Abstract
Elevation of the chromatin repression factor EZH2 is associated with progression and poor prognosis in several human cancers, including prostate cancer. However, the mechanisms driving EZH2 expression are not fully understood. In this study, we investigated the functional synergy in prostate cancers in mice resulting from activation of the androgen receptor (AR), Kras and Akt, which drive three of the most frequently activated oncogenic signaling pathways in prostate cancer. While any two of these three events were sufficient to promote the formation and progression of prostate cancer, only the synergy of AR and Kras signaling could elevate EZH2 expression and expand prostate cancer progenitor cells in vivo. Our findings have revealed a genetic mechanism resulting in enhanced EZH2 expression during the progression of aggressive prostate cancer, with important implications for understanding how to target advanced disease where cancer progenitor cells may be critical.
Keywords: Kras, Androgen receptor, EZH2, Tumor propagating cells, Prostate cancer
Introduction
Prostate cancer is a heterogeneous and multifocal disease. Disease progression is believed to develop through defined pathological states, beginning with prostatic intraepithelial neoplasia (PIN), progressing to invasive carcinoma, and metastatic cancer representing the most aggressive phase (1). During progression, genetic and epigenetic alterations and the tumor micro-environment play important roles in the etiology of prostate cancer. Numerous reports suggest that multiple genetic changes occur in advanced prostate cancer, but no single major oncogenic event determines a large fraction of the disease (2). Therefore, a detailed understanding of how commonly occurring oncogenic events may synergize will be helpful for elucidating the mechanistic basis of tumor progression, as well as for the identification of therapeutic targets.
Aggressive prostate cancers exhibit a multiplicity of genetic mutations (3) and a high incidence of genetic aberrations (4). A common theme among these genetic alterations are events contributing to the expression or activation of the androgen-androgen receptor (AR) signaling axis (5). This trend is highlighted in that over 80% of patients with castration resistant disease maintain high AR expression or exhibit AR signaling (6, 7). Targeting the androgen-AR signaling axis remains an effective strategy in the treatment of prostate cancer (5, 8, 9). In addition to AR signaling, loss of PTEN or activation of AKT and activation of Ras/Raf pathways are among the most frequently occurring genetic changes in prostate cancer (4). Enhanced PTEN/PI3K/Akt signaling occurs in almost all prostate cancers, and aberrations in the Ras signaling pathway occur in over 40% of prostate primary tumors and 90% of prostate metastases (4). However, how these signaling pathways collaborate to promote prostate tumor progression is not well understood.
The progression of metastatic prostate cancer is coupled with enhanced expression levels of enhancer of zeste homolog (EZH2) (10). EZH2 is one of the essential components in Polycomb Repressive Complex 2 (PRC2) protein complex (11). Together with Suppressor of Zeste 12 (SUZ12) and Embryonic Ectoderm Development (EED) protein, EZH2 catalyzes the methylation of histone H3 via its methyltransferase activity (12). EZH2 further recruits DNA methyltransferases (DNMTs) to hypermethylate the promoter region of its target genes and subsequently mediates epigenetic transcriptional repression (13). The epigenetic silencing function of EZH2 regulates stem cell pluripotency, early embryogenesis, lymphopoiesis, and other normal developmental events (14-17). Numerous reports have also implicated EZH2 in the regulation of tumor cell growth, proliferation, invasion, and metastasis in a variety of human cancers, including prostate cancer (18, 19). The expression of EZH2 is up-regulated by aberrant expression of Myc, ETS transcription factor ERG, or by suppression of microRNA (20-23). Further investigation of whether activated signaling pathways in prostate cancer may similarly up-regulate EZH2 expression will be helpful in identifying potential targets for treating aggressive prostate cancer.
We have previously demonstrated that primary prostate cells from adult prostate tissue could regenerate into prostate tissue in vivo (24, 25). Utilizing this prostate tissue regeneration system, we have reported that collaboration of some oncogenic signaling pathways recapitulate the progression of invasive prostate cancer in vivo, including the process of epithelial to mesenchymal transition (26, 27). Therefore, we utilized this in vivo regeneration system to determine whether the frequently occurring oncogenic events in prostate cancer, including activation of Kras and Akt, and over-expression of AR can synergize to enhance EZH2 expression and subsequently promote the progression of prostate cancer. We demonstrate that any two of these three oncogenic events synergize in promoting the progression of prostate carcinoma. However, these three related tumors, mAkt+AR, Kras(G12D)+mAkt, and Kras(G12D)+AR, showed a diversity in prostate lineage expansion and tumorigenic cell renewal capacity. In particular, primary Kras(G12D)+AR tumors harbor tumor-propagating cells that maintain the tumorigenic re-initiation capability and primary cancer type in sequential passage. Importantly, Kras(G12D)+AR tumors exhibited higher expression of EZH2, concomitant with their propensity for increased prostate tumor propagating cells. Our study reveals a genetic mechanism of enhanced expression of EZH2 and may have important implications for targeting advanced prostate cancer.
Materials and Methods
Prostate regeneration and prostate epithelial viral infections
The prostate regeneration process, lentivirus preparation, titering, and infection of dissociated prostate cells were performed as described previously in compliance with the safety regulations for lentivirus usage at the University of California, Los Angeles or Medical University of South Carolina (24). Housing, maintenance, and all surgical and experimental procedures were undertaken in fulfillment with the regulations of the Division of Laboratory Animal Medicine at the University of California, Los Angeles or Medical University of South Carolina. Dissociated prostate cell suspensions were prepared fro m 6- to 10-week-old Kras(G12D) mice. Dissociated cells or cells fractionated with surface markers (Lin-CD49f+Sca1+) were infected with lentivirus FUCRW-Cre, FUCRW-mAkt, FUCRW-mAkt-Ires-Cre, FUCGW-AR, or co-infected with combinations of lentiviruses according to the experimental setup. Infected cells (1-2 × 105) were mixed with urogenital sinus mesenchymal cells (1-2 × 105). Grafts were implanted under the kidney capsule in SCID mice and allowed to regenerate for 8 weeks. The plasmids, mice strains, immunohistochemistry analysis of regenerated tissues, and the use of antibodies are presented in the Supplemental Material and Methods.
