-adjusted prevalence of moderate/severe CI consistent with dementia in the subgroup with the greatest probability of HF was 15% (95% confidence interval: 12–18%; p ≤ .001 when compared to no HF and low probability HF subgroups) and for mild CI it was 24% (95% confidence interval: 21–28%). The age-adjusted prevalence of mild CI among the different heart condition subgroups was not significantly different (Wald test p=0.11 comparing the low probability HF subgroup to the no HF group; p=0.34 when comparing the low probability HF group to the moderate/high probability HF subgroup.)
The association of cognitive impairment (CI) with heart failure
In a series of multivariate logistic regression models shown in Table 3 predicting the likelihood of moderate/severe CI, by heart condition category, HF was consistently associated with significantly increased odds of moderate/severe CI. Age had the greatest effect size in the model, as expected, given its strong association with mild CI and dementia. In the final model, the effect of HF on dementia was further attenuated with the addition of race, educational level, net worth, and self-reported prior stroke(OR: 1.52; 95% confidence interval: 1.14–2.02). Gender and self-reported diabetes were not significant predictors of moderate/severe CI in the final model. The low probability HF subgroup had higher odds of moderate/severe CI when age was the only covariate in the model. However, the odds were not significantly different compared to the no HF subgroup in the full model (OR: 1.19; 95% confidence interval: 0.94–1.51).
DISCUSSION
Using a unique nationally-representative sample from the HRS- Medicare claims linked data, we found that approximately 40% of older adults ≥ 67 years old with HF are cognitively impaired, with 15% demonstrating moderate/severe impairment consistent with dementia. HF was independently associated with an increased risk for cognitive impairment (CI). Our prevalence estimates of CI fell within the range (25–46%) most commonly reported by prior studies, but the prevalence of moderate/severe CI consistent with dementia was lower than previously reported. We also found that HFhas a significant, independent association with dementia after adjustment for important sociodemographic and clinical predictors.
Comparing prevalence estimates across studies is difficult because of differences in patient samples (in HF type and study setting) and the large variability in cognitive tests utilized across studies. Our study’s strengths are its use of a broad case-mix of HF cases by use of an algorithm which likely captures the mild-severe stages of HF, the presence of a comparison groups within the cohort, and its use of standardized cognitive measures that are validated from clinically diagnosed cognitively impaired cases from ADAMS, an epidemiologic substudy of dementia in the HRS(26). The TICS-m and informant assessment of cognitive performance discriminate well between demented and mild cognitively impaired cases (22).
Several limitations of our study warrant mention. First, both self-report and Medicare claims data have limited validity as accurate measurements of HF. Multiple factors can influence a patient’s awareness of a heart condition such as illness severity, including socioeconomic background, race, patient health awareness gained through encounters with healthcare providers and effective self-management skills. The validity of Medicare administrative data is also highly dependent on many factors: ICD-9 coding practices, organizational culture, coder experience, and the type of administrative file utilized(28). For these reasons, our prevalence estimates may be an over- or underestimate of HF cases in the population. Using HRS-Medicare linked data in prior work, self-report prevalence of HF at 5% is an undercount; claims-based prevalence at 16%, an over count (21). Thus, our algorithm which combines self- and proxy-report of a heart problem with ≥ 1 Medicare diagnostic HF claim is likely more accurate than use of self- or proxy-report or claims data alone in this data source.
Second, medical records were not available to further adjudicate cases, ascertain HF severity, or determine the use of medications that might affect cognition. Third, approximately 11% of the HRS respondents did not consent to the linkage of their survey data to Medicare claims. These factors may introduce selection bias since the population enrolled in managed care programs differs from those with traditional Medicare fee-for-service plans with respect to health and socioeconomic status (29). Fourth, the cognitive measures utilized in our study may insufficiently probe the cognitive domains most commonly affected in HF patients leading to inaccuracies in our findings. Our estimates of moderate/severe impairment are lower than prior epidemiological reports likely due to the exclusion of nursing home residents.(30) Nonetheless, this work provides among the first estimates of global cognitive function in a nationally representative sample.
