Abstract
Purpose
Cowden syndrome (CS), a Mendelian autosomal-dominant disorder, predisposes to breast, thyroid, and other cancers. Germline variations in succinate dehydrogenase genes (SDHx) occur in ~10% PTEN mutation-negative CS and CS-like (CSL) individuals (SDHvar+). We previously showed that SDHx variants result in elevated reactive oxygen species (ROS), disruption of nicotinamide adenine dinucleotide (NAD) equilibrium, and destabilization of p53 hence apoptosis resistance in CS/CSL patient-derived lymphoblastoid cells. In the present study, we sought to address the tumorigenic impacts of increased ROS and the potential of protecting SDHvar+ cells with antioxidants.
Experimental Design
We measured the lipid peroxidation levels in patient-derived SDHvar+ lymphoblastoid cells and sequenced 74 controls or SDHvar+ germline DNA samples for mitochondrial hypervariable region II (HVRII) polymorphisms. SDHvar+ lymphoblastoid cells were treated with various antioxidants to check p53 expression and SubG1 cell population with cell cycle analysis.
Results
We demonstrated that elevated ROS results in higher lipid peroxidation in SDHvar+ cells. Accumulation of polymorphisms in mitochondrial HVRII were observed in SDHvar+ samples. Interestingly, α-tocopherol (vitamin E) treatment, but not other antioxidants, rescued SDHvar+ cells from apoptosis resistance and protected SDHvar+ cells from oxidative damage such as decreased lipid peroxidation as well as partially recovered p53 expression and NAD/NADH levels.
Conclusions
We conclude that disruption of complex II due to SDHx variants leads to increased ROS generation, specifically accompanied by lipid peroxidation. The lipid soluble antioxidant α-tocopherol can selectively protect SDHxvar+ cells from oxidative damage, apoptosis resistance, and rebalance redox metabolites NAD/NADH.
Keywords: SDH, CS/CSL, α-tocopherol, ROS, mitochondria
Introduction
Cowden syndrome (CS, [MIM 158350]) is an autosomal dominant disorder with lifetime risks of up to 85% for developing female breast cancer, 35% for epithelial thyroid cancer, and increased risks of developing other cancers (1). Germline mutations in the phosphatase and tensin homolog deleted on chromosome ten tumor suppressor gene (PTEN [MIM 601728]) are found in 25% of classic CS patients accrued from the community (2). When individuals have features of CS but do not meet these criteria, they are referred to as CS-like (CSL) and necessarily represent a heterogeneous series. Only up to 5% of CSL individuals have germline PTEN mutations (2, 3). Other than PTEN, we have recently uncovered alternative mechanisms, germline hypermethylation of the tumor suppressor gene KLLN (encoding KILLIN) and germline variants in succinate dehydrogenase (SDH) genes SDHB-D, accounting for ~35% and ~10%, respectively, of PTEN mutation negative CS/CSL (4–6). Germline KLLN hypermethylation is associated with increased prevalence of breast and renal cancers, while SDHB-D variants show increased prevalence of breast and thyroid cancers, over those with PTEN mutations.
Mitochondrial respiratory enzyme succinate dehydrogenase (SDH or complex II) is involved in both electron transport and the Krebs tricarboxylic-acid cycle, catalyzing FAD-dependent oxidation of succinate to fumarate. Germline homozygous or compound heterozygous mutations in mitochondrial complex genes, including SDH, result in Leigh syndrome, a rare but fatal neurodegenerative disease. Germline heterozygous SDHB/C/D mutations result in hereditary pheochromocytoma-paraganglioma (PCC/PGL) syndrome (7–10). Functionally, we discovered that SDHx variants resulted in elevated reactive oxygen species (ROS), hyperactivated hypoxia inducible factor (HIF), and in disruption of such mitochondrial metabolites as flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NAD) homeostasis in CS/CSL patient-derived lymphoblastoid cells. Consequently, improper NADH quinone oxidoreductase 1 (NQO1)-p53 interaction results in destabilization of p53 and apoptosis resistance in variant carrier cells (5, 6, 11, 12).
ROS, which can be generated during cell metabolism, especially during mitochondrial respiration (13, 14), plays an important role in cell redox control, signaling regulation, which has long been implicated in tumorigenesis (15). ROS-mediated lipid peroxidation stimulates additional ROS formation and DNA damage (16, 17). In the present study, we sought to address our hypotheses that increased ROS may specifically induce lipid peroxidation in germline SDHx variants carrier cells, and that by treating cells with antioxidant, we should be able to rescue ROS induced tumorigenic phenotypes.
Materials and Methods
Research Participants
CS or CSL patients were prospectively enrolled in accordance with our research protocol IRB8458-PTEN, which was approved by the Cleveland Clinic and respective Institutional Review Boards for Human Subjects Protection. All research participants provided written informed consent. To be enrolled in the IRB8458-PTEN, individuals are eligible if he/she meets the full CS diagnostic criteria established by the International Cowden Consortium (Supplemental Table 1) or the relaxed criteria (criteria minus one) according to version 2006 NCCN Guidelines (18). Patients meeting the relaxed criteria are referred to as individuals with CS-like phenotypes or CSL. In other words, CSL was diagnosed when an individual did not fully meet the strict diagnostic criteria but had features with one or two criteria short of the operational diagnostic criteria. Matching the subjects, normal (population) controls are from northern and western European origin and were anonymized prior to storage and analysis.
Germline SDHx variants were detected in both PTEN mutation negative and PTEN mutation positive CS/CSL individuals as we reported previously (6). The updated SDHx variant lists in both patient subsets are summarized in Supplemental Table 2.
