Abstract
The immunotherapy of cancer has made significant strides in the past few years due to improved understanding of the underlying principles of tumor biology and immunology. These principles have been critical in the development of immunotherapy in the laboratory and in the implementation of immunotherapy in the clinic. This improved understanding of immunotherapy, enhanced by increased insights into the mechanism of tumor immune response and its evasion by tumors, now permits manipulation of this interaction and elucidates the therapeutic role of immunity in cancer. Also important, this improved understanding of immunotherapy and the mechanisms underlying immunity in cancer has fueled an expanding array of new therapeutic agents for a variety of cancers. Pegylated interferon-α2b as an adjuvant therapy and ipilimumab as therapy for advanced disease, both of which were approved by the United States Food and Drug Administration for melanoma in March 2011, are 2 prime examples of how an increased understanding of the principles of tumor biology and immunology have been translated successfully from the laboratory to the clinical setting. Principles that guide the development and application of immunotherapy include antibodies, cytokines, vaccines, and cellular therapies. The identification and further elucidation of the role of immunotherapy in different tumor types, and the development of strategies for combining immunotherapy with cytotoxic and molecularly targeted agents for future multimodal therapy for cancer will enable even greater progress and ultimately lead to improved outcomes for patients receiving cancer immunotherapy.
Introduction
Historic Overview
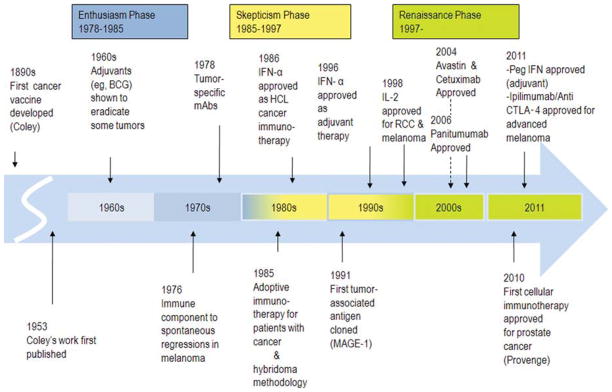
The pillars of human cancer therapy have historically been surgery, radiotherapy, and chemotherapy, but a fourth modality of immunotherapy has been well documented since 1890 when Coley demonstrated that bacterial products (Coley toxins) had benefits for inoperable cancers and the subsequent application of Bacillus Calmette-Guerin (BCG) and other crude immunostimulants showed benefits that led to regulatory approval of their use in some solid tumors such as bladder cancer (Fig. 1).7,8 Cancer immunotherapy was generally ignored until the middle of the last century, when chemically induced tumors of inbred mice were found to elicit predictable transplantation resistance, and “spontaneous” regressions of several human solid tumors suggested that tumor regression might be achieved using emerging new immunological approaches.9 In the 1970s and 1980s, immunologists searched for antibodies that would bind to tumors in the serum of cancer patients, and lymphocytes activated with lectins or with interleukin-2 (IL-2) were found to target tumor cells in vitro.10–12 Cytokines were then investigated in large-scale clinical trials for breast cancer, renal cell cancer (RCC), glioblastoma, lymphoma, and melanoma in the 1980s.1–6 It was during this same period of discovery and early clinical use that interferon-α (IFN-α) was first investigated. Initial experiments with IFN-α were predicated on the erroneous belief that human sarcomas were of viral origin; however, at this same time, IFN-α had demonstrated antitumor activity in hairy cell leukemia, melanoma, RCC, and other solid tumors.13,14 Recombinant IFN-α2, a member of the type I IFN family, was shown to be highly pleiotropic, demonstrating immunoregulatory, antiproliferative, differentiation-inducing, apoptotic, and antiangiogenic properties in multiple malignancies,15–21 and objective tumor response rates of 10% to 20% were observed in phase 1/2 trials for metastatic disease.9,14 In 1986, IFN-α2 (Intron-A [Merck, Whitehouse Station, NJ]; Roferon-A [Roche, Nutley, NJ]) was approved as therapy for hairy cell leukemia and in 1995 it became the first immunotherapy approved by the US Food and Drug Administration (FDA) for the adjuvant treatment of stage IIB/III melanoma.15–21
FIGURE 1.
Key Events in the History of Cancer Immunotherapy.1–7 An earlier publication for Coley can be found at: Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc R Soc Med. 1910;3(Surg Sect):1–48. BCG indicates Bacillus Calmette-Guerin; mAbs, monoclonal antibodies; IFN-α, interferon-α; HCL, hairy cell leukemia; IL-2, interleukin-2; RCC, renal cell carcinoma; Peg IFN, pegylated IFN; CTLA-4, cytotoxic T lymphocyte antigen-4; MAGE-1, melanoma-associated antigen 1. Adapted from Kirkwood JM, Tarhini AA, Panelli MC, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445–3455.
IL-2 (aldesleukin [Proleukin; Prometheus Inc, San Diego, Calif]) was the second exogenous cytokine to demonstrate antitumor activity against solid tumors, including melanoma and RCC, and was approved by the FDA in 1998 for the treatment of metastatic melanoma. IL-2, a glycoprotein first described in 1976 as a T-cellgrowth factor,22 plays a central role in immune regulation and T-cell proliferation.23 High-dose bolus intravenous IL-2 antitumor effects were observed with and without lymphokine-activated killer cells in 8 clinical trials that took place between 1983 and 1995 and involved 270 patients with advanced metastatic melanoma. Retrospective long-term analysis of these phase 2 studies demonstrated an objective response rate of 16% (median duration, 8.9 months; range, 4 months-106+ months) and durable in 4%,2–6 suggesting a memory T-cell response. The toxicities associated with high-dose IL-2 were severe but reversible; such toxicities sometimes included hemodynamic complications that required hospitalization in specialized or intensive care units.2–6 Autoimmunity and the appearance of thyroid dysfunction were identified early as correlates of improved outcome for patients receiving this therapy, although the correlation of autoimmunity and therapeutic outcome has not been rigorously analyzed and reported. Autoimmunity induced as a collateral event in association with antitumor effects has been noted with IL-2, but more carefully correlated with the adjuvant antitumor effects of IFN-α24 and most recently with the anticytotoxic T lymphocyte antigen-4 (CTLA-4)–blocking antibody ipilimumab, the newest FDA-approved immunotherapy for advanced metastatic melanoma.25–27 Ipilimumab has provided the first phase 3 trial evidence of a survival benefit in advanced melanoma, further suggesting that autoimmunity against nontumor antigens (non-TAs) in the host may accompany antitumor responses and may be related to the abrogation of host immune tolerance to the tumor (Fig. 2).
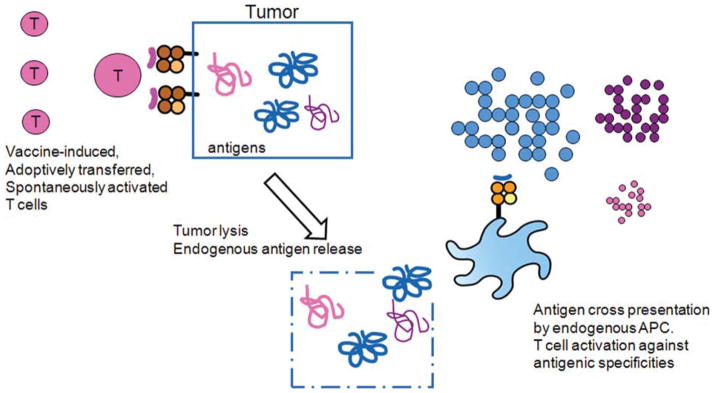
FIGURE 2.
Cross-Presentation/Determinant Spreading. Self-antigen cross-presentation is thought to be a mechanism for inducing autoimmunity, which can involve tumor antigens (and determinant spreading from an antigen immunized against to self-antigens not specifically immunized against) and normal self-antigens (such as thyroid antigens). Cell lysis in an immunogenic milieu allows endogenous antigen-presenting cells (APCs) to take up these self-antigens, cross-present them, and activate T cells with new specificities.
This review will discuss the role of multiple modalities of immunotherapy in a series of signal solid tumors. It will focus specifically on our current understanding of TAs that mark human cancers, which enable the therapeutic manipulation of immune reactivity to achieve durable antitumor responses, with the likelihood of cure for some solid tumors. The analysis of tumor-infiltrating lymphocyte (TIL) responses to cancer antigens has served to define many of the relevant TAs, and adoptive cellular immunotherapy with TILs has achieved some of the most remarkable immunotherapy results to date.28,29 However, these will not specifically be reviewed in detail here, since their reproducibility and applicability outside research institutions has not yet been established.28,29
Harnessing Peptides and Proteins for Cancer Vaccination as Immunotherapy
The first successful identification of TAs recognized by T cells occurred in 1991, when multiple TAs were identified in a range of solid tumors.30 TAs, identified by different approaches, may be classified into 5 categories: differentiation antigens, cancer-testis or cancer-germline antigens, mutated antigens, overexpressed antigens, and viral antigens. TAs stimulate cellular and/or humoral responses in cancer patients,31 and give rise to epitopes or fragments that are presented at the surface of tumor cells in the context of the major histocompatibility complex (MHC) class I molecules, and may stimulate CD8+ T cells. They also give rise to MHC class II epitopes that are presented in the context of MHC class II molecules by antigen-presenting cells (APCs) and sometimes by tumor cells, where they may be recognized by CD4+ T cells. Unlike full-length proteins, which contain all potential MHC class I and MHC class II epitopes capable of stimulating CD8+ and CD4+ T cells, respectively, each peptide binds to a well-defined MHC molecule and is of interest only for patients expressing the specific MHC molecule to which the peptide is bound. One exception is the so-called “promiscuous” MHC class II epitopes, which are capable of binding to multiple MHC class II epitopes.32–34 A large number of peptides derived from multiple TAs and capable of binding to MHC class I and II molecules have now been identified,35 allowing the development of peptide- and protein-based vaccines for a variety of different cancers. Peptides represent short amino acid portions of tumor proteins that may be manufactured under Current Good Manufacturing Practices (cGMP) conditions that allow their use as pharmaceuticals. They are easy to produce and generally have been safe. A large number of vaccines comprised of peptides and proteins targeting multiple types of cancers have been tested to date. Collectively, the first generations of peptide and protein vaccines have shown evidence of clinical benefit in a minority of patients with advanced cancers.28,36 The development and use of such vaccines, with varying clinical effectiveness, have enabled an improved understanding of the specific mechanisms tumor cells use to counteract vaccine-induced immune responses. Such information will be critical for the optimization of future vaccines, providing knowledge critical to improving each of the components (ie, the antigen, the immune adjuvants, and potential combinations) needed to develop and implement more effective vaccines/immunotherapies.
Peptides can be modified to increase their binding to MHC molecules to stimulate stronger CTL responses. This has successfully been performed with the melanocyte-specific GP100 melanoma antigen and melanoma antigen recognized by T cell (MART-1) analog peptides for melanoma.37,38 However, these peptide analogues often stimulate peptide-reactive but not tumor-reactive CTLs, which do not improve tumor rejection.39,40 Because CD4+ T cells play an important role in promoting the persistence of memory CD8+ T cells that recognize and destroy tumor cells,41,42 the introduction of T-helper (Th) epitopes in addition to CTL epitopes appears to represent a major improvement over previous peptide vaccines and is currently under clinical investigation. Although CD4 epitopes derived from TAs may provide help to APCs, which cross-present TA to CD8+ T cells, their in vivo superiority to general “helper” epitopes such as tetanus toxoid, keyhole limpet hemocyanin (KLH), or pan DR (PADRE) remains to be determined. In support of the critical role of TA-derived CD4 epitopes, a clinical trial with long peptides spanning the complete sequence of the oncogenic proteins E6 and E7 of human papillomavirus type 16 (HPV-16) has induced HPV-16–specific CD8+ and CD4+ T cells and generated clinical responses in women with HPV-16–positive, grade 3 vulvar intraepithelial neoplasia.43 It is noteworthy that the same tumor epitopes can stimulate not only CD4+ Th but also regulatory T cells that can decrease the expansion of CTLs.44 Therefore, additional strategies to preferentially promote the expansion of TA-specific Th cells are needed. One major weakness of protein-based vaccines is their inconsistent ability to stimulate effector T cells; specifically, protein vaccines tend to elicit incomplete responses because although they are quite capable of stimulating TA-specific CD4+ T cells, they are only poorly able to stimulate TA-specific CD8+ T cells.45,46 To increase the capability of APCs loaded with protein to give rise to MHC class I epitopes (ie, to cross-present), new approaches are being investigated in pilot trials; one example is the use of protein coupled with antibody to the mannose receptor or DEC-205.47,48
Peptides and proteins given alone as vaccines elicit weak immune responses in vivo. The use of immunological adjuvants allowing the slow release of antigen and increasing the presentation of antigens by APCs to immune cells has been recognized as a critical method in the induction of more effective immune responses. Among the adjuvants in current use with cancer vaccines are aluminum salts, oil-in-water emulsion (MF59), and nontoxic derivatives from Salmonella (MPL), as well as water-in-oil emulsions (Montanide ISA 51 and ISA 720) and the saponins (ISCOM, QS-21, AS01 and AS02).
A major new advance in the field of peptide and protein vaccines has been the introduction of toll-like receptor ligands (TLRL), which potently activate APCs in vivo. These include TLR3L, TLR4L, TLR7/8L (imiquimod, resiquimod), and TLR9L (CpG). Notably, some TLRLs such as TLR3L have pleiotropic effects, activating APCs as well as natural killer (NK) cells, and mediating tumor cell death.44 Several of these TLRL adjuvants are currently under investigation in combination with new cancer vaccines. CpG is a potent adjuvant for peptide and protein-based cancer vaccines, stimulating ex vivo detectable TA-specific CD8+ T cells in patients with advanced cancers.38,39 In contrast, granulocyte-macrophage–colony-stimulating factor (GM-CSF) appears to be less effective as an adjuvant, decreasing vaccine-induced immune responses to multipeptide vaccines.49
Although a number of peptide vaccines and adjuvants have suggested increased TA-specific immune responses and modest clinical benefits,36,50 there is ample evidence of high levels of CTL responses to TA in patients with progressive cancer.39,51 This observation stresses the need to better understand the mechanisms of tumor-induced immunosuppression that may impede vaccine-induced T cells in promoting tumor rejection.52 A number of combinatorial therapeutic strategies to counteract immunosuppression in vivo are currently under investigation. One promising area is the development of monoclonal antibodies (mAbs) that target coinhibitory molecules such as the programmed death receptor 1 (PD-1) expressed by TA-specific T cells in patients with advanced cancers.53,54 We have observed that spontaneous and vaccine-induced CD8+ T cells and TA-specific CD8+ T cells upregulate PD-1 and that PD-1 blockade enhances the antitumor functions of such immune cells.55 In addition, we have observed that highly dysfunctional TA-specific CD8+ T cells in patients with advanced melanoma upregulate coinhibitory molecules such as the T cell immunoglobulin and mucin-domain–containing molecule 3 (T cell immunoglobulin mucin-3 [TIM-3]) in addition to PD-1. Important, blockade of PD-1 and TIM-3, appears to restore TA-specific T cell functions.56 Of particular interest, a recent trial with anti–PD-1 antibodies has shown evidence of prolonged antitumor responses in patients with advanced cancers.57 Our findings in the laboratory as well as in the clinic strongly support the potential efficacy of CPG-based vaccines combined with PD-1 and TIM-3 blockade to enhance vaccine-induced T cell immune responses and reverse tumor-induced T cell dysfunction. Increasing the likelihood of clinical benefits in patients with advanced cancers.