Prostate sphere assay
The sphere assay was performed as previously described (28). To examine if sphere formation of Kras(G12D)+AR tumorigenic cells was inhibited by suberoylanilide hydroxamic acid (SAHA), or 3-Deazaneplanocin A (DZNep), after plating normal primary prostate cells, Kras(G12D) or Kras(G12D)+AR tumorigenic cells in matrigel, 1 μM of SAHA, 5 μM of DZNep, or the same volume of DMSO was added to the PrEGM medium. Half of the culture medium was replaced with fresh PrEGM containing the same concentration of SAHA or DZNep every 2 days in the experiment.
Prostate cell culture
Normal prostate basal epithelial cell line (PEB) cell line (29) was maintained in PrEGM with 10% serum. Cells were transduced with lentivirus carrying over-expression of AR (GFP marker), or Kras(G12V) (RFP marker), or in combination. To exclude the un-infected cell population, cells were sorted by FACS based on GFP and/or RFP markers and were further cultured for protein analysis. The AR+Kras(G12V) transduced PEB cells were also treated with 10 μM U0126 or 5 μM DZNep, or the same volume of DMSO for 1 day. The treated cells were lysed for analysis of protein expression.
Subcutaneous implantation to passage the Kras(G12D)+AR tumor
The regenerated tumor tissues generated from the prostate regeneration assay were minced into small pieces and digested under 1 mg/ml of collagenase in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS) and 1% Penicillin and Streptomycin (P/S). 1×106 dissociated tumor cells were preserved in 10% DMSO of FBS and stored at −80 °C. When performing experiments, the frozen cells were revived in a 37 °C water bath and spun down in 600 rpm. The cells were washed once with DMEM 10% FBS P/S medium and gently resuspended in 20 μL of collagen, and then injected into the left flank of SCID mice. The Kras(G12D)+AR tumors were re-grown after 2 months of subcutaneous implantation.
Results
1) Synergy among over-expression of AR, activation of Kras and Akt signaling pathways in the progression of prostate cancer
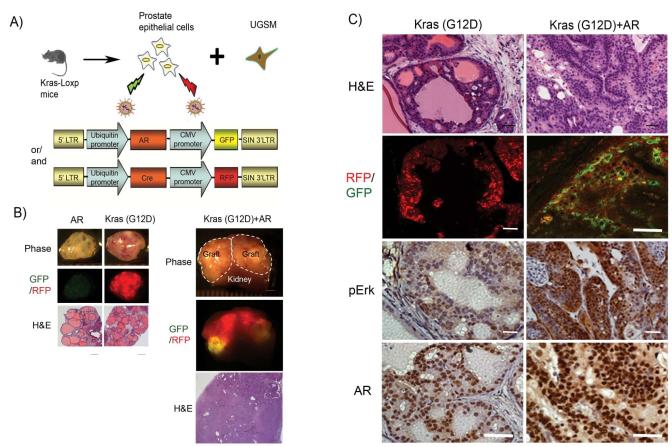
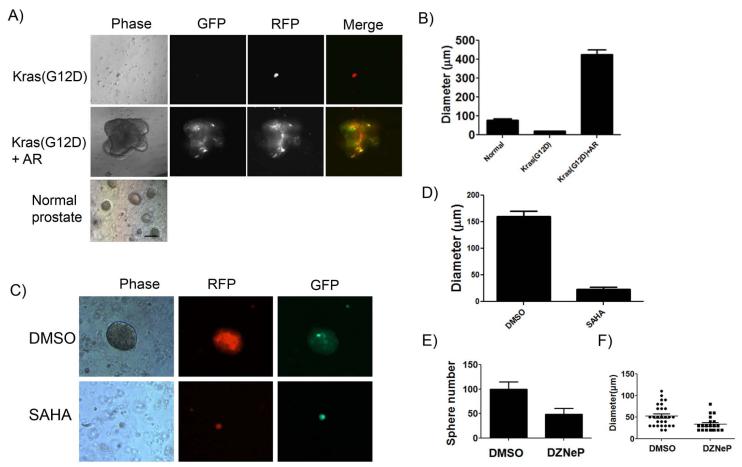
To investigate the combination of oncogenic events on the progression of prostate cancer, we studied the synergy of three frequently occurring oncogenic events in prostate cancer, over-expression of AR, activation of Akt (mAkt), and activated Kras(G12D), and observed the diversity of tumor characteristics from these combinations. Myristoylated Akt and point mutation (G to D) of Kras at position 12 were used as a surrogate for activation of PTEN/Akt and Ras signaling pathways, respectively. To assess the effects of Kras signaling in tandem with AR over-expression, we used mice bearing Kras(G12D)-loxp. Dissociated prostate cells from these mice were infected with a lentivirus over-expressing Cre and/or AR (Fig. 1A). Although over-expression of AR alone inhibited the prostate regeneration as previously reported (27), over-expression of Cre recombinase leads to activation of Kras (30). Tissues regenerated from activation of Kras alone displayed prostatic intraepithelial neoplasia (PIN) (Fig. 1B and C). In striking contrast, grafts regenerated from activated Kras(G12D) and over-expression of AR grew larger in size, and tumors invaded into the host kidney tissue (Fig. 1B). The tumor tissue derived from the AR and Kras(G12D) combination were confirmed by the overlap of RFP and GFP signals (Fig. 1B and C). Tumors derived from Kras(G12D)+AR displayed poorly differentiated or undifferentiated carcinoma (Fig. 1 C), and expression of both AR and phospho-Erk were enhanced in the Kras(G12D)+AR tumor (Fig. 1C). Collectively, our results indicate that activation of Kras synergizes with over-expression of AR, and the crosstalk of these two signaling pathways appears to significantly contribute to the progression of advanced prostate cancer.