Mitochondrial Mutation Analysis
Germline DNA was extracted from peripheral blood samples from patients and healthy controls by the Genomic Medicine Biorepository (GMB), Genomic Medicine Institute, Cleveland Clinic (protocols are available at GMB website, http://www.lerner.ccf.org/gmi/gmb/methods.php). PCR amplification and direct sequencing (ABI3730xl) of mitochondrial hyper variable region II were performed with primer L16340 5’-AGCCATTTACCGTACATAGCACA-3’ and H408 5’-TGTTAAAAGTGCATACCGCCA-3’. Revised Cambridge Reference Sequence (NC_012920.1) was used as reference mitochondrial sequence.
Cell Lines and Cell Cultures
Human immortalized lyphoblastoid cell lines (LCLs) derived from patients and normal healthy controls were generated by Genomic Medicine Biorepository, Genomic Medicine Institute, Cleveland Clinic (protocols are available at GMB website, http://www.lerner.ccf.org/gmi/gmb/methods.php). LCLs were cultured in RPMI 1640 supplemented with 20% fetal bovine serum (FBS) and 100units/ml each of Penicillin and Streptomycin. All cell lines were cultured at 37°C with 5% CO2. NQO1 inducer dimethyl fumarate (DMF), dimethyl sulfoxide (DMSO), N-acetyl-cysteine (NAC), ascorbic acid, and α-tocopherol (Sigma-Aldrich Co., St. Louis, MO) were added into cell culture at different doses as described in figure legend.
Protein Analysis
Whole-cell lysates were prepared as described previously (19) with M-PER Mammalian Protein Extraction Reagent (ThermoFisher Scientific, Waltham, MA) supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Lysates were either separated by SDS-PAGE and transferred to nitrocellulose (BioRad, Hercules, CA). The resulting blots were subjected to Western blot analysis for PTEN (6H2.1, Cascade Bioscience, Portland, OR), NQO1, p53 (Santa Cruz Biotechnology, Santa Cruz, CA), GAPDH (Cell Signaling, Danver, MA) and α-tubulin (Sigma-Aldrich, St. Louis, MO) protein levels.
ROS and Lipid Peroxidation Measurement
The measurement of ROS was performed using carboxy-dichlorodihydrofluorescein diacetate (Carboxy-H2DCFDA), a reliable fluorogenic marker for ROS in live cells (Molecular Probes, Invitrogen, Carlsbad, CA). The cells were washed with HBSS/Ca/Mg buffer, centrifuged, resuspended in HBSS/Ca/Mg and incubated with 25 uM carboxy-H2DCFDA for 30 minutes at 37°C. Hoechst 33342 was added at a final concentration of 1 uM to the carboxy-H2DCFDA staining solution during the last 5 minutes of the incubation. For flow cytometry measurement, cells were washed and resuspended in HBSS/Ca/Mg buffer after incubation and count with FACScans (Becton-Dickinson) immediately.
The measurement of lipid peroxidation was performed using Lipid Peroxidation Microplate Assay Kit (Oxford Biomedical Research, Oxford, MI) according to the manufacturer’s protocol to measure malondiadehyde (MDA) and 4-hydroxyalkenals (HAE), the products upon decomposition of polyunsaturated fatty acid peroxides. In brief, cells were washed and lysed with ice-cold 20mM PBS with 5mM butylated hydroxytoluene (BHT) to prevent sample oxidation during preparation. Lysed protein were incubated with Reagent R1 and 37% HCl (for MDA only) or R2 (for MDA + HAE) for 60 minutes at 45°C. The incubated products were then read at 586 nm wavelength to calculate the concentration based on the standard curve and normalized to the protein concentration measured using separate protein aliquots.
Cell Cycle Analysis by FACS Flow Cytometry
LCLs were serum starved overnight and allowed to grow under 0.2% FBS condition for 36 hours before 70% ethanol fixation for cell cycle analysis using FACScan flow cytometer (Becton-Dickinson).
NAD+/NADH Quantification
NAD and NADH concentrations were measured using NAD+/NADH quantification kit #337-100 (BioVision, Mountain View, CA) following product protocol. In brief, cells were extracted by freeze/thaw two cycles (20 minutes on dry ice, then 10 minutes at room temperature). Extracted samples were filtered through 10Kd molecular weight cut off filters (BioVison #1997-25) to remove enzymes consuming NADH before performing the assay. To detect total NADt (NADH and NAD) the samples and NADH standard were incubated directly with NAD cycling mix (cycling buffer and enzyme mix). To detect NADH, samples were heated to 60°C for 30 minutes to decompose NAD before incubating with NAD cycling mix. Duplicated samples were then mixed with NADH developer and incubate at room temperature for 1 to 4 hours before colorimetric reading at OD450 nm. The amount of NAD in samples was calculated by subtracting NADH from NADt.
Statistical Analysis
The results are presented as means ± Standard Errors of the Mean (S.E.M.) of the indicated number of samples in each mutation/variant group. Significant differences (p<0.05) were evaluated with 2-tailed Student’s t-test.