Role of Dendritic Cells in Cancer Immunotherapy
Dendritic cells (DCs), sometimes called “nature’s adjuvant,” are induced in the course of immunization with cancer vaccines, but are potentially subject to the same immunoregulatory mechanisms that have restrained vaccination as discussed in relation to T cells. The development of approaches to generate DCs ex vivo has circumvented issues relating to the dysfunction of endogenous DCs in patients with cancer, allowing controlled “loading” of DCs with antigens to ensure delivery of the proper signals for effective immunization. These signals required for effective immunization are, initially, antigen uptake and T-cell selection (“signal 1”), which determines the specificity of T-cell response, and costimulatory molecule-mediated expansion (“signal 2”), which determines the magnitude of response57–59 of the selected tumor-specific T cells (Fig. 3). Ex vivo production of DCs also allows the imprinting of additional features critical for DCs to induce effective cancer immunity, such as preferential interaction with selected subsets of effector immune cells (rather than regulatory T cells), and imprinting of desirable effector mechanisms in CD4+ and CD8+ T cells60 to selectively enhance Th-1 (Th1)-, CTL-, and NK cell-mediated type 1 immunity (polarizing “signal 3”, which determines effector function [reactive or suppressive] and immunity type [Th1 or Th2]). Additionally, ex vivo production of DCs allows the imprinting of tumor-relevant homing properties of the activated T cells (ie, induction of TA-targeting pattern [“signal 4”], which governs trafficking of immune cells and potentially guides T cells to tumor tissue/sites of metastasis).61
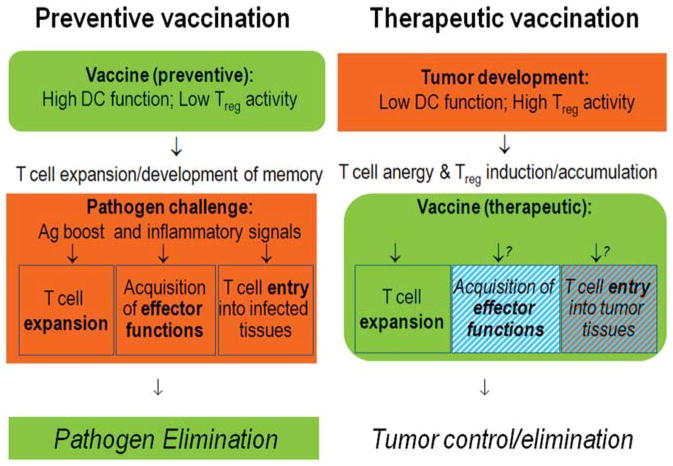
FIGURE 3.
Different Tasks of Preventive Versus Therapeutic Vaccines. Therapeutic vaccines need to function despite tumor-induced dysfunction of endogenous dendritic cells (DCs) and in the presence of tumor-induced suppressive cells such as regulatory T cells (Treg). Their roles go beyond the induction of long-lived memory cells, because cancer is a poor source of proinflammatory alarm signals capable of inducing effector functions and peripheral homing potential in antigen (Ag)-specific T cells. The effectiveness of therapeutic vaccines may require the provision of such signals by the vaccines themselves or by additional factors used in combination with the vaccines. Some tumors show limited production of the chemokines capable of attracting effector cells (cytotoxic T lymphocytes [CTLs], T helper-1 [Th1-], and natural killer [NK] cells), and rather produce Treg-attracting chemokines. Effective immunotherapies for cancer may benefit from the combination of vaccines with additional modulation of the production of the effector cell-attracting versus Treg-attracting chemokines within tumor tissues.
While the goals of “therapeutic vaccines” requiring the induction of large numbers of T cells specific for unique TAs (delivery of “signal 1” and “signal 2”)62–66 are shared in part with protective vaccines, several aspects of therapeutic vaccination against cancer pose additional challenges (Fig. 4). Since therapeutic cancer vaccines need to function in the presence of established tumor and tumor-associated immune dysfunction, including the expansion of regulatory T(reg) cells and myeloid-derived suppressor cells (MDSCs),67–70 the use of ex vivo-matured DCs, which acquire significant resistance to inhibitory factors,71–73 has become a therapeutic option. Unfortunately, while preexisting Tregs are known to limit the effectiveness of cancer vaccines,61,67–70 their numbers can be even further expanded by some of the currently applied DC vaccines.70,74,75
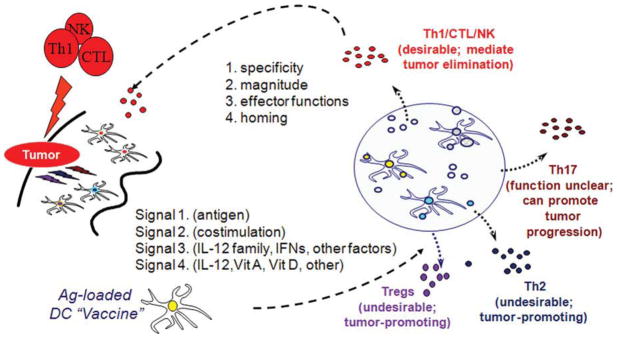
FIGURE 4.
Dendritic Cells Provide Different Types of Information to Tumor-Specific T Cells. Dendritic cells (DCs) provide T cells with antigenic “signal 1” and costimulatory “signal 2,” which are needed for the activation and expansion of pathogen-specific T cells. DCs have also been shown to provide T cells with an additional polarizing “signal 3,” driving the development of different effector mechanisms and activating various subsets of immune cells with different abilities to induce cancer rejection. Recent studies have indicated that DCs also provide T cells with an additional signal (“signal 4”) regulating the organ-specific trafficking of immune cells. DCs regulate the expansion and acquisition of effector functions, as well as tumor-relevant homing properties for the development of effective immunotherapy. Th indicates T helper; NK, natural killer; CTLs, cytotoxic T lymphocytes; IL-12, interleukin-12; IFNs, interferons; Vit A, vitamin A; Vit D, vitamin D; Tregs, regulatory T cells.
The second challenge to therapeutic vaccination is the need to substitute for the pathogen-induced acute inflammatory response (“booster” immunostimulatory signals to T cells in preventive vaccines for infectious agents), which induces associated effector functions and enables the acquisition of peripheral homing function76–82 by tumor-specific T cells. In contrast to viral and bacterial infections, which act as sources of effector cell-attracting chemokines and direct effector cells to the sites of pathogen entry,82–89 therapeutic cancer vaccines need to be particularly effective in inducing T cells that respond to chemokines spontaneously expressed by tumors (which use chemokines themselves for growth, metastasis, and survival),85–89 and/or they need to be combined with additional factors able to modulate expression of tumor-produced chemoattractants.
The early promise of the therapeutic vaccines involving partially mature “first-generation” DCs in follicular lymphoma and melanoma in the 1990s90,91 led to the exploration of DCs for the treatment of patients with numerous other malignancies. These DCs expressed suboptimal levels of costimulatory molecules, at least when assessed directly after removal from culture, and constituted weaker immunogens than the mature DCs used in “second-generation” DC vaccines.92,93 They showed limited ability to induce “objective” clinical responses as assessed by Response Evaluation Criteria In Solid Tumors (RECIST) or World Health Organization (WHO) criteria,28,64,94–97 but 2 recent phase 3 trials98 of “first-generation” DC-based vaccines (sipuleucel-T [Provenge; Dendreon Corporation, Seattle, Wash]) have demonstrated the prolonged overall survival (OS) of patients with advanced hormone-refractory prostate cancer.95–103
These data demonstrate that immature DCs have a potential therapeutic role, even in advanced cancer, and raise the question of whether RECIST criteria of clinical response, which were developed to assess the cytotoxic effects of chemotherapeutic agents, are an optimal reference frame for the prediction of the long-term benefits of cancer vaccines.104 This question is particularly relevant in view of essential differences between the modes of action for cancer vaccines and for cytostatic drugs that directly target tumor cells. In contrast to chemotherapeutic agents, and newer molecularly targeted inhibitors of oncogenes that are cytotoxic, cancer vaccines and other immunotherapies that target the patient’s immune system need first to reprogram the pattern of interactions between the immune system and the tumor. This may explain the delay and frequent lack of early “acute” tumor rejection, despite later evidence of reduced tumor progression and improved patient survival.
To overcome the limitations of immature or partially mature DCs constituting the “first generation” of DC vaccines, numerous approaches to induce fully mature DCs for clinical use were undertaken. Initially, 2 modalities involving prostaglandin E2 (PGE2) were advanced: macrophage-conditioned medium105,106 and a cytokine cocktail involving IL-1α, tumor necrosis factor-α (TNF-α), IL-6, and PGE2107 to induce mature DCs with high expression of costimulatory molecules and high surface expression of CCR7 and the associated high migratory responsiveness to the lymph node-produced chemokines CCL19 and CCL21.108,109 This cocktail108 showed enhanced immunogenic function in vitro and in vivo in healthy volunteers,92,93 as well as improved migratory responses to lymph node-associated chemokines compared with immature DCs,108–110 and has been applied in numerous clinical trials. Unexpectedly, a randomized comparison (vs dacarbazine) in a phase 3 trial for advanced melanoma showed limited clinical responses (<5%) and no survival advantage.111 While the heterogeneous quality of the DCs produced in different laboratories might have affected the results of this multicenter trial, the negative impact of PGE2 on the production of IL-12p70,71–78,112 the factor central to the induction and survival of type 1 immune cells,113 and its ability to promote the interaction of DCs with Treg cells present in cancer patients70 are possible culprits.
To circumvent the limitations of the first 2 generations of DC vaccines, several groups, including ours, demonstrated the feasibility of inducing mature DCs with an elevated rather than “exhausted” ability to produce IL-12 and Th1-, CTL-, and NK cell-activating factors. This was accomplished by exposing immature DCs to type 1 and type 2 IFNs combined with TNF-α or TLRLs,72,114–127 or exposing DCs to activated NK cells or memory CD8+ T cells.116–119 The resulting “type 1 polarized” DCs (DC1s; for DCs inducing Th1-polarized responses) show a strongly enhanced capacity to induce durable antitumor CTLs, TH1, and NK cells in the human model in vitro and in mouse models in vivo. When compared with DCs matured with IL-1α, TNF-α, IL-6, and PGE2, polarized DC1s loaded with tumor peptides or whole tumor cells induce an average of 20-fold to 70-fold higher numbers of functional tumor-specific CD8+ T cells than PGE2-matured DCs.114,115 Our data relevant to melanoma,114,125 chronic lymphocytic leukemia (CLL),115 head and neck cancer,114,125 and several other forms of cancer uniformly demonstrate the feasibility of generating DC1s from patients with advanced cancer, their effective loading with peptide antigens115 or apoptotic tumor cells,115,125 and their high effectiveness in inducing tumor-specific CTLs. While our recent work focused on IFN-α–supported DC1s (αDC1s)114,115 and DC1s induced by autologous NK cells or memory-type CD8+ T cells,116–119,125–128 the data from several other groups123 show the feasibility of generating analogous DC1s with the combination of IFN-γ with lipopolysaccharide (LPS) (including its clinical grade “detoxified” form, monophosphoryl lipid A).119–121 Additional ways of enhancing the desirable properties of DCs129–131 (that can potentially be combined with DC1 polarization) include the use of IL-15 (instead of the usually applied IL-4) to promote early DC development,127 B7-DC cross-linking,128 inhibition of p38MAPK,132,133 or T-bet transduction of DCs.134
Of interest, while polarized and nonpolarized DCs both induce the expansion and CD45RA to CD45RO conversion of naive CD8+ T cells, the induction of effector T cells expressing granzyme B and perforin, and able to mediate cytolytic CTL activity, strongly benefits from priming with polarized DC1s.135 Moreover, the MART-1–specific136–144 CD8+ T cells from patients with melanoma positive for human leukocyte antigen A2 (HLA-A2+) activated by polarized DC1 also showed elevated levels of CCR5 (receptor for CCR1, CCR2, and CCR5) and CXCR3 (receptor for CXCL9, CXCL10, and CXCL11),135 the chemokine receptors involved in T cell traffic into melanomas and other tumors.84,136–138,145 An additional advantage of eliminating PGE2 and including IFN-α in the DC1-inducing maturation cocktails is the enhanced production of CXCL9, CXCL10, CXCL11, and CCL5 by resulting mature DCs and their decreased production of CCL22, allowing preferential interaction with CXCR3- and CCR5-expressing CTLs, Th1, and NK cells. This would allow selective expansion of these subsets and support their functions in avoiding CCR4 (receptor for CCL22)-expressing suppressor/regulatory cells.70
The clinical activity of IFN-α–supported, type 1 polarized DCs (αDC1s) is currently being evaluated in various cancers, including melanoma, glioma, colon cancer, and prostate cancer (NCT00390338, NCT00099593, NCT00766753, NCT00558051, and NCT00970203, respectively), with clinical trials of “third-generation” DC-based vaccines ongoing in other centers.140 In a recently completed phase 1/2 trial for glioma, HLA-A2+ patients with recurrent disease received intranodal injections of ephrin type-A receptor 2 (EphA2),141 IL-13Rα2,142 YKL-40-, and gp100-loaded αDC1s. Among the 22 patients who completed the trial, 9 displayed prolonged progression-free survival (PFS) at 12 months (compared with an expected PFS of 2 months-4 months for this group of patients).140 OS analysis is pending, but the ability of DC1 vaccines from the individual patients to produce IL-12p70 proved to be the predictive marker of prolonged PFS in vaccinated individuals in accordance with the key role of IL-12 and type-1 immunity in resistance against intracellular infectious and cancer.143–148
Harnessing Antitumor Antibodies for Cancer Therapy
The development of the hybridoma technology149 in the 1970s overcame past difficulties. Hybridoma technology enabled the production of highly specific antibodies against human TAs in quantities sufficient for therapy, and with a reliability that would allow its application by multiple investigators for the production of mouse mAbs to many human TAs. Some, such as the carcinoembryonic antigen (CEA), were known TAs that had been already extensively characterized; others, such as chondroitin sulfate proteoglycan 4 (CSPG4), were newly identified TAs.150 The high degree of mAb specificity and the availability of large amounts of purified, well-standardized mAb preparations stimulated their enthusiastic use by tumor immunologists and clinical oncologists and ultimately led to TA-specific and subsequent immunoregulatory mAb-based immunotherapy trials in patients with various types of cancer. Facilitated by the less stringent regulatory requirements compared with the current era, many clinical trials were implemented at various centers and a large number of patients were treated.151 Contrary to expectations, the results were initially disappointing, most likely due to the immunogenicity of mouse mAbs and their poor ability to recruit human immune effector mechanisms. These problems have now been overcome through the replacement of mouse mAbs with chimeric, humanized murine and fully human mAbs, which display limited, if any, immunogenicity in the human, and improved recruitment of human antitumor effector mechanisms.
A growing body of clinical evidence indicates that mAb-based immunotherapy against TAs is an effective treatment method for both hematological malignancies and solid tumors. As single agents, mAbs specific for TAs yield response rates of 8% to 10% in patients with advanced stage, heavily pretreated cancer, and in patients with recurrent disease.151–156 The response rates increase to 30% when TA-specific mAbs are combined with chemotherapy and/or radiotherapy. As a result, mAbs have become part of the therapeutic repertoire for lymphoma, with the anti-CD20 mAb rituximab and the anti-human epidermal growth factor 2 (HER-2) trastuzumab routinely used for the treatment of breast cancer, and the anti-human epidermal growth factor receptor (EGFR) cetuximab routinely used for the treatment of head and neck cancer and colorectal carcinoma. The statistically significant improvements in response, disease-free interval, and survival are well documented.151 Responses are usually observed weeks following the administration of TA-specific mAbs. Hematologic malignancies are in general more responsive than solid tumors to mAb-based immunotherapies, with higher tumor penetration and lower doses of mAbs required to achieve therapeutic response. Furthermore, radioimmunotherapy has been effective in hematologic malignancies. In general, side effects of TA-specific mAb immunotherapy have been mild. Most toxicities have been allergic or hypersensitivity reactions caused by the foreign protein. Rare, but more serious, side effects such as tumor lysis syndrome and renal failure result from a therapeutic antibody binding to its target antigen (Fig. 5).
FIGURE 5.
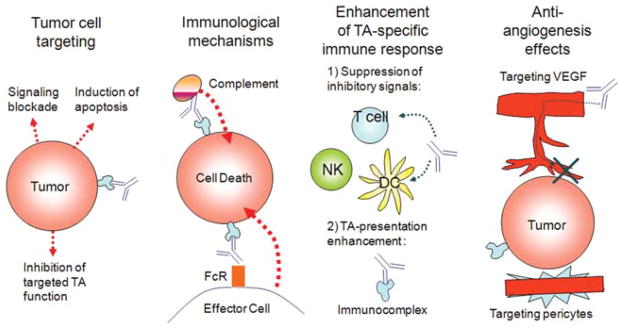
Mechanisms Used by Monoclonal Antibodies to Mediate Antitumor Effects. Multiple roles of monoclonal antibodies (mAbs) in cancer therapy are shown.
