Abstract
Purpose
Although studies have shown health benefits for moderate-intensity physical activity, there is limited evidence to support beneficial effects for high amounts of vigorous activity among middle-aged and older men. The objective of this study was to examine the relationship between vigorous-intensity physical activity, compared to moderate-intensity activity, and risk of major chronic disease in men.
Methods
We prospectively examined the associations between vigorous- and moderate-intensity physical activity and risk of major chronic disease among 44,551 men aged 40–75 years in 1986. Leisure-time physical activity was assessed biennially by questionnaire. During 22 years of follow-up, we documented 14,162 incident cases of major chronic disease, including 4769 cardiovascular events, 6449 cancer events, and 2944 deaths from other causes.
Results
The hazard ratio (HR) of major chronic disease comparing ≥ 21 to 0 MET-hours/week of exercise was 0.86 (95% CI: 0.81, 0.91) for vigorous-intensity activity and 0.85 (95% CI: 0.80, 0.90) for moderate activity. For CVD, the corresponding HR were 0.78 (95% CI: 0.70, 0.86) and 0.80 (95% CI: 0.72, 0.88), respectively. When examined separately, running, tennis, and brisk walking were inversely associated with CVD risk. Furthermore, more vigorous activity was associated with lower disease risk; the HR comparing >70 to 0 MET-hours/week of vigorous-intensity exercise was 0.79 (95% CI: 0.68, 0.92; P <0.0001 for trend) for major chronic disease and 0.73 (95% CI: 0.56, 0.96; P <0.0001 for trend) for CVD.
Conclusions
Vigorous- and moderate-intensity physical activity were associated with lower risk of major chronic disease and cardiovascular disease. Increasing amounts of vigorous activity remained inversely associated with disease risk, even among men in the highest categories of exercise.
Keywords: exercise, epidemiology, cardiovascular disease, cancer, risk factors
INTRODUCTION
The importance of physical activity in disease prevention has been widely studied and is generally well accepted. There is substantial evidence that suggests that active individuals have a 30% reduced risk for developing cardiovascular disease (CVD) compared to inactive individuals(18, 27, 30, 35).
The most consistent associations between physical activity and reduced cancer risk have been observed for breast and colon cancers(6, 8, 16, 27, 32). Growing evidence now also supports reduced risks of lung and pancreatic cancer in physically active persons(2, 15 –17, 21, 26, 27, 34).
Several studies have reported reduced risks of CVD and mortality for vigorous-intensity activity compared with lesser-intensity physical activity(13, 27, 35). However, few have accounted adequately for duration and total energy expended. Therefore, it is uncertain whether reduced risk of disease attributed to physical activity is due to higher intensity exercise or simply more energy expended.
For CVD and cancer, greater overall activity provides greater benefit but the shape of the dose-response curve specifically for vigorous-intensity activity is not well defined(27). The dose-response curve between exercise and health risk at high levels of intensity and duration is important to determine especially if too much exercise causes harm. Several studies have demonstrated biochemical or echocardiographic evidence of cardiac dysfunction and injury in recreational marathon runners and endurance athletes(5, 11, 25, 28, 31).
The purpose of this study was to assess the relationship between vigorous-intensity physical activity, compared to moderate-intensity activity, and risk of major chronic disease in men. Additionally, we specifically investigated the dose-response relationship between total amount of vigorous-intensity activity and risk of disease. The overall associations of moderate- and vigorous-intensity physical activity with major chronic disease provide insight into total public health effects, including whether harm could occur due to performing too much exercise.
METHODS
Study Population
The Health Professionals Follow-up Study (HPFS) began in 1986, when 51,529 predominately white, male podiatrists, optometrists, pharmacists, dentists, and veterinarians, aged 40 to 75 years, completed the baseline questionnaire that included lifestyle assessment and medical history. Follow-up questionnaires were sent biennially to update information on potential risk factors and medical conditions. Follow-up was complete for over 90% of participants in each 2-year cycle. After exclusion of men with CVD or cancer prior to 1986 or missing physical activity at baseline, 44,551 men were included in these analyses. Responses to questionnaires constituted written informed consent and the protocol was approved by the Institutional Review Board at the Harvard School of Public Health.
Assessment of Physical Activity
Leisure-time physical activity was assessed every two years between 1986 and 2006 through questions on average total time per week spent on each of ten activities over the previous year. Walking pace, categorized as casual (<2 miles/hour), normal (2–2.9 miles/hour), brisk (3–3.9 miles/hour), or striding (≥4 miles/hour), was also assessed. A metabolic equivalent task (MET) score was assigned to each activity based on its energy cost (1). To calculate the amount of energy expended, the time spent at each activity in hours per week was multiplied by its MET score then summed over all activities to yield total MET-hours/week. Vigorous activities, defined as requiring MET values ≥ 6, were jogging (>10 minutes/mile), running (≤10 minutes/mile), bicycling, swimming, tennis, squash or racquetball, and rowing. Moderate activities (3 ≤ METs < 6) were brisk walking, heavy outdoor work, and weight training. To represent long-term levels of exercise more accurately and to reduce measurement error(9), we calculated the cumulative average of physical activity levels from all available questionnaires up to the start of each 2-year follow-up interval(10).
The validity and reproducibility of the physical activity questionnaire have been reported in detail elsewhere(3). Briefly, Pearson correlations between four 1-week diaries and the questionnaire was 0.65 for total activity, 0.58 for vigorous-intensity activity, and 0.28 for non-vigorous activity. The Spearman correlation between questionnaire-derived vigorous activity and resting pulse rate was −0.45.
Ascertainment of End Points
The primary endpoint of this analysis was incident major chronic disease, defined as the sum of total CVD, total cancer, or other non-traumatic death(19). We also examined the associations of physical activity with CVD and cancer separately. When an outcome of interest was reported, we sought permission to obtain medical records, which were used to confirm and classify self-reported diagnoses by physicians blinded to exposure data.