Figure 1. Synergy of the activation Kras signaling with AR promotes prostate cancer progression.
A) Schematic outline for obtaining prostate epithelial cells from Kras(G12D)-loxp mice, lentiviral infection of Cre (with the fluorescent marker RFP) and/or AR (with the fluorescent marker GFP), and combination with UGSM for prostate regeneration.
B) Regenerated prostate grafts, RFP/GFP signals (scale bar, 2 mm), and H&E staining of regenerated tissues (low magnification) (scale bar, 400 μm) derived from primary prostate cells transformed by AR, Kras(G12D), or Kras(G12D)+AR. The dashed lines show the regenerated prostate grafts on mice kidney.
C) Histological analysis of Kras(G12D) or Kras(G12D)+AR tumor tissues by H&E (high magnification), RFP and/or GFP, and IHC for AR and phospho-Erk. Scale bar, 50 μm.
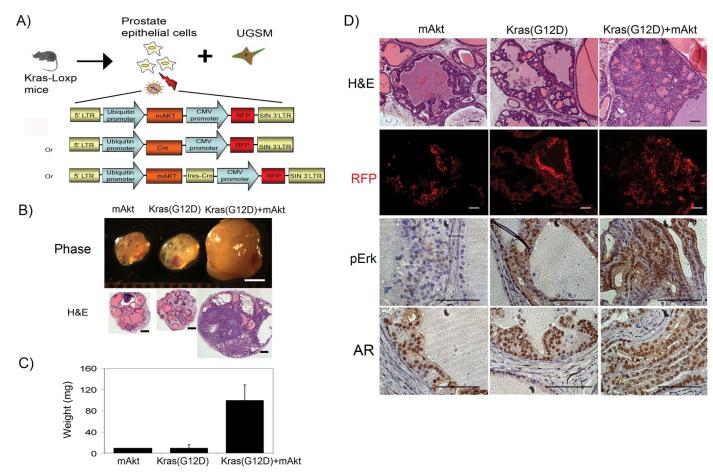
We next investigated the potential synergy of the PI3K-Akt and Kras signaling pathways. Prostate cells isolated from mice carrying Kras(G12D)-Loxp were infected with a lentivirus over-expressing Cre, mAkt, or mAkt-ires-Cre gene (Fig. 2A). Strikingly, tumor grafts derived from the combination of Kras(G12D)+mAkt weighed ~10 times more than those derived from either mAkt or Kras(G12D) alone (Fig. 2B and C). Similar to mAkt, regenerated prostate tissues from over-expression of Cre, and thus activated Kras, showed PIN lesions as described in Figure 1 (Fig. 2D). In contrast, tissues regenerated from co-overexpression of Kras and mAkt exhibited prostate adenocarcinoma (Fig. 2D). While similar expression levels of AR were observed in the tissues regenerated from over-expression of mAkt, Kras(G12D), or Kras(G12D)+mAkt, activated Kras signaling was confirmed by the elevated expression levels of phospho-Erk in Kras(G12D) or Kras(G12D)+mAkt tumors (Fig. 2D). Collectively, our results indicate that activation of Kras synergizes with the Akt signaling pathway to promote prostate cancer progression.
Figure 2. Synergy of the activation Kras signaling with mAkt promotes the progression of prostate cancer.
A) Schematic representation of the procedure for obtaining prostate epithelial cells from Kras(G12D)-loxp mice, lentiviral infection of Cre (with the fluorescent marker RFP), mAkt (with the fluorescent marker RFP), or mAkt-ires-Cre (with the fluorescent marker RFP), and combined with UGSM for prostate regeneration. The expression of Cre recombinase leads to activation of Kras(G12D) (30).
B-C) Regenerated prostate grafts (scale bar, 2 mm), H&E staining (low magnification, scale bar, 400 μm) (B), and the weight of regenerated prostate grafts (C) derived from primary prostate cells transformed by activation of Kras(G12D), mAkt, or Kras(G12D)+mAkt.
D) Histological analysis of Kras(G12D), mAkt, or Kras(G12D)+mAkt tumor tissues by H&E (high magnification), RFP and IHC for AR and phospho-Erk. Scale bar, 100μm.
We have previously reported that synergy of mAkt and AR can initiate frank carcinoma in naïve adult murine prostatic epithelium (26). Although mAkt induced tumors showed PIN lesions with secretion in the center of glandular structure, tumor tissues derived from prostate epithelia infected with AR+mAkt exhibited sheets of undifferentiated carcinoma cells (26) (Supplemental Fig. S1A and B). Taken together, we demonstrated that any two of three oncogenic events, activation of Kras and Akt signaling pathways, or over-expression of AR, synergize leading to the progression of prostate cancer.
2) Phenotypic diversity in expansion of epithelial lineages in Kras(G12D)+AR, Kras(G12D)+mAkt, and mAkt+AR prostate tumors
We further analyzed the phenotypic diversity of epithelial lineages in Kras(G12D)+AR, Kras(G12D)+mAkt, and mAkt+AR prostate tumors tissues. Tissues derived from mAkt or Kras(G12D) alone displayed predominant proliferation of CK8+ cells, a marker of luminal cells, in PIN lesions (supplemental Fig.S2A). Similarly, tissues derived from Kras(G12D)+mAkt tumors showed predominant proliferation of CK8+ cells with CK5+ cells, a marker of basal cells, or p63+ cells, a marker of basal and/or progenitor cells, localized at the basal membrane of the glandular structure (Fig. 3A). In some cases, a glandular structure contained predominantly CK5+ and p63+ with fewer of CK8+ cells, and some cells were both CK5 and CK8 positive. Alternatively, the tumor cells from the mAkt+AR tumor displayed weak expression levels of CK8 with no expression of CK5 and p63 (Fig. 3A). Expression of E-cadherin in mAkt+AR transformed cells suggests these cells were derived from the epithelia (Supplemental Fig. S1B). In striking contrast, tissues derived from Kras(G12D)+AR tumors exhibited multiple layers of CK5+ cells in the basal compartment with fewer CK8+ cells located at the luminal layer (Fig. 3A and B). In addition, CK5+ cells localized on the top of a distinct p63+ basal layer in tumorigenic tubules (Fig. 3A and B, and Supplemental Fig. S3). Collectively, our data suggest dramatic diversity of lineage expansion in three related tumors. Particularly, synergy of Kras(G12D) with AR promotes proliferation of progenitor cells in prostate tumors.