Results
Increased Lipid Peroxidation in SDHx Variant-Positive Cells
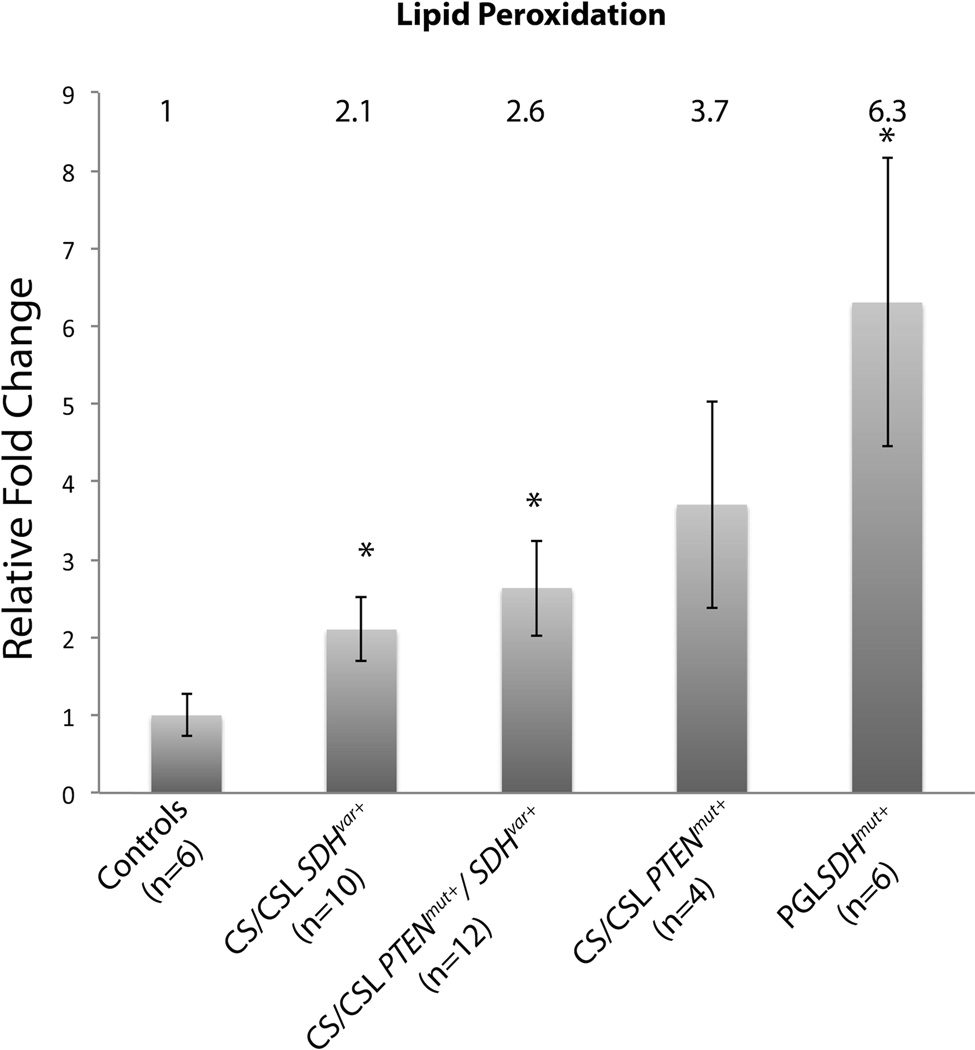
We have previously reported that the cellular ROS is significantly increased in CS/CSL patient samples harboring SDHx variants compared to normal controls. To investigate if the elevated ROS generated can cause damage to lipids such as lipid peroxidation, we measured the byproducts of polyunsaturated fatty acid peroxides upon decomposition - malondialdehyde (MDA) and 4-hydroxyalkenals (HAE), in lymphoblastoid cells derived from normal control and SDHx variant carrier patients. Compared to controls, CS/CSL samples with SDHx variants showed 2.1-fold increase in lipid peroxidation, while patients with both PTEN mutations and SDHx variants had the highest level, 2.6-fold increase, which correlates with overall ROS increase patterns in these three groups as we noted previously (6). Interestingly, CS/CSL patients with PTEN pathogenic truncation mutations (R130X or R335X) also had 3.7-fold increased lipid peroxidation compared to controls. As positive controls, paraganglioma patients with SDHx pathogenic mutations were included and had the highest levels of lipid peroxidation among all five groups, a mean 6.3-fold increase (Figure 1).
Figure 1.
Elevated lipid peroxidation levels in patient-derived lymphoblastoid cells with SDHx variants. Lipid peroxidation levels were measured in SDHxVar+ CS/CSL samples (n=10), PTENmut+/SDHxVar+ CS/CSL samples (n=12), PTENmut+ CS/CSL samples (n=4) SDHxmut+ PGL samples (n=6), and normalized to normal controls (n=6, mean±SEM). The relative fold change is noted above each column. * on each bar indicates two-tail student t-test with p<0.05 compared to control group.