Total CVD included fatal or nonfatal myocardial infarction (MI) and fatal or nonfatal stroke. MI was confirmed according to World Health Organization criteria: symptoms plus either diagnostic ECG changes or elevated cardiac enzymes(29). Stroke was confirmed by diagnosis of a typical neurological defect of sudden or rapid onset lasting ≥ 24 hours that was attributable to a cerebrovascular event (36). We included all cancers except non-melanoma skin cancer and low-grade, organ-confined prostate cancer because of the relatively low mortality from these highly prevalent lesions.
Deaths were reported by next of kin or the postal service or through the National Death Index. We estimated that follow-up for deaths was more than 98% complete (33). Cause of death was confirmed by reviewing medical records or autopsy reports. All causes of death, except those resulting from external causes (e.g. injuries and suicides), were included.
Statistical Analysis
All analyses were performed using SAS statistical software, version 9.2 (SAS Institute Inc, Cary, North Carolina). Each eligible participant contributed person-time until the first diagnosis of CVD, cancer, or death or until January 31st, 2008.
For certain conditions (e.g., dementia, respiratory disease), there may not be an isolated “date” of onset and exercise is probably limited before a clinical diagnosis is made. To minimize bias due to reverse causation for these conditions, we stopped updating physical activity for a period in which individuals reported difficulty climbing a flight of stairs or walking. Additionally, we performed analyses with a 2- and 4-year lag to exclude preclinical cases at baseline. For example, in a 2-year lag analysis, the cumulative average of activity reported in 1986 and 1988 would be used for the 1990–1992 follow-up period.
Since diagnosis of intermediate events or conditions may lead to systematic changes in physical activity, we examined whether participants changed their level of exercise after such diagnoses. We found that participants changed their level of exercise after angina, CABG, and TIA, and as such we stopped updating physical activity after new diagnosis of these conditions.
Cox proportional hazards models were used to estimate hazard ratios (HR) of outcomes over each 2-year follow-up interval. Tests for linear trend were computed by using the medians for categories modeled as a continuous variable. We also examined the possibly non-linear relation between vigorous activity and major chronic disease non-parametrically with restricted cubic splines(4). Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with linear and cubic spline terms. The proportional hazards assumptions were tested by including interaction terms between exposure and time or age and comparing the difference in −2 log likelihood between the interaction model and the model without the interaction terms. In all cases, the interactions were not significant, indicating that the proportional hazards assumptions were met.
Participants were divided into categories of physical activity (0, 0.1 –3.5, 3.6 – 8.8, 8.9 – 21, > 21 MET-hours/week) based on the distribution of vigorous- and moderate-intensity activity and informative cut points. For example, 3.5 MET-hrs/wk corresponds to 1 hour of moderate or 0.5 hours of vigorous activity, 8.8 MET-hrs/wk to 2.5 hours of moderate or 1.25 hours of vigorous activity (the current recommendations based on the Physical Activity Guidelines for Americans) and 21 MET-hrs/wk to 6 – 7 hours of moderate or 3 hours of vigorous activity. To assess if exercise intensity was related to major chronic disease independent of amount of energy expended, we included vigorous-, moderate-, and low-intensity activity in MET-hours/week in the same model(22). In this model, the coefficient for each intensity represents the effect of increasing activity of this intensity while holding other activity types constant. In secondary analyses, we used a substitution model to estimate the effect of replacing moderate-intensity physical activity with vigorous activity, keeping total MET-hours constant (22).
In addition, we looked at each type of activity separately while adjusting for all other activities using categories of 0, 0.1 – 0.9, 1.0 – 1.9, 2.0 – 4.9, and 5+ hours per week. For this analysis we used hours rather than MET-hours to examine high levels of all activities, both those requiring more METs (e.g. running) and fewer METs (e.g. walking). Yard work and weight training were not assessed at baseline; follow-up for these activities began in 1988 and 1990, respectively. Finally, to evaluate the upper end of the dose-response curve for vigorous-intensity physical activity, we divided the highest category (> 21 MET-hours/week) into smaller groups while adjusting for low- and moderate-intensity activity.
In all multivariable models, we stratified on age in months and included the following covariates: smoking (five categories), aspirin use, vitamin E supplement use, parental history of MI or cancer, alcohol consumption (five categories), energy-adjusted intake of polyunsaturated fat, trans fatty acids, omega-3 fatty acids, and fiber (quintiles), as well as diabetes, hypertension, and hypercholesterolemia at baseline. All covariates were updated over time, except for diabetes, hypertension, or hypercholesterolemia because the incidence of these conditions may be in the causal pathway relating physical activity to CVD. Information from previous questionnaires was used when covariate data in a given cycle were missing. For dietary covariates, the cumulative average intake was used to reduce measurement error(10). In secondary analyses we additionally adjusted for body mass index (BMI), also a potential intermediate.
The interaction between vigorous and moderate physical activity was assessed by the difference in −2 log likelihood between the model containing the interaction with moderate activity in two categories (≤ 1 hour/week vs. >1 hour/week moderate activity) and the main effects model. The interaction between vigorous activity and age (< 70 years, ≥ 70 years) was similarly assessed.
RESULTS
We examined vigorous-intensity physical activity in relation to other potential risk factors for major chronic disease at baseline (Table 1). Men who reported more vigorous activity tended to have lower BMI, were less likely to smoke, and had higher intakes of omega-3 fatty acids and fiber.
Table 1.