Several molecular mechanisms were shown to underlie the therapeutic efficacy of TA-specific mAb-based immunotherapy.151 These mechanisms interact with one another extensively and can broadly be divided into those that utilize immune effector mechanisms and those that do not (Figs. 5 and 6). Among the latter mechanisms is the ability of TA-specific mAbs to block the activation signals required for continued malignant cell growth and/or viability. These effects are mediated by inhibition of the interactions of ligand with its receptor, induction of modulation of the receptor, and/or dimerization of the receptor. Anti–CTLA-4–blocking antibodies recently approved for melanoma therapy and anti–PD-1 antibodies that have shown benefits in non-small cell lung cancer (NSCLC) and melanoma are recent examples of this class of immune checkpoint-blocking antibodies. The therapeutic efficacy of several other clinically approved TA-specific mAbs such as rituximab and cetuximab is mediated by more classical immunologic mechanisms; their efficacy as therapeutic agents is specifically reflected in their ability to mediate complement- and cell-dependent lysis of TA-expressing target cells, both in vitro and in animal model systems. The clinical significance of these findings is indicated by the association found between the clinical course of the disease and polymorphisms of the Fcγ receptors (FcγR), which mediate the interactions of effector cells such as monocytes and NK cells with tumor cells coated with therapeutic mAbs.155–159 This association may reflect the impact of the FcγR polymorphism on the extent of lysis of tumor cells coated with TA-specific mAbs by effector cells in vitro and on their ability to control growth of human tumors in immunodeficient mice. An additional strategy that relies on the ability of TA-specific mAbs to target T cells to tumor cells is represented in bispecific antibodies. This approach, first described over 20 years ago, links the variable region of a TA-specific mAb to the variable region of a mAb that recognizes a cell surface molecule of T cells. As a result, bispecific antibodies can trigger the T cell compartment, overcoming the restriction of clonotypic specificity. Many bispecific antibody formats are being developed to overcome the difficulties in producing these reagents at a clinically relevant quantity and purity. Among the various formats tested, the bispecific T cell engagers have attracted much interest, since they are relatively stable and easy to produce, and are very potent at low doses.
FIGURE 6.
Mechanisms Underlying the Antitumor Activity of Monoclonal Antibody-Based Immunotherapy. TA indicates tumor antigens; FcR, Fc receptor; NK, natural killer; DC, dendritic cell; VEGF, vascular endothelial growth factor.
Growing evidence suggests that an important therapeutic role of TA-specific immunotherapy is played by the TA-specific T cell immunity induced by TA-specific mAbs.160–162 The injected TA-specific mAb may enhance antigen uptake through FcγR on DCs in the tumor microenvironment or draining lymph nodes. TAs are then presented on HLA class I antigens to CD8+ T cells, which can recognize and destroy them.
As previously stated, TA-specific mAb-based immunotherapy is effective in up to 30% of treated patients; however, the effect is frequently limited in duration. Additionally, some cancers are not responsive to TA-specific mAb-based immunotherapy, even though the targeted TA is expressed in malignant lesions. Currently, little is known about the mechanisms underlying patients’ differential clinical responses to TA-specific mAb-based immunotherapy. However, the discovery of specific immune response mechanisms is a critical area for current and future immunology/tumor biology research and will, as it unfolds, lead to the development of improved patient selection criteria for these treatments, ultimately helping clinicians better match patients to the immunologic treatments most likely to benefit them.
Additionally, as already mentioned, the polymorphism of FcγR expressed on monocytes and NK cells appears to have clinical significance, since the affinity of the receptors for the Fc portion of TA-specific mAbs is associated with the clinical course of the disease. However, these associations have not been found in all diseases. Furthermore, when they are found, while statistically significant, they are not absolute. Moreover, the association between FcγR polymorphism and the clinical course of the disease has been found only in some subsets of cancer, and a pattern as to which cancers (or cancer subsets) it appears in has yet to be clearly identified. An example of one cancer subset in which such an association has been identified is colorectal carcinoma, in which the association between FcγRIIIa polymorphism and clinical response to immunotherapy with the (EGFR)-specific mAb cetuximab is statistically significant only in patients with colorectal carcinoma who do not harbor activating KRAS mutations in their tumors.163 In vitro experiments suggest that the targeted TA expression level and patients’ disease status may also play a role in the differential clinical response to TA-specific mAb-based immunotherapy.
One area of resistance to mAb-based immunotherapy that has not been adequately studied is the role of the idiotypic network and escape mechanisms used by tumor cells to avoid recognition and destruction by the host immune system. According to the idiotypic network theory,164 for which Jerne was awarded the Nobel Prize in Medicine in 1984, the administration of antibodies to a host induces antibodies to epitopes (ie, idiotopes) expressed in the variable region of the injected antibodies. Some of the idiotopes are located in the antigen-combining site of the injected antibodies. The resulting binding of the antiidiotypic antibodies to the antigen-combining site of the injected antibodies inhibits their binding to the corresponding antigens and thereby neutralizes their effects on tumor cells. Furthermore, some antiidiotypic antibodies may mimic the nominal TA and therefore may induce TA-specific antibodies. The potential clinical relevance of the idiotypic network in patients treated with TA-specific mAbs is suggested by the role of the idiotypic network in the modulation of the immune response165 and by the association between triggering of the idiotypic network and favorable clinical course of the disease in patients with neuroblastoma treated with disialoganglioside (GD2)-specific mAb-based immunotherapy.166 The potential role of TA-specific cellular immunity in the therapeutic efficacy of TA-specific mAb-based immunotherapy raises the possibility that defects in the expression and functional properties of HLA antigen-processing machinery components167 may play a role in patients’ differential clinical response to this type of therapy.
Antibodies as Vehicles for Therapy for Cancer and as Targeted Pathway Inhibitors
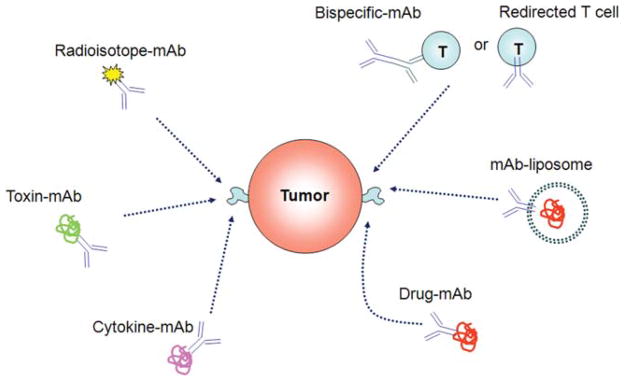
One approach that has revolutionized the treatment of cancer involves mAbs targeting tumor-specific antigens, receptors, or their ligands to block major pathways central to tumor cell proliferation and survival. Some mAbs are modified to deliver toxins, radioisotopes, cytokines, or other active conjugates, while other mAbs are designed as biospecific antibodies that bind with their fragment antigen-binding (Fab) regions both to an antigen and to a conjugate or effector cell. Following is a brief discussion of some of these mAbs tested successfully for the treatment of cancer.
Bevacizumab is a recombinant, humanized mAb that binds to, and neutralizes, vascular endothelial growth factor (VEGF), preventing its association with endothelial receptors, Flt-1, and KDR.168 VEGF neutralization inhibits angiogenesis (endothelial proliferation and the formation of new blood vessels). Bevacizumab is approved for the indications of non-squamous NSCLC, colorectal cancer, glioblastoma, and breast cancer.168–170
Cetuximab is a recombinant human/mouse chimeric mAb that targets EGFR (EGFR, HER-1, c-ErbB-1) and competitively inhibits the binding of EGF and other ligands. Binding to EGFR blocks phosphorylation and activation of receptor-associated kinases, resulting in inhibition of cell growth leading to apoptosis. Cetuximab is indicated for the treatment of colorectal cancer and head and neck cancer.171–173 Panitumumab is a recombinant human anti-EGFR immunoglobulin (Ig) G2 mAb.174 Similar to cetuximab, it competitively inhibits the binding of EGF and other ligands to EGFR.174 Panitumumab is indicated for the treatment of metastatic colorectal cancer.175 In metastatic colorectal cancer, the benefits of cetuximab and panitumumab are confined only to the subset of patients whose tumors have wild-type and not mutated K-ras (about 40% of patients).176
Trastuzumab is a mAb that binds to the extracellular domain of EGFR 2 protein (HER-2). This mAb mediates antibody-dependent cellular cytotoxicity by inhibiting proliferation of cells that overexpress the HER-2 protein.177 Trastuzumab is indicated for the treatment of breast cancer.177,178 Rituximab is a mAb directed against the CD20 antigen on B lymphocytes, activating complement-dependent B-cell cytotoxicity, and to human Fc receptors, mediating cell killing through an antibody-dependent cellular toxicity. Rituximab is indicated for the treatment of non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma and CLL.179,180
Ibritumomab (Zevalin; Spectrum Pharmaceuticals, Henderson, Nev) and tositumomab radioconjugate (Bexxar; GlaxoSmithKline, Research Triangle Park, NC) are radioisotope-linked mAbs that act as delivery systems to direct a radioactive isotope to the targeted cells and are indicated for the treatment of B-cell NHL. Tositumomab radioconjugate is a murine IgG2a-λ mAb that binds to the CD20 antigen, expressed on B lymphocytes and on greater than 90% of B-cell NHLs. Iodine-131 tositumomab is a radio-iodinated derivative of tositumomab covalently linked to iodine-131.181 Ibritumomab is an anti-CD20 mAb that is linked with the chelator tiuxetan, which acts as a specific chelation site for either indium-111 or yttrium-90.182
Alemtuzumab is a mAb that binds CD52, leading to antibody-dependent cellular lysis.183 It is indicated for the treatment of B-cell CLL,184 but also has clinical use in cutaneous T-cell lymphoma,185 peripheral T-cell lymphoma,186 and T-cell prolymphocytic leukemia.187 CD52 is expressed on the surface of B and T lymphocytes, macrophages, different types of monocytes, NK cells, and a sub-population of granulocytes.183
Blockade of Immune Checkpoints in the Therapy for Cancer
Tumors are able to evade detection and destruction by the immune system, despite the fact that many tumors such as melanoma appear to elicit a strong immune response that is evident in lymphocyte infiltrates of the primary lesion.188 Tumor immune evasion can be divided into 2 main mechanisms: 1) the induction of immune tolerance and 2) resistance to killing by activated immune effector cells.189 The concept of “immunoediting” relates to the manner in which tumors manipulate their microenvironment through tumor-derived cytokines, chemokines, and other soluble factors.190 Therefore, by the time tumors have become clinically detectable, they have already evolved mechanisms to evade immune response mounted by the host that must be overcome to create effective and durable antitumor immunity.
Monoclonal antibodies that block the immunoregulatory damping mechanisms of host responses to tumor-associated antigens have recently become a practical reality with the first approval of the antibody ipilimumab, directed against CTLA-4, a molecule that downregulates T-cell activation via a homeostatic feedback loop. In normal physiology, this prevents autoimmunity and allows the body to establish tolerance to self-antigens.191 Anti–CTLA-4 mAbs including ipilimumab and tremelimumab block CTLA-4 signaling, prolonging T-cell activation and restoring T-cell proliferation, thus amplifying T-cell–mediated immunity and the patient’s capacity to mount an effective antitumor immune response.191,192 Clinical testing of ipilimumab has now yielded significant new results that have led to the regulatory approval of this agent.
Several phase 3 trials with ipilimumab and tremelimumab in patients with advanced, inoperable, American Joint Committee on Cancer stage III and stage IV melanoma have been reported. The phase 3 trial of tremelimumab was presented at the American Society of Clinical Oncology annual meeting in 2008,193 and was a large, open-label (n =655) comparison of tremelimumab versus chemotherapy with dacarbazine or temozolomide that was interpreted as negative ipilimumab with a hazard ratio (HR) of 1.04 (P =.729). The first trial (MDX-010), which led to the FDA approval of ipilimumab, tested the combination of ipilimumab with gp100 peptide vaccine versus gp100 vaccine alone and versus ipilimumab monotherapy in the second-line setting.194 This study randomized 676 patients who had failed prior therapy and tested ipilimumab induction therapy given at a dose of 3 mg/kg every 3 weeks for 4 doses without maintenance therapy and with responding patients eligible for reinduction with ipilimumab if they relapsed. The 1-year and 2-year survival rates for ipilimumab and gp100 were 44% and 22%, respectively; for ipilimumab and placebo, these corresponding rates were 46% and 24%, respectively, while for gp100 and placebo, the corresponding rates were 25% and 14%, respectively. The overall response rates for ipilimumab and gp100, ipilimumab and placebo, and gp100 and placebo were 5.7%, 10.9%, and 1.5%, respectively. The disease control rates were 20.1% (ipilimumab and gp100), 28.5% (ipilimumab and placebo), and 11% (gp100 and placebo). The median OS was increased from 6.4 months to 10.0 months with the addition of ipilimumab to gp100 vaccine (HR, 0.68; P <.0001) and long-term survival rates improved.194 The survival improvement among patients assigned to the ipilimumab treatment arms was significant (HR, 0.66; P =.68) compared with the control arm of gp100 alone.194 More recently (June 2011), the MDX-024 phase 3 trial was reported. This trial randomly assigned 502 patients with previously untreated metastatic melanoma, in a 1:1 ratio, to ipilimumab (at a dose of 10 mg/kg) plus dacarbazine (at a dose of 850 mg/m2 of body surface area) or dacarbazine (at a dose of 850 mg/m2) plus placebo given at weeks 1, 4, 7, and 10, followed by dacarbazine alone every 3 weeks through week 22. Patients with stable disease or who achieved an objective response and with no dose-limiting toxic effects received ipilimumab or placebo every 12 weeks thereafter as maintenance therapy.195 This study showed that ipilimumab plus dacarbazine has significant survival benefit over dacarbazine alone as first-line treatment in metastatic melanoma (median OS, 11.2 months vs 9.1 months; median PFS, 2.8 months vs 2.6 months; response rate, 15% vs 10%). While this advance in therapy creates substantial new interest in immunotherapy and hope among patients, at the same time this therapeutic strategy presents new challenges in the management of the unique drug toxicities that are associated with the release of immune checkpoints.
Tremelimumab has shown promising clinical activity in advanced melanoma, which led to a phase 3 clinical trial (A3671009) that was conducted in patients with treatment-naive advanced melanoma as mentioned earlier. This study randomized patients to therapy with single-agent tremelimumab (n =328) or standard-of-care chemotherapy (n =327) with either dacarbazine or temozolomide.193 The primary endpoint was OS. At second interim analysis, the log-rank test statistic (P =.729) crossed the prespecified O’Brien-Fleming futility boundary and consequently the trial was halted. Nevertheless, the majority of responses to tremelimumab were durable. The 1-year survival rate of greater than 50% for tremelimumab and the median survival of 12.02 months (compared with 10.45 months for chemotherapy) are notable, although this may have been the result of the selection criteria for this study.
Ongoing clinical trials are building on these studies to refine the immunotherapeutic strategies through more effective combinations to overcome tumor-induced immune suppression and tumor evasion and further, to identify biomarkers of prognostic and therapeutic predictive value. A phase 2 study of anti–CTLA-4–blocking antibodies and high-dose IFN-α2b has already shown benefit that appears to be at least additive.196,197 Several other mAbs targeting important immunoregulatory checkpoints such as PD-1 appear to be promising as monotherapy but are in earlier stages of development and are awaited in combinations.
