Abstract
MicroRNA-21 (miR-21) is an oncomir overexpressed in most human tumors where it promotes malignant growth and progression by acting on multiple targets. Here we broaden the impact of miR-21 in cancer by demonstrating that it regulates formation of reactive oxygen species (ROS) that promote tumorigenesis. Key targets of miR-21 in mediating this function were SOD3 and TNF-a. We found that miR-21 inhibited the metabolism of superoxide to hydrogen peroxide, produced either by endogenous basal activities or exposure to ionizing radiation, by directing attenuating SOD3 or by an indirect mechanism that limited TNF-a production, thereby reducing SOD2 levels. Importantly, both effects contributed to an elevation of IR-induced cell transformation. Our findings therefore establish that miR-21 promotes tumorigenesis to a large extent through its regulation of cellular ROS levels.
Keywords: miR-21, SOD, TNFα, Reactive Oxygen Species (ROS), ionizing radiation
Introduction
MicroRNAs (miRs) are a class of small non-coding RNAs (ncRNAs) comprising ~22 nucleotides in length. In general, miRs negatively regulate gene expression post-transcriptionally by binding to the 3′-untranslated region (UTR) of the targeted messenger RNA (mRNA) to inhibit gene translation. One miR is capable of targeting multiple mRNAs and one mRNA can be targeted by multiple miRs. Most mammalian mRNAs are conserved targets of miRs. Among the more than 1,400 miRs that have been documented, miR-21 is overexpressed in multiple human cancers (1) and miR-21 upregulation induced tumorigenesis in a mouse model (2, 3) and in human cells (4), indicating a central role of miR-21 in carcinogenesis. Although multiple targets of miR-21 have been identified, including tumor suppressors: PTEN, PDCD4 and TPM1 (5–9), the whole picture for miR-21-induced tumorigenesis remains unclear.
Reactive oxygen species (ROS) generated from endogenous metabolic processes or exogenous ionizing radiation (IR) exposure, are mutagenic and it is widely accepted that ROS promotes tumorigenesis (10, 11). Superoxide (O2−) and hydrogen peroxide (H2O2) are the major ROS species. The human superoxide dismutase (SOD) family includes three members SOD1, SOD2 and SOD3, and are the major enzymes metabolizing ROS by converting superoxide to hydrogen peroxide (Mn+ − SOD + 2O2− + 2H+ → M(n+1)+ − SOD + H2O2 + O2; where M = Cu (n=1), Mn (n=2), Fe (n=2), Ni (n=2)). Among the family members, SOD1 is a copper and zinc-containing homodimer, CuZn-SOD primarily localized in cytoplasm; SOD2 is a manganese-containing enzyme, Mn-SOD is exclusively localized in mitochondria; SOD3 is a copper and zinc-containing tetramer, containing a signal peptide directing SOD3 primarily to the extracellular space (EC-SOD), and is produced by lung tissue cells (12). We investigated whether there is a functional link between miR-21 and the regulation of ROS levels through targeting a member of the SOD family, thereby contributing to miR-21-induced tumorigenesis. In this study, we found that miR-21 affected the levels of superoxide to hydrogen peroxide in human bronchial epithelial cells by directly targeting SOD3 and TNFα decreasing SOD2 levels, and that these changes are linked to increased IR-induced cell transformation in miR-21 expressing cells. Our findings reveal a new mechanism to explain the role of miR-21 in promoting tumorigenesis via regulating cellular ROS levels.
Materials and Methods
Cell lines and radiation
Immortalized human bronchial epithelial cells (NL20) were purchased from ATCC and cultured according to the manufacturer’s instructions. The cell line was verified using a soft agar colony forming assay. Low-LET radiation was carried out using an x-ray machine (X-RAD 320, N. Branford 320 kV, 10 mA, 2-mm aluminum filtration) in our laboratory and high-LET radiation was carried out using an alternating-gradient synchrotron (Fe ions, 1 GeV/amu) at Brookhaven National Laboratory (BNL). The dose rates for both high-LET IR and low-LET IR were about 1 Gy/min.