Figure 3. Distinct lineage expansion of prostate epithelia in tumors from mAkt+AR, Kras(G12D)+mAkt, and Kras(G12D)+AR prostate tumors.
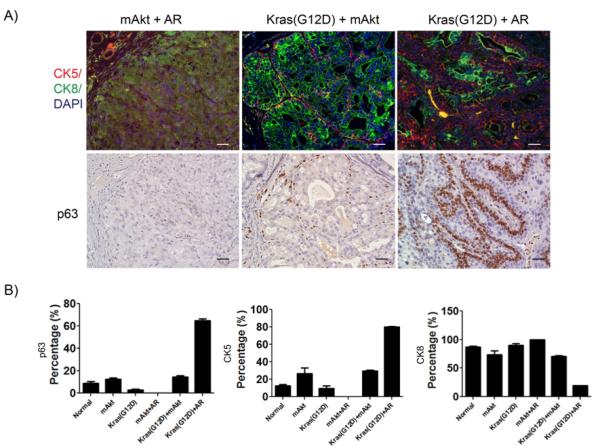
A) Lineage analysis of tumors from three mutually synergistic oncogenic events. IHC analysis of mAkt+AR, Kras(G12D)+mAkt, and Kras(G12D)+AR tumors for CK5 (red), CK8 (Green), and p63. Scale bar, 50μm.
B) The percentage of p63, CK5, and CK8 cells in prostate epithelia of normal regenerated prostate tissue, mAkt, Kras(G12D), mAkt+AR, Kras(G12D)+mAkt, and Kras(G12D)+AR tumors. Normal regenerated tissue and tumors were stained for CK5/CK8 or p63 by IHC. The number and percentage of CK5, CK8, and p63 positive cells in regenerated tubules were measured
3) Tumorigenic cells derived from Kras(G12D)+AR prostate tumors, b u t n o t Kras(G12D)+mAkt or mAkt+AR, maintain self-renewal potential to re-initiate secondary tumors
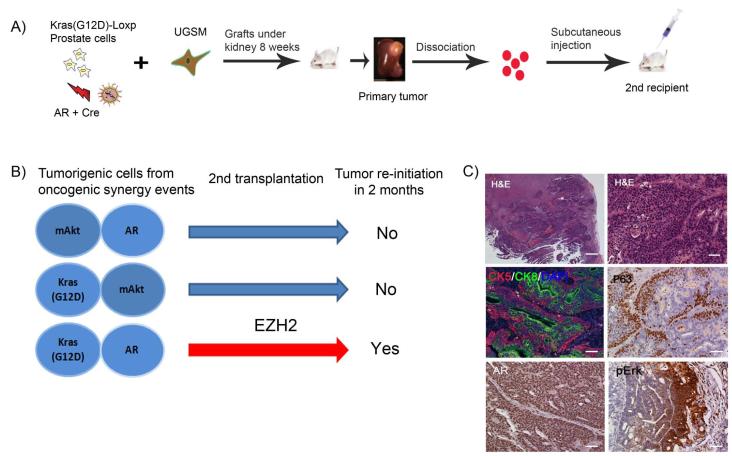
As Kras(G12D)+AR primary tumors harbor an expansion of progenitor cells, we asked if dissociated tumorigenic cells had self-renewal potential, exemplified by their ability to re-initiate secondary tumors. Kras(G12D)+AR, Kras(G12D)+mAkt, and mAkt+AR tumors were dissociated into single cells. After mixing with collagen, the same number of tumor cells was re-implanted subcutaneously into the flank side of SCID mice (Fig. 4A). Only dissociated tumor cells derived from Kras(G12D)+AR primary tumors gave rise to a visible secondary tumor after 2 months incubation (Fig. 4B). We further examined if properties of the secondary tumor tissues recapitulated characteristics of parental tumors. Indeed, lineage analysis showed that the secondary tumor resembled the primary tumor, which contained glandular tubules with an expansion of CK5+ and p63+ cells located at the prominent basal layer, and CK8+ cells localized toward the luminal layer (Fig. 4C). In addition, expression levels of both phospho-Erk and AR were increased in the secondary tumor (Fig. 4C). In summary, our results show that cells from Kras(G12D)+AR primary tumors exhibited the capacity to regenerate secondary tumors with characteristics resembling the parental tumors.
Figure 4. Dissociated tumorigenic cells from Kras(G12D)+AR primary tumors re-initiate secondary tumors, which recapitulate characteristics of parental tumors.
(A) Schematic of experimental procedure for secondary transplantation. Primary naïve prostate cells from dissociated Kras(G12D)-Loxp mice were infected with Cre and AR lentivirus and combined with UGSM cells. The grafts were implanted in the sub-renal capsule of SCID mice and incubated for 8 weeks. The grafts were harvested and dissociated into single cells. 1×106 of the dissociated primary tumorigenic cells were revived and mixed with 20 μl of collagen and implanted subcutaneously in SCID mice. Mice were sacrificed after 2 months and the secondary tumor was recovered and analyzed for histology and IHC.
(B) Transplantation of primary tumorigenic cells derived from three tumor types. Only tumorigenic cells from Kras(G12D)+AR primary tumor were able to re-initiate visible tumors in 2 months.
(C) H&E (low magnification, scale bar, 400 μm; and high magnification, scale bar, 50 μm) and IHC analysis of Kras(G12D)+AR secondary tumor tissues for AR, phospho-Erk, CK5 (red), CK8 (Green), and p63 (scale bar, 50 μm).