Accumulated Mitochondrial Polymorphisms in SDHx Variant-Positive Cells
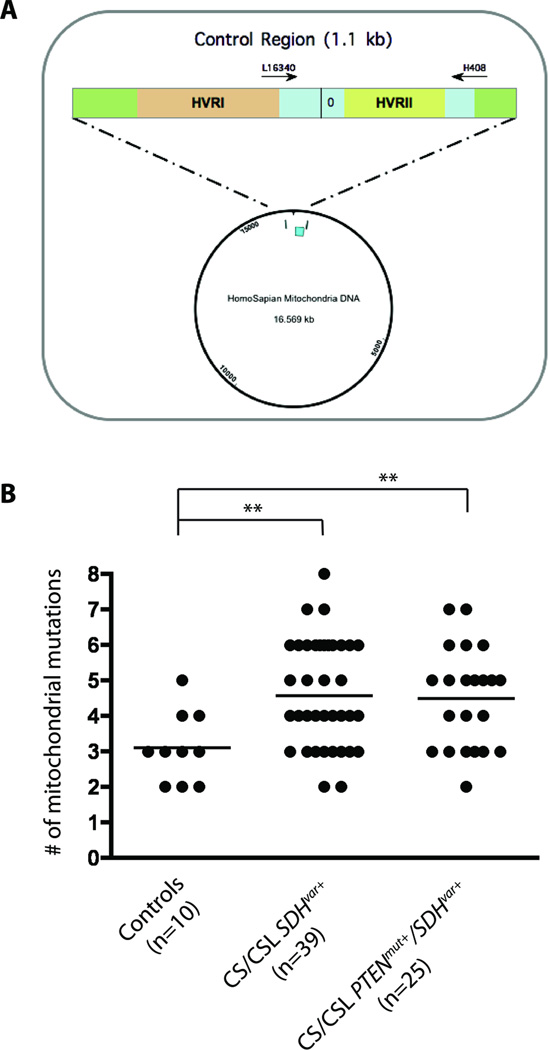
Exposure of cells to high levels of ROS leads to oxidative stress and may cause DNA damage, especially to the fragile mitochondrial genome that lacks DNA repair machinery and has greatest proximity to the mitochondrial electron transport chain(20). To explore if SDHx variants may accumulate more mitochondrial polymorphisms, 10 control genomic DNA samples and 64 SDHx variant positive genomic DNA samples (39 with SDHx variant alone, 25 with both PTEN and SDHx variant) were sequenced for mitochondrial hypervariable region II (HVRII), which is the most polymorphic region in the human mitochondrial genome. In total, 38 different alterations were found in both controls and patients screened, 30 of which were unique in cases not seen in controls (Supplemental Table 3). Out of these 38 polymorphisms, 34 are reported in Mitomap database (http://www.mitomap.org/bin/view.pl/MITOMAP/PolymorphismsControl) while 4 are novel and only presented in SDHx variant positive patients. Compared to controls (median=3, range 2–5), we observed significantly more genetic polymorphisms in both SDHx variant carriers (median=5 polymorphisms/patient, range 2–8; p=0.004) and PTEN/SDHx double variant carriers (median=4, range 2–7; p=0.011) [Figure 2].
Figure 2.
Accumulated mitochondrial HVRII polymorphisms in SDHxVar+ CS/CSL samples. (A) Schematic diagram of mitochondrial HVRII region amplified and sequenced with primers L16340 and H408. (B) Number of HVRII polymorphisms in SDHxVar+ CS/CSL samples (n=39), PTENmut+/SDHxVar+ CS/CSL samples (n=25), and normal controls (n=10). * p<0.05 and ** p<0.01 with two-tailed student t-test.
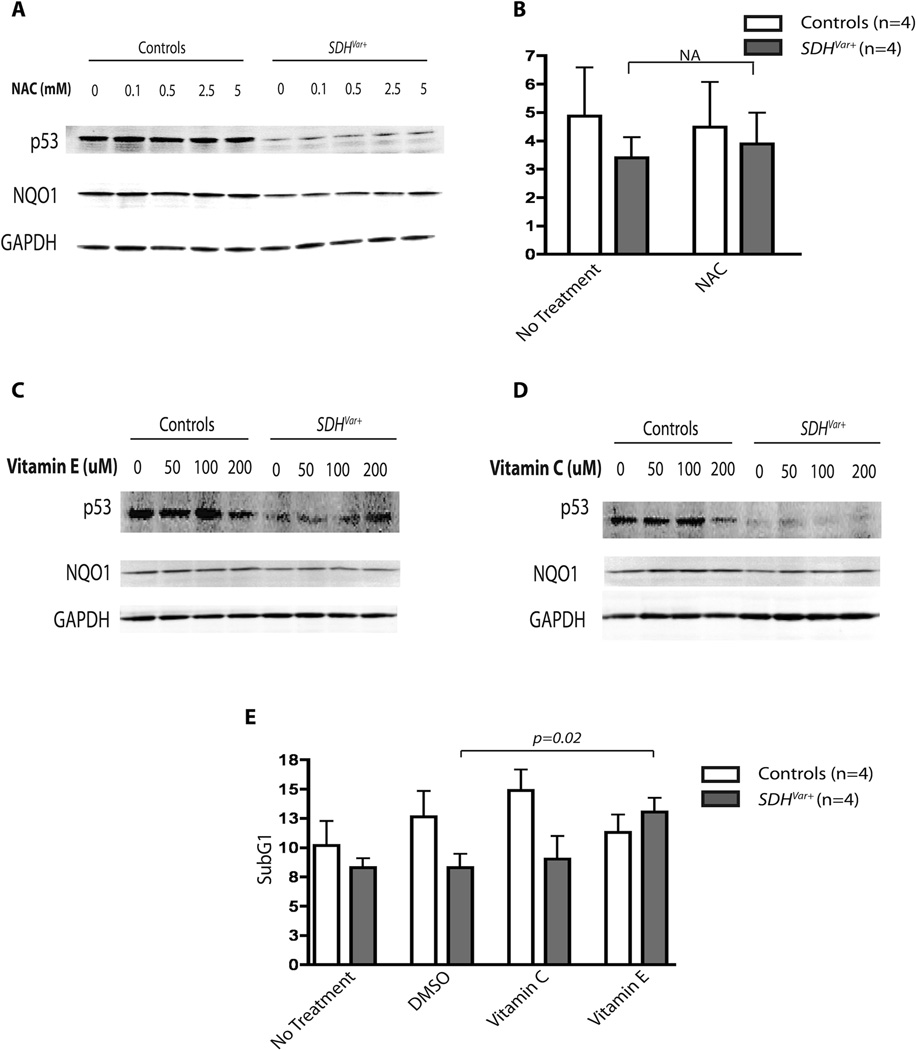
Alpha-tocopherol, but Not Other Anitioxidants, Rescues p53 Loss and Apoptosis Resistance in SDHx Variant Positive Cells
We previously showed the loss of steady state p53 expression in SDHx variants previously (6), due to reduced NQO1 and p53 interaction, most likely caused by disrupted mitochondrial metabolism. To test if ROS relief by antioxidant treatment could reverse the apoptosis-resistance phenotype, we treated both control and SDHx variant positive cells with various antioxidants and measured p53 protein expression and percentage of cells in the sub-G1 phase of the cell cycle. Treating cells with the most direct water-soluble antioxidant N-acetyl-cysteine (NAC) induced dose-dependent increase of NQO1 expression, but not p53 in both control and SDHx variant positive cells (Figure 3A). Cell cycle analysis did not reveal significant changes in cell number in the sub-G1 phase after treatment (Figure 3B). When we tested water-soluble vitamin C (ascorbic acid) and lipid-soluble vitamin E (α-tocopherol) exposures, interestingly, neither of these two vitamins had any effect on NQO1 expression, unlike NAC or DMF. Only lipid-soluble α-tocopherol, but not vitamin C, treatment recovered p53 expression in SDHx variant-positive cells (Figure 3C, D). Correlating with the relative levels of p53 expression, only α-tocopherol completely rescued SDHx variant-positive cells from apoptosis resistance to the level of control cells (Figure 3E).