PD-1 is an inhibitory receptor belonging to the CD28/CTLA-4 receptor family that is expressed on activated T cells, B cells, and monocytes.198,199 PD-1 is also expressed on Tregs, where it interacts with DCs and NK T cells, and has been shown to be associated with anergy and tumor immune escape.200–202 PD-1 is induced by T cell receptor signaling and is upregulated on nonfunctional, exhausted T cells in chronic viral infection and cancers. The ligands for PD-1, are PD ligands (PD-Ls) 1 and 2 (also known as B7-H1 and B7-H2 based on their similarity to other B7 family molecules). PD-L1 is expressed on T cells, B cells, macrophages, DCs, and some nonimmune cells and is upregulated after their activation. Type 1 and type 2 IFNs upregulate PD-L1 expression by tumor cells, including melanoma cells. PD-L2 is regulated more tightly and is expressed mainly on activated macrophages and DCs. Interestingly, PD-L1 ligation has been implicated in escape from the host immune system as well as tumor antiapoptotic activity.199,203,204 PD-L1 and PD-L2 expressed on APCs have been shown to induce T cell anergy or apoptosis via PD-1 on T cells, whereas PD-L1 expressed on peripheral tissues directly suppress self-reactive lymphocytes.205,206 PD-Ls expressed on tumors regulate the generation of adaptive Tregs, resulting in tumor-induced immune suppression including the suppression of the effector function of CD8+ T cells.202 A significant inverse correlation has been observed between PD-L1 expression and the intraepithelial CD8+ T lymphocyte count, suggesting that PD-L1 on tumor cells directly suppresses antitumor CD8+ T cells.207 PD-1 blockade has been shown to enhance the expansion and functional capacity of human melanoma antigen-specific cytotoxic T cells.208 In animal models, PD-1 receptor-deficient mice (PD-1 genetic knockout mice) develop autoimmunity manifestations such as lupus-like autoimmune diseases including nephritis and arthritic changes and autoimmune-dilated cardiomyopathy.209,210 PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces Tregs and myeloid cells within B16 melanoma tumors.211 Anti–PD-1 synergizes with GM-CSF–secreting tumor cell immunotherapy, providing therapeutic benefit to mice with established tumors.212 Clinically, the expression levels of PD-L1 on tumors have been shown to correlate with poor clinical outcome for patients with cancers of the esophagus, kidney, lung, pancreas, brain, ovary, and head and neck, as well as melanoma.207,213–217
These data strongly support a central role for PD-1 and its ligands in tumor immune escape and support the clinical targeting of PD-1 as an antitumor strategy that would overcome the PD-1–PD-L axes and potentiate the function of tumor-specific T cells. They further support the use of PD-1/PD-Ls pathway blockade in cancer patients to partially restore tumor-specific CD8 (+) T cell numbers and functions, thereby increasing the likelihood of tumor response. Anti–PD-1 mAbs have been developed for clinical applications. CT-011 is a humanized IgG1 mAb that has been tested in a phase 1 study in patients with advanced hematologic malignancies (N =17).218 This study has shown one complete response (a patient with follicular NHL), and one minimal response (in a patient with acute myeloid leukemia [AML]) at the dose levels tested. Several studies with CT-011 used as monotherapy or in combination are ongoing in multiple malignancies.219
MDX-1106 (ONO-4538) is a fully human IgG4 anti–PD-1–blocking mAb that has been tested in a phase 1 study in patients with refractory or relapsed solid tumors (N = 39).220 No maximum tolerated dose (MTD) was reached up to a dose of 10 mg/kg. Two objective responses and 3 cases of stable disease were observed at the dose levels tested. In a subsequent phase 2 study, 21 patients with treatment-refractory solid tumors received a single infusion of MDX-1106 at a dose of 10 mg/kg, including 6 patients who received retreatment.221 One patient with RCC had a durable partial response after 3 doses. Mixed responses with regression of individual lesions were seen in 2 patients with melanoma. Biopsy of a regressing lymph node metastasis showed a moderately increased and selective CD8+ T-cell infiltrate. Subsequent studies include phase 2 testing in melanoma including combinations with ipilimumab and gp100 peptide vaccine as well as monotherapy in other malignancies.222
MDX-1105 (BMS-936559) is a human IgG4 mAb that targets PD-L1 and is designed to disrupt the interaction of PD-L1 on tumor cells with PD-1 on effector T cells. For example, PD-L1 tumor expression has been shown to be present in up to 80% of metastatic melanoma lesions, making melanoma an ideal therapeutic target with this mAb. The blockade of PD-1 and PD-L1 binding with an anti–PD-L1 antibody in a murine AML model has been reported to decrease tumor burden and prolong survival.223 A phase 1, multidose study of MDX-1105 (BMS-936559) administered every 14 days is ongoing in subjects with solid tumors.224
Members of the TNF super family are involved in the regulation of diverse immune functions. CD40 is a costimulatory molecule that is one such member and is widely expressed by immune cells and by cancer cells of different histologies. CD40 expression on immune cells has been implicated in the regulation of humoral and cellular immunity while CD40 expression on certain tumor cell types has been implicated in proapoptotic and antiproliferative activity.225–228 CD40 is broadly expressed on DCs and its activation by CD40 ligand, found on activated T cells, appears to “license” the antigen-presenting cell for T-cell activation.229 CD40 stimulation leads to effective therapy of CD40-deficient tumors through strong induction of systemic CTL immunity.230 Therefore, the targeting of CD40 may have antitumor effects both indirectly through the activation of immune cells and/or directly by provoking tumor cell apoptosis and impaired tumor growth.225 CP-870,893 is a fully human IgG2 agonist mAb targeting CD40. Preclinical testing has shown its ability to activate DCs and B cells as well as antitumor activity in human xenograft models.231 In a phase 1 dose escalation study of a single intravenous infusion in 29 patients, the single-dose MTD was estimated at 0.2 mg/kg, with a dose-limiting cytokine release syndrome found to be associated with acute increases of serum levels of TNF-α and IL-6 and characterized clinically by fevers, chills, and rigors. This was associated with a transient depletion in circulating CD40+ CD19+ B cells and, among B cells remaining in the blood, a dose-related upregulation of costimulatory molecules (CD86) after treatment. This was also associated with the induction of melanoma antigen-specific T cells and clinically, objective partial responses were noted in 4 patients with metastatic melanoma.231
A multiple-dose phase 1 trial of weekly dosing of CP-870,893 for up to 8 doses was conducted in 27 patients. The MTD was again estimated at a dose of 0.2 mg/kg limited by a cytokine-release syndrome.232 A phase 1 study testing the combination of CP-870,893 with carboplatin and paclitaxel in patients with solid tumors has been completed. Others are ongoing, including monotherapy in patients with pancreatic cancer and combinations with tremelimumab and peptide vaccination in patients with melanoma.233
Dacetuzumab (SGN-40) is a humanized IgG1 agonist mAb that also targets CD40.234 A phase 1 single-dose study in patients (N = 17) with lymphoid malignancies, AML, and multiple myeloma demonstrated safety up to a dose of 6 mg/kg, with no MTD declared.235 One complete response was observed and 5 patients with stable disease were reported. A subsequent study tested a dose of 2 mg/kg weekly for 4 weeks in 50 patients with refractory NHL, with additional cohorts of patients receiving dacetuzumab with an intrapatient dose escalation up to 8 mg/kg.236 Six of 50 patients had a response, with one complete response and 5 partial responses. Two phase 1 studies were conducted in patients with multiple myeloma237 and CLL.238 Other trials with SGN-40 were conducted in patients with hematologic malignancies.239
OX40 and its ligand, OX40L, are key TNF members that augment T-cell expansion, cytokine production, and survival. OX40 is best described as a costimulatory molecule that is expressed transiently at the surface of CD4+ and CD8+ T cells upon activation. OX40 is also expressed by CD4+ CD25+ Tregs and controls Treg differentiation and suppressive function. Engagement of OX40 on Tregs appears to abrogate Treg suppressive function.240–242 In murine models, engagement of OX40 in vivo with mAb agonist OX40L:Ig during tumor priming has been shown to have antitumor activity.243 A murine IgG1 agonist mAb targeting OX40 was tested in a phase 1 dose escalation trial. The trial was designed to test 0.1, 0.4, and 2 mg/kg dose levels administered on days 1, 3, and 5 in 3 cohorts of 10 patients each. The results of the first 2 cohorts testing doses of 0.1 and 0.4 mg/kg of antibody have been reported at the 2009 meeting of the Society for Immunotherapy of Cancer (SITC) (formerly the International Society for Biological Therapy of Cancer [iSBTc]) demonstrating acceptable toxicity and with 5 of 20 patients reported to have stable disease.244 More recent studies of CD40 agonists have shown promise against pancreatic cancer.245
CD137 (4-1BB) and its ligand are members of the TNF receptor and TNF families, respectively, and are involved in the regulation of a wide range of immune activities. CD137 ligand cross-links its receptor, CD137, which is expressed on activated T cells, and costimulates T cell activities. The costimulatory function induced by the cross-linking of CD137 on activated T cells enhances T cell proliferation and the memory and cytotoxic activity of T cells.246–248 BMS-663513, a fully human anti-CD137 agonist mAb has been tested in a phase 1 dose escalation study administered at doses of 0.3, 1, 3, 6, 10, and 15 mg/kg and administered every 3 weeks for 4 injections, with retreatment for patients demonstrating stable disease or response.249 Eighty-three patients (54 with melanoma, 15 with RCC, 13 with ovarian cancer, and 1 with prostate cancer) were treated. Three responded and had durable stable disease. A randomized, multidose, phase 2 study of BMS-663513 as a second-line monotherapy in subjects with previously treated advanced melanoma was completed. Two other studies tested combinations of chemotherapy in solid tumors and chemoradiation in patients with NSCLC.250
Genetic Strategies for Cancer Immunotherapy
Many approaches for the genetic immunization of solid tumors have been investigated. Early work attempted to directly inject tumors with plasmid DNA and vectors encoding cytokines and allogeneic MHC molecules, designed to promote an immune response to the tumor but resulting in minimal efficiency (generally only affecting the injected tumor) and minimal (systemic) immunologic impact. Another genetic approach is to use plasmid and/or viral vector DNA to systemically immunize subjects. This approach is inexpensive, simple, and allows for immunization with multiple genes (Fig. 7).
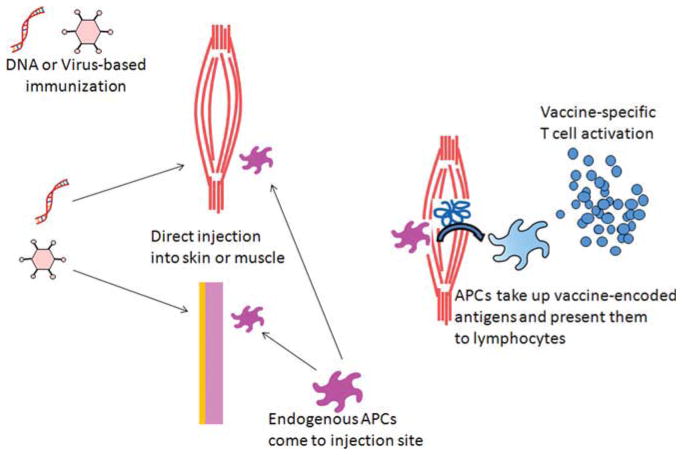
FIGURE 7.
Genetic Immunization. Plasmid DNA and viral vectors can be utilized for vaccination by direct injection, often into muscle or skin. While direct transfection/transduction of antigen-presenting cells (APCs) at the injection site can occur, the transfected tissue serves as a source of vaccine protein that can be taken up as cross-presented by host APCs to activate antitumor immunity.
Naked DNA Immunization
Plasmid-based DNA immunization is a powerful method of immunization against microbial and viral antigens, capable of generating both antibody and cellular responses, particularly in mice.251–253 Uptake of protein antigens produced by locally transfected cells, generally muscle, subsequently taken up by the patients’ endogenous APCs, (“cross-presentation”) is thought to be the main mechanism of generating T cell immunity,254–257 although direct gene transfer to local APCs has demonstrated similar T cell immunity.258 In several animal models immunized with “self”-TAs, this approach has been shown to generate weak antitumor responses.259–261 Therefore, strategies to enhance the ability of naked DNA immunization to generate more potent immune responses have been tested, including the coinjection of muscle-damaging agents, the coadministration of GM-CSF to enhance the attraction of endogenous APCs, or the coadministration of B7-1/B7-2 (CD80/CD86) to add a costimulatory “signal 2,”262 and the coinjection of plasmids carrying other immunostimulatory molecules (CD40-L, IL-2, and IL-12).263,264
A plasmid DNA-based clinical trial tested the self-TA CEA265 for immunizing patients with colorectal cancer. The CEA complementary DNA (cDNA) was embedded in the hepatitis B virus (HBV) surface antigen “helper” cDNA, and this construct was administered intramuscularly to 17 patients. The DNA immunization was safe, and 6 of 8 tested patients became immunized against the foreign viral helper HBV protein, but only 4 of the 17 patients had immune responses to the CEA antigen, indicating that plasmid DNA immunization alone, to a self-antigen, could stimulate a T cell response in some, but not all, patients. A similar study testing a MART-1 plasmid vaccine injected intramuscularly in patients with melanoma did not result in any increased immunity.266 Plasmid immunization into skin via a gene gun was also only minimally immunogenic.267 Injection into lymph nodes may be more immunogenic.268 Together, these studies show the limitations of naked DNA immunization in humans.
Viral Vectors
Viral vectors can be very efficient gene transfer vehicles and many classes have been tested in clinical trials, often with the goal of achieving long-term replacement of defective genes. Each of these viruses differs in important areas of transgene size, capability, host genomic integration, encoded viral genes, and virus immunogenicity.269,270
Retroviruses
These well-studied, small, integrating viruses were the first explored for replacement gene therapy. They transduce only dividing cells and can undergo silencing of the transgene. They are not very immunogenic, and have been produced at clinical grade for many years.
Lentiviruses
These retroviruses also integrate, but they transduce nondividing cells, including APCs, more efficiently, and are less immunogenic than adenoviruses.
Adenovirus
The adenovirus (AdV) is a highly immunogenic vector that can carry 2 to 3 transgenes and has a long safety record. It does not integrate. Direct injection of antigen-encoding AdV is limited by potent induction of neutralizing antibodies.271 These are easily produced at clinical grade.
Adeno-associated virus
This low-immunogenicity vector is small and has a low efficiency of APC transduction. Early studies indicated the potential for long-term trans-gene expression in vivo.
Vaccinia
These large, complex, immunogenic viruses are often lytic to transduced cells. Different subtypes exist, which allows for serial vaccination without cross-reactive neutralizing antibodies. They have been produced at clinical grade for many years. Both viral vectors and plasmid DNA have been used to transduce/transfect immunogenic molecules into cells for genetic immunotherapy vaccines.
DNA and Virally Transfected Cells
Transfected cells (either cell lines or patient autologous tumor) are vaccines able to produce both the full array of TAs in those cells and an immunogenic transgene such as GM-CSF. These have been tested in several formats and cancer settings. Both viral vectors and plasmid DNA have been used to transduce/transfect immunogenic molecules into cells for genetic immunotherapy vaccines. Both virus-transduced autologous tumor and transfected allogeneic cell lines have been tested with GM-CSF (based on preclinical murine model data)272 and are referred to as “GVAX (BioSante Pharmaceuticals, Inc., Lincolnshire, Ill)”. Early studies272–274 demonstrated the immunogenicity of this platform and the potential for some clinical responses, but also the technical obstacles in vaccines based on engineering autologous tumors in sufficient number for vaccination.
Prime-Boost Strategies
Further enhancement of the immunogenicity of plasmid DNA has been achieved by providing a “boost” with a viral vector expressing the same antigen. While we focused on genetic immunization, protein was also used in heterologous prime-boost approaches. It has been postulated that the plasmid DNA “prime” expands TA-reactive T cell precursors (which may have higher avidity due to the limited plasmid-derived antigen), and the viral vector “boost,” by virtue of providing high levels of TA expression in the setting of strong viral immunogenicity, greatly enhances T cell activation and expansion.275–282
In 2 early pilot clinical trials using prime-boost to induce antitumor immunity in human subjects, plasmid prostate-specific membrane antigen (PSMA) (as well as a plasmid encoding B7-2 [CD86], with or without recombinant GM-CSF) was delivered intradermally and then boosted with a PSMA AdV vector. All subjects developed a delayed-type hypersensitivity reaction to PSMA.263 The second trial tested CEA, expressed by each of 2 different viral vectors, vaccinia and avipox. CEA-specific T cells and antibodies were detected. The immune response could be further enhanced by the codelivery of recombinant GM-CSF.283 In these trials, detectable levels of neutralizing antibodies to the viral vectors did not preclude the stimulation of antigen-specific responses. Most recently, a prime-boost trial testing a TAc and “TriCOM” (trio of costimulatory molecules) in pox-based vectors has shown positive clinical outcomes in a large phase 2 trial.284
Evaluation of the Effectiveness of Immunotherapies: Immunologic Monitoring
The primary goal of immunotherapy is promotion of an effective antitumor immune response. These can include promotion of TA-specific CTL responses, tumor-specific antibody, CD4+ helper T cell responses, or non–antigen-specific responses from NK cells; reduction of suppressive Treg or MDSC cells; modulation of signaling cascades; or improvement of the cytokine, chemokine, or growth factor milieu at the tumor or throughout the body. To determine whether the intervention had the hypothesized impact on the cell population or analyte, immunologic monitoring is critical. In addition to addressing the primary immunologic endpoints of an intervention, such studies are also central to identifying prognostic and predictive immunologic biomarkers of response to immunotherapy (Fig. 8).285–288
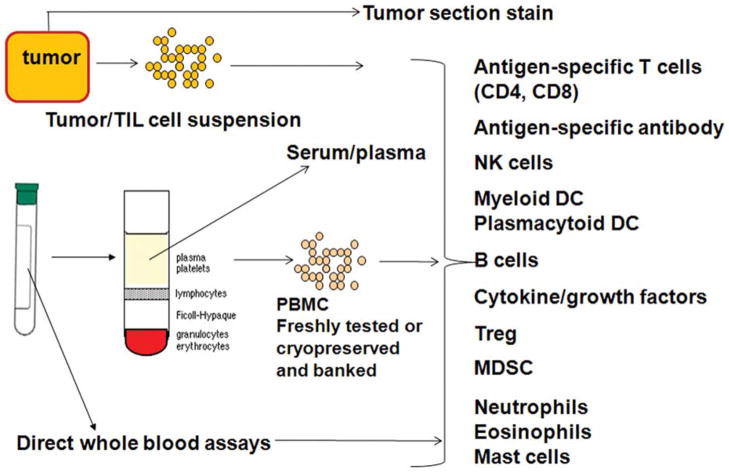
FIGURE 8.