CI commonly co-occurs with HF in older adults, yet assessing cognition is not routinely incorporated into traditional HF disease management models of care. Awareness of cognitive status by healthcare providers, in particular HF care providers, is important for setting expectations for patient participation in a HF treatment plan and achieving optimal care. We found an independent association of HF with the presence of moderate/severe CI-a cognitive performance level most consistent with dementia. CI is an under-recognized comorbid condition in community-dwelling older adults with HF which will have important effects on prognosis, physical function, quality of life, healthcare utilization, and mortality.
Table 2.
Predictors of Moderate/Severe CI, Logistic Regression, Odds Ratio (95% Confidence Interval), n=6,189
| Heart Condition Status | Univariate | Age-adjusted | Multivariate adjustment* |
|---|---|---|---|
| Not Heart Failure | Ref. | Ref. | Ref. |
| Low Probability of HF | 1.55 (1.24–1.99) | 1.33 (1.05–1.68) | 1.19 (0.94–1.51) |
| Moderate/High probabilityof HF | 2.50 (1.99–3.14) | 1.95 (1.50–2.54) | 1.52 (1.14–2.02) |
Covariates are age, race, educational level, net worth, and self-reported prior stroke
ACKNOWLEDGMENT
The authors appreciate the assistance of Yuo-Yu Lee, MS and Jinkyung Ha, PhD with data management and statistical analysis in the completion of this work.
Funding Sources: The National Institute on Aging (NIA) provides funding for the Health and Retirement Study (U01 AG09740) which is performed at the Institute for Social Research, University of Michigan. Dr. Langa was supported by NIA grant (R01 AG030155). Dr. Blaum was supported by the Ann Arbor VA GRECC. Dr. Koelling was supported by Medtronic, Inc. and MESPERE, Inc. Dr. Hummel was supported by NHLBI grant (K23 HL109176). Dr. Gure was supported by an NIA Diversity Supplement award (R01 AG027010-02S1), John A. Hartford Foundation Center of Excellence, the NIA Claude D. Pepper Center Research Career Development Core, and the National Center for Research Resources (UL1 RR024986). The content is solely the responsibility of the authors and does not necessarily represent the official views of NCRR or the National Institutes of Health.
Sponsor’s Role: None
The authors appreciate the thoughtful input of the participating faculty in the Junior Faculty Career Development Writing Workshop, sponsored by the Division of Geriatric and Palliative Medicine at the University of Michigan.
Footnotes
An earlier version of this work was presented at the National Pepper Center meeting April 11, 2011.
Conflict of Interest
Dr. Koelling received research funding from Medtronic, Inc. and MESPERE, Inc. He received an honorarium (< $10,000) from Abbott Vascular. He also served as apro bono expert witness in a myocardial infarction case.
Author Contributions:
Gure: Study concept and design, data analysis and interpretation, manuscript preparation.
Blaum: Data analysis and interpretation, manuscript preparation
Giordani: Data interpretation, manuscript preparation.
Koelling: Data interpretation and manuscript preparation.
Galecki: Data interpretation, manuscript preparation
Pressler: Data interpretation, manuscript preparation.
Hummel: Data interpretation and manuscript preparation.
Langa: Study concept and design, data interpretation, manuscript preparation.