Reagents
The plasmids for miR-21 expression, pCDH-CMV-MCS-EF1-copGFP (with GFP) and pCDH-CMV-MCS-EF1-Puro (without GFP) were purchased from SBI. The plasmid for the luciferase assay, psiCHECK™-2, was purchased from Promega. The SOD3 or TNFα encoding plasmid was purchased from GeneCopoeia. The miR-21 mimic (double strand sense mature miR-21) and miR-21 antisense inhibitor (50 nM) were purchased from Thermo Fisher Scientific. The siRNA against SOD2, SOD3, TNFα and the control RNA were purchased from Santa Cruz. The antibody against SOD1, SOD2 or β-Actin was purchased from Santa Cruz Biotech Inc. The antibody against SOD3 was purchased from ENZO Life Sciences and the antibody against TNFα was purchased from Cell Signaling.
ROS detection
0.5×106 cells were plated in a 60 mm dish and grown over night at 37°C in a CO2 incubator. For some transient gene-regulation experiments, the cells were transfected with the proper vector or small RNAs and incubated at 37°C for 48 h. The cells were collected, washed with PBS and re-suspended in CM-H2DCFDA (Invitrogen, for H2O2 detection) or Dihydroethidium (DHE) (Sigma, for O2− detection) at a final concentration of 10 μM. The cells were incubated at 37°C for 30 min, and then kept on ice. The cells were irradiated on ice and re-suspended in medium after IR and kept at 37 °C for different times, the cells were then washed with PBS and measured against the ROS level using a BD FACSCanto II flow cytometer.
Real time PCR
Real time PCR was performed as described in our previous publication (13) with the proper primers as described in supplementary Table 1.
Luciferase assay
A luciferase assay was performed as described in our previous publication (13). Briefly, 293FT cells were transfected with the plasmid (psiCHECK™-2) containing different 3′UTRs from different genes with or without 100 nM hsa-miR-21 mimics in 48-well plates. The cells were harvested 48 h after transfection, the cells were then lysed with a luciferase assay kit (Promega) according to the manufacturer’s protocol and measured on a LUMIstar Galaxy luminescent microplate reader (BMG labtechnologies). β-galactosidase or renilla luciferase was used for normalization.
Cell transformation
Cell transformation was measured by using a soft agar colony forming assay. 4% low melting temperature agarose and 1x complete NL 20 complete medium were mixed to obtain a 0.5% agarose concentration, then 2 ml of 0.5% agarose -NL 20 complete medium mixture was added to each well in 6 well plates, and the agar was solidified at 4°C. These plates were kept in the incubator until the next day. Cells were harvested and mixed (500, 1000, 1500 cells per well) with tissue culture medium containing 0.7% agar to a final agar concentration of 0.35%. Then 2 ml of the cell suspension were immediately plated in six-well plates coated with 2 ml of 0.5% agar in tissue culture medium per well (in triplicate) and the cells were cultured at 37°C with 5% CO2 for 3 weeks. The culture was stained with 0.2% p-iodonitrotetrazolium violet (Sigma) or scanned for colony counting and colonies larger than 100 μm in diameter were counted.
Statistical analysis
Statistical analysis of data was done using the Student’s t test. Differences with p < 0.05 are considered significant.
Results
miR-21 over-expression increases IR-induced cellular ROS levels
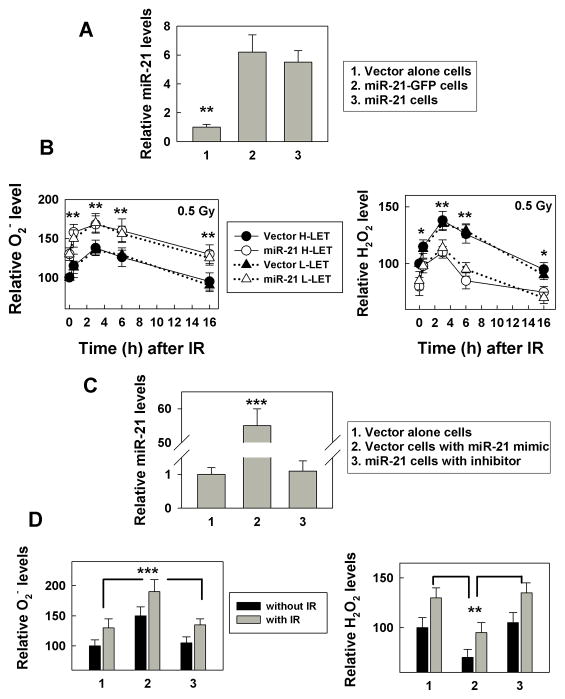