Age-standardized characteristics* according to weekly vigorous activity at baseline (1986), Health Professionals Follow-up Study
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Categories of Vigorous-Intensity Activity, MET-hours/week (1986) | |||||||
|
| |||||||
| 1 (0) (n = 16197) |
2 (0.1 – 21.0) (n = 19894) |
3 (21.1 – 31.5) (n = 2726) |
4 (31.6 – 42.0) (n = 2291) |
5 (42.1 – 56.0) (n = 1045) |
6 (56.1 – 70.0) (n = 1273) |
7 (> 70) (n = 1125) |
|
| Vigorous activity, hrs/wk | 0.0 (0.0) | 1.1 (0.8) | 3.2 (0.7) | 4.7 (0.9) | 6.3 (1.1) | 7.1 (1.8) | 11.8 (3.5) |
| Moderate activity, hrs/wk | 1.0 (2.7) | 1.0 (2.5) | 1.1 (2.4) | 1.3 (2.8) | 1.3 (2.8) | 1.0 (2.4) | 1.8 (3.7) |
| Low activity, hrs/wk | 1.2 (2.9) | 1.0 (2.4) | 0.8 (2.3) | 0.8 (2.2) | 0.9 (2.4) | 0.8 (2.3) | 1.0 (3.0) |
| Alcohol, g/d | 11.7 (16.9) | 11.0 (14.5) | 11.0 (14.1) | 11.6 (14.5) | 11.2 (14.8) | 12.3 (14.7) | 10.8 (14.4) |
| Current smoker, % | 14 | 8 | 5 | 5 | 5 | 4 | 4 |
| Body mass index, kg/m2 | 26.1 (3.7) | 25.4 (3.2) | 24.7 (2.7) | 24.8 (2.6) | 24.6 (2.6) | 24.5 (2.7) | 24.4 (3.4) |
| Hypercholesterolemia, % | 10 | 11 | 12 | 11 | 12 | 10 | 12 |
| Hypertension, % | 22 | 20 | 17 | 19 | 15 | 16 | 15 |
| Diabetes, % | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| Aspirin use, % | 27 | 27 | 25 | 25 | 28 | 23 | 24 |
| Omega 3 fatty acids, g/d | 0.26 (0.25) | 0.30 (0.26) | 0.35 (0.28) | 0.35 (0.29) | 0.38 (0.31) | 0.35 (0.30) | 0.39 (0.48) |
| Fiber, g/d | 19.2 (8.2) | 20.9 (8.8) | 22.5 (9.2) | 22.7 (10.3) | 23.7 (11.6) | 23.1 (10.2) | 25.4 (11.6) |
All values are means (SD) for continuous variables or frequencies for categorical variables, adjusted for age.
During 22 years of follow-up, a total of 14,162 men (31%) developed a major chronic disease event. These included 4769 CVD events, 6449 cancer events, and 2944 deaths from other causes (e.g. pneumonia, kidney, or liver disease). There was a significant inverse association between total physical activity and risk of major chronic disease and total CVD, but no association with total cancer after adjusting for covariates (Table 2).
Table 2.
Hazard ratios of major chronic disease, cardiovascular disease, or cancer according to categories of vigorous-intensity and moderate-intensity activity, 1986 – 2008
| Categories of Physical Activity, MET-hrs/wk | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 (0) |
2 (0.1 – 3.5) |
3 (3.6 – 8.8) |
4 (8.9 – 21.0) |
5 (>21.0) |
p-value for linear trend | HR for 10 MET-hr/wk increase | |
| All events (n = 14162) | |||||||
| Total activity | |||||||
| Cases/PY | 331/15,149 | 1597/73,462 | 2065/106,911 | 3716/211,409 | 6453/410,116 | ||
| Basic* | 1.00 | 0.96 (0.85, 1.09) | 0.82 (0.73, 0.92) | 0.73 (0.65, 0.82) | 0.64 (0.57, 0.72) | <0.0001 | 0.92 (0.91, 0.93) |
| Multivariable† | 1.00 | 1.06 (0.93, 1.19) | 0.94 (0.83, 1.05) | 0.87 (0.78, 0.98) | 0.80 (0.72, 0.90) | <0.0001 | 0.95 (0.94, 0.96) |
| Vigorous activity | |||||||
| Cases/PY | 3767/160,655 | 3578/184,399 | 2216/127,778 | 2434/164,749 | 2167/179,466 | ||
| Basic* | 1.00 | 0.91 (0.87, 0.95) | 0.85 (0.81, 0.90) | 0.80 (0.76, 0.84) | 0.75 (0.71, 0.80) | <0.0001 | 0.93 (0.91, 0.94) |
| Multivariable† | 1.00 | 0.97 (0.92, 1.01) | 0.93 (0.88, 0.98) | 0.89 (0.84, 0.94) | 0.86 (0.81, 0.91) | <0.0001 | 0.96 (0.94, 0.97) |
| Moderate activity | |||||||
| Cases/PY | 3491/170,281 | 3018/177,576 | 2544/162,349 | 2838/176,986 | 2271/129,855 | ||
| Basic* | 1.00 | 0.88 (0.84, 0.93) | 0.84 (0.79, 0.88) | 0.82 (0.78, 0.87) | 0.77 (0.72, 0.81) | <0.0001 | 0.93 (0.92, 0.95) |
| Multivariable† | 1.00 | 0.93 (0.88, 0.98) | 0.91 (0.86, 0.96) | 0.90 (0.85, 0.95) | 0.85 (0.80, 0.90) | <0.0001 | 0.96 (0.94, 0.97) |
|
| |||||||
| Total CVD (n = 4769) | |||||||
| Total activity | |||||||
| Cases/PY | 124/15,253 | 574/74,038 | 742/107,727 | 1262/213,021 | 2067/413,138 | ||
| Basic* | 1.00 | 0.96 (0.79, 1.17) | 0.84 (0.69, 1.02) | 0.72 (0.59, 0.86) | 0.61 (0.50, 0.73) | <0.0001 | 0.91 (0.89, 0.92) |
| Multivariable† | 1.00 | 1.06 (0.87, 1.30) | 0.98 (0.80, 1.19) | 0.89 (0.73, 1.07) | 0.80 (0.66, 0.97) | <0.0001 | 0.94 (0.93, 0.96) |
| Vigorous activity | |||||||
| Cases/PY | 1326/162,044 | 1231/185,939 | 729/128,786 | 820/165,852 | 663/180,556 | ||
| Basic* | 1.00 | 0.92 (0.85, 1.00) | 0.83 (0.76, 0.91) | 0.78 (0.72, 0.86) | 0.67 (0.60, 0.73) | <0.0001 | 0.89 (0.87, 0.92) |
| Multivariable† | 1.00 | 0.98 (0.90, 1.06) | 0.91 (0.82, 1.00) | 0.88 (0.80, 0.96) | 0.78 (0.70, 0.86) | <0.0001 | 0.93 (0.91, 0.95 ) |