Immunologic Monitoring. Cross-talk between the tumor and the immune system can be examined in tumor tissue and blood. From tumor tissue, infiltrating cells can be identified. From blood, a variety of assays can be performed, some of which require fresh blood. Others can be performed from frozen/cryopreserved samples and batched for directly compared analysis. TIL indicates tumor-infiltrating lymphocyte; PBMC, peripheral blood mononuclear cells; NK, natural killer; DC, dendritic cell; Treg, regulatory T cells; MDSC, myeloid-derived suppressor cells.
Primary Immunological Endpoints
Antigen-specific T cells
There are several methods to test for the presence, frequency, and function of antigen-specific T cells. CD8+ T cells can most commonly be interrogated by peptide-bearing MHC multimers, cytokine enzyme-linked immunospot assays, and/or intracellular cytokine staining and flow cytometry.
Most murine tumor models support the central role of tumor-specific CTLs in tumor protection and eradication, and because of their critical function in immune processes and responses, they are a major immunologic monitoring focus. When the antigen of interest is known, the CD8+ T cells’ ability to recognize MHC class I-restricted peptide epitopes or antigen-expressing, HLA-matched but allogeneic or autologous cells can be tested, and the magnitude and nature of the response can be characterized. “Type I” cytokine (IFN-γ, IL-2) and cytotoxic responses are generally best correlated to antitumor activity.
The precise role of CD4+ T cells (as they are not often directly cytotoxic) and the requirement for cognate, antigen-specific CD4+ T cell responses versus a nonspecifically activated T cell response (agonist tetanus toxoid, KLH, and PADRE) is debated, but the frequency and function of these cells are also important to assess. When a specific immunizing antigen is not part of an intervention, antigen-specific immunity can still be assessed based on knowledge of shared tumor-specific antigens in different tumors or by responses to autologous tumor.
Non–antigen-specific lymphocyte assessment
NK cells have proven to be important antitumor effectors, exhibiting both direct cytotoxicity or more regulatory, cytokine-mediated effects. These CD56+ CD16+/− cells can be tested for frequency, activation, and a variety of cell surface-activating and inhibitory receptors that may impact overall antitumor immunity. The overall state of different T, B, NK, monocytic, and other cell types can be tracked with single-platform detection of absolute counts and relative percentages of multiple subsets.
Regulatory cells
Established tumors often also have established regulatory cell populations that serve to inhibit effective immunity. Assessment of these cells (such as Tregs [particularly CD3/CD4/CD25hi/FOXP3-expressing natural Tregs] and MDSCs [several monocytic and granulocytic subtypes]) by frequency, location, and function can be informative.
Antibodies
Igs specific to tumor-specific proteins can play a role in antitumor immunity. Binding to cell surface proteins on tumors can induce antibody-dependent cytotoxicity via the Ig Fc receptors. Signal transduction pathways can also be triggered. Specific antibodies can be detected via direct enzyme-linked immunoabsorbent assay or protein arrays. Serum can also be used in SERological EXpression cloning technology to identify the Ig specificity.
Systemic Milieu and Microenvironment
Cytokines and chemokines (characterizing the environment for Th1, Th2, Th17, and regulatory and proinflammatory molecules) can be detected singly or in multiplex assays (eg, Luminex [Invitrogen Corporation, Carlsbad, Calif] and SearchLight [Aushon Biosystems, Billerica, Mass]) to fully characterize the broad expression of multiple proteins that provide insights into the type of response and trafficking of cells. Increased angiogenesis is a common hallmark of tumor growth (including VEGF). Additional growth factors can impact tumors, effector cells, and regulatory cells. Detection of these factors can provide important insights into the mechanisms of tumor growth.
The most important site to assess is the tumor. However, the location of most tumors and the demands of patient care can make tissue access difficult or impossible. More commonly accessible is the original diagnostic biopsy, which may be accessible and used to understand the earliest detectable tumor cells, any immune infiltration, vascularization, signaling pathways activated, and specific mutations. When tumor sampling can be performed, a surgical biopsy of sufficient size can yield: 1) snap frozen cells for molecular studies; 2) single-cell suspensions for functional testing of tumor cells and TIL; and 3) tissue to paraffin embed and study by immunohistochemistry or immunoflourescence.
Standardization and Validation
Despite substantial effort, we do not yet know which parameters of antitumor immunity are appropriate to measure, nor which assays are optimal for those measurements. The FDA and National Cancer Institute (NCI) partnered with the SITC to address these issues for immunotherapy for cancer. While specific immune parameters and assays are not yet validated as predictive or prognostic immune biomarkers, the SITC/FDA/NCI workshop recommended following standardized (accurate, precise, and reproducible) procedures and the use of functional assays for the primary immunologic readouts of a clinical trial and consideration of central laboratories for immune monitoring of large, multiinstitutional trials. To promote broader analysis of multiple aspects of immunity and gather data on variability, they also recommend that in addition to cells and serum, RNA and DNA samples are banked for later testing. Sufficient blood should be drawn to allow for planned testing of the primary hypothesis being addressed in the trial, and additional baseline and posttreatment blood samples should be banked for testing novel hypotheses (or generating new hypotheses) that arise in the field.286–288 With a thorough and standardized approach, the field is better poised to understand the full positive and negative effects of immunotherapy, identify complementary combinations to test, and identify early responders and those capable of responding to these interventions.
Cancer Immunotherapy in the Clinic
Following are brief summaries of the solid tumors for which immunotherapy has been most intensely pursued, and for which the data now are promising or already have warranted FDA approval of therapies.
Hepatocellular Cancer
Hepatocellular cancer (HCC) is one of the main causes of cancer deaths worldwide. As the incidence of HCC and hepatic steatosis continues to rise, it will become a progressively greater health problem. Small, localized tumors are potentially curable with surgery, including resection and liver transplantation.289,290 Unfortunately, most patients have advanced disease at diagnosis.
Results from several clinical trials demonstrate that immune-based therapy can improve outcomes for patients with HCC.291–297 The character of the tumor infiltrate (CTL, Treg) correlates with clinical outcome.291,292 In one large trial, 150 patients received either IL-2 and anti–CD3-activated peripheral blood lymphocytes (or observation) after curative resection.295 There were statistically significant improvements in time to recurrence as well as OS (P =.09). Thirty-one patients with HCC received DC pulsed with autologous tumor lysate,296 and there were 14 partial responses and 17 disease stabilizations. Patients had an improved 1-year survival rate (63% vs 10%; P =.038). A randomized phase 2 trial tested formalin-fixed autologous tumor mixed with GM-CSF, IL-2, and BCG.292 After curative resection was performed, 41 subjects with stage I-IIIA disease were enrolled and randomized; 19 received vaccine. Overall, treated patients had statistically significant improvements in risk of recurrence and time to recurrence as well as OS (P =.01). In a follow-up trial,293 60 patients were randomized to observation or fixed autologous HCC vaccine plus GM-CSF plus IL-2 plus tuberculin. Patients with stage I/II disease were immunized intradermally, and 3-year recurrence rates were 33% for immunized patients and 61% for controls, again suggesting the clinical activity of this approach.
NK Cells in HCC
NK cells are critical innate effectors with direct killing and regulatory roles, and their function is impaired in cancer patients,298 including those with HCC, where the defect is in CD56dimCD16+ cells.299 In HCC, the TILs and peritumoral lymphocytes are primarily T and NK cells.300 IL-12 and IL-18-activated NK cells are important for tumor regression in an HCC model.301
α-Fetoprotein–Based Immunotherapy in HCC
α-Fetoprotein (AFP) serves as the major serum protein (1–3 mg/mL) in the fetus. It is also an oncofetal antigen,302 transcriptionally repressed shortly after birth. Most HCCs express AFP, and serum assays play an important role in diagnosis and in monitoring responses to treatment.303 AFP expression in HCC is associated with increased tumor proliferation and apoptosis resistance,304,305 and it is expressed in CD45-CD90+ HCC cancer stem cells.306 Two clinical trials have been conducted testing MHC class I-restricted peptides in Montanide307 and pulsed into DC.308 The immunological responses demonstrated that AFP peptide epitopes were immunogenic in vivo and were able to stimulate antigen-specific T cells in patients with HCC with very high serum levels of AFP. In the second trial, 10 patients (with stage III-IV disease) were immunized and 6 of them showed MHC tetramer AFP-specific T cell increases and 6 had increased frequency of IFN-γ–producing, AFP-specific T cells,297,308 demonstrating immunological activity of the AFP-based vaccine. Two patients experienced transient decreases in serum AFP. Radio-frequency ablation was demonstrated to unmask AFP-specific T cells.309 Hence, combinations of ablation or resection with immunotherapy may be a more efficacious approach than either alone.
Immunotherapy for Prostate Cancer
Several immunotherapeutic approaches have shown promise in prostate cancer phase 2 and phase 3 trials. The first, “GVAX,” tested a combination of 2 prostate cancer cell lines (PC3 and LNCaP), which were engineered via AdV to produce GM-CSF.310–312 While it showed promise in single-arm phase 2 studies, the subsequent 2 phase 3 studies did not reach the expected survival improvements. In contrast, phase 3 trials testing sipuleucel-T (Provenge; Dendreon) in patients with advanced hormone-refractory prostate cancer have demonstrated prolonged OS (25.8 months vs 21.7 months in the placebo-controlled arm; P =.017), and the agent has been approved by the FDA for treatment of this group of patients.313
This therapy consists of autologous patient peripheral blood mononuclear cells (PBMCs) activated with the prostatic acid phosphatase prostate cancer-associated antigen fused to GM-CSF, leading to the enrichment and activation of APCs, such as DCs, and includes a broad mixture of lymphocytic and monocytic cells. The mechanism of action is not yet completely clear, but may involve CD54 expression. The third promising approach is a heterologous viral prime-boost regimen named ProstVac (Bavarian Nordic, Mountain View, Calif). This product is a recombinant fowlpox and vaccinia virus encoding prostate-specific antigen in addition to a trio of costimulatory molecules to increase immunogenicity (B7-1, intercellular adhesion molecule 1 [ICAM-1], and lymphocyte function-associated antigen 3 [LFA-3]). A series of clinical studies have shown promise, and a recent randomized phase 2 trial demonstrated a survival benefit of several months.284
Immunotherapy for Ovarian Cancer
Recent work has shown a correlation between increased survival and the presence of tumor-infiltrating effector-type lymphocytes in a given patient. The absence of tumor-infiltrating regulatory cells has supported the role of immune surveillance in the progression of ovarian cancer and provided additional rationale for immunotherapy for this aggressive disease.314 While attempts using non–antigen-specific treatments of ovarian cancers included the local administration of cytokines and antibodies,314 more recently, several groups initiated vaccination trials involving whole tumor cells,315 either autologous or allogeneic, and tumor cells loaded on autologous DCs316,317 to provide the immune system with the wide panels of the ovarian carcinoma-specific antigens and to avoid the obstacles related to the unclear ability of the individual OvCa-associated antigens (CA 125, HER-2/neu, folate receptor, or mucin antigen 1 [MUC1]) to serve as common tumor-restricted antigenic targets.318 A clinical trial of DC-enriched PBMCs loaded with HER-2/neu–GM-CSF (lapuleucel-T [APC8024]; Dendreon’s product analogous to Provenge) showed modest clinical activity against HER-2/neu–overexpressing tumors, including ovarian cancer, but it remains to be tested whether such a strategy will have survival-prolonging effects analogous to sipuleucel-T for this group of patients.
Immunotherapy for Lung Cancer
Small cell lung cancer (SCLC) cells have been shown to exhibit decreased expression of class I (HLA-A, HLA-B, or HLA-C) and class II (HLA-DR) antigens due to reduced gene transcription, with diminished immunogenicity.319–321 As discussed earlier, these MHC antigens are critical for T cell recognition, and represent the first step in initiating an immune response against the tumor (“signal 1”). Agents that could increase MHC antigen expression such as IFN-α have been shown to induce the expression of these MHC antigens on SCLC cells both in vitro and in vivo.320 These data supported the clinical investigation of IFN-α and IFN-γ in patients with SCLC. Despite the lack of efficacy of IFNs in patients with SCLC with extensive (advanced) disease, these agents appeared to show promise in patients with minimal disease; in these patients, additional testing was pursued.322 Three randomized trials have evaluated maintenance IFN-α after response to chemotherapy, and one study tested recombinant IFN-γ in complete responders with SCLC. Overall, these studies failed to demonstrate a favorable survival impact.323–325 Toxicities also limited acceptance of these agents and the clinical development of IFNs for SCLC was subsequently halted. Other immunotherapeutic agents have been evaluated without significant toxicity in patients with SCLC; these include cancer vaccines, supported by tumor cellular expression of several ganglioside antigens that are not expressed on most normal tissues, which further suggests these might be targets for vaccination.326 Such vaccines include fucosyl monosialoganglioside (GM1), polysialic acid, disialoganglioside (GM2), and the gangliosides (GD2 and GD3).326 The antiidiotypic antibody BEC-2, mimicking GD3, was tested in a randomized phase 3 trial in patients with limited stage SCLC following chemoradiation therapy.327,328 However, these trials failed to demonstrate significant improvement in OS or PFS compared with observation.327,328 Other vaccines in clinical development in SCLC target polysialic acid and fucosyl GM1 ganglioside.329,330 Other immunotherapeutic approaches in clinical development include the combination of the p53 cancer vaccine (DCs transduced with the p53 gene and delivered via an AdV) with chemotherapy in patients with extensive stage SCLC,331 and the immunological adjuvant SRL-172 (suspension of heat-killed mycobacterium vaccae), which also failed to improve survival in a phase 3 trial of standard chemotherapy with or without SRL-172.332
Tumor vaccination strategies have also been tested in NSCLC. These include a phase 2 randomized trial of the BLP25 liposome vaccine immunizing against the MUC1 antigen, a cell surface glycoprotein that is overexpressed in NSCLC, where it was associated with a trend toward improved survival.333 CTLA-4 blockade with ipilimumab has been tested in NSCLC also. A randomized, double-blind, placebo-controlled, multicenter, phase 2 trial compared the efficacy and safety of ipilimumab (10 mg/kg every 3 weeks) in combination with paclitaxel and carboplatin against the chemotherapy doublet alone in patients with untreated lung cancer. Preliminary analysis showed that the combination of ipilimumab and chemotherapy was well tolerated, with trends toward improved PFS and OS.334 The TLR9 agonist oligodeoxynucleotide, PF-3512676, has shown promising results when tested in a randomized phase 2 trial in combination with first-line taxane plus platinum chemotherapy for patients with advanced stage NSCLC, leading to 2 phase 3 trials in NSCLC.335 However, these studies in patients with advanced NSCLC showed no survival benefit and a safety review identified an imbalance in the number of sepsis and sepsis-related serious adverse events (including some resulting in deaths) for patients treated with PF-3512676 in combination with platinum-based doublet chemotherapy compared with those patients treated with chemotherapy alone.336 A current international trial is testing the role of the cancer germline melanoma-associated antigen (MAGE)-A3 as a vaccination in NSCLC (MAGRIT study) on the basis of phase 2 studies that suggest a potential benefit of vaccination with this antigen.7
Immunotherapy for Melanoma
Melanoma has been the flagship of solid tumors for the evaluation of immunotherapy, and with its epidemic rise in incidence across the world it has become the focus of multiple immunotherapy trials over the years. The evidence that increased numbers of TILs correlate to improved prognosis of melanoma and other solid tumors337,338 have argued for the importance of the immune response in the clinical outcome of melanoma. Some of these elegant approaches to immunotherapy have led to striking antitumor effects using modified TILs as applied for adoptive therapy for melanoma at the NCI, although they are not yet applicable to patients in larger multicenter clinical trials that are required to understand their applicability to treatment in general.339
Advanced Stage IV Melanoma
Studies of nonspecific immunotherapy in the 1970s led to large multicenter trials that ultimately have failed to show a role for BCG in the treatment of melanoma. The exploration of nonrecombinant and then recombinant IFN-α2b in advanced melanoma showed antitumor effects in approximately 16% of patients with stage IV melanoma, a rate that is very similar to that subsequently reported for high-dose IL-2 in a series of phase 2 trials that were compiled, leading to the approval of this therapy for metastatic melanoma in 1998. The benefits of anti–CTLA-4–blocking antibodies for the treatment of stage IV melanoma treated in the second-line setting were studied more recently in trials initiated by Medarex Inc with ipilimumab, with the first statistically significant improvement reported in OS (reduction in the hazard of mortality to 0.67). Similar studies undertaken by Pfizer Inc with a different anti–CTLA-4 antibody known as tremelimumab have also shown durable benefits in a subset of patients that appear to have changed the course of disease.340,341 These agents, as discussed earlier in regard to the general modality of anti–CTLA-4–blocking antibodies, have been associated with significant issues of autoimmunity, including colitis, rash, and endocrinopathy, and will need to be addressed as applied more widely for melanoma therapy (and possibly for other tumor types) following approval by the US FDA in March 2011. Studies of the role of combinations of these immunotherapies, the new small molecule therapies targeting mutated BRAF, and mutated C-Kit with the anti–CTLA-4–blocking antibodies are now in planning.