To establish a stable miR-21 expressing cell model, we inserted a 362 base pair (bp) sequence comprising pri-miR-21 (4) into a plasmid encoding the GFP gene (or vector control), transfected these plasmids into the human bronchial epithelial cell line NL20, and selected for miR-21 expressing cells (Fig. 1A). The purpose of establishing two sets of cells overexpressing miR-21 (with or without GFP) is: 1) to confirm the relationship between miR-21 and its targets in two independently generated cell lines; 2) to monitor miR-21 expression by GFP co-expression; and 3) to detect ROS levels by flow cytometry in miR-21 expressing cells without GFP signal interference. We measured ROS (superoxide and hydrogen peroxide) levels in the cells at different times following exposure to IR (either high linear energy (LET) iron or low-LET x-rays). The results show that miR-21 expression increased superoxide (O2−) levels, while reducing hydrogen peroxide (H2O2) levels (Fig. 1B, 0 h time point). To examine whether our measurements for the superoxide or hydrogen peroxide were specific, we measured the superoxide levels in cells with PEG-SOD and the hydrogen peroxide levels with additional H2O2. The results showed that the (O2−) levels measured by DHE flow cytometry decreased with additional PEG-SOD (Supplementary Fig. S1A), and the (H2O2) levels measured by DCFDA flow cytometry increased with additional hydrogen peroxide (Supplementary Fig. S1B). These results further supported that our measurements reflected the (O2−) or (H2O2) levels. IR (0.5 Gy) stimulated the generation of superoxide and hydrogen peroxide in the cells; although, returning to non-irradiated levels at 16 h after IR (Fig. 1B). There was no apparent difference in the ROS levels between the cells exposed to iron (high-LET) or x-ray (low-LET) IR (Fig. 1B). Furthermore, increasing the IR dose to 5 Gy did not further increase the ROS response in these cells (Supplementary Fig. S2). These results indicate that ROS generation in the cells is sensitive to IR; the ROS levels reach a maximal level at 3 h and return to pre-irradiated levels within 16 h. Although IR increased miR-21 expression after 3 h in cells transfected with vector alone, it did not additively increase the miR-21 levels in cells already over-expressing miR-21 (Supplementary Fig. S3), supporting the previous observation that IR-induced miR-21 expression peaks over a short time period, (2–4). To exclude the possibility that the increased ROS level in the miR-21 overexpressing cells was due to miR-21-independent effects, we used either a miR-21 antisense inhibitor (for miR-21 over-expressing cells) or a miR-21 mimic (for control cells) and measured ROS levels. The results show that after miR-21 was upregulated in these cells (Fig. 1C); the ROS levels changed and IR enhanced this phenotype (Fig. 1D), In contrast, the miR-21 inhibitor abrogated the miR-21-induced ROS phenotypes. (Fig. 1D). These results confirm that miR-21 increases the basal and IR–induced superoxide level in the cells. The higher superoxide level and lower hydrogen peroxide level in the miR-21 overexpressing cells suggest that miR-21 might be targeting SODs.
Figure 1.
miR-21 over-expression increases basal and IR-induced ROS level. A, MiR-21 levels were measured in the cells with or without stable miR-21 overexpression using a real time PCR assay. The relative miR-21 levels are expressed as a ratio of the miR-21 level in mimic transfected cells over the vector transfected control cells, **, p< 0.01. B, The ROS level (left panel: superoxide (O2−); right panel: hydrogen peroxide (H2O2)) was measured in cells with or without stable miR-21 overexpression at the indicated time after exposure to 0.5 Gy high- or low-LET IR using a flow cytometer. The results are expressed as a percentage of the level in non-irradiated control cells and are the mean ± SE obtained from two separate experiments, *: p< 0.05, **: p< 0.01. C, MiR-21 levels were measured using the cells transfected with the miR-21 mimic, inhibitor or control RNA (50 nM) at 48 h after transfection with a real time PCR assay, ***, p< 0.001. D, The ROS level (left panel: O2−; right panel: H2O2) was measured at 3 h after exposure the cells (at 48 h after transfected with miR-21 mimic, inhibitor or control RNA) to 0.5 Gy low-LET IR by flow cytometry as described in Materials and Methods. Numbers 1, 2 and 3 reflect the data from the same cells as described in C. The data are the mean ± SE and obtained from three separated experiments, **: p< 0.01; ***: p< 0.001.