4) Kras(G12D)+AR, but not Kras(G12D)+mAkt or mAkt+AR prostate tumors enhances expression levels of EZH2
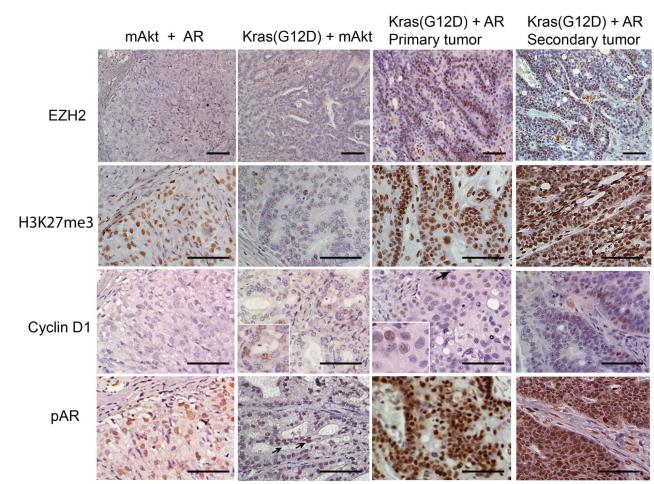
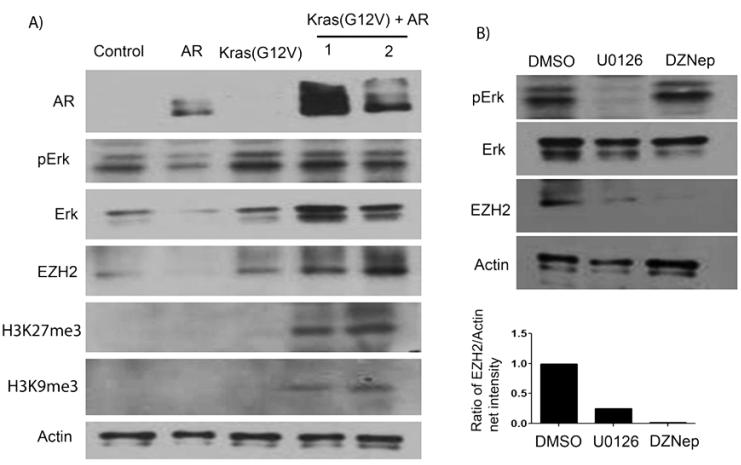
EZH2 plays an important role in suppression of epithelial differentiation and maintenance of stem cell pluripotency by epigenetic silencing (14). We have observed that epithelia in Kras(G12D)+AR primary tumors and secondary tumors were composed of more un-differentiated progenitor cells. We hypothesized that the expansion of prostate primitive cells, such as increasing CK5+ basal cells and p63+ cells in Kras(G12D)+AR primary and secondary tumors, may be correlated with inhibition of the ability to differentiate due to epigenetic silencing. We therefore examined the expressio n l e v e l s o f E Z H 2 i n mAkt+AR, Kras(G12D)+mAkt, and primary and secondary Kras(G12D)+AR tumors and found enhanced expression levels of EZH2 only in Kras(G12D)+AR primary and secondary tumors (Fig.5 and Supplemental Fig.S2B). EZH2, an essential component of the polycomb group (PcG) protein complex 2, catalyzes the tri-methylation of histone H3 (Lys27) (H3K27me3) (13). Consistently, Kras(G12D)+AR primary and secondary tumors displayed higher levels of H3K27me3 (Fig.5), while mAkt+AR showed only basal expression of H3K27me3 similar to mAkt, Kras(G12D), or normal regenerated tubules (Supplemental Fig. S2B). In contrast, Kras(G12D)+mAkt tumors exhibited a down-regulation of H3K27me3 expression (Fig. 5). Additionally, tissues derived from Kras(G12D)+mAkt and Kras(G12D)+AR primary and secondary tumors exhibited increased expression of cyclin D1. Expression levels of phospho-AR were elevated in mAkt+AR and dramatically increased in Kras(G12D)+AR primary and secondary tumors, but, to a less extent, in Kras(G12D)+mAkt tumors compared to Kras(G12D) or mAkt tumors (Fig. 5). Collectively, our results demonstrated that Kras(G12D)+AR, Kras(G12D)+mAkt, and mAkt+AR prostate tumors exhibited distinct molecular features. Additionally, epigenetic silencing by increased EZH2 activity may inhibit tumorigenic cell differentiation and enhance cell renewal capacity in Kras(G12D)+AR tumors.
Figure 5. Distinct molecular features of prostate epithelia in mAkt+AR, Kras(G12D)+mAkt primary tumors, and primary and secondary Kras(G12D)+AR tumors.
IHC analysis of regenerated tumor tissues for the expression of EZH2, H3K27me3, phospho-AR, and cyclin D1. Scale bar, 200 μm.
5) The expression of EZH2 is important for the enhanced sphere forming capacity in tumorigenic cells derived from Kras(G12D)+AR prostate tumors
Sphere formation capacity has been utilized to measure primitive prostate cell activity in vitro (25). To examine whether cells derived from Kras(G12D)+AR tumors possess enhanced regeneration capacity, we generated Kras(G12D)+AR tumors from enriched basal prostate cells. Prostate basal cells were sorted based on Lin-CD49f+Sca1+ markers (Supplemental Fig. S4A) from prostate tissues of transgenic mice carrying Kras(G12D)-loxp (27). The isolated basal cells were infected with either FUCRW-Cre and/or FUCGW-AR. The basal cells transformed by Kras(G12D)+AR showed increased tumor size and advanced adenocarcinoma in comparison with that of Kras(G12D) alone which showed PIN lesions as previously described (Supplemental Fig. S4B, C, D and Fig. 1D). To further examine if tumorigenic cells of Kras(G12D)+AR had enhanced capacity to form spheres, the regenerated Kras(G12D)+AR and Kras(G12D) tumors or primary prostate tissue from adult mice (as a control) were subjected for sphere formation assay (28). Oncogene-transformed cells usually become more differentiated (31), and subsequently could lose the capability to form spheres. Tumorigenic cells derived from Kras(G12D) did not form spheres, however cells from Kras(G12D)+AR tumors grew spheres that were approximately 4 times larger in diameter than spheres derived from normal prostate cells (Fig. 6 A and B). Interestingly, in some cases, daughter spheres divided out from parental spheres (Supplemental Fig. S5). These results further support our finding that tumorigenic cells from Kras(G12D)+AR demonstrate enhanced cell renewal capacity.