| Moderate activity | |||||||
| Cases/PY | 1305/171,495 | 971/178,887 | 868/163,504 | 923/178,343 | 702/130,948 | ||
| Basic* | 1.00 | 0.80 (0.73, 0.88) | 0.82 (0.74, 0.89) | 0.77 (0.70, 0.84) | 0.70 (0.63, 0.78) | <0.0001 | 0.92 (0.89, 0.94) |
| Multivariable† | 1.00 | 0.85 (0.78, 0.93) | 0.90 (0.82, 0.98) | 0.86 (0.78, 0.95) | 0.80 (0.72, 0.88) | 0.0001 | 0.95 (0.92, 0.97) |
|
| |||||||
| Total cancer (n = 6449) | |||||||
| Total activity | |||||||
| Cases/PY | 116/15,264 | 618/73,999 | 852/107,604 | 1680/212,591 | 3183/412,128 | ||
| Basic* | 1.00 | 1.08 (0.88, 1.32) | 0.99 (0.82, 1.21) | 0.98 (0.81, 1.18) | 0.95 (0.78, 1.15) | 0.02 | 0.98 (0.96, 1.00) |
| Multivariable† | 1.00 | 1.13 (0.92, 1.38) | 1.04 (0.85, 1.27) | 1.03 (0.85, 1.25) | 1.01 (0.84, 1.23) | 0.12 | 0.99 (0.97, 1.00) |
| Vigorous activity | |||||||
| Cases/PY | 1513/161,901 | 1591/185,536 | 1031/128,457 | 1170/165,569 | 1144/180,128 | ||
| Basic* | 1.00 | 0.98 (0.91, 1.06) | 0.96 (0.88, 1.04) | 0.91 (0.84, 0.98) | 0.91 (0.84, 0.99) | 0.009 | 0.97 (0.95, 0.99) |
| Multivariable† | 1.00 | 1.01 (0.93, 1.08) | 0.99 (0.91, 1.07) | 0.94 (0.87, 1.02) | 0.97 (0.89, 1.05) | 0.23 | 0.99 (0.97, 1.01) |
| Moderate activity | |||||||
| Cases/PY | 1304/171,479 | 1394/178,506 | 1191/163,188 | 1408/177,880 | 1152/130,533 | ||
| Basic* | 1.00 | 1.09 (1.01, 1.18) | 1.03 (0.95, 1.12) | 1.07 (0.99, 1.16) | 1.05 (0.96, 1.15) | 0.81 | 1.00 (0.98, 1.02) |
| Multivariable† | 1.00 | 1.10 (1.01, 1.19) | 1.04 (0.96, 1.13) | 1.09 (1.00, 1.18) | 1.07 (0.97, 1.16) | 0.95 | 1.00 (0.98, 1.02) |
The basic model is age-adjusted and includes vigorous-intensity, moderate-intensity, and low-intensity activities simultaneously
The multivariable model adjusts for parental history of MI at or before age 60 years, parental history of cancer at or before age 60 years, smoking, aspirin, vitamin E supplement use, intake of polyunsaturated fat, trans fat, EPA+DHA, and fiber, as well as alcohol intake and pre-existing disease including a diagnosis of diabetes, hypertension, or hypercholesterolemia
In multivariable-adjusted analyses, moderate-and vigorous-intensity activity were inversely associated with risk of major chronic disease (P for trend <0.0001 for both; Table 2). The hazard ratio (HR) for major chronic disease comparing ≥ 21 to 0 MET-hours/week of physical activity was 0.86 (95% confidence interval [CI]: 0.81, 0.91) for vigorous activity and 0.85 (95% CI: 0.80, 0.90) for moderate activity (Table 2). The results for total CVD were stronger, with HR comparing extreme categories of 0.78 (95% CI: 0.70, 0.86; P for trend < 0.0001) for vigorous activity and 0.80 (95% CI: 0.72, 0.88; P for trend < 0.0001) for moderate activity (Table 2). All associations were mildly attenuated after adjustment for BMI, but remained statistically significant. Using category medians, the HR for CVD corresponding to 10 MET-hr/wk of energy expended in vigorous activity was 0.93 (95% CI: 0.91, 0.95) compared to a HR of 0.95 (95% CI: 0.92, 0.97) for moderate activity (Table 2). In secondary analyses, we found replacing 10 MET-hr/wk of moderate-intensity activity with 10 MET-hr/wk of vigorous-intensity activity was associated with lower CVD risk (HR: 0.96, 95% CI: 0.93, 0.99, p = 0.02). Similar results were obtained for vigorous-intensity activity in the 2- and 4-year lag analyses, but results for moderate-intensity activity were attenuated suggesting benefits of moderate activity may be short-term. Vigorous activity was inversely associated with age-adjusted risk of total cancer (P for trend = 0.009; Table 2), but no longer significant after covariate adjustment. Moderate-intensity activity was not associated with total cancer risk. We also repeated this analysis using “net” MET levels (i.e. subtracting MET-hours of resting metabolism from total MET-hours of activity) and obtained similar results.
We examined the association between individual activities and CVD risk (Table 3). In multivariable-adjusted analyses including all activities simultaneously in the model, running, tennis, and brisk walking were significantly associated with CVD (P for trend <0.0001, 0.004, and <0.0001 respectively) (Table 3). Running ≥ 5 hours/week was associated with a 46% risk reduction (HR: 0.54; 95% CI: 0.33, 0.89), tennis with a 28% risk reduction (HR: 0.72; 95% CI: 0.56, 0.92), and brisk walking with a 23% risk reduction (HR: 0.77, 95% CI: 0.68, 0.88) compared to men not participating in these activities (Table 3).