miR-21 directly targets SOD3
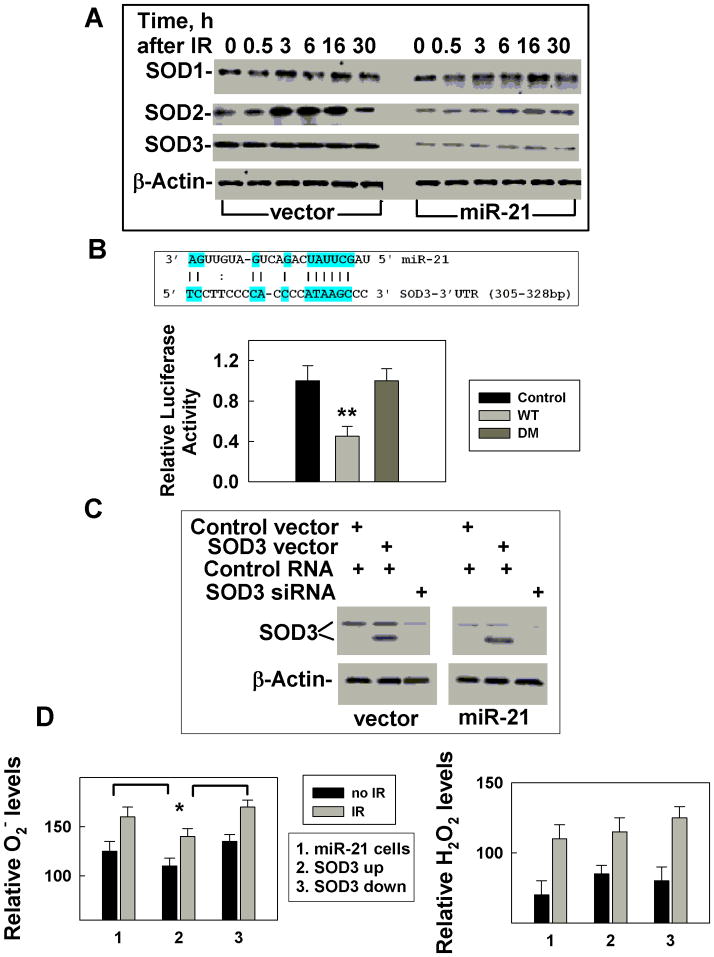
To address whether miR-21 targets SODs, we measured the levels of SOD1, SOD2 and SOD3 in cells with or without miR-21 over-expression at different times following IR exposure. The results show that non-irradiated cells, overexpressing miR-21 have unchanged SOD1 levels but reduced SOD2 and SOD3 levels (Fig. 2A). IR (0.5 Gy) induced a transient increase in SOD1 and SOD2 expression in control cells, while miR-21 over-expression dramatically reduced this effect (Fig. 2A). Cells exposed to a higher dose (5 Gy) did not respond differently (Supplementary Fig. S4). The lower levels of SOD2/SOD3 in the miR-21 over-expressing cells suggested that miR-21 might target SOD2 and SOD3. To test this hypothesis, we performed a matching search by using PicTar, TargetScan3.1, MiRanda and miRGen tools and identified one potential miR-21 binding site in the 3′UTR of SOD3 (Fig. 2B, Supplementary Fig. S5). To demonstrate that miR-21 targets SOD3 directly, the SOD3 complementary site was cloned into the 3′UTR of the firefly luciferase gene and co-transfected with miR-21 in 293FT cells. The results showed that placing miR-21 the binding site resulted in a substantial inhibition of luciferase activity. This inhibitory effect was lost when the binding site was deleted (Fig. 2B), indicating that the site in the 3′UTR of SOD3 represents an authentic miR-21 binding site and confirming that SOD3 is a direct target of miR-21. To examine whether miR-21 affected ROS through targeting SOD3, we up-regulated SOD3 expression by transfecting a cDNA without 3′UTR region in cells overexpressing miR-21 and compared them to cells with reduced levels of SOD3 expression by siRNA. Transfection of the SOD3 cDNA generated a non-glycosylated form (30kD) of SOD3 (14) that is recognized by the SOD3 antibody (Fig. 2C). This non-glycosilated form of SOD3 appears to be active, because overexpressing it upregulated SOD3 activity (Supplementary S6) and IR increased ROS levels in cells ectopically expressing SOD3 (Fig. 2D). Conversely, reducing the endogenous SOD3 levels by siRNA (Fig. 2C), was sufficient to change the basal and IR induced ROS levels (Fig. 2D). These results support a model where miR-21 overexpression results in the ROS change in irradiated cells by targeting SOD3. SOD3 is an extracellular enzyme, and it has been established that the extracellular environment also plays an important role in influencing genomic integrity (15), Thus SOD3 may contribute to protecting cells from ROS-induced genetic instability.