Adjuvant Therapy
The antitumor effects of IFN-α2b are greater in patients with a smaller burden of disease, and several groups pursued the hypothesis that postoperative adjuvant therapy with IFN-α2 might improve the recurrence-free survival and OS of patients with resectable disease who are at a high risk of recurrence. The first statistically significant improvement in the recurrence-free survival and OS of patients with melanoma was demonstrated in the Eastern Cooperative Oncology Group (ECOG) study E1684, testing high-dose IFN-α2b given intravenously at a dose of 20 MU/m2/day for 20 days over 4 weeks, and then subcutaneously 3 times a week at a dose of 10 MU/m2/day for 48 weeks versus observation in patients with deep primary melanoma or lymph node metastasis. The 40% reduction in the hazard of recurrence and 28% reduction in the hazard of mortality in this trial were corroborated in ECOG Intergroup trial E1694, where IFN-α2b demonstrated a 33% reduction in both recurrence and mortality compared with a ganglioside vaccine (GMK; Progenics Pharmaceuticals; Tarrytown, NY). Several meta-analyses of 12 to 14 trials worldwide suggest the benefits of IFN therapy upon both recurrence and mortality, with the magnitude of recurrence reduction being several-fold the reduction in mortality.341–343 A significant reduction in mortality has only been observed to date with high-dose IFN for 1 year in the Intergroup E1684 and E1694 trials.337,338 Most recently, the US FDA has approved pegylated IFN given in a weekly regimen for up to 5 years (median, 13 months) at a dose of 3 to 6 μg/kg as adjuvant therapy for resectable microscopic lymph node metastatic disease. This regimen has shown a 17% reduction in the hazard of recurrence, without any improvement in mortality. The adjuvant benefits of anti–CTLA-4–blocking antibody are now under evaluation in European Organization for Research and Treatment of Cancer (EORTC) trials compared with placebo, and in ECOG-Intergroup trials compared with high-dose IFN. Data from these studies are not expected for several years.344–348
Conclusions
The modality of immunotherapy has come of age, with a demonstrable impact upon a wide range of solid tumors, and with a level of understanding that promises significant new advances for cancer therapy in the near term. The progress summarized above has come from careful evaluation of TAs that are immunogenic in patients with cancer, and also from understanding the complicated dialogue between the immune system and cancer. The impact of immunotherapy was initially demonstrated in patients with advanced cancer, and then translated to the adjuvant setting of patients with operable disease at high risk for postoperative recurrence. The relative efficacy of immunotherapy has been greater in the adjuvant setting than in the setting of advanced disease. Vaccines have remained the ultimate goal for immunotherapy, but to date have had relatively little impact on the outcome of patients with advanced solid tumors. Sipuleucel-T for prostate cancer is the first example of a vaccine associated with modest, but significant, prolongation of survival in patients with advanced cancer. Monoclonal antibodies to immune checkpoint molecules, as well as to tumor-signaling molecules, have each achieved a significant impact upon cancer, with improved survival noted in 2 trials of anti–CTLA-4–blocking antibodies for advanced melanoma as reviewed above. The unimpressive effects of vaccines for cancer observed in the past are not surprising given that vaccines have had their greatest impact in medicine in the setting of prevention against infectious disease, not in the treatment of active disease, as most vaccine-based treatments of cancer have been tested. Apart from the prevention setting of vaccines for HPV-associated cervical carcinoma, the application of specific immunotherapy vaccines for cancer has been limited; the understanding of tumor progression will pave the way for the improved application of immunotherapy for cancer, and of more potent vaccine-based methods of cancer prevention, where the rewards may be even greater still. The pursuit of immunotherapy has followed our understanding of the immunomodulatory effects of solid tumors upon the immune system of patients with cancer; circulating cytokines and chemokines, and the modulation of antigen processing by DCs, along with the exhaustion or dysfunction of effector T cells are the effects of cancer in the local milieu that represent complex interactions of tumor and host. This understanding has opened multiple avenues for immunotherapy, beginning with the application of multiple antitumor antibodies that target TAs, and cytokines and IFNs that modulate DC and T cell activation and functions, which have achieved durable antitumor effects and led to FDA approval of multiple promising agents over the past 20 years. The recent FDA approval of pegylated IFN for the adjuvant therapy of melanoma, requiring only weekly administration, has improved the quality of life for patients in the adjuvant treatment setting, while anti–CTLA-4–blocking antibodies that target checkpoint molecules that improve the T cell response to cancer and appear to abrogate tolerance have improved the survival of patients with melanoma for the first time in history. Antibodies blocking other checkpoint molecules including PD-1 and TIM-3 are on the horizon, and will potentially add to the benefits of immunotherapy, alone and in combination with other modalities. The future of immunotherapy will likely involve combinations of immunotherapy, such as vaccines and the immunomodulators, and immunotherapy with new potent inhibitors of the tumor signaling pathways that remit disease in more than one-half of treated subjects. The careful evaluation of immune responses to tumors during the combined application of immunotherapy and other modalities will be critical to defining biomarkers of response and in providing patients with the optimal benefits from rational combinations of more specific immunotherapies and combinations.
Acknowledgments
The authors would like to acknowledge the help of Bonita Mislanovich.
Footnotes
DISCLOSURES: Supported by National Institutes of Health (NIH) grants CA39229, CA21115, CA47904, CA121973, and CA132714 (to J.M.K.); grants CA138635, CA121973, and P30CA47904 (to L.B.); grants CA109688, CA110249, CA138188, and CA105500 (to S.F.); grants CA095128, CA114931, CA121973, and CA132714 (to P.K.); grant CA121973 (to A.T.); and grants CA90360, CA112198, CA121973, and CA157467 (to H.Z.).
References
- 1.Kim CJ, Dessureault S, Gabrilovich D, Reintgen DS, Slingluff CL., Jr Immunotherapy for melanoma. Cancer Control. 2002;9:22–30. doi: 10.1177/107327480200900104. [DOI] [PubMed] [Google Scholar]
- 2.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(suppl 1):S11–S14. [PubMed] [Google Scholar]
- 3.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 4.Dutcher JP, Creekmore S, Weiss GR, et al. A phase II study of interleukin-2 and lymphokine-activated killer cells in patients with metastatic malignant melanoma. J Clin Oncol. 1989;7:477–485. doi: 10.1200/JCO.1989.7.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson DR, Abrams JS, Wiernik PH, et al. Interleukin-2 therapy in patients with metastatic malignant melanoma: a phase II study. J Clin Oncol. 1990;8:1650–1656. doi: 10.1200/JCO.1990.8.10.1650. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- 7.Ding M, Beck RJ, Keller CJ, Fenton RG. Cloning and analysis of MAGE-1-related genes. Biochem Biophys Res Commun. 1994;202:549–555. doi: 10.1006/bbrc.1994.1963. [DOI] [PubMed] [Google Scholar]
- 8.Nauts HC, Fowler GA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley’s mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl. 1953;276:1–103. [PubMed] [Google Scholar]
- 9.Nathanson L. Spontaneous regression of malignant melanoma: a review of the literature on incidence, clinical features, and possible mechanisms. Natl Cancer Inst Monogr. 1976;44:67–76. [PubMed] [Google Scholar]
- 10.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazumder A, Grimm EA, Zhang HZ, Rosenberg SA. Lysis of fresh human solid tumors by autologous lymphocytes activated in vitro with lectins. Cancer Res. 1982;42:913–918. [PubMed] [Google Scholar]
- 12.Strausser JL, Mazumder A, Grimm EA, Lotze MT, Rosenberg SA. Lysis of human solid tumors by autologous cells sensitized in vitro to alloantigens. J Immunol. 1981;127:266–271. [PubMed] [Google Scholar]
- 13.Krown SE, Burk MW, Kirkwood JM, Kerr D, Morton DL, Oettgen HF. Human leukocyte (alpha) interferon in metastatic malignant melanoma: The American Cancer Society phase II trial. Cancer Treat Rep. 1984;68:723–726. [PubMed] [Google Scholar]
- 14.Kirkwood JM, Ernstoff MS. Interferons in the treatment of human cancer. J Clin Oncol. 1984;2:336–352. doi: 10.1200/JCO.1984.2.4.336. [DOI] [PubMed] [Google Scholar]
- 15.Kirkwood JM, Ernstoff M. Melanoma: therapeutic options with recombinant interferons. Semin Oncol. 1985;12 (4 suppl 5):7–12. [PubMed] [Google Scholar]
- 16.Kirkwood JM, Ernstoff MS, Davis CA, Reiss M, Ferraresi R, Rudnick SA. Comparison of intramuscular and intravenous recombinant alpha-2 interferon in melanoma and other cancers. Ann Intern Med. 1985;103:32–36. doi: 10.7326/0003-4819-103-1-32. [DOI] [PubMed] [Google Scholar]
- 17.Kirkwood J. Cancer immunotherapy: the interferon-alpha experience. Semin Oncol. 2002;29(3 suppl 7):18–26. doi: 10.1053/sonc.2002.33078. [DOI] [PubMed] [Google Scholar]
- 18.Kirkwood JM, Strawderman MH, Ernst-off MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 20.Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 21.Kirkwood JM, Bender C, Agarwala S, et al. Mechanisms and management of toxicities associated with high-dose inter-feron alfa-2b therapy. J Clin Oncol. 2002;20:3703–3718. doi: 10.1200/JCO.2002.03.052. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi T, Matsui H, Fujita T, et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983;302:305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- 23.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 24.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 25.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarhini AA, Edington H, Butterfield LH, et al. Neoadjuvant ipilimumab in patients with stage IIIB/C melanoma: immunogenicity and biomarker analysis [abstract] J Clin Oncol. 2011;29(suppl):Abstract 8536. [Google Scholar]
- 27.Tarhini AA, Kirkwood JM. CTLA-4-blocking immunotherapy with ipilimumab for advanced melanoma. Oncology (Williston Park) 2010;24:1302, 1304. [PubMed] [Google Scholar]
- 28.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 31.Sahin U, Tureci O, Schmitt H, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandic M, Almunia C, Vicel S, et al. The alternative open reading frame of LAGE-1 gives rise to multiple promiscuous HLA-helper 1-type tumor-reactive CD4+ T cells. Cancer Res. 2003;63:6506–6515. [PubMed] [Google Scholar]
- 33.Mandic M, Castelli F, Janjic B, et al. One NY-ESO-1-derived epitope that promiscuously binds to multiple HLA-DR and HLA-DP4 molecules and stimulates autologous CD4+ T cells from patients with NY-ESO-1-expressing melanoma. J Immunol. 2005;174:1751–1759. doi: 10.4049/jimmunol.174.3.1751. [DOI] [PubMed] [Google Scholar]
- 34.Zarour HM, Maillere B, Brusic V, et al. NY-ESO-1 119–143 is a promiscuous major histocompatibility complex class II T-helper epitope recognized by Th1- and Th2-type tumor-reactive CD4+ T cells. Cancer Res. 2002;62:213–218. [PubMed] [Google Scholar]
- 35.Cancer Immunity. [Accessed September 6, 2011.];Mapping and binding analysis of peptides derived from the tumor-associated antigen surviving for eight HLA alleles. Available at: http://www.cancerimmunity.org/v5p6/050106.pdf. [PubMed]
- 36.Kirkwood JM, Lee S, Moschos SJ, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine ± granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speiser DE, Lienard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fourcade J, Kudela P, Andrade Filho PA, et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother. 2008;31:781–791. doi: 10.1097/CJI.0b013e318183af0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speiser DE, Baumgaertner P, Voelter V, et al. Unmodified self antigen triggers human CD8 T cells with stronger tumor reactivity than altered antigen. Proc Natl Acad Sci U S A. 2008;105:3849–3854. doi: 10.1073/pnas.0800080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 42.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 onco-proteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 44.Fourcade J, Sun Z, Kudela P, et al. Human tumor antigen-specific helper and regulatory T cells share common epitope specificity but exhibit distinct T cell repertoire. J Immunol. 2010;184:6709–6718. doi: 10.4049/jimmunol.0903612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams S, O’Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–784. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valmori D, Souleimanian NE, Tosello V, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bozzacco L, Trumpfheller C, Siegal FP, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuji T, Matsuzaki J, Kelly MP, et al. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J Immunol. 2011;186:1218–1227. doi: 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
- 49.Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P. Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin Cancer Res. 2007;13(15 pt 1):4565–4574. doi: 10.1158/1078-0432.CCR-07-0274. [DOI] [PubMed] [Google Scholar]
- 50.Slingluff CL, Jr, Petroni GR, Olson WC, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg SA, Sherry RM, Morton KE, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 53.Gajewski TF. Identifying and overcoming immune resistance mechanisms in the melanoma tumor microenvironment. Clin Cancer Res. 2006;12(7 pt 2):2326s–2330s. doi: 10.1158/1078-0432.CCR-05-2517. [DOI] [PubMed] [Google Scholar]
- 54.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fourcade J, Kudela P, Sun Z, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 58.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 59.Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 61.Kalinski P, Okada H. Polarized dendritic cells as cancer vaccines: directing effector-type T cells to tumors. Semin Immunol. 2010;22:173–182. doi: 10.1016/j.smim.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 63.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13:3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 65.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the micro-environment. Lancet. 2008;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 66.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 68.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 69.Vieweg J, Su Z, Dahm P, Kusmartsev S. Reversal of tumor-mediated immunosuppression. Clin Cancer Res. 2007;13(2 pt 2):727s–732s. doi: 10.1158/1078-0432.CCR-06-1924. [DOI] [PubMed] [Google Scholar]
- 70.Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalinski P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162:3231–3236. [PubMed] [Google Scholar]
- 72.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 73.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–2809. [PubMed] [Google Scholar]
- 74.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity. Immunol Rev. 2006;212:314–329. doi: 10.1111/j.0105-2896.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 76.Pearce EL, Shen H. Making sense of inflammation, epigenetics, and memory CD8+ T-cell differentiation in the context of infection. Immunol Rev. 2006;211:197–202. doi: 10.1111/j.0105-2896.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 77.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harty JT, Badovinac VP. Influence of effector molecules on the CD8(+) T cell response to infection. Curr Opin Immunol. 2002;14:360–365. doi: 10.1016/s0952-7915(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 79.van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T cells. Curr Opin Immunol. 2009;21:167–172. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 82.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 83.Hartl D, Krauss-Etschmann S, Koller B, et al. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol. 2008;181:8053–8067. doi: 10.4049/jimmunol.181.11.8053. [DOI] [PubMed] [Google Scholar]
- 84.Mantovani A, Allavena P, Sozzani S, Vecchi A, Locati M, Sica A. Chemokines in the recruitment and shaping of the leukocyte infiltrate of tumors. Semin Cancer Biol. 2004;14:155–160. doi: 10.1016/j.semcancer.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Mrowietz U, Schwenk U, Maune S, et al. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br J Cancer. 1999;79:1025–1031. doi: 10.1038/sj.bjc.6690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 87.Bonfil RD, Chinni S, Fridman R, Kim HR, Cher ML. Proteases, growth factors, chemokines, and the microenvironment in prostate cancer bone metastasis. Urol Oncol. 2007;25:407–411. doi: 10.1016/j.urolonc.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walser TC, Fulton AM. The role of chemokines in the biology and therapy of breast cancer. Breast Dis. 2004;20:137–143. doi: 10.3233/bd-2004-20114. [DOI] [PubMed] [Google Scholar]
- 90.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 91.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 92.Dhodapkar MV, Steinman RM, Sapp M, et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Vries IJ, Lesterhuis WJ, Scharenborg NM, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- 94.Pilla L, Patuzzo R, Rivoltini L, et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-alpha in metastatic melanoma patients. Cancer Immunol Immunother. 2006;55:958–968. doi: 10.1007/s00262-005-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 96.Beer TM, Bernstein GT, Corman JM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin Cancer Res. 2011;17:4558–4567. doi: 10.1158/1078-0432.CCR-10-3223. [DOI] [PubMed] [Google Scholar]
- 97.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 98.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 99.Sipuleucel-T: APC APC-8015 prostate cancer vaccine—Dendreon. Drugs R D. 2006;7:197–201. doi: 10.2165/00126839-200607030-00006. [DOI] [PubMed] [Google Scholar]
- 100.Harzstark AL, Small EJ. Immunotherapy for prostate cancer using antigen-loaded antigen-presenting cells: APC8015 (Pro-venge) Expert Opin Biol Ther. 2007;7:1275–1280. doi: 10.1517/14712598.7.8.1275. [DOI] [PubMed] [Google Scholar]