Table 3.
Hazard ratios for cardiovascular disease associated with average weekly hours of individual activities, 1986 – 2008
|
|
||||||
|---|---|---|---|---|---|---|
| Categories of Physical Activity, hours/week | ||||||
|
| ||||||
| 0 | 0.1 – 0.9 | 1.0 – 1.9 | 2.0 – 4.9 | ≥ 5.0 | p-value for trend | |
| Vigorous Activities | ||||||
|
| ||||||
| Jogging | (3679/548,349 PY) | (915/228,354 PY) | (113/32,958 PY) | (55/11,596 PY) | (7/1920 PY) | |
| Multivariable* | 1.00 | 0.84 (0.77, 0.90) | 0.74 (0.61, 0.90) | 0.98 (0.75, 1.28) | 0.69 (0.33, 1.48) | 0.01 |
| MV + activities† | 1.00 | 0.90 (0.82, 0.98) | 0.82 (0.67, 0.99) | 1.09 (0.83, 1.43) | 0.69 (0.32, 1.48) | 0.10 |
|
| ||||||
| Running | (4072/602,474 PY) | (531/145,407 PY) | (89/36,297 PY) | (61/29,469 PY) | (16/9530 PY) | |
| Multivariable* | 1.00 | 0.83 (0.75, 0.91) | 0.75 (0.61, 0.93) | 0.68 (0.52, 0.88) | 0.55 (0.34, 0.90) | <0.0001 |
| MV + activities† | 1.00 | 0.92 (0.83, 1.02) | 0.80 (0.64, 0.99) | 0.70 (0.54, 0.90) | 0.54 (0.33, 0.89) | <0.0001 |
|
| ||||||
| Cycling | (2563/395,264 PY) | (1670/324,816 PY) | (355/64,297 PY) | (145/31,524 PY) | (36/7276 PY) | |
| Multivariable* | 1.00 | 0.96 (0.90, 1.02) | 1.00 (0.89, 1.12) | 0.87 (0.74, 1.03) | 0.93 (0.67, 1.30) | 0.28 |
| MV + activities† | 1.00 | 1.01 (0.94, 1.08) | 1.04 (0.93, 1.17) | 0.93 (0.78, 1.10) | 0.95 (0.68, 1.33) | 0.61 |
|
| ||||||
| Swimming | (3832/655,760 PY) | (753/141,015 PY) | (117/16,020 PY) | (51/8928 PY) | (16/1454 PY) | |
| Multivariable* | 1.00 | 0.99 (0.91, 1.07) | 1.23 (1.02, 1.48) | 0.88 (0.66, 1.16) | 1.79 (1.08, 2.97) | 0.12 |
| MV + activities† | 1.00 | 1.03 (0.95, 1.12) | 1.25 (1.03, 1.51) | 0.87 (0.66, 1.16) | 1.76 (1.06, 2.91) | 0.09 |
|
| ||||||
| Tennis | (4071/656,900 PY) | (346/89,155 PY) | (148/28,718 PY) | (139/32,619 PY) | (65/15,785 PY) | |
| Multivariable* | 1.00 | 0.93 (0.83, 1.05) | 1.09 (0.92, 1.29) | 0.83 (0.69, 0.98) | 0.72 (0.56, 0.93) | 0.004 |
| MV + activities† | 1.00 | 0.99 (0.88, 1.11) | 1.11 (0.94, 1.32) | 0.84 (0.71, 1.00) | 0.72 (0.56, 0.92) | 0.004 |
|
| ||||||
| Rowing | (2763/418,933 PY) | (1601/318,089 PY) | (292/60,250 PY) | (97/22,488 PY) | (16/3417 PY) | |
| Multivariable* | 1.00 | 0.89 (0.84, 0.95) | 0.88 (0.78, 1.00) | 0.79 (0.64, 0.97) | 1.15 (0.70, 1.89) | 0.03 |
| MV + activities† | 1.00 | 0.95 (0.88, 1.02) | 0.94 (0.83, 1.07) | 0.83 (0.68, 1.03) | 1.16 (0.70, 1.90) | 0.17 |
|
| ||||||
| Racquetball | (4497/744,373 PY) | (178/50,307 PY) | (54/13,906 PY) | (33/11,789 PY) | (7/2802 PY) | |
| Multivariable* | 1.00 | 0.97 (0.83, 1.13) | 1.09 (0.83, 1.43) | 0.75 (0.53, 1.06) | 0.69 (0.33, 1.45) | 0.12 |
| MV + activities† | 1.00 | 1.04 (0.89, 1.22) | 1.15 (0.88, 1.52) | 0.76 (0.54, 1.07) | 0.68 (0.32, 1.42) | 0.16 |
|
| ||||||
| Moderate Activities | ||||||
|
| ||||||
| Brisk Walking | (2461/345,949 PY) | (879/186,316 PY) | (544/106,539 PY) | (553/117,622 PY) | (332/66,751 PY) | |
| Multivariable* | 1.00 | 0.89 (0.82, 0.97) | 0.92 (0.83, 1.02) | 0.77 (0.70, 0.85) | 0.75 (0.66, 0.85) | <0.0001 |
| MV + activities† | 1.00 | 0.93 (0.85, 1.01) | 0.96 (0.87, 1.06) | 0.80 (0.72, 0.89) | 0.77 (0.68, 0.88) | <0.0001 |
|
| ||||||
| Weight training | (2123/322,564 PY) | (539/119,861 PY) | (113/32,748 PY) | (67/16,574 PY) | (15/3591 PY) | |
| Multivariable* | 1.00 | 0.89 (0.81, 0.99) | 0.80 (0.66, 0.97) | 0.97 (0.76, 1.25) | 1.14 (0.68, 1.91) | 0.40 |
| MV + activities† | 1.00 | 0.97 (0.87, 1.08) | 0.91 (0.74, 1.11) | 1.08 (0.83, 1.40) | 1.24 (0.73, 2.09) | 0.80 |
|
| ||||||
| Heavy Yard Work | (950/134,927 PY) | (1266/245,972 PY) | (438/76,563 PY) | (375/62,437 PY) | (262/42,368 PY) | |