Figure 2.
miR-21 directly targets SOD3 and indirectly affects SOD2. A, SOD levels were measured in cells with or without stable miR-21 overexpression at different times after 0.5 Gy low- or high-LET IR by Western blot. β-Actin was used as an internal loading control. B, Potential miR-21 binding site in the 3′UTR of SOD3 and the effects of the binding site on luciferase activity. 293T cells were transfected with a firefly luciferase reporter plasmid containing a partial 3′-UTR of SOD3 with the putative miR-21 binding site (WT) or without the binding site, deleted mutation (DM). Luciferase activity was assayed 48 h after transfection with miR-21 mimic (miR-21) or control RNA (mock) and are standardized by beta galactosidase activity, **: p < 0.01. C, SOD3 levels were measured in cells with up- or down-regulation of SOD3 expression in cells overexpressing miR-21 or a vector by Western blot. β-Actin was used as an internal loading control. D, ROS levels (left panel: O2−; right panel: H2O2) were measured in cells with up-or down-regulation of SOD3 at 3h after 0.5 Gy low-LET IR. The data is an average of three separate experiments, *: p < 0.05.
miR-21 directly targets TNFα and influences SOD2 levels
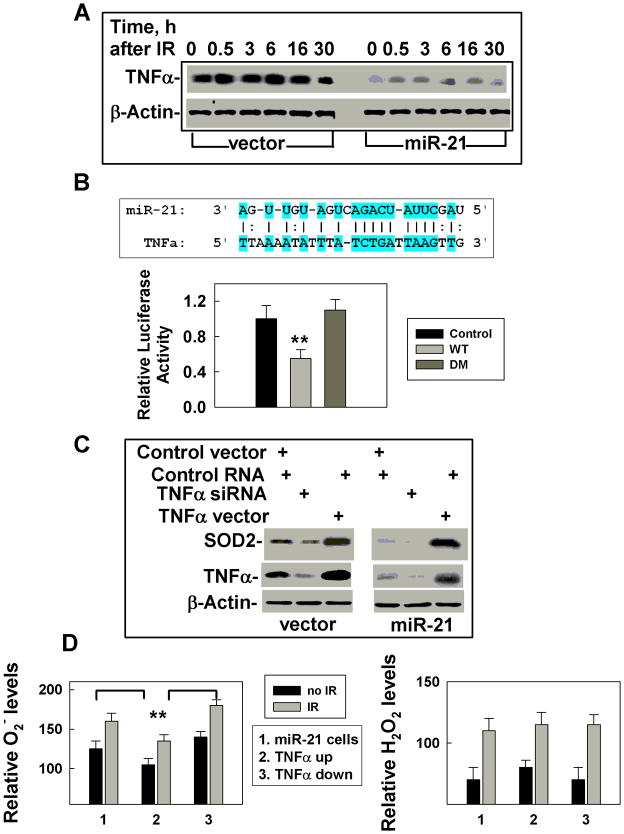
Reduced SOD2 protein levels following miR-21 overexpression has been previously reported by another group (16), but the mechanism leading to this phenotype is unknown since there was no predictable miR-21 binding site in 3′-UTR of SOD2. Next, we sought the candidates that could up-regulate SOD2 expression and be targeted by miR-21. TNFα came to our focus because it could induce SOD2 expression (17, 18) and the level of TNFα expression decreased in miR-21 overexpressing cells, compared to vector-transfected control cells (Fig. 3A). IR increased the levels of TNFα, while miR-21 overexpression dramatically reduced both the basal and the IR-induced increase (Fig. 3A). In addition, the low TNFα levels in 5 Gy irradiated cells (Supplementary Fig. S4) further support the concept that TNFα might be a target of miR-21. In addition, the 3′UTR of TNFα contains a potential miR-21 binding site (Fig 3B, Supplementary Fig. S7). To verify that TNFα is a miR-21 target, we tested luciferase activity driven by the 3′ UTR region of TNFα. These results indicate that TNFα is a target of miR-21 (Fig. 3B). Our results also confirm that TNFα stimulates transcription of SOD2 (18). In addition, up-regulating TNFα (without 3′ UTR) in cells overexpressing miR-21 or down-regulating TNFα by using siRNA in vector transfected control cells (Fig. 3C), altered SOD2 mRNA levels (Supplementary Fig. S8) and TNFα protein levels (Fig. 3C), as well as the SOD2 activities (Supplementary Fig. S9). More importantly, such changes in TNFα expression affected basal and IR-induced increases in ROS (Fig. 3D). Since there were no apparent differences in the levels and activities of catalase or glutathione peroxidases between miR-21 upregulated cells and their counterpart control cells (Supplementary Fig. S10), these results exclude the possibility that miR-21 affecting the ROS level is through other pathways. These results strongly support the conclusion that the IR–induced increase in superoxide levels in miR-21 overexpressing cells is at least due in part to miR-21 targeting of TNFα, thereby decreasing SOD2 levels.