Figure 6. Dissociated tumorigenic cells derived from Kras(G12D)+AR primary tumors form distinctively large spheres and sphere formation is suppressed by SAHA and DZNep.
A) The regenerated prostate tumors, Kras(G12D) or Kras(G12D)+AR, or primary mouse prostate tissues (control) were dissociated into single cells and mixed with Matrigel. After 10 days incubation, images of prostate spheres were taken. Scale bar, 50 μm. B) The diameters of spheres derived from normal prostate primary cells, Kras(G12D), and Kras(G12D)+AR tumors were measured by micro-scale. C-F) The Kras(G12D)+AR tumor cells were treated with DMSO,1μM SAHA (C and D), or 5 μM DZNep (E andF) for 10 days in sphere assay. Number or diameter of sphere was measured.
HDAC activity is important for EZH2 function and DNA methylation (19). To examine if EZH2 plays a critical role in the increased cell renewal capacity in Kras(G12D)+AR tumorigenic cells, we examined if the HDAC and EZH2 inhibitors, suberoylanilide hydroxamic acid (SAHA) and Deazaneplanocin A (DZNep), can suppress the elevated sphere formation capacity. As expected, DZNep inhibits prostate sphere formation of normal prostate cells (Fig. S6) (32). The size or number of spheres derived from Kras(G12D)+AR tumorigenic cells was similarly suppressed by the SAHA or DZNep treatment (Fig. 6 C-F), suggesting enhanced sphere formation capacity in Kras(G12D)+AR tumorigenic cells is likely mediated by EZH2 activity.
6) Erk signaling partially facilitates the enhanced EZH2 expression in PEB cells overexpressing Kras(G12V) and AR
To study how co-overexpression of constitutively active Kras and AR promotes the expression of EZH2, we established an in vitro model. Normal prostate epithelial basal cells (PEB) (29) were transduced with constitutively active Kras(G12V) [similar to Kras(G12D) mutant] (33) and/or AR. While, as expected, PEB cells with co-overexpression of Kras(G12V) and/or AR exhibited elevated expression levels of AR and phospho-Erk, over-expression of AR alone led to a repression of EZH2 expression (Fig. 7A). This is consistent with reported studies, in which addition of androgen represses EZH2 expression, while knockdown of AR increases EZH2 expression in LNCaP cells (34, 35). However, similar to the previously described in vivo regeneration model, Kras(G12V)+AR transduced PEB cells displayed increased expression levels of EZH2 and histone 3 Lys 27 tri-methylation (H3K27Me3) and histone 3 Lys 9 tri-methylation (H3K9Me3) (Fig. 7A), suggesting that co-overexpression of Kras(G12V) and AR in PEB cells leads to an acquired epigenetic alteration.
Figure 7. Co-overexpression of AR and constitutively active Kras(G12V) induced epigenetic alterations in normal prostate cells.
Normal prostate basal cells (PEB) were transduced with a lentivus for over-expression of AR, Kras(G12V), or AR+Kras(G12V). A) Protein expression of AR, phospho-Erk, total Erk, EZH2, H3K27Me3, H3K9Me3, and actin (loading control) were measured by Western blot analysis. B) PEB cells transduced with Kras(G12V)+AR were treated with DMSO, 10 μM Erk signaling inhibitor U0126, or 5 μM DZNep for 1 day. The expression levels of total Erk, phospho-Erk, EZH2, and actin were examined by Western blot analysis. The net intensity of EZH2 and actin bands were measured by Carestream Molecular Imaging software (New Haven), and the ratio of EZH2/Actin expression was calculated.
To determine if Erk signaling plays an important role in the regulation of EZH2 expression, Kras(G12V)+AR transduced PEB cells were treated with the Erk signaling inhibitor U0126 or EZH2 inhibitor DZNep (control). As expected, U0126, but not DZNep, significantly suppressed phospho-Erk levels (Fig. 7B). Expression of EZH2 was suppressed by DZNep treatment, while treatment with U0126 attenuated EZH2 expression to a lesser extent (Fig. 7B). The data suggest that the enhanced expression of EZH2 may partially mediate by Erk signaling in co-overexpression of Kras(G12V)+AR tumorigenic cells.
Discussion
In this study, we have demonstrated that any two of three frequently occurring oncogenic events, overexpression of AR and activation of Akt or Kras, synergize to promote the progression of prostate cancer. Using our regeneration model, tumors derived from the combinations, Kras(G12D)+AR, Kras(G12D)+mAkt, and mAkt+AR, show distinctive features in the expression pattern of EZH2, the expansion of prostate lineages and tumor re-initiation capacity (Supplemental Table 1). Although synergy of Kras(G12D) and mAkt promotes tumor progression, we found this combination down-regulated EZH2 expression. This may be due to activation of Akt in promoting EZH2 phosphorylation at Serine 21 (36), which down-regulates its methyltransferase activity by blocking EZH2 binding to histone H3, decreasing trimethylation at Lys 27 and de-repressing epigenetic silencing (36). In contrast, synergy of Kras(G12D) and AR signaling uniquely recapitulates the elevated EZH2 expression profile observed in the combination of the studied oncogenic events. Despite the complexity of genetic alterations in aggressive human prostate cancer, persistent AR signaling and activation of Kras signal transduction pathways represent two of the most commonly occurring oncogenic events in prostate tumorigenesis (4). Our results demonstrate a genetic mechanism by which oncogenic signaling pathways cooperate to increase the expression of EZH2 and drive a feed forward loop to support the progression of advanced prostate cancer.