- 101.Rethinking therapeutic cancer vaccines. Nat Rev Drug Discov. 2009;8:685–686. doi: 10.1038/nrd2994. [DOI] [PubMed] [Google Scholar]
- 102.Drake CG. Immunotherapy for prostate cancer: walk, don’t run. J Clin Oncol. 2009;27:4035–4037. doi: 10.1200/JCO.2009.22.2299. [DOI] [PubMed] [Google Scholar]
- 103.Lassi K, Dawson NA. Emerging therapies in castrate-resistant prostate cancer. Curr Opin Oncol. 2009;21:260–265. doi: 10.1097/CCO.0b013e32832a1868. [DOI] [PubMed] [Google Scholar]
- 104.Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med (Maywood) 2008;233:522–534. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from non-proliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 106.Reddy A, Sapp M, Feldman M, Subklewe M, Bhardwaj N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997;90:3640–3646. [PubMed] [Google Scholar]
- 107.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prosta-glandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 108.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–1372. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 109.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 110.de Vries IJ, Krooshoop DJ, Scharenborg NM, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- 111.Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 112.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 113.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 114.Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 115.Lee JJ, Foon KA, Mailliard RB, Muthuswamy R, Kalinski P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J Leukoc Biol. 2008;84:319–325. doi: 10.1189/jlb.1107737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kalinski P, Giermasz A, Nakamura Y, et al. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42:535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 117.Kalinski P, Mailliard RB, Giermasz A, et al. Natural killer-dendritic cell cross-talk in cancer immunotherapy. Expert Opin Biol Ther. 2005;5:1303–1315. doi: 10.1517/14712598.5.10.1303. [DOI] [PubMed] [Google Scholar]
- 118.Mailliard RB, Egawa S, Cai Q, et al. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195:473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mailliard RB, Son YI, Redlinger R, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 120.Xu S, Koski GK, Faries M, et al. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171:2251–2261. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 121.Wesa A, Kalinski P, Kirkwood JM, Tatsumi T, Storkus WJ. Polarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4+ T cell responses in vitro. J Immunother. 2007;30:75–82. doi: 10.1097/01.cji.0000211316.15278.6e. [DOI] [PubMed] [Google Scholar]
- 122.Kalinski P, Nakamura Y, Watchmaker P, Giermasz A, Muthuswamy R, Mailliard RB. Helper roles of NK and CD8+ T cells in the induction of tumor immunity. Polarized dendritic cells as cancer vaccines. Immunol Res. 2006;36:137–146. doi: 10.1385/IR:36:1:137. [DOI] [PubMed] [Google Scholar]
- 123.Thomas MJ, Noble A, Sawicka E, Askenase PW, Kemeny DM. CD8 T cells inhibit IgE via dendritic cell IL-12 induction that promotes Th1 T cell counter-regulation. J Immunol. 2002;168:216–223. doi: 10.4049/jimmunol.168.1.216. [DOI] [PubMed] [Google Scholar]
- 124.Wong KL, Tang LF, Lew FC, et al. CD44high memory CD8 T cells synergize with CpG DNA to activate dendritic cell IL-12p70 production. J Immunol. 2009;183:41–50. doi: 10.4049/jimmunol.0803473. [DOI] [PubMed] [Google Scholar]
- 125.Lopez-Albaitero A, Mailliard R, Hackman T, et al. Maturation pathways of dendritic cells determine TAP1 and TAP2 levels and cross-presenting function. J Immunother. 2009;32:465–473. doi: 10.1097/CJI.0b013e3181a1c24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007;25:7145–7152. doi: 10.1016/j.vaccine.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 127.Dubsky P, Saito H, Leogier M, et al. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen LT, Radhakrishnan S, Ciric B, et al. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J Exp Med. 2002;196:1393–1398. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Kalinski P, Moser M. Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat Rev Immunol. 2005;5:251–260. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 130.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 131.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang S, Yang J, Qian J, Wezeman M, Kwak LW, Yi Q. Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006;107:2432–2439. doi: 10.1182/blood-2005-06-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jarnicki AG, Conroy H, Brereton C, et al. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008;180:3797–3806. doi: 10.4049/jimmunol.180.6.3797. [DOI] [PubMed] [Google Scholar]
- 134.Lipscomb MW, Chen L, Taylor JL, et al. Ectopic T-bet expression licenses dendritic cells for IL-12-independent priming of type 1 T cells in vitro. J Immunol. 2008;84:319–325. doi: 10.4049/jimmunol.0901477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Watchmaker P, Berk E, Muthuswamy R, et al. Independent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cells. J Immunol. 2010;184:591–597. doi: 10.4049/jimmunol.0902062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kunz M, Toksoy A, Goebeler M, Engel-hardt E, Brocker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J Pathol. 1999;189:552–558. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 137.Wenzel J, Bekisch B, Uerlich M, Haller O, Bieber T, Tuting T. Type I interferon-associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesions. Am J Clin Pathol. 2005;124:37–48. doi: 10.1309/4EJ9KL7CGDENVVLE. [DOI] [PubMed] [Google Scholar]
- 138.Mullins IM, Slingluff CL, Lee JK, et al. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 139.Gustafsson K, Ingelsten M, Bergqvist L, Nystrom J, Andersson B, Karlsson-Parra A. Recruitment and activation of natural killer cells in vitro by a human dendritic cell vaccine. Cancer Res. 2008;68:5965–5971. doi: 10.1158/0008-5472.CAN-07-6494. [DOI] [PubMed] [Google Scholar]
- 140.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hatano M, Eguchi J, Tatsumi T, et al. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eguchi J, Hatano M, Nishimura F, et al. Identification of interleukin-13 receptor alpha2 peptide analogues capable of inducing improved antiglioma CTL responses. Cancer Res. 2006;66:5883–5891. doi: 10.1158/0008-5472.CAN-06-0363. [DOI] [PubMed] [Google Scholar]
- 143.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 144.Ikeda H, Chamoto K, Tsuji T, et al. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 2004;95:697–703. doi: 10.1111/j.1349-7006.2004.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994;56:853–857. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- 146.Pulendran B. Modulating TH1/TH2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol Res. 2004;29:187–196. doi: 10.1385/IR:29:1-3:187. [DOI] [PubMed] [Google Scholar]
- 147.Palucka K, Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol. 2002;14:420–431. doi: 10.1016/s0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 148.Czerniecki BJ, Cohen PA, Faries M, Xu S, Roros JG, Bedrosian I. Diverse functional activity of CD83+ monocyte-derived dendritic cells and the implications for cancer vaccines. Crit Rev Immunol. 2001;21:157–178. [PubMed] [Google Scholar]
- 149.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 150.Campoli M, Ferrone S, Wang X. Functional and clinical relevance of chondroitin sulfate proteoglycan 4. Adv Can Res. 2010;109:73–121. doi: 10.1016/B978-0-12-380890-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 151.Campoli M, Ferrone S. Immunotherapy of malignant disease: the coming age of therapeutic monoclonal antibodies. In: DeVita V, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams and Wilkins; 2009. pp. 21–18. [Google Scholar]
- 152.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 153.Winter MC, Hancock BW. Ten years of rituximab in NHL. Expert Opin Drug Saf. 2009;8:223–235. doi: 10.1517/14740330902750114. [DOI] [PubMed] [Google Scholar]
- 154.Rasul KI, Kerr DJ. Targeted therapies: cetuximab plus chemotherapy in patients with advanced NSCLC. Nat Rev Clin Oncol. 2009;6:499–500. doi: 10.1038/nrclinonc.2009.108. [DOI] [PubMed] [Google Scholar]
- 155.Hall PS, Cameron DA. Current perspective—trastuzumab. Eur J Cancer. 2009;45:12–18. doi: 10.1016/j.ejca.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 156.Mariani G, Fasolo A, De Benedictis E, Gianni L. Trastuzumab as adjuvant systemic therapy for HER2-positive breast cancer. Nat Clin Pract Oncol. 2009;6:93–104. doi: 10.1038/ncponc1298. [DOI] [PubMed] [Google Scholar]
- 157.William WN, Jr, Kim ES, Herbst RS. Cetuximab therapy for patients with advanced squamous cell carcinomas of the head and neck. Nat Clin Pract Oncol. 2009;6:132–133. doi: 10.1038/ncponc1321. [DOI] [PubMed] [Google Scholar]
- 158.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 160.Taylor C, Hershman D, Shah N, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 161.Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116:926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- 162.Campoli M, Ferris R, Ferrone S, Wang X. Immunotherapy of malignant disease with tumor antigen-specific monoclonal antibodies. Clin Cancer Res. 2010;16:11–20. doi: 10.1158/1078-0432.CCR-09-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 164.Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris) 1974;125C:373–389. [PubMed] [Google Scholar]
- 165.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cheung NK, Cheung IY, Canete A, et al. Antibody response to murine anti-GD2 monoclonal antibodies: correlation with patient survival. Cancer Res. 1994;54:2228–2233. [PubMed] [Google Scholar]
- 167.Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 168.Sandler A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 169.Vredenburgh JJ, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 170.Miller K, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 171.Pfeiffer P, et al. Biweekly cetuximab and irinotecan as third-line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Ann Oncol. 2008;19:1141–1145. doi: 10.1093/annonc/mdn020. [DOI] [PubMed] [Google Scholar]
- 172.Cunningham D, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 173.Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 174.Lynch DH, Yang XD. Therapeutic potential of ABX-EGF: a fully human anti-epidermal growth factor receptor monoclonal antibody for cancer treatment. Semin Oncol. 2002;29(1 suppl 4):47–50. doi: 10.1053/sonc.2002.31522. [DOI] [PubMed] [Google Scholar]
- 175.Van Cutsem E. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 176.Allegra CJ. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 177.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 178.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 179.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 180.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 181.Kaminski MS, Zelenetz AD, Press OW, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 182.Gordon LI, et al. Durable responses after ibritumomab tiuxetan radioimmunotherapy for CD20+ B-cell lymphoma: long-term follow-up of a phase 1/2 study. Blood. 2004;103:4429–4431. doi: 10.1182/blood-2003-11-3883. [DOI] [PubMed] [Google Scholar]
- 183.Kennedy B, Hillmen P. Immunological effects and safe administration of alemtuzumab (MabCampath) in advanced B-cLL. Med Oncol. 2002;19(suppl):S49–S55. doi: 10.1385/mo:19:2s:s49. [DOI] [PubMed] [Google Scholar]
- 184.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 185.Lundin J, Hagberg H, Repp R, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101:4267–4272. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- 186.Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103:2920–2924. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- 187.Dearden CE, Matutes E, Cazin B, et al. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98:1721–1726. doi: 10.1182/blood.v98.6.1721. [DOI] [PubMed] [Google Scholar]
- 188.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 190.Swann JB, Vesely MD, Silva A, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Engelhardt JJ, Sullivan TJ, Allison JP. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006;177:1052–1061. doi: 10.4049/jimmunol.177.2.1052. [DOI] [PubMed] [Google Scholar]
- 192.Robert C, Ghiringhelli F. What is the role of cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma? Oncologist. 2009;14:848–861. doi: 10.1634/theoncologist.2009-0028. [DOI] [PubMed] [Google Scholar]
- 193.Ribas A, Hauschild A, Kefford R, et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma [oral] [abstract]. Presented at: 44th Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2008; Chicago, IL. p. Abstract LBA9011. [Google Scholar]
- 194.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 196.Tarhini AA, Moschos SJ, Tawbi H, et al. Phase II evaluation of tremelimumab (Treme) combined with high-dose interferon alpha-2b (HDI) for metastatic melanoma [abstract] J Clin Oncol. 2010;28(15 suppl):Abstract 8524. [Google Scholar]
- 197.Tarhini AA, Cherian J, Moschos SJ, et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol. 2012;30:322–328. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–2826. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Talay O, Shen CH, Chen L, Chen J. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc Natl Acad Sci U S A. 2009;106:2741–2746. doi: 10.1073/pnas.0813367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 204.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 206.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 207.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 209.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 210.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Auto-immune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 211.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 214.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 215.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 216.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 218.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 219.ClinicalTrials.gov. 2011 Available at: http://www.clinicaltrials.gov/ct2/results?term=CT-011+
- 220.Brahmer JRTS, Wollner I. Safety and activity of MDX-1106 (ONO-4538), an anti–PD-1 monoclonal antibody, in patients with selected refractory or relapsed malignancies. J Clin Oncol. 2008;26(suppl):abstract 3006. [Google Scholar]
- 221.Brahmer JR, Topalian SL, Powderly J, et al. Phase II experience with MDX-1106 (ONO-4538), an anti–PD-1 monoclonal antibody, in patients with selected refractory or relapsed malignancies. J Clin Oncol. 2008;27(suppl):abstract 3015. [Google Scholar]
- 222.ClinicalTrials.gov. 2011 Available at: http://www.clinicaltrial.gov/ct2/results?term=MDX-1106.
- 223.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.ClinicalTrials.gov. 2011 Available at: http://www.clinicaltrials.gov/ct2/show/NCT00729664?term=MDX-1105&rank=1.
- 225.Fonsatti E, Maio M, Altomonte M, Hersey P. Biology and clinical applications of CD40 in cancer treatment. Semin Oncol. 2010;37:517–523. doi: 10.1053/j.seminoncol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 226.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 227.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–749. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 228.Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70:99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.van Mierlo GJ, Boonman ZF, Dumortier HM, den Boer AT, Fransen MF, Nouta J, et al. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol. 2004;173:6753–6759. doi: 10.4049/jimmunol.173.11.6753. [DOI] [PubMed] [Google Scholar]
- 230.van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, et al. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci U S A. 2002;99:5561–5566. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 232.Ruter J, Antonia SJ, Burris HA, Huhn RD, Vonderheide RH. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther. 2010;10:983–993. doi: 10.4161/cbt.10.10.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.ClinicalTrials.gov. 2011 Available at: http://www.clinicaltrials.gov/ct2/results?term=CP870%2C893.
- 234.Law CL, Gordon KA, Collier J, Klussman K, McEarchern JA, Cerveny CG, et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65:8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- 235.Khubchandani S, Czuczman MS, Hernandez-Ilizaliturri FJ. Dacetuzumab, a humanized mAb against CD40 for the treatment of hematological malignancies. Curr Opin Investig Drugs. 2009;10:579–587. [PubMed] [Google Scholar]
- 236.Advani R, Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Ren H, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 237.Hussein M, Berenson JR, Niesvizky R, Munshi N, Matous J, Sobecks R, et al. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010;95:845–848. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Furman RR, Forero-Torres A, Shustov A, Drachman JG. A phase I study of dacetuzumab (SGN-40, a humanized anti-CD40 monoclonal antibody) in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2010;51:228–235. doi: 10.3109/10428190903440946. [DOI] [PubMed] [Google Scholar]
- 239.ClinicalTrials.gov. 2011 Available at: http://www.clinicaltrials.gov/ct2/results?term=SGN-40.
- 240.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J Immunol. 2007;179:7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 241.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 242.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167:6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 243.Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 244.Kovacsovics-Bankowski M, Walker E, Floyd K. Increased CD4 and CD8 memory T cell proliferation following anti-OX40 administration to cancer patients: immunologic assessment of a phase I clinical trial. ISBTC. 2009 [Google Scholar]
- 245.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, et al. 4–1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179:4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, et al. 4–1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 248.May KF, Jr, Chen L, Zheng P, Liu Y. Anti-4–1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res. 2002;62:3459–3465. [PubMed] [Google Scholar]
- 249.Sznol M, Hodi FS, Margolin K. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA) J Clin Oncol. 2008;26(20 suppl):abstract 3007. [Google Scholar]
- 250.ClinicalTrials.gov. 2011 Available at: http://www.clinicaltrials.gov/ct2/results?term=BMS-663513.