Figure 3.
miR-21 directly targets TNFα, an SOD2 inducer. A. TNFα levels were measured by Western blot in cells with or without stable miR-21 overexpression at different times after 0.5 Gy low-LET IR. β-Actin was used as an internal loading control. B. Potential miR-21 binding site in the 3′UTR of TNFα and the effects of the binding site on the luciferase activity. Luciferase activity was assayed 48h after transfection with the miR-21 mimic (miR-21) or control RNA (mock), **: p < 0.01. (D) Effects of TNFα on SOD2 expression. Cells overexpressing miR-21 or vector transfected cells were transiently transfected with the TNFα cDNA (without 3’-UTR) or siRNA. At 48 h after transfection, the cells were collected and analyzed by Western blot. β-Actin was used as an internal loading control. D. ROS levels (left panel: O2−; right panel: H2O2) were measured using the cells (as described in figure 3C), with up- or down-regulated TNFα at 3h after 0.5 Gy low-LET IR. The data is an average of three separate experiments, **: p < 0.01.
miR-21 increases IR-induced cell transformation via targeting SOD3 and TNFα
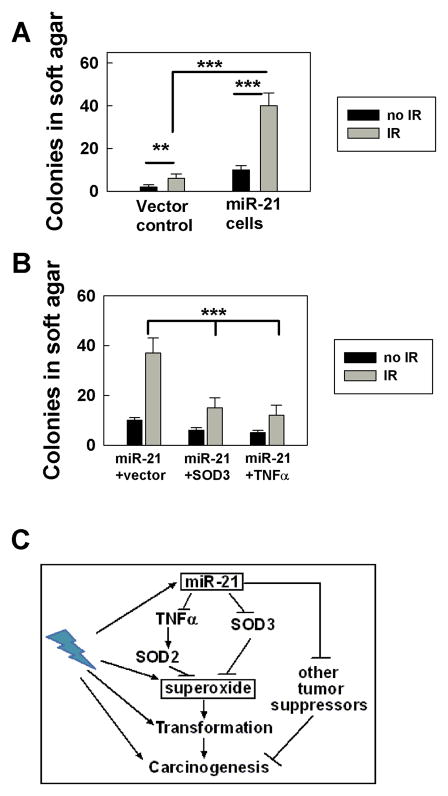
It is known that increased levels of intracellular ROS induce a cellular genotoxic stress response (19) and promote cellular transformation (10). Previously, we reported that over-expressing miR-21 can result in conversion of non-tumorigenic human cells to tumorigenic cells and that exposure to IR enhanced this conversion (4). We also demonstrated in this study that hydrogen peroxide levels were decreased but superoxide levels were increased in miR-21 over-expressing cells. Since superoxide has been shown to be a more powerful factor than hydrogen peroxide at stimulating cell proliferation (20), it is possible that miR-21-induced tumorigenesis (2, 4) is partially due to the high superoxide level generated in the cells. To test this hypothesis, we used cell-colony forming in soft agar to examine the effects of miR-21 on IR-induced transformation. We compared the cell transformation among the cells with or without miR-21 overexpression, with or without SOD3 or TNFα up-regulation in the miR-21 overexpressing cells four months following exposure to a low dose (0.5 Gy) of x-ray exposure. The results showed that non-irradiated cells lacking miR-21 over-expression generated very few colonies while cells over-expressing miR-21 displayed significant colony formation (Fig. 4A). Following IR exposure, both cell lines generated elevated numbers of colonies compared to non irradiated cells. Furthermore, miR-21 overexpressing cells displayed elevated colony formation compared to cells transfected with vector alone (Fig. 4A). These results suggest that miR-21 promotes IR-induced cell transformation.