Figure 3.
Loss of p53 expression and apoptosis resistance rescued by α-tocopherol treatment. Control cell lines or SDHvar+ cell lines were treated with 0, 0.1, 0.5, 2.5 or 5 mM NAC (A) for 48 hours, and 0, 50, 100, 200 µM of vitamin E (C) or vitamin C (D) for 72 hours. Whole cell lysates were then blotted for p53, NQO1, and GAPDH as loading control. B) The comparison of percentage of cells in sub-G1 phases between control (n=4) and SDHvar+ cells (n=4) with 5µM NAC treatment for 48 hours; water mock treatment was used as control. E) The comparison of percentage of cells in sub-G1 phases between control (n=4) and SDHvar+ cells (n=4) with 100µM vitamin C or vitamin E treatment for 72 hours; DMSO mock treatment was used as control for vitamin E, and water mock treatment was used as control for vitamin C.
Alpha-tocopherol Protects SDHx Variant-Positive Cells from Redox Imbalance
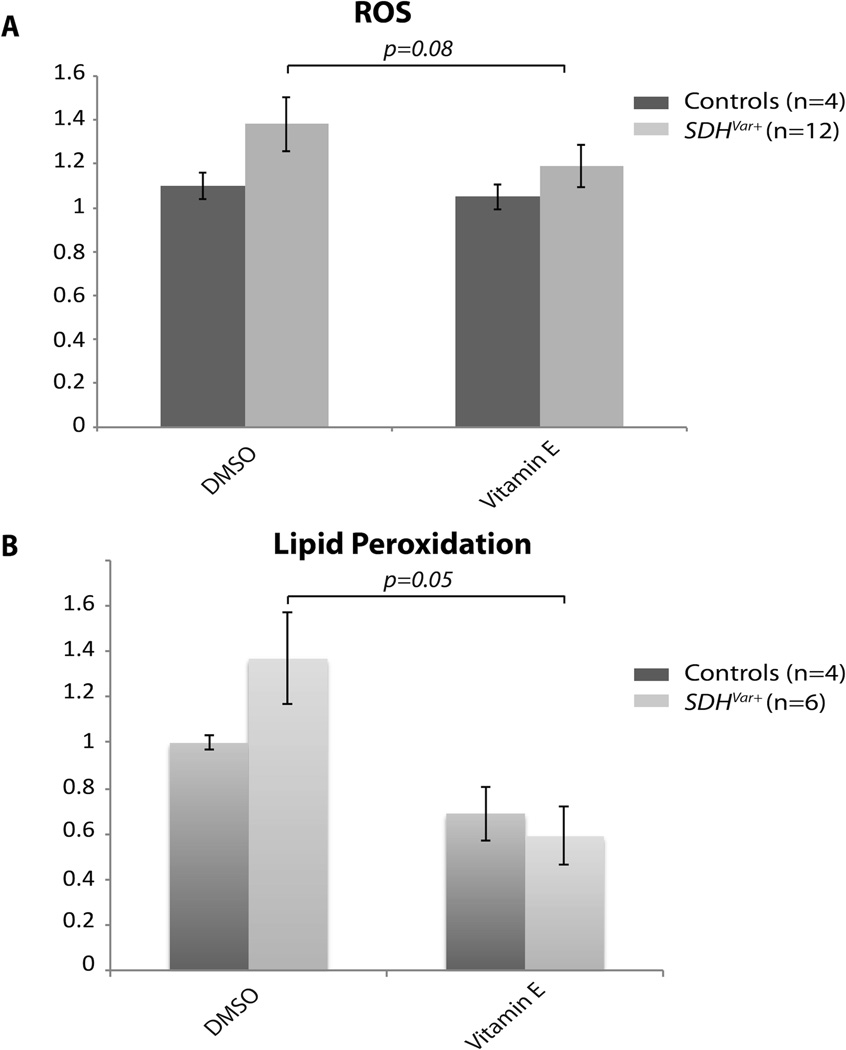
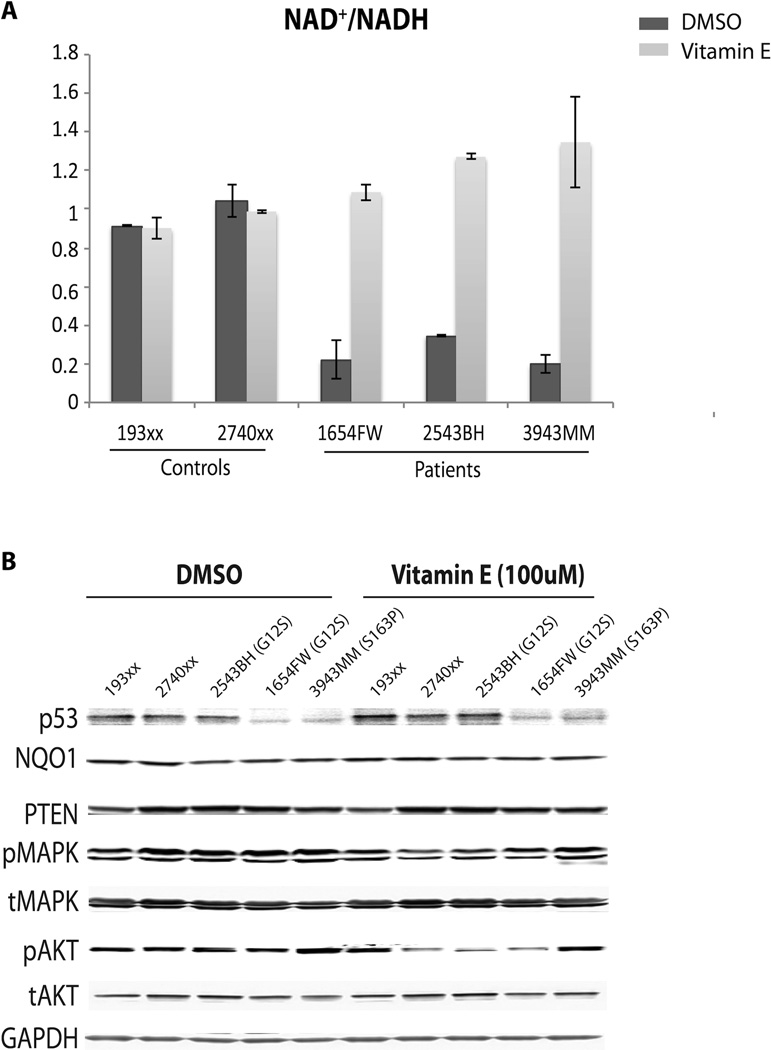
Since α-tocopherol is lipid-soluble, which is the most distinguishable characteristic compared to the other antioxidants we tested, we hypothesized that α-tocopherol may directly protect cells from ROS damage, specifically lipid peroxidation. As shown in Figure 4A, α-tocopherol treatment significantly reduced overall ROS levels in SDHx variant cells. As we expected, lipid peroxidation in CS/CSL patients’ cells with SDHx variation was also reduced, as well as in two positive control groups, namely CS/CSL patients’ cells with PTEN pathogenic mutations and PGL patients’ cells with SDHx pathogenic mutations (Figure 4B). To further investigate weather α-tocopherol is involved in NAD metabolic regulation, we measured NAD/NADH levels after α-tocopherol treatment. As shown in Figure 5A, α-tocopherol treatment had no additional effect on the NAD/NADH ratio in control cells. In contrast, α-tocopherol treatment led to complete recovery of this NAD/NADH reduction back to normal levels. This was true despite DMSO treatment being associated with a significant decrease of the NAD/NADH ratio in SDHx variant positive cells. Western blot showed slight increases of p53 expression in SDHx variant-positive samples compared to controls after treatment, which was accompanied by decreased pAKT and pMAPK (Figure 5B).
Figure 4.
Vitamin E protected SDHvar+ cells from ROS stress. A) Normalized ROS levels in control (n=4) and SDHvar+ cells (n=12) with DMSO or 100µM vitamin E treatment for 72 hours. B) Normalized lipid peroxidation levels in control (n=4) and SDHvar+ CS/CSL (n=6) cells with DMSO or 100uM vitamin E treatment for 72 hours.
Figure 5.