Elevated Ras-signaling plays an important role in epigenetic inactivation of multiple genes during cancer progression (37). An involvement for Kras in epigenetic regulation is further supported by a prior report wherein an in vitro genome-wide RNA interference screen demonstrated that the polycomb group proteins EZH2 and BMI1 facilitated Ras-mediated epigenetic silencing in Kras transformed NIH 3T3 cells (38). Our in vivo and in vitro experiments suggest that the collaboration of AR and active Kras, but not active Kras alone, is required in the regulation of the epigenetic silencing in primary prostate cells or a normal prostate cell line. Although the mutation of Kras leading to constitutive activation is rarely observed in prostate cancer, aberrant Ras signaling could occur via autocrine and paracrine growth factor stimulation or by gene fusions in prostate primary tumor and metastatic disease (39). For example, several gene fusion events, UBE2L3-KRAS (a fusion of Kras with ubiquitin-conjugating enzyme), SLC45A3-BRAF (solute carrier family 45, member3-v-raf murine sarcoma viral oncogene homolog B1) or ESRP1-RAF1 (epithelial splicing regulatory protein-1-v-raf-1 murine leukemia viral oncogene homolog 1) have been described in prostate tumors (40, 41). Cross-talk of activated RAS-RAF-MAPK signaling with other oncogenic signaling pathways could dramatically change the tyrosine kinase phosphorylation profile in prostate cancer progression (33). Therefore, dissecting Ras mediated epigenetic changes will be important in understanding prostate cancer progression.
Elevated Ras down-stream Erk signaling may be important in mediating enhanced expression of EZH2 and initiating epigenetic changes. Indeed, we observed increased expression of phospho-Erk in early passage PEB cells transduced with Kras and AR (data not shown). Furthermore, our in vitro data demonstrate that suppression of Erk signaling modulates the expression levels of EZH2. Erk signaling in the regulation of EZH2 expression has also been reported in a study in which the Mek-Erk1/2-Elk-1 pathway can induce EZH2 over-expression in breast cancer (42), and elevation of Erk phosphorylation by platelet-derived growth factor (PDGF) modulates EZH2 expression levels and, subsequently, regulates adult pancreatic beta-cells proliferation (43).
It is still debated as to whether basal or luminal cells are the targets for tumorigenesis in prostate cancer (44). By a lineage tracing approach, Choi et al. (2012) recently showed that both adult murine prostate basal and luminal cells could serve as targets for tumorigenesis. Although basal cells are more resistant to oncogenesis (such as loss of PTEN), transformed basal cells could differentiate and establish luminal tumor cells (31). Different to PTEN loss or activated Akt induced tumorigenesis, basal cells transformed by the combination of Kras and AR signaling could elevate the expression of EZH2, and subsequently EZH2 mediated epigenetic silencing could suppress the differentiation of transformed basal cells into luminal cells. This is further supported by the experimental evidence that isolated human prostate basal cells can be transformed to produce luminal cancer with combinations of the most common genetic changes in human cancer (45). Despite the known observation that basal cell carcinoma of the prostate is not common (46), our study implies that tumor propagating cells might be induced through the synergy of activation of Kras and AR signaling during prostate cancer progression.
Enhanced expression of EZH2 is a marker of prostate metastatic disease (47) and is associated with poor prognosis in a variety of other human cancers (10). We have shown herein that over-expression of AR alone suppresses EZH2 expression and prostate regeneration (26, 27). This AR mediated EZH2 suppression might be regulated by the RB-E2F1 pathway (48) and require the RB-related protein P130 (35). However, Kras(G12D)+AR tumors are associated with increased expression of EZH2 and possess tumor propagating cells which highly enhance cancer re-initiation capacity. Aberrant expression of EZH2 may play an important role in maintaining expanded tumorigenic cells in the progenitor stage as confirmed by the proliferation of CK5+/p63+ progenitor cells in the Kras(G12D)+AR primary tumors. EZH2 is an essential component for maintaining stem cell properties (14) and suppressing lineage genes that are required for the differentiation of stem or progenitor cells (18). The enhanced EZH2 expression could further interact with AR in the process of epigenetic silencing as it has been shown that EZH2 can cooperate with AR to regulate AR-repressed genes (49). A better understanding of the emerging relationships between cooperating oncogenic events and signaling pathways within the context of EZH2 expression may have important implications for therapeutically targeting aggressive prostate cancer.
Supplementary Material
Acknowledgements
We thank Deanna Janzen and Shirley Quan for technical help and maintaining the Kras(G12D) mice colony. We thank Dr. Jennifer Isaacs and Dr. Shannon Richards-Slaughter, for reading and correcting the manuscript and giving valuable suggestions and comments. This work was supported by NIH/NCRR Grant UL1RR029882 to H.C.. TS is supported by the California Institute for Regenerative Medicine Training Grant TG2-01169. ASK is supported by the Department of Defense Grant W81XWH-08-PCRP-IDA and the NIH 1P30-CA138313. SM is supported by the PCF Young Investigators Award, BIRCWH grant (NIH/NICHHD 5 K12 HD001400), UCLA Jonsson Comprehensive Cancer Center Foundation Seed Grant, The Eli & Edythe Broad Center of Regenerative Medicine and Stem Cell Research Award. O.N.W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Author Contributions H.C. and O.N.W. designed research; H.C., T. S., Z.B and S.M. performed research; H.C., S.M., T.S., A.S.K, and O.N.W. analyzed data; and H.C. wrote the paper.
All authors declare no conflicts of interests in this manuscript.