Figure 4.
miR-21 increases IR-induced cell transformation partially via targeting SOD3 and TNFα. A. The data reflect the number of colonies that were grown from cells with or without miR-21 overexpression in soft-agar for 3 weeks. The results were obtained from two separate experiments with triple dishes per sample in each experiment, **: p< 0.01; ***: p< 0.001. B. Similar experiments were carried out as described in A but with the cells upregulated either by SOD3 or TNFα cDNAs. The results were confirmed in two separate experiments with triple dishes per sample in each experiment, **: p< 0.01. C. A model proposed to explain how miR-21 could stimulate cell transformation partially through increasing superoxide, thereby contributing to the role of miR-21 in carcinogenesis. miR-21 increased IR-induced superoxide levels through targeting SOD3 and TNFα (a transcriptional activator of SOD2), which contributes to IR-induced cell transformation and carcinogenesis.
To examine whether the miR-21-increased IR-induced cell transformation was linked to miR-21-increased superoxide, we irradiated cells overexpressed with miR-21 and upregulated SOD3 or TNFα expression, and compared soft-agar colony formation among the cells after four months of subculture. Increased expression of SOD3 or TNFα at the time of radiation, decreased the number of IR-induced soft-agar colonies in the miR-21 overexpressing cells (Fig. 4B), suggesting that miR-21-stimulated cell transformation is linked to miR-21-increased superoxide. Since miR-21 plays an important role in initiating and promoting tumorigenesis (2–4), our results indicate that a component of miR-21-stimulated cell transformation occurs via increasing superoxide and may be associated with its role in tumor development (Fig. 4C).
Discussion
In this study, we reported for the first time that miR-21 affects ROS levels through targeting SOD3 and TNFα (indirectly affecting SOD2), which might be linked to the role of miR-21 in carcinogenesis. Although IR stimulated an increase of the ROS levels in the cells, we did not observe the IR dose response effects: 5 Gy irradiated cells did not show higher ROS levels than 0.5 Gy irradiated cells. In addition, we did not find any dramatic difference in ROS levels between high-LET and low-LET irradiated cells. These phenotypes might be due to the fact that the amount of ROS generation per cell is relatively consistent, which does not change with a radiation dose increase or quality change (21, 22). As mentioned in these papers, their results are consistent with a threshold of a all-or-nothing response (21, 22). After 0.5 Gy exposure, we observed a clear increase of ROS, indicating that such a dose is enough to initiate the change in the ROS levels; although at 24 h after IR the ROS level reverted back to the levels similar to that in non-irradiated cells, and we believe that the transient change would induce some consequences that affect the cell biological functions. Of course, these predictions need future experiments to elucidate.
The difference in the ROS levels between miR-21 upregulated cells and their control counterparts are consistent, which provides additional evidence that the change in ROS might contribute to the role of miR-21 in carcinogenesis. Carcinogenesis is a complicated process and involves more proteins and pathways. Up-regulating miR-21 could result in non-tumorigenesis human cells becoming tumorigenesis (4) and mouse lymphomas (2), confirming the tumorigenesis of miR-21. The role of mir-21 in carcinogenesis is believed to be linked to the multiple targets of miR-21 such as PTEN, PDCD4 and TPM1 (5–9), however, the whole picture for miR-21-induced tumorigenesis remains unclear. We previously reported that radiation enhanced the role of miR-21 in tumorigenesis (4) and the transformation results in this study also support it. However, how radiation enhances the miR-21’s tumorigenesis needs more studies to explore. Our results shown in this study strongly support that the effects of miR-21 on the ROS level to cooperate with the effects of other miR-21 targets contribute to the miR-21’s carcinogenesis.