Vitamin E recovered NAD/NADH ratio in SDHvar+ cells. Two control cell lines and 3 SDHvar+ cell lines were treated with wither DMSO or 100µM vitamin E for 72 hours. A) Normalized NAD/NADH ratio in each cell line after the treatment. Data were presented as mean ± SEM of three replicates. B) Whole cell lysate was blotted for p53, NQO1, PTEN, pMAPK, tMAPK, pAKT, tAKT, and GAPDH as loading control.
Discussion
Mitochondrial dysfunction has long been observed in cancer cells, known as the Warburg effect (21). Decades of research implicated the important roles of mitochondrial abnormalities contributing to tumorigenesis, including mitochondrial DNA mutations, oxidative stress, loss of p53, and aberrant expression of metabolic enzymes. Many studies have shown that cancer cells tend to have elevated levels of ROS, compared to normal cells (22). Even though the exact mechanisms of the ROS generation in cancer cells are not clear, we and others proposed mitochondrial dysfunction, such as mitochondrial mutations or the imbalance of redox system, as one of the plausible reasons (5, 6, 11, 23, 24).
Among all the targets ROS could impact, lipid peroxidation is particularly harmful because it facilitates the propagation of free radical reactions. Lipid peroxidation has been reported in numerous human cancers (25–27). We previously presented data that lymphoblastoid cells derived from CS/CSL patient with SDHx variants showed elevated ROS compared to the controls (5, 6, 11). In this study, we further proved that as a consequence of ROS on polyunsaturated fatty acids (PUFA), lipid peroxidation was also elevated accordingly in these SDHx variant-positive cells. ROS is not a prominent cellular phenotype with PTEN mutations alone (Ni and Eng, unpublished data). However, germline mutations within the ATP-binding motifs of PTEN showed enhanced ROS production especially with cellular senesence (12), suggesting that only certain PTEN mutations may have similar cellular phenotypes as those with SDH variant which may explain the observed increased lipid peroxidation in the PTEN mutation positive group. Thus, we believe that the increased oxidative stress is not a universal cellular phenotype in PTEN mutation carriers, as we observed in SDH mutation/variant carrier cells. The fact that the PGL patients with SDHx pathogenic mutations presented with extremely high lipid peroxidation suggests that complex II abnormalities had a more severe impact on membrane redox homeostasis, notably mitochondrial membranes. The most abundant aldehydes identified as products of PUFA decomposition upon lipid peroxidation are 4-HNE and MDA. Other than being used as a measure of lipid peroxidation status, both of them form adducts with DNA and are mutagens (28, 29). As the most direct target of mitochondrial free radicals, the mitochondrial genome is prone to DNA damage, as we observed in SDHx variants cells that accumulated more mitochondrial DNA mutations than controls.