References
- 1.Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39(1):41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 2.Heng HH, Bremer SW, Stevens JB, Ye KJ, Liu G, Ye CJ. Genetic and epigenetic heterogeneity in cancer: a genome-centric perspective. J Cell Physiol. 2009;220(3):538–47. doi: 10.1002/jcp.21799. [DOI] [PubMed] [Google Scholar]
- 3.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470(7333):214–20. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadar MD. Small molecule inhibitors targeting the “achilles’ heel” of androgen receptor activity. Cancer Res. 2011;71(4):1208–13. doi: 10.1158/0008-5472.CAN_10-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9(4):401–6. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 7.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Current opinion in pharmacology. 2008;8(4):440–8. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 11.Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell. 2004;15(1):57–67. doi: 10.1016/j.molcel.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298(5595):1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 13.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439(7078):871–4. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 14.Shen X, Liu Y, Hsu YJ, Fujiwara Y, Kim J, Mao X, et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32(4):491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21(13):4330–6. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su IH, Basavaraj A, Krutchinsky AN, Hobert O, Ullrich A, Chait BT, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4(2):124–31. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 17.Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14(2):155–64. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Sellers WR, Loda M. The EZH2 polycomb transcriptional repressor--a marker or mover of metastatic prostate cancer? Cancer cell. 2002;2(5):349–50. doi: 10.1016/s1535-6108(02)00187-3. [DOI] [PubMed] [Google Scholar]
- 19.Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR, et al. Coordinated Regulation of Polycomb Group Complexes through microRNAs in Cancer. Cancer cell. 2011;20(2):187–99. doi: 10.1016/j.ccr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322(5908):1695–9. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer cell. 2010;17(5):443–54. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh CM, Iwata T, Zheng Q, Bethel C, Yegnasubramanian S, De Marzo AM. Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget. 2011;2(9):669–83. doi: 10.18632/oncotarget.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunderfranco P, Mello-Grand M, Cangemi R, Pellini S, Mensah A, Albertini V, et al. ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancer. PLoS One. 2010;5(5):e10547. doi: 10.1371/journal.pone.0010547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11896–903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson DA, Zong Y, Memarzadeh S, Xin L, Huang J, Witte ON. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(6):2610–5. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xin L, Teitell MA, Lawson DA, Kwon A, Mellinghoff IK, Witte ON. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci U S A. 2006;103(20):7789–94. doi: 10.1073/pnas.0602567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H, Babic I, Wei X, Huang J, Witte ON. Invasive Prostate Carcinoma Driven by c-Src and Androgen Receptor Synergy. Cancer Res. 2011;71(3):862–72. doi: 10.1158/0008-5472.CAN-10-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukacs RU, Goldstein AS, Lawson DA, Cheng D, Witte ON. Isolation, cultivation and characterization of adult murine prostate stem cells. Nat Protoc. 2010;5(4):702–13. doi: 10.1038/nprot.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salm SN, Takao T, Tsujimura A, Coetzee S, Moscatelli D, Wilson EL. Differentiation and stromal-induced growth promotion of murine prostatic tumors. Prostate. 2002;51(3):175–88. doi: 10.1002/pros.10075. [DOI] [PubMed] [Google Scholar]
- 30.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15(24):3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer cell. 2012;21(2):253–65. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiba T, Suzuki E, Negishi M, Saraya A, Miyagi S, Konuma T, et al. 3-Deazaneplanocin A is a promising therapeutic agent for the eradication of tumor-initiating hepatocellular carcinoma cells. Int J Cancer. 2012;130(11):2557–67. doi: 10.1002/ijc.26264. [DOI] [PubMed] [Google Scholar]
- 33.Drake JM, Graham NA, Stoyanova T, Sedghi A, Goldstein AS, Cai H, et al. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(5):1643–8. doi: 10.1073/pnas.1120985109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Li W, Liu XS, Carroll JS, Janne OA, Keeton EK, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27(3):380–92. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohrer LR, Chen S, Hallstrom TC, Huang H. Androgens suppress EZH2 expression via retinoblastoma (RB) and p130-dependent pathways: a potential mechanism of androgen-refractory progression of prostate cancer. Endocrinology. 2010;151(11):5136–45. doi: 10.1210/en.2010-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, et al. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310(5746):306–10. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- 37.Patra SK. Ras regulation of DNA-methylation and cancer. Exp Cell Res. 2008;314(6):1193–201. doi: 10.1016/j.yexcr.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Gazin C, Wajapeyee N, Gobeil S, Virbasius CM, Green MR. An elaborate pathway required for Ras-mediated epigenetic silencing. Nature. 2007;449(7165):1073–7. doi: 10.1038/nature06251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91(1):13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- 40.Wang XS, Shankar S, Dhanasekaran SM, Ateeq B, Sasaki AT, Jing X, et al. Characterization of KRAS Rearrangements in Metastatic Prostate Cancer. Cancer Discov. 2011;1(1):35–43. doi: 10.1158/2159-8274.CD-10-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nature medicine. 2010;16(7):793–8. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujii S, Tokita K, Wada N, Ito K, Yamauchi C, Ito Y, et al. MEK-ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene. 2011;30(39):4118–28. doi: 10.1038/onc.2011.118. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, et al. PDGF signalling controls age-dependent proliferation in pancreatic beta-cells. Nature. 2011;478(7369):349–55. doi: 10.1038/nature10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Witte ON. A seminal finding for prostate cancer? N Engl J Med. 2010;362(2):175–6. doi: 10.1056/NEJMcibr0909965. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329(5991):568–71. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali TZ, Epstein JI. Basal cell carcinoma of the prostate: a clinicopathologic study of 29 cases. Am J Surg Pathol. 2007;31(5):697–705. doi: 10.1097/01.pas.0000213395.42075.86. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R, et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer research. 2007;67(22):10657–63. doi: 10.1158/0008-5472.CAN-07-2498. [DOI] [PubMed] [Google Scholar]
- 48.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. Embo J. 2003;22(20):5323–35. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao JC, Yu J, Runkle C, Wu L, Hu M, Wu D, et al. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome Res. 2012;22(2):322–31. doi: 10.1101/gr.131508.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.