REFERENCES
- 1.Jessup M, Abraham WT, Casey DE, et al. 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 3.Gure TR, Kabeto MU, Blaum CS, et al. Degree of disability and patterns of caregiving among older Americans with congestive heart failure. J Gen Intern Med. 2008;23:70–76. doi: 10.1007/s11606-007-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 5.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 6.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, et al. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol B Psychol Sci Soc Sci. 2004;59:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry SI, Wang Y, Gill TM, et al. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55:309–316. doi: 10.1016/j.jacc.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure - Derivation and validation of a clinical model. J Am Med Assoc. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 9.Pressler SJ, Kim J, Riley P, et al. Memory dysfunction, psychomotor slowing, and decreased executive function predict mortality in patients with heart failure and low ejection fraction. J Card Fail. 2010;16:750–760. doi: 10.1016/j.cardfail.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horowitz C, Rein S, Leventhal H. A story of maladies, misconceptions and mishaps: effective management of heart failure. Soc Sci Med. 2004;58:631–643. doi: 10.1016/s0277-9536(03)00232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granger BB, Swedberg K, Ekman I, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: Double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 12.Trojano L, Antonelli Incalzi R, Acanfora D, et al. Cognitive impairment: A key feature of congestive heart failure in the elderly. J Neurol. 2003;250:1456–1463. doi: 10.1007/s00415-003-0249-3. [DOI] [PubMed] [Google Scholar]
- 13.Qiu C, Winblad B, Marengoni A, et al. Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 14.Vogels RL, Scheltens P, Schroeder-Tanka JM, et al. Cognitive impairment in heart failure: A systematic review of the literature. Eur J Heart Failure. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Zuccala G, Onder G, Pedone C, et al. Hypotension and cognitive impairment - Selective association in patients with heart failure. Neurology. 2001;57:1986–1992. doi: 10.1212/wnl.57.11.1986. [DOI] [PubMed] [Google Scholar]
- 16.Vogels RL, Oosterman JM, van Harten B, et al. Profile of cognitive impairment in chronic heart failure. J Am Geriatr Soc. 2007;55:1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 17.Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juster FT, Suzman R. An Overview of the Health and Retirement Study. J Hum Resources. 1995;30:S7–S56. [Google Scholar]
- 19.Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare plus Choice health plans that have chronic medical conditions. Health Serv Res. 2004;39:1839–1857. doi: 10.1111/j.1475-6773.2004.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birman-Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 21.Gure TR, McCammon RA, Cigolle CT, et al. Predictors of self-report of heart failure in a population-based survey of older adults. Circ Cardiovasc Qual Outcomes. 2012;5:396–402. doi: 10.1161/CIRCOUTCOMES.111.963116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plassman BL, Newman TT, Welsh KA, et al. Properties of the telephone interview for cognitive status - Application in Epidemiologic and Longitudinal Studies. Neuropsychiatr Neuropsychol Behav Neurol. 1994;7:235–241. [Google Scholar]
- 23.Welsh KA, Breitner JCS, Magruderhabib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatr Neuropsychol Behav Neurol. 1993;6:103–110. [Google Scholar]
- 24.de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18:318–324. doi: 10.1002/gps.830. [DOI] [PubMed] [Google Scholar]
- 25.Langa KM, Kabeto MU, Weir D. Percentage of Americans Aged 55 and Older with Selected Diseases by Race/Ethnicity and Cognitive Status, Health and Retirement Study, 2006. 2010 Alzheimer's Disease Facts and Figures. [Provided under contract to the Alzheimer's Association] 2010 In press. [Google Scholar]
- 26.Langa KM, Plassman BL, Wallace RB, et al. The aging, demographics, and memory study: Study design and methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 27.Crimmins EM, Kim JK, Langa KM, et al. Assessment of Cognition Using Surveys and Neuropsychological Assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 2011. 2011;66B(suppl 1):162–171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quach S, Blais C, Quan H. Administrative data have high variation in validity for recording heart failure. Can J Cardiol. 2010;26:306–312. doi: 10.1016/s0828-282x(10)70438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada SL, Zaslavsky AM, Zaborski LB, et al. Market and beneficiary characteristics associated with enrollment in Medicare managed care plans and fee-for-service. Med Care. 2009;47:517–523. doi: 10.1097/MLR.0b013e318195f86e. [DOI] [PubMed] [Google Scholar]
- 30.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the united states: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]