We previously showed the NQO1 inducer dimethyl fumarate (DMF) increased NQO1 protein expression in a dose-dependent manner, without any impact on p53 expression and cell apoptosis resistance (6). These data are consistent with our previous report that the p53 degradation is enhanced by loss of interaction with NQO1 but not loss of absolute NQO1 expression. The most interesting, at first puzzling, observation was that only vitamin E treatment, specifically α-tocopherol, rescued the cell apoptosis resistance phenotype, but others such as vitamin C and NAC had no effect. Given that α-tocopherol is lipid-soluble compared to the other two, which are water-soluble, we suspect that protection of cells from lipid peroxidation could play a critical role. Vitamin E is a well-known antioxidant which functions as a peroxyl radical scavenger, and has been reported to be co-localized to the mitochondrial membrane (30). Indeed, our data showed that α-tocopherol treatment not only inhibited overall ROS generation but also reduced lipid peroxidation in SDHx variant-positive cells, thus protecting cells from oxidative damages. Redox-silent analog of vitamin E such as α-tocopheryl succinate (α-TOS) has been reported to specifically target ubiquinone-binding sites in the SDH complex (31, 32) and causes rapid production of ROS in cancer cells triggering apoptosis. However, in our case, the existing complex II abnormality may silence or even saturate the response to α-TOS, while antioxidant α-tocopherol works by a completely distinct mechanism from α-TOS. We and others reported that altered metabolic intermediates such as FAD and NAD/NADH could inactivate PTEN/PI3K pathways with elevated phosphorylation of AKT and MAPK (6, 33, 34). Recovered NAD/NADH ratio upon α-tocopherol treatment in SDHx variants cells suggest the protection from α-tocopherol may function even at the redox metabolite level. The inhibition of AKT/MAPK activation corroborates that the intervention of α-tocopherol on PTEN/PI3K signaling. Therefore, we think α-tocopherol may have intriguing clinical implications such as utility as a potential preventive adjunct for CS/CSL individuals with SDHx variants. In addition to all the preclinical and epidemiology evidence of its anticancer property, supplemental vitamin E for prostate cancer prevention has also been investigated by several clinical trials, such as the α-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) and Selenium and Vitamin E Cancer Prevention Trial (SELECT). Compared to a 32% prostate cancer risk reduction by α-tocopherol in the ATBC study, SELECT did not show any preventive effectiveness but paradoxically had higher risks of prostate cancer with vitamin E supplementation (35–37). However, the dose (400IU/d) used in the SELECT trial is 8-fold higher than that in ATBC, with the latter the dosage we chose to use in this current study, which speaks to dosage effect. Indeed, treating SDHx variant-positive cells with 800uM (8-fold higher dose) α-tocopherol showed the opposite effect, ie, further reduction of p53 expression and even slight activation of AKT and MAPK pathways (Supplementary Figure 1).
In summary, we report that disruption of complex II due to SDHx variants leads to increased ROS generation, specifically accompanied by lipid peroxidation. The lipid soluble antioxidant α-tocopherol can protect SDHx variant positive cells from oxidative damage, apoptosis resistance, and rebalance redox metabolites NAD/NADH. The protective effect from α-tocopherol most likely is due to its lipid peroxyl radical scavenger property preventing ROS damage and PTEN inactivation. Our study supports the notion that α-tocopherol may be useful as a therapeutic adjunct or preventative agent, especially for individuals with germline SDHx variants/mutations or cancers with somatic mutations in complex II.
Supplementary Material
Translational Relevance.
Whereas the involvement of germline SDHx mutations is well known in hereditary pheochromocytomas/paragangliomas, the role of impaired SDH in other tumors are still unclear. We reported SDHx variants could be important risk factors for breast cancer or thyroid cancer in CS/CSL. Our data suggest that disruption of complex II due to SDHx variants leads to increased ROS generation, specifically accompanied by lipid peroxidation. The lipid-soluble antioxidant α-tocopherol functions as a lipid peroxyl radical scavenger protecting SDHxvar+ cells from oxidative damage, apoptosis resistance, and rebalance redox metabolites NAD/NADH. Our study supports the notion that α-tocopherol may be useful as a therapeutic adjunct or preventative agent, especially for individuals with germline SDHx variants/mutations or cancers with somatic mutations in complex II.
Acknowledgments
These data were presented, in part, at the Annual Meeting of the American Association for Cancer Research, April 2, 2012. CE is the Sondra J. and Stephen R. Hardis Chair of Cancer Genomic Medicine at the Cleveland Clinic, and is an American Cancer Society Clinical Research Professor, generously funded, in part, by the F.M. Kirby Foundation.
Financial Support: This work was funded, in part, by the Breast Cancer Research Foundation, the William Randolf Hearst Foundations and NCI grant P01CA124570-04S1 (all to CE). YN is a recipient of the USARMC Department of Defense Breast Cancer Research Program Predoctoral Fellowship (W81XWH-10-1-0088).