| Multivariable* | 1.00 | 0.89 (0.82, 0.98) | 0.99 (0.87, 1.11) | 0.96 (0.84, 1.09) | 0.89 (0.77, 1.03) | 0.54 |
| MV + activities† | 1.00 | 0.95 (0.87, 1.04) | 1.04 (0.92, 1.17) | 1.03 (0.90, 1.17) | 0.94 (0.81, 1.09) | 0.76 |
The multivariable model is age-adjusted and additionally adjusts for low-intensity activities, parental history of MI at or before age 60 years, parental history of cancer at or before age 60 years, smoking, aspirin, vitamin E supplement use, intake of polyunsaturated fat, trans fat, EPA+DHA, and fiber, as well as alcohol intake and pre-existing disease including a diagnosis of diabetes, hypertension, or hypercholesterolemia
This model adjusts for all covariates in multivariable model and all other types of physical activity
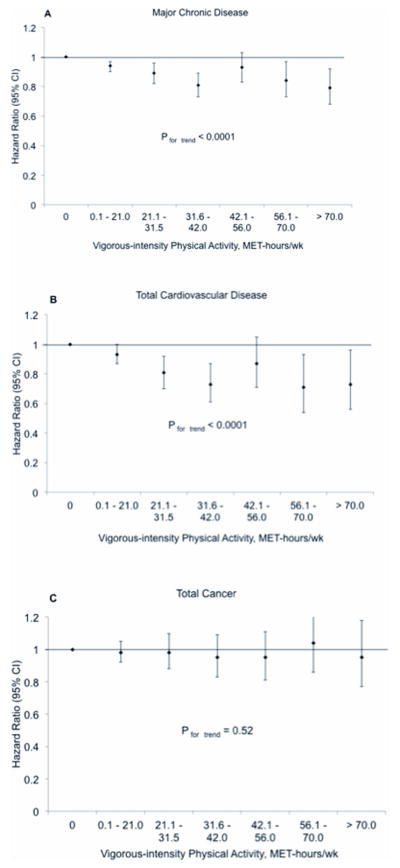
When we examined the more extreme categories of vigorous-intensity activity, higher amounts of vigorous activity were associated with a lower risk of major chronic disease and total CVD (Figure 1). The multivariable-adjusted HR comparing ≥ 70 MET-hrs/wk of vigorous-intensity activity, which is equivalent to 10+ hours per week of vigorous exercise, to 0 MET-hrs/wk was 0.79 (95% CI: 0.68, 0.92; P for trend < 0.0001) for major chronic disease and 0.73 (95% CI: 0.56, 0.96; P for trend < 0.0001) for CVD (Figure 1). In models further adjusted for BMI, the corresponding HR were 0.80 (95% CI: 0.69, 0.93; P for trend < 0.0001) for major chronic disease and 0.78 (95% CI: 0.60, 1.02; P for trend < 0.0001) for CVD. There was no association between vigorous activity and risk of total cancer.
Figure 1. Hazard ratios and 95% confidence intervals (CI) of major chronic disease (A), cardiovascular disease (B), and cancer (C) associated with weekly MET-hours of vigorous-intensity physical activity.
The hazard ratios are adjusted for age, moderate-intensity and low-intensity activities, parental history of MI at or before age 60 years, parental history of cancer at or before age 60 years, smoking, aspirin, vitamin E supplement use, intake of polyunsaturated fat, trans fat, EPA+DHA, and fiber, as well as alcohol intake and pre-existing disease including a diagnosis of diabetes, hypertension, or hypercholesterolemia.
We did find evidence of non-linearity when applying restricted cubic splines to the association between vigorous activity and major chronic disease (p < 0.0001) and CVD (p = 0.03). As shown in Figure 1, for major chronic disease and CVD, much of the risk reduction is achieved in lower categories of vigorous activity; additional risk reduction is seen in higher categories, but with smaller magnitude.
We also investigated the association between vigorous-intensity physical activity and major chronic disease within subgroups defined by age (< 70 years, ≥ 70 years) and concurrent moderate-intensity exercise (≤ 1 hour/week, >1 hour/week moderate activity). An inverse association was observed in men younger than 70 and in men 70 years and older. There was, however, evidence of an interaction between participation in moderate activity and vigorous activity. Although significant in both groups, the inverse association between vigorous-intensity activity and major chronic disease was stronger among men reporting ≤ 1 hour/week of moderate activity compared to men reporting > 1 hour/week (P for interaction = 0.01) (Figure 2).
Figure 2. Hazard ratios and 95% confidence intervals (CI) of major chronic disease associated with weekly MET-hours of vigorous-intensity physical activity, stratified by participation in ≤ 1 hour of moderate activity per week (A) or > 1 hour of moderate activity per week (B).