- 251.Kumar V, Sercarz E. Genetic vaccination: the advantages of going naked. Nat Med. 1996;2:857–859. doi: 10.1038/nm0896-857. [DOI] [PubMed] [Google Scholar]
- 252.Lowrie DB, Tascon RE, Bonato VL, et al. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 253.Shiver JW, Fu TM, Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 254.Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999;18:765–777. doi: 10.1016/s0264-410x(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Doe B, Selby M, Barnett S, Baenziger J, Walker CM. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc Natl Acad Sci U S A. 1996;93:8578–8583. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Iwasaki A, Torres CA, Ohashi PS, Robinson HL, Barber BH. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol. 1997;159:11–14. [PubMed] [Google Scholar]
- 258.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Vollmer CM, Jr, Eilber FC, Butterfield LH, et al. Alpha-fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res. 1999;59:3064–3067. [PubMed] [Google Scholar]
- 260.Ribas A, Butterfield LH, McBride WH, et al. Genetic immunization for the melanoma antigen MART-1/Melan-A using recombinant adenovirus-transduced murine dendritic cells. Cancer Res. 1997;57:2865–2869. [PubMed] [Google Scholar]
- 261.Conry RM, LoBuglio AF, Kantor J, et al. Immune response to a carcinoembryonic antigen polynucleotide vaccine. Cancer Res. 1994;54:1164–1168. [PubMed] [Google Scholar]
- 262.Chan K, Lee DJ, Schubert A, et al. The roles of MHC class II, CD40, and B7 costimulation in CTL induction by plasmid DNA. J Immunol. 2001;166:3061–3066. doi: 10.4049/jimmunol.166.5.3061. [DOI] [PubMed] [Google Scholar]
- 263.Mincheff M, Tchakarov S, Zoubak S, et al. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur Urol. 2000;38:208–217. doi: 10.1159/000020281. [DOI] [PubMed] [Google Scholar]
- 264.Barouch DH, Santra S, Schmitz JE, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 265.Conry RM, Curiel DT, Strong TV, et al. Safety and immunogenicity of a DNA vaccine encoding carcinoembryonic antigen and hepatitis B surface antigen in colorectal carcinoma patients. Clin Cancer Res. 2002;8:2782–2787. [PubMed] [Google Scholar]
- 266.Triozzi PL, Aldrich W, Allen KO, Carlisle RR, LoBuglio AF, Conry RM. Phase I study of a plasmid DNA vaccine encoding MART-1 in patients with resected melanoma at risk for relapse. J Immunother. 2005;28:382–388. doi: 10.1097/01.cji.0000162779.88687.4c. [DOI] [PubMed] [Google Scholar]
- 267.Cassaday RD, Sondel PM, King DM, et al. A phase I study of immunization using particle-mediated epidermal delivery of genes for gp100 and GM-CSF into uninvolved skin of melanoma patients. Clin Cancer Res. 2007;13(2 pt 1):540–549. doi: 10.1158/1078-0432.CCR-06-2039. [DOI] [PubMed] [Google Scholar]
- 268.Weber J, Boswell W, Smith J, et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 269.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors [erratum appears in: Gene Ther. 2010;17:294] Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Liu L, Wang S, Shan B, Sang M, Liu S, Wang G. Advances in viral-vector systemic cytokine gene therapy against cancer. Vaccine. 2010;28:3883–3887. doi: 10.1016/j.vaccine.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 271.Rosenberg SA, Zhai Y, Yang JC, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Nemunaitis J, Sterman D, Jablons D, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst. 2004;96:326–331. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- 274.Luiten RM, Kueter EW, Mooi W, et al. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cells in metastatic melanoma patients. J Clin Oncol. 2005;23:8978–8991. doi: 10.1200/JCO.2005.01.6816. [DOI] [PubMed] [Google Scholar]
- 275.Meng WS, Butterfield LH, Ribas A, et al. alpha-Fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res. 2001;61:8782–8786. [PubMed] [Google Scholar]
- 276.Schneider J, Gilbert SC, Blanchard TJ, et al. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 277.Schneider J, Gilbert SC, Hannan CM, et al. Induction of CD8+ T cells using heterologous prime-boost immunisation strategies. Immunol Rev. 1999;170:29–38. doi: 10.1111/j.1600-065x.1999.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 278.Sedegah M, Weiss W, Sacci JB, Jr, et al. Improving protective immunity induced by DNA-based immunization: priming with antigen and GM-CSF-encoding plasmid DNA and boosting with antigen-expressing recombinant poxvirus. J Immunol. 2000;164:5905–5912. doi: 10.4049/jimmunol.164.11.5905. [DOI] [PubMed] [Google Scholar]
- 279.Plebanski M, Gilbert SC, Schneider J, et al. Protection from Plasmodium berghei infection by priming and boosting T cells to a single class I-restricted epitope with recombinant carriers suitable for human use. Eur J Immunol. 1998;28:4345–4355. doi: 10.1002/(SICI)1521-4141(199812)28:12<4345::AID-IMMU4345>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 280.McMichael AJ, Hanke T. Is an HIV vaccine possible? Nat Med. 1999;5:612–614. doi: 10.1038/9455. [DOI] [PubMed] [Google Scholar]
- 281.Hanke T, Blanchard TJ, Schneider J, et al. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine. 1998;16:439–445. doi: 10.1016/s0264-410x(97)00226-0. [DOI] [PubMed] [Google Scholar]
- 282.Hanke T, McMichael A. Pre-clinical development of a multi-CTL epitope-based DNA prime MVA boost vaccine for AIDS. Immunol Lett. 1999;66:177–181. doi: 10.1016/s0165-2478(98)00164-3. [DOI] [PubMed] [Google Scholar]
- 283.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avi-pox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 284.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Butterfield LH, Disis ML, Fox BA, et al. A systematic approach to biomarker discovery; preamble to “the iSBTc-FDA taskforce on immunotherapy biomarkers”. J Transl Med. 2008;6:81. doi: 10.1186/1479-5876-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tahara H, Sato M, Thurin M, et al. Emerging concepts in biomarker discovery; the US-Japan Workshop on Immunological Molecular Markers in Oncology. J Transl Med. 2009;7:45. doi: 10.1186/1479-5876-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Butterfield LH, Disis ML, Khleif SN, Balwit JM, Marincola FM. Immunooncology biomarkers 2010 and beyond: perspectives from the iSBTc/SITC biomarker task force. J Transl Med. 2010;8:130. doi: 10.1186/1479-5876-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Butterfield LH, Palucka AK, Britten CM, et al. Recommendations from the iSBTc-SITC/FDA/NCI Workshop on Immunotherapy Biomarkers. Clin Cancer Res. 2011;17:3064–3076. doi: 10.1158/1078-0432.CCR-10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Levin B, Amos C. Therapy of unresectable hepatocellular carcinoma [erratum appears in N Engl J Med. 1995;333:675] N Engl J Med. 1995;332:1294–1296. doi: 10.1056/NEJM199505113321910. [DOI] [PubMed] [Google Scholar]
- 290.Venook AP. Treatment of hepatocellular carcinoma: too many options? J Clin Oncol. 1994;12:1323–1334. doi: 10.1200/JCO.1994.12.6.1323. [DOI] [PubMed] [Google Scholar]
- 291.Kuang M, Peng BG, Lu MD, et al. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res. 2004;10:1574–1579. doi: 10.1158/1078-0432.ccr-03-0071. [DOI] [PubMed] [Google Scholar]
- 292.Peng BG, Liang LJ, He Q, et al. Tumor vaccine against recurrence of hepatocellular carcinoma. World J Gastroenterol. 2005;11:700–704. doi: 10.3748/wjg.v11.i5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gao Q, Siu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 294.Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 295.Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower post-surgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 296.Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2005;28:496–504. doi: 10.1097/01.cji.0000171291.72039.e2. [DOI] [PubMed] [Google Scholar]
- 297.Butterfield LH, Ribas A, Potter DM, Economou JS. Spontaneous and vaccine induced AFP-specific T cell phenotypes in subjects with AFP-positive hepatocellular cancer. Cancer Immunol Immunother. 2007;56:1931–1943. doi: 10.1007/s00262-007-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 299.Cai L, Zhang Z, Zhou L, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 300.Liu Y, Poon RT, Hughes J, Feng X, Yu WC, Fan ST. Chemokine receptors support infiltration of lymphocyte subpopulations in human hepatocellular carcinoma. Clin Immunol. 2005;114:174–182. doi: 10.1016/j.clim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 301.Subleski JJ, Hall VL, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 2006;66:11005–11112. doi: 10.1158/0008-5472.CAN-06-0811. [DOI] [PubMed] [Google Scholar]
- 302.Tilghman SM. The structure and regulation of the alpha-fetoprotein and albumin genes. Oxf Surv Eukaryot Genes. 1985;2:160–206. [PubMed] [Google Scholar]
- 303.Kirkwood JM, Lotze MT. Melanoma. In: Kirkwood JM, Lotze MT, Yasko JM, editors. Current Cancer Therapeutics. Vol. 1. Philadelphia: Current Medicine; 1994. pp. 131–136. [Google Scholar]
- 304.Li M, Zhou S, Liu X, Li P, McNutt MA, Li G. alpha-Fetoprotein shields hepatocellular carcinoma cells from apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand. Cancer Lett. 2007;249:227–234. doi: 10.1016/j.canlet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 305.Yang X, Zhang Y, Zhang L, Zhang L, Mao J. Silencing alpha-fetoprotein expression induces growth arrest and apoptosis in human hepatocellular cancer cell. Cancer Lett. 2008;271:281–293. doi: 10.1016/j.canlet.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 306.Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 307.Butterfield LH, Ribas A, Meng WS, et al. T-cell responses to HLA-A*0201 immuno-dominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9(16 pt 1):5902–5908. [PubMed] [Google Scholar]
- 308.Butterfield LH, Ribas A, Dissette VB, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 309.Zerbini A, Pilli M, Penna A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139–1146. doi: 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]
- 310.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol. 2006;24:419–424. doi: 10.1016/j.urolonc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 311.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor–secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 312.Higano CS, Corman JM, Smith DC, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 313.Kantoff PW, Higano CS, Shore ND, et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 314.Agarwal R, Linch M, Kaye SB. Novel therapeutic agents in ovarian cancer. Eur J Surg Oncol. 2006;32:875–886. doi: 10.1016/j.ejso.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 315.Cannon MJ, O’Brien TJ. Cellular immunotherapy for ovarian cancer. Expert Opin Biol Ther. 2009;9:677–688. doi: 10.1517/14712590902932897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sabbatini P, Odunsi K. Immunologic approaches to ovarian cancer treatment. J Clin Oncol. 2007;25:2884–2893. doi: 10.1200/JCO.2007.11.0775. [DOI] [PubMed] [Google Scholar]
- 317.Chiang CL, Benencia F, Coukos G. Whole tumor antigen vaccines. Semin Immunol. 2010;22:132–143. doi: 10.1016/j.smim.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Hernando JJ, Park T, Kubler K, Offergeld R, Schlebusch H, Bauknecht T. Vaccination with autologous tumour antigen-pulsed dendritic cells in advanced gynaecological malignancies: clinical and immunological evaluation of a phase I trial. Cancer Immunol Immunother. 2002;51:45–52. doi: 10.1007/s00262-001-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ball ED, Sorenson GD, Pettengill OS. Expression of myeloid and major histocompatibility antigens on small cell carcinoma of the lung cell lines analyzed by cytofluorography: modulation by gamma-interferon. Cancer Res. 1986;46:2335–2339. [PubMed] [Google Scholar]
- 320.Marley GM, Doyle LA, Ordonez JV, Sisk A, Hussain A, Yen RW. Potentiation of interferon induction of class I major histocompatibility complex antigen expression by human tumor necrosis factor in small cell lung cancer cell lines. Cancer Res. 1989;49:6232–6236. [PubMed] [Google Scholar]
- 321.Doyle LA, Giangiulo D, Hussain A, Park HJ, Yen RW, Borges M. Differentiation of human variant small cell lung cancer cell lines to a classic morphology by retinoic acid. Cancer Res. 1989;49:6745–6751. [PubMed] [Google Scholar]
- 322.Jaffe HS, Herberman RB. Rationale for recombinant human interferon-gamma adjuvant immunotherapy for cancer. J Natl Cancer Inst. 1988;80:616–618. doi: 10.1093/jnci/80.9.616. [DOI] [PubMed] [Google Scholar]
- 323.Mattson K, Niiranen A, Pyrhonen S, et al. Natural interferon alfa as maintenance therapy for small cell lung cancer. Eur J Cancer. 1992;28A:1387–1391. doi: 10.1016/0959-8049(92)90526-8. [DOI] [PubMed] [Google Scholar]
- 324.Kelly K, Crowley JJ, Bunn PA, Jr, et al. Role of recombinant interferon alfa-2a maintenance in patients with limited-stage small-cell lung cancer responding to concurrent chemoradiation: a Southwest Oncology Group study. J Clin Oncol. 1995;13:2924–2930. doi: 10.1200/JCO.1995.13.12.2924. [DOI] [PubMed] [Google Scholar]
- 325.Jett JR, Maksymiuk AW, Su JQ, et al. Phase III trial of recombinant interferon gamma in complete responders with small-cell lung cancer. J Clin Oncol. 1994;12:2321–2326. doi: 10.1200/JCO.1994.12.11.2321. [DOI] [PubMed] [Google Scholar]
- 326.Zhang S, Cordon-Cardo C, Zhang HS, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 327.Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971–08971B; Silva Study) J Clin Oncol. 2005;23:6854–6864. doi: 10.1200/JCO.2005.17.186. [DOI] [PubMed] [Google Scholar]
- 328.Bottomley A, Debruyne C, Felip E, et al. Symptom and quality of life results of an international randomised phase III study of adjuvant vaccination with Bec2/BCG in responding patients with limited disease small-cell lung cancer. Eur J Cancer. 2008;44:2178–2184. doi: 10.1016/j.ejca.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 329.Krug LM, Ragupathi G, Hood C, et al. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]
- 330.Krug LM, Ragupathi G, Ng KK, et al. Vaccination of small cell lung cancer patients with polysialic acid or N-propionylated polysialic acid conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:916–923. doi: 10.1158/1078-0432.ccr-03-0101. [DOI] [PubMed] [Google Scholar]
- 331.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 332.Harper-Wynne CL, Sumpter K, Ryan C, et al. Addition of SRL 172 to standard chemotherapy in small cell lung cancer (SCLC) improves symptom control. Lung Cancer. 2005;47:289–290. doi: 10.1016/j.lungcan.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 333.Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 334.Lynch TJ, Bondarenko IN, Luft A, et al. Phase II trial of ipilimumab (IPI) and paclitaxel/carboplatin (P/C) in first-line stage IIIb/IV non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28(suppl):7s (abstract 7531). [Google Scholar]
- 335.Manegold C, Gravenor D, Woytowitz D, et al. Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3979–3986. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 336.Hirsch V, Boyer M, Rosell R, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 as first line treatment of advanced non-small cell lung cancer (NSCLC). Program and abstracts of the 44th American Society of Clinical Oncology Annual Meeting; 2008. p. Abstract 8016. [Google Scholar]
- 337.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 338.Rao UN, Lee SJ, Luo W, Mihm MC, Jr, Kirkwood JM. Presence of tumor-infiltrating lymphocytes and a dominant nodule within primary melanoma are prognostic factors for relapse-free survival of patients with thick (t4) primary melanoma: pathologic analysis of the e1690 and e1694 intergroup trials. Am J Clin Pathol. 2010;133:646–653. doi: 10.1309/AJCPTXMEFOVYWDA6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Jaffe HS, Herberman RB. Rationale for recombinant human interferon-gamma adjuvant immunotherapy for cancer. J Natl Cancer Inst. 1988;80:616–618. doi: 10.1093/jnci/80.9.616. [DOI] [PubMed] [Google Scholar]
- 340.Mattson K, Niiranen A, Pyrhonen S, et al. Natural interferon alfa as maintenance therapy for small cell lung cancer. Eur J Cancer. 1992;28A:1387–1391. doi: 10.1016/0959-8049(92)90526-8. [DOI] [PubMed] [Google Scholar]
- 341.Kelly K, Crowley JJ, Bunn PA, Jr, et al. Role of recombinant interferon alfa-2a maintenance in patients with limited-stage small-cell lung cancer responding to concurrent chemoradiation: a Southwest Oncology Group study. J Clin Oncol. 1995;13:2924–2930. doi: 10.1200/JCO.1995.13.12.2924. [DOI] [PubMed] [Google Scholar]
- 342.Hirsch V, Boyer M, Rosell R, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 as first line treatment of advanced non-small cell lung cancer (NSCLC) [abstract]. Paper presented at: 44th American Society of Clinical Oncology Annual Meeting; May 30-June 3, 2008; Chicago, IL. p. Abstract 8016. [Google Scholar]
- 343.Morgan RA, Dudley ME, Rosenberg SA. Adoptive cell therapy: genetic modification to redirect effector cell specificity. Cancer J. 2010;16:336–341. doi: 10.1097/PPO.0b013e3181eb3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Hong JJ, Rosenberg SA, Dudley ME, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010;16:4892–4898. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kirkwood JM, Tarhini AA, Panelli MC, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445–3455. doi: 10.1200/JCO.2007.14.6423. [DOI] [PubMed] [Google Scholar]
- 346.Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemo-therapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol. 2007;25:5426–5434. doi: 10.1200/JCO.2007.12.0253. [DOI] [PubMed] [Google Scholar]
- 347.Wheatley K, Ives N, Eggermont A, et al. on behalf of International Malignant Melanoma Collaborative. Interferon-α as adjuvant therapy for melanoma: an individual patient data meta-analysis of randomised trials [abstract] J Clin Oncol. 2007;25(suppl 18):Abstract 8526. [Google Scholar]
- 348.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]