Supplementary Material
Acknowledgments
We thank the support team at Brookhaven National Laboratory for helping with the high-LET IR, members of the Wang laboratory for helpful discussion and Ms Doreen Theune for editing the manuscript.
Grant Support
This work is supported by grants from NIH (GM080771), NASA (NNX11AC30G) to Y.W.
Footnotes
Disclosure of Potential Conflict of Interest
No potential conflict of interest disclosed.
References
- 1.Croce CM. miRNAs in the spotlight: Understanding cancer gene dependency. Nat Med. 2011;17:935–6. doi: 10.1038/nm0811-935. [DOI] [PubMed] [Google Scholar]
- 2.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 3.Hatley ME, Patrick DM, Garcia MR, Richardson JA, Bassel-Duby R, van Rooij E, et al. Modulation of K-Ras-Dependent Lung Tumorigenesis by MicroRNA-21. Cancer Cell. 2010;18:282–93. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Yu X, Fu H, Wang H, Wang P, Zheng X, et al. MicroRNA-21 involves radiation-promoted liver carcinogenesis. Int J Clin Exp Med. 2010;3:211–22. [PMC free article] [PubMed] [Google Scholar]
- 5.Asangani IA, Rasheed SAK, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2007;27:2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 6.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo Y-Y. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–9. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 7.Lu Z, Liu M, Stribinskis V, Klinge C, Ramos K, Colburn N. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–9. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 Targets a Network of Key Tumor-Suppressive Pathways in Glioblastoma Cells. Cancer Res. 2008;68:8164–72. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 9.Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Aca Sci USA. 2009;106:12085–90. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441–4. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 11.Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–17. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biology and Medicine. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 13.Yan D, Ng W, Zhang X, Wang P, Zhang Z, Mo Y, et al. Targeting DNA-PK and ATM with miR-101 sensitizes tumors to radiation. PLoS ONE. 2010:e11397. doi: 10.1371/journal.pone.0011397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marklund S. Human copper-containing superoxide dismutase of high molecular weight. Proc NatL Acad Sci USA. 1982;79:7634–8. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen David H, Oketch-Rabah Hellen A, Illa-Bochaca I, Geyer Felipe C, Reis-Filho Jorge S, Mao J-H, et al. Radiation Acts on the Microenvironment to Affect Breast Carcinogenesis by Distinct Mechanisms that Decrease Cancer Latency and Affect Tumor Type. Cancer Cell. 2011;19:640–51. doi: 10.1016/j.ccr.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleissner F, Jazbutyte V, Fiedler J, Gupta SK, Yin X, Xu Q, et al. Short Communication: Asymmetric Dimethylarginine Impairs Angiogenic Progenitor Cell Function in Patients With Coronary Artery Disease Through a MicroRNA-21 –Dependent Mechanism. Circulat Res. 2010;107:138–43. doi: 10.1161/CIRCRESAHA.110.216770. [DOI] [PubMed] [Google Scholar]
- 17.Wispé JR, Clark JC, Warner BB, Fajardo D, Hull WE, Holtzman RB, et al. Tumor necrosis factor-alpha inhibits expression of pulmonary surfactant protein. J Clin Invest. 1990;86:1954–60. doi: 10.1172/JCI114929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Kiningham KK, Devalaraja MN, Yeh C-C, Majima H, Kasarskis EJ, et al. An Intronic NF-kappaB Element Is Essential for Induction of the Human Manganese Superoxide Dismutase Gene by Tumor Necrosis Factor-alpha and Interleukin-1beta. DNA and Cell Biology. 1999;18:709–22. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 19.Rowe L, Degtyareva N, Doetsch P. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radical Biology & Medicine. 2008;45:1167–77. doi: 10.1016/j.freeradbiomed.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radical Biology and Medicine. 1995;18:775–94. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 21.Leach JK, Van Tuyle G, Lin P-S, Schmidt-Ullrich R, Mikkelsen RB. Ionizing Radiation-induced, Mitochondria-dependent Generation of Reactive Oxygen/Nitrogen. Cancer Res. 2001;61:3894–901. [PubMed] [Google Scholar]
- 22.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–54. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.