Footnotes
Conflict of Interest: CE is co-PI of a sponsored research agreement from IntegraGen for autism genomic markers, receives royalties from Quest Diagnostics for test to differentiate benign from malignant thyroid neoplasia, is an unpaid member of the External Scientific Advisory Boards of Ecoeos.com and of GenomOncology, and is an unpaid member of the Genomic Medicine Advisory Board of Complete Genomics, Inc. YN declares no conflict of interest.
References
- 1.Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime Cancer Risks in Individuals with Germline PTEN Mutations. Clin Cancer Res. 2012;18:400–407. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan MH, Mester J, Peterson C, Yang Y, Chen JL, Rybicki LA, et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am J Hum Genet. 2011;88:42–56. doi: 10.1016/j.ajhg.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh DJ, Dahia PL, Caron S, Kum JB, Frayling IM, Tomlinson IP, et al. Germline PTEN mutations in Cowden syndrome-like families. J Med Genet. 1998;35:881–885. doi: 10.1136/jmg.35.11.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA. 2010;304:2724–2731. doi: 10.1001/jama.2010.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni Y, He X, Chen J, Moline J, Mester J, Orloff MS, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependant destabilization of p53. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ni Y, He X, Chen J, Moline J, Mester J, Orloff MS, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependant destabilization of p53. Hum Mol Genet. 2012;21:300–310. doi: 10.1093/hmg/ddr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 8.Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–1466. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- 9.Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- 10.Eng C, Kiuru M, Fernandez MJ, Aaltonen LA. A role for mitochondrial enzymes in inherited neoplasia and beyond. Nat Rev Cancer. 2003;3:193–202. doi: 10.1038/nrc1013. [DOI] [PubMed] [Google Scholar]
- 11.Ni Y, Zbuk KM, Sadler T, Patocs A, Lobo G, Edelman E, et al. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am J Hum Genet. 2008;83:261–268. doi: 10.1016/j.ajhg.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X, Ni Y, Wang Y, Romigh T, Eng C. Naturally occurring germline and tumor-associated mutations within the ATP-binding motifs of PTEN lead to oxidative damage of DNA associated with decreased nuclear p53. Hum Mol Genet. 2011;20:80–89. doi: 10.1093/hmg/ddq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saybasili H, Yuksel M, Haklar G, Yalcin AS. Effect of mitochondrial electron transport chain inhibitors on superoxide radical generation in rat hippocampal and striatal slices. Antioxid Redox Signal. 2001;3:1099–1104. doi: 10.1089/152308601317203602. [DOI] [PubMed] [Google Scholar]
- 14.Staniek K, Gille L, Kozlov AV, Nohl H. Mitochondrial superoxide radical formation is controlled by electron bifurcation to the high and low potential pathways. Free Radic Res. 2002;36:381–387. doi: 10.1080/10715760290021225. [DOI] [PubMed] [Google Scholar]
- 15.Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 16.Carew JS, Huang P. Mitochondrial defects in cancer. Mol Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siems WG, Grune T, Esterbauer H. 4-Hydroxynonenal formation during ischemia and reperfusion of rat small intestine. Life Sci. 1995;57:785–789. doi: 10.1016/0024-3205(95)02006-5. [DOI] [PubMed] [Google Scholar]
- 18.Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000;37:828–830. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng LP, Brown JL, Baker KM, Ostrowski MC, Eng C. PTEN blocks insulin-mediated ETS-2 phosphorylation through MAP kinase, independently of the phosphoinositide 3-kinase pathway. Hum Mol Genet. 2002;11:1687–1696. doi: 10.1093/hmg/11.15.1687. [DOI] [PubMed] [Google Scholar]
- 20.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 22.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 23.Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25:4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 24.Pani G, Koch OR, Galeotti T. The p53-p66shc-Manganese Superoxide Dismutase (MnSOD) network: a mitochondrial intrigue to generate reactive oxygen species. Int J Biochem Cell Biol. 2009;41:1002–1005. doi: 10.1016/j.biocel.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Gago-Dominguez M, Castelao JE, Yuan JM, Ross RK, Yu MC. Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma (United States) Cancer Causes Control. 2002;13:287–293. doi: 10.1023/a:1015044518505. [DOI] [PubMed] [Google Scholar]
- 26.Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, et al. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781–1789. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- 27.Sander CS, Hamm F, Elsner P, Thiele JJ. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br J Dermatol. 2003;148:913–922. doi: 10.1046/j.1365-2133.2003.05303.x. [DOI] [PubMed] [Google Scholar]
- 28.Mao H, Schnetz-Boutaud NC, Weisenseel JP, Marnett LJ, Stone MP. Duplex DNA catalyzes the chemical rearrangement of a malondialdehyde deoxyguanosine adduct. Proc Natl Acad Sci U S A. 1999;96:6615–6620. doi: 10.1073/pnas.96.12.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 30.Morley S, Thakur V, Danielpour D, Parker R, Arai H, Atkinson J, et al. Tocopherol transfer protein sensitizes prostate cancer cells to vitamin E. J Biol Chem. 2010;285:35578–35589. doi: 10.1074/jbc.M110.169664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong LF, Swettenham E, Eliasson J, Wang XF, Gold M, Medunic Y, et al. Vitamin E analogues inhibit angiogenesis by selective induction of apoptosis in proliferating endothelial cells: the role of oxidative stress. Cancer Res. 2007;67:11906–11913. doi: 10.1158/0008-5472.CAN-07-3034. [DOI] [PubMed] [Google Scholar]
- 32.Dong LF, Low P, Dyason JC, Wang XF, Prochazka L, Witting PK, et al. Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene. 2008;27:4324–4335. doi: 10.1038/onc.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui ST, Andres AM, Miller AK, Spann NJ, Potter DW, Post NM, et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci U S A. 2008;105:3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Alpha-Tocopherol BCCPSG. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 36.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.