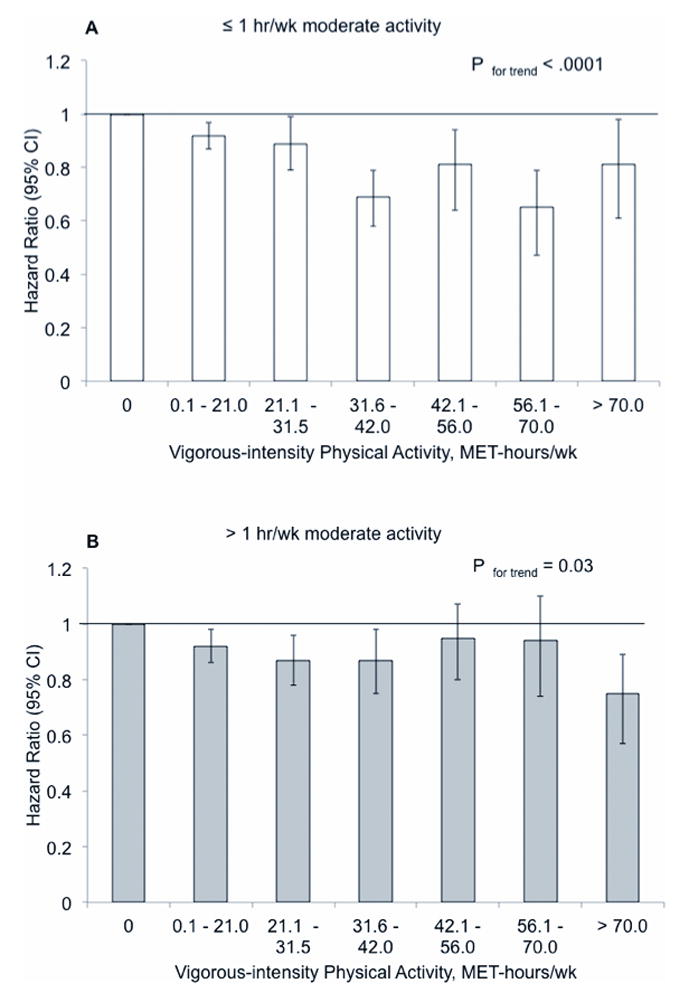
P = 0.01 for interaction between vigorous activity and participation in moderate activity. The height of the bars represents the hazard ratios adjusted for age, moderate-intensity and low-intensity activities, parental history of MI at or before age 60 years, parental history of cancer at or before age 60 years, smoking, aspirin, vitamin E supplement use, intake of polyunsaturated fat, trans fat, EPA+DHA, and fiber, as well as alcohol intake and pre-existing disease including a diagnosis of diabetes, hypertension, or hypercholesterolemia.
DISCUSSION
In this large, prospective study of U.S. men, vigorous-intensity physical activity, even at ≥ 70 MET-hours per week, was associated with decreased risk of incident major chronic disease and total cardiovascular disease. Moderate-intensity physical activity was also associated with decreased risk, albeit weaker. When examined individually, running, tennis, and brisk walking were each associated with reduced CVD risk. We observed no association between vigorous- or moderate-intensity physical activity and risk of total cancer.
Although moderate-intensity physical activity was associated with decreased risk of total CVD, we found that vigorous exercise was modestly more protective. When 10 MET-hours per week of moderate exercise was substituted for an identical amount of energy expended on vigorous exercise, CVD risk was 4% lower (p = 0.02). This result is consistent with previous studies; among studies that investigated the association between exercise intensity and CHD while adjusting for amount of energy expended, all reported inverse associations between intensity of physical activity and CHD risk(14, 35). This may be due to a true biological advantage of vigorous activity or because vigorous activity is measured with greater validity than non-vigorous activity(3).
A limitation of the questionnaire used to assess physical activity in this study is that it does not include an assessment of the intensity at which participants perform many of the activities. Thus, some participants may perform vigorous activities, like bicycling and swimming, at a truly vigorous intensity while others may be performing these same activities at a much lower intensity. The inability to distinguish between the same activity performed at different intensities may have diminished our ability to observe a larger difference between vigorous- and moderate-intensity activity and risk of disease. In contrast, for activities where we do have an estimate of intensity because we assess pace (e.g. brisk walking and running) we found strong inverse associations with CVD.
We investigated the high end of the dose-response curve for vigorous-intensity activity to determine if too much activity is harmful(5, 11, 25, 28, 31). A study on coronary artery calcification (CAC) in marathon runners found that a CAC score ≥ 100 was present in 36% of runners and CAC score among marathoners exceeded that in controls matched for age and Framingham Risk Score. Additionally, CAC burden and frequent marathon running were correlated with subclinical myocardial damage, indicating a potentially higher coronary risk than may be anticipated(23). Another study, using cardiovascular magnetic resonance imaging, found 50% of older lifelong endurance athletes had evidence of myocardial fibrosis whereas age-matched controls or young athletes had none(37). We found no evidence of increased cardiovascular risk for high amounts of vigorous-intensity physical activity even among men in the top 1–2% of vigorous exercise (corresponding to 10+ hours per week). Furthermore, when each vigorous activity was examined separately, all but one was inversely associated with CVD. Specifically, running ≥ 5 hours per week was associated with the lowest cardiovascular risk. This result is reassuring as much of the discussion regarding health risk at high levels of exercise has resulted from adverse outcomes in runners and endurance athletes. We cannot, however, exclude the possibility that more extreme levels of physical activity than reported in our population of middle-aged and older men may be harmful.
There are multiple mechanisms through which physical activity may decrease risk of disease. Physical activity reduces CVD risk through improvements in blood pressure, lipoprotein levels, and glucose tolerance(12, 24); activity also enhances cardiac mechanical and metabolic function(7); and improves hemostatic factors(24). Exercise may affect cancer risk through effects on obesity, with resulting changes to circulating levels of adipokines, cytokines, insulin, and sex hormones(15, 20). Other mechanisms may involve direct effects on target organs and tissues(20). We did not find an inverse association for physical activity and total cancer, but that does not rule out lower risk for several specific cancer sites(6, 8, 16, 17, 21, 27, 32).
Strengths of our study include the prospective design, detailed information on physical activity and covariates, the large number of major chronic disease events, and minimal loss to follow-up.
Our study also has several limitations that should be considered. As in any observational study, the possibility of residual confounding cannot be eliminated. Our study population, consisting of predominantly white, male health professionals, is not representative of the general population. Thus, we cannot necessarily generalize our results to women or other populations with different educational levels, incomes, or distributions of race and ethnicity. Physical activity was self-reported in this study, but this method has been previously validated in this population(3). Measurement error is unlikely to bias our results because physical activity was assessed prospectively and would be non-differential with respect to subsequent disease status.
In conclusion, vigorous- and moderate-intensity physical activity was associated with lower risk of major chronic disease and total cardiovascular disease. Running, playing tennis, and brisk walking were associated with significantly reduced risk of cardiovascular disease. Increasing amounts of vigorous activity remained inversely associated with risk of major chronic disease and cardiovascular disease; even among men in the highest categories of vigorous activity, there was not an increased risk of CVD events.
Acknowledgments
Funding/support: This study was supported National Institute of Health grants CA055075 and HL35464. Dr. Chomistek was supported by an institutional training grant (HL07575) from the National Heart, Lung, and Blood Institute.
The authors thank Dr. Walter Willett for his comments and suggestions in the preparation of this manuscript and Lydia Liu for her helpful statistical assistance.
Footnotes
CONFLICT OF INTEREST
None. The results of the present study do not constitute endorsement by ACSM.
References
- 1.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Calton BA, Stolzenberg-Solomon RZ, Moore SC, Schatzkin A, Schairer C, Albanes D, Leitzmann MF. A prospective study of physical activity and the risk of pancreatic cancer among women (United States) BMC Cancer. 2008;8:63. doi: 10.1186/1471-2407-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 5.Fortescue EB, Shin AY, Greenes DS, et al. Cardiac troponin increases among runners in the Boston Marathon. Ann Emerg Med. 2007;49(2):137–143. doi: 10.1016/j.annemergmed.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122(5):327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hambrecht R, Wolf A, Gielen S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342(7):454–60. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 8.Howard RA, Freedman DM, Park Y, Hollenbeck A, Schatzkin A, Leitzmann MF. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2008;19(9):939–53. doi: 10.1007/s10552-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu FB, Stampfer MJ, Colditz GA, et al. Physical activity and risk of stroke in women. JAMA. 2000;283(22):2961–7. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 10.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 11.Jaworski CA. Medical concerns of marathons. Curr Sports Med Rep. 2005;4 (3):137–43. doi: 10.1097/01.csmr.0000306196.51994.5f. [DOI] [PubMed] [Google Scholar]
- 12.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–92. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 13.Lee IM, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol. 2000;151(3):293–9. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 14.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS., Jr Relative intensity of physical activity and risk of coronary heart disease. Circulation. 2003;107(8):1100–1116. doi: 10.1161/01.cir.0000052626.63602.58. [DOI] [PubMed] [Google Scholar]
- 15.Lee IM, Oguma Y. Physical Activity. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2006. pp. 449–467. [Google Scholar]
- 16.Lee IM. Epidemiologic Methods in Physical Activity Studies. Oxford: Oxford University Press; 2009. p. 328. [Google Scholar]
- 17.Leitzmann MF, Koebnick C, Abnet CC, Freedman ND, Park Y, Hollenbeck A, Ballard-Barbash R, Schatzkin A. Prospective study of physical activity and lung cancer by histologic type in current, former, and never smokers. Am J Epidemiol. 2009;169(5):542–53. doi: 10.1093/aje/kwn371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347(10):716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 19.McCullough ML, Feskanich D, Rimm EB, et al. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr. 2000;72(5):1223–31. doi: 10.1093/ajcn/72.5.1223. [DOI] [PubMed] [Google Scholar]
- 20.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8(3):205–11. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 21.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286 (8):921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 22.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–527. doi: 10.1093/aje/kwp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Möhlenkamp S, Lehmann N, Breuckmann F, et al. Marathon Study Investigators; Heinz Nixdorf Recall Study Investigators. Running: the risk of coronary events: Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J. 2008;29(15):1903–10. doi: 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- 24.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295(12):1412–9. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 25.Neilan TG, Januzzi JL, Lee-Lewandrowski E, et al. Myocardial injury and ventricular dysfunction related to training levels among non-elite participants in the Boston Marathon. Circulation. 2006;114(22):2325–2333. doi: 10.1161/CIRCULATIONAHA.106.647461. [DOI] [PubMed] [Google Scholar]
- 26.Parent ME, Rousseau MC, El-Zein M, Latreille B, Desy M, Siemiatycki J. Occupational and recreational physical activity during adult life and the risk of cancer among men. Cancer Epidemiol. 2011;35(2):151–159. doi: 10.1016/j.canep.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. p. 683. [Google Scholar]
- 28.Rifai N, Douglas PS, O’Toole M, Rimm E, Ginsburg GS. Cardiac troponin T and I, electrocardiographic wall motion analyses, and ejection fractions in athletes participating in the Hawaii ironman triathlon. Am J Cardiol. 1999;83(7):1085–1089. doi: 10.1016/s0002-9149(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 29.Rose GA, Blackburn H, Gillum R, Prineas RJ. Cardiovascular Survey Methods: WHO Monograph Series No 56. Geneva, Switzerland: World Health Organization; 1982. pp. 162–165. [PubMed] [Google Scholar]
- 30.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose-response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–95. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scharhag J, Herrmann M, Urhausen A, Haschke M, Herrmann W, Kindermann W. Independent elevations of N-terminal pro-BNP and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am Heart J. 2005;150(6):1128–1134. doi: 10.1016/j.ahj.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 32.Sesso HD, Paffenbarger RS, Jr, Lee IM. Physical activity and breast cancer risk in the College Alumni Health Study (United States) Cancer Causes Control. 1998;9(4):433–9. doi: 10.1023/a:1008827903302. [DOI] [PubMed] [Google Scholar]
- 33.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–9. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 34.Sui X, Lee DC, Matthews CE, Adams SA, Hebert JR, Church TS, Lee CD, Blair SN. Influence of cardiorespiratory fitness on lung cancer mortality. Med Sci Sports Exerc. 2010;42(5):872–878. doi: 10.1249/MSS.0b013e3181c47b65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288(16):1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 36.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12 (2 Pt 2 Suppl 1):I13–44. [PubMed] [Google Scholar]
- 37.Wilson M, O’Hanlon R, Prasad S, et al. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol. 2011;110(6):1622